Construction of an Ec-LDP-D5 fusion protein that targets human epidermal growth factor receptor and its anti-pancreatic cancer activity
Abstract
Objective
It is well known that α-defensins are mainly packaged in neutrophil granules (HNP1, HNP2, and HNP3) or secreted by intestinal Paneth cells (human defensin [HD]5 and HD6). HNP1–3 peptides are also secreted, and their accumulation in biological fluids was proposed as a tumor biomarker. It has been reported that α-defensins promote tumor cell growth, but also provoke cell death at higher concentrations. The aim of the present study was to construct a novel fusion protein consisting of oligopeptides specific for human epidermal growth factor receptor (EGFR), lidamycin apoprotein (LDP), and HD5, and investigate its anti-pancreatic cancer activities.
Methods
HD5 was fused to the Ec-LDP protein to obtain the Ec-LDP-D5 fusion protein by DNA recombination. Then, immunofluorescence and enzyme-linked immunosorbent assays were used to investigate the binding activities of Ec-LDP-D5 to EGFR-overexpressed cancer cells. CCK-8 assay was used to measure Ec-LDP-D5 in vitro cytotoxicity, and AnnexinV-FITC/PI staining assay was used to analyze its apoptosis-inducing efficacy.
Results
The Ec-LDP-D5 fusion protein was constructed correctly and expressed in Escherichia coli in insoluble inclusion bodies. The production of Ec-LDP-D5 was 1 mg/L fermentation broth, and the purity of the fusion protein was 85% as analyzed by high-performance liquid chromatography. Ec-LDP-D5 showed strong binding activities for pancreatic cancer cells that highly express EGFR, including PNAC-1 and Aspc-1 cells. The Ec-LDP-D5 fusion protein showed more potent cytotoxicity to cancer cells compared with Ec-LDP. The results from the AnnexinV-FITC/PI staining assay also revealed that Ec-LDP-D5 significantly induced cell apoptosis.
Conclusion
The novel Ec-LDP-D5 fusion protein binds to EGFR specifically, and shows potent cytotoxicity and apoptosis-inducing activities towards pancreatic cancer cells, which suggests that it would be a promising cancer therapeutics candidate.
1 INTRODUCTION
Defensins typically have broad-spectrum antimicrobial activities against Gram-positive and -negative bacteria, viruses, fungi, and protozoa.1, 2 In humans, defensins are classified into one of two families depending on their disulfide bridging pattern, either as α-defensins or β-defensins.1 Defensins play important roles in innate immune defense by neutralizing bacterial toxins or disrupting the cytoplasmic membrane to kill microbial pathogens.1 Additionally, they can also be involved in adaptive immunity by serving as chemoattractants and activators for immune cells.1 Previous studies have shown that human defensins show antibacterial and antiviral activities and tumor cell cytotoxicity.3 Human defensin 5 (HD5) could execute the killing of BxPC3 cancer cells.4 The overexpression of epidermal growth factor receptor (EGFR) has been observed in many human tumors, and the receptor has been evaluated as an important target for the development of new targeted therapeutics.5, 6 Lidamycin (LDM), an antitumor antibiotic, shows extremely potent cytotoxicity to cultured cancer cells in vitro, and high efficacy against various experimental tumors in vivo. LDM contains an active enediyne (AE) responsible for its highly potent cytotoxicity and a non-covalently bound apoprotein (LDP).5, 6 Notably, LDP and AE can be dissociated and reconstituted in vitro.8, 9 Our previous tissue microarray studies have shown that LDP could bind to various human tumors with significant differences compared with the corresponding normal tissues.7 In the present study, we generated the HD5-based fusion protein, Ec-LDP-D5, and its corresponding AE-integrated analog, Ec-LDM-D5, to enhance antitumor efficacy. The study provides evidence that Ec-LDP-D5 is highly effective against pancreatic cancer.
2 METHODS
2.1 Cell culture
The human pancreatic carcinoma PNAC-1 and Aspc-1 cell lines were routinely grown in RPMI-1640 (Gibco, Thermo Fisher Scientific, Grand Island, USA) supplemented with 10% fetal bovine serum (Gibco), penicillin (100 IU/mL) and streptomycin (100 μg/mL).
2.2 Construction of the pET-EC-LDP-D5 expression vector
As shown in Figure 1, the full gene included the Ec-LDP-D5 fusion protein (from 5′ to 3′), the C-loop from EGF (22 amino acids from the EGF COOH terminal region), apoprotein LDP (110 amino acids), and HD5 (32 amino acids). Two (GGGGS)2 linkers were separately inserted into the spaces between the EGF C-loop and LDP coding sequences, and the LDP and HD5 coding sequences. After polymerase chain reaction and DNA cloning, the resultant 558-bp fragment was digested by NdeI/XhoI and inserted into the pET30a expression vector to generate the pET-Ec-ldp-D5 plasmid.
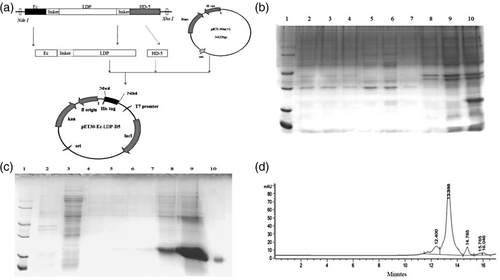
2.3 Expression and purification of the fusion proteins
The pET-Ec-ldp-D5 expression plasmid was transformed into the Escherichia coli BL21 (DE3) star strain (Novagen, Merck Millipore, USA). After overnight culture, the bacteria were grown at 37°C until the OD600 reached 1.0. Gene expression was induced by the addition of Isopropyl β-D-1-Thiogalactopyranoside (IPTG) at 1 mmol/L at 37°C for 8 h. The fusion proteins were purified by affinity chromatography (HisTrap HP; GE Healthcare, USA) through a C-terminal 6 × His-tag according the manufacturer's instructions. The purified proteins were refolded through stepwise dialysis as reported, and the purity of the Ec-LDP-D5 fusion proteins was analyzed by high-performance liquid chromatography on a S3000 column (Tosoh, Tosoh Corporation, North America).
2.4 Binding specificity with cancer cells
The binding activities of Ec-LDP-D5 were detected by enzyme-linked immunosorbent assay, as described previously. Briefly, serial dilutions of refolded Ec-LDP-D5 in phosphate-buffered saline were added to 1% bovine serum albumin phosphate-buffered saline pre-coated plates, incubated, and then washed. Then, the plate was incubated with anti-His-tag HRP-conjugate and washed. Thereafter, 3.3′5.5′-tetramethylbenzidine was used as a chromogen for color development, and absorbance values were measured on a microplate reader (Bio-Rad, Berkeley, California) at 450 nm.
The binding specificity of the fusion protein Ec-LDP-D5 for PNAC-1 and Aspc-1 cells was evaluated with a fluorescence microscope. This assay was carried out as previously reported.
2.5 Cytotoxicity assay with cell counting kit-8
Cells were plated in 96-well plates at a density of 3 × 103 cells per well. After 24 h, the cells were serum-starved for 8 h, and then further incubated with various concentrations of Ec-LDP-TF, Ec-LDP, or HD5 for 24 h at 37°C. After adding 10 μL of CCK-8 solution (Dojindo, Kumamoto, Japan) to 100 μL of culture media, the optical density was measured at 450 nm.
2.6 Flow cytometry analysis
Flow cytometry was used to analyze the influence of the recombinant proteins on PNAC-1 cells. A total of 2.5 × 106 cells cultured on six-well plates were treated with different concentrations of HD5, Ec-LDP-D5, and Ec-LDP for 24 h. The cells were then collected, resuspended in annexin V-FITC and propidium iodide, and subjected to analysis on a BD Calibur flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA).
2.7 Statistical analysis
Statistical differences between the experimental groups were determined with the unpaired t-test using the Prism software (GraphPad, La Jolla, CA, USA). Bar graphs show the means, and error bars represent the SD.
3 RESULTS
3.1 Construction, preparation, and characterization of the HD5-based EGFR-targeting fusion protein
Construction of the gene encoding the defensin-based EGFR-targeting fusion protein Ec-LDP-D5 is shown in Figure 1a. The DNA fragments encoding the Ec (EGF c-coop), LDP (apoprotein of lidamycin), and HD5 (D5) targeting oligopeptides were obtained by genetic engineering. The engineered proteins were successfully expressed in BL21 cells, and secreted into the culture in an insoluble form with a six-histidine tag at the carboxyl-terminus. The purity of the fusion proteins was analyzed by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Figure 1b,c). The purified proteins migrated as a single band at approximately 18.6 kDa, and the purity of both proteins was >85%, as assessed by high-performance liquid chromatography (Figure 1d). The Ec-LDP-D5 final yield of was 1 mg/L.
3.2 Binding affinity of the Ec-LDP-D5 fusion protein to cancer cells
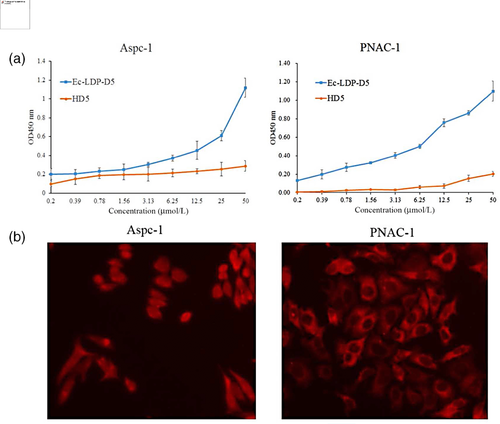
To assess the specificity, Ec-LDP-D5 binding to a panel of proteins was determined by enzyme-linked immunosorbent assay. As expected, Ec-LDP-D5 bound to human EGFR (Figure 2a,b). The binding specificity of Ec-LDP-D5 to cancer cells was also characterized by immunofluorescence. The expression of EGFR on different carcinoma cell lines was analyzed by western blot. Ec-LDP-hBD1 protein was observed to bind specifically to the membrane of EGFR-expressing cancer cells (Figure 2c).
3.3 Ec-LDP-D5 fusion protein cytotoxicity to cancer cells
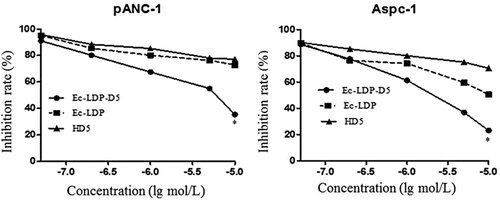
The CCK-8 assay showed that Ec-LDP-D5 markedly inhibited cancer cell proliferation (Figure 3). Ec-LDP-D5 was more potent than equivalent concentrations of free Ec-LDP and HD5, suggesting that HD5 potency in cancer cells was increased by tethering to Ec-LDP. Ec-LDP-D5 was also more potent than Ec-LDP and LDP. Ec-LDP-D5 and Ec-LDP treatments showed inhibition rates of 76.8% and 49.4% (P = 0.038), respectively, at 10–5 mol/L (Figure 3). Ec-LDP-D5 and Ec-LDP treatments of the human pancreatic carcinoma cell line PNAC-1 at 10–5 mol/L also showed inhibition rates of 64.6% and 28.3% (P = 0.042), respectively.
3.4 Induction of apoptosis by the fusion protein Ec-LDP-hBD1
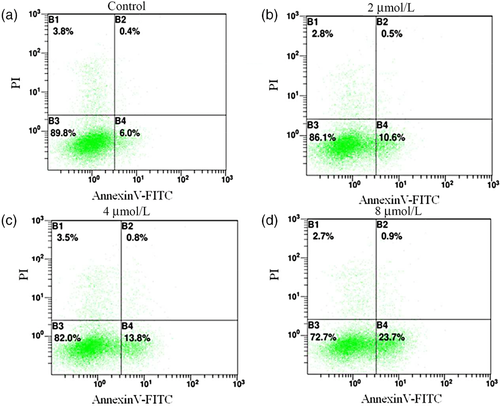
Flow cytometry analysis showed that the ratio of early- and late-stage apoptosis in PNAC-1 cells treated with 2 μmol/L, 4 μmol/L, and 8 μmol/L Ec-LDP-D5 was 11.1 ± 4.23% (P = 0.521), 14.6 ± 2.31% (P = 0.433), and 24.6 ± 0.56% (P = 0.222), respectively, which is far more effective than treatment with the control (P < 0.05; Figure 4).
4 DISCUSSION
Human defensins, which act as important mediators of the innate immune system, are small cationic peptides produced by epithelial cells and neutrophils that occur in two genetically distinct forms, α- and β-defensins.2, 10 Through neutralizing bacterial toxins or disrupting the cytoplasmic membrane, defensins can kill microbial pathogens.11 Additionally, they might also be involved in adaptive immunity by serving as chemoattractants and activators of immune cells.12, 13 Previous studies have shown that human defensins display antibacterial and antiviral activities, and tumor cell cytotoxicity. HD5 is predominantly expressed in intestinal Paneth cells.14 Like other defensins, HD5 is stored in a precursor form that does not have any antimicrobial activity, and processing of the precursor to a mature form occurs when it is secreted.13, 15 The cytotoxic effects of defensins on cancer cells, including both solid tumors and leukemias, have also stimulated interest in these peptides as possible leads for new cytotoxic drugs.16 HD5 could kill tumor cells by a unique mechanism that involves membrane lysis and DNA damage. The membrane lysis mechanisms by host defense peptides have the following two issues: (1) defensins that are not cell-selective bind to all types of membranes and form transmembrane pores through the “barrel-stave” mechanism; and (2) defensins might also be involved in adaptive immunity by serving as chemoattractants and activators of immune cells. In the past few years, human defensins have been successfully expressed in heterologous host cells, including E. coli, which is the most commonly used host cell because of its fast growth rate, easy purification procedure, high yield of target proteins, and low cost.
The recombinant pET30a(+)/Ec-LDP-D5 plasmid, which was verified by enzyme-digestion and sequencing, was transformed into E. coli BL21 (DE3) cells.15, 17 The fusion protein mainly existed as insoluble inclusion bodies. This fusion protein was easily expressed in E. coli, and was insoluble in inclusion bodies; thus, we can obtain many proteins from the purification. After purification by IMAC under denatured conditions, the Ec-LDP-D5 fusion protein was refolded by stepwise dialysis, meaning that approximately 1 mg of soluble functional Ec-LDP-D5 could be obtained from 1 L of fermentation broth; the protein had a purity >85%. Initially, the protein could not be correctly refolded, and showed no activity. To resolve this problem, we first changed the host bacteria and vector conditions to increase the soluble expression of the fusion protein; however, the Ec-LDP-D5 protein was not expressed in an inclusion body. We next changed to another bacterial host, but could still not obtain soluble Ec-LDP-D5 protein expression. In the future, we will try a vector or yeast expression system to obtain soluble Ec-LDP-D5 protein.
During the past decade, biodegradable polymers or oligopeptides that recognize specific cell-surface receptors have been shown to increase drug specificity while lowering systemic drug toxicity.6, 18 Thus, for the construction of the defensin-based fusion protein, we used a ligand-based oligopeptide (Ec) for EGFR binding, an apoprotein LDP as a protein scaffold and specific carrier for assembly with AE, and HD5 as the “effector” agent. This genetically engineered EGFR-targeting fusion protein, Ec-LDP-TF, efficiently bound to carcinoma PNAC-1 and Aspc-1 cells (Figure 2). Furthermore, the proliferation capacities of PNAC-1 and Aspc-1 cells was markedly inhibited by Ec-LDP-D5, whereas few effects were observed by treatment with the Ec-LDP protein, showing that the defensin HD5 molecule played an active role in the inhibition (Figure 3). Furthermore, AnnexinV-FITC/PI staining combined with flow cytometry detected PNAC-1 cell apoptosis at 24 h (Figure 4). This induction of apoptosis was in a concentration-dependent manner.
In summary, the newly designed and genetically engineered fusion protein, Ec-LDP-D5, shows both selectivity and enhanced cytotoxic potency against relevant pancreatic carcinoma cells. The novel format of HD5 might play an active role in pancreatic carcinoma targeting therapy.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.