Chinese clinical practice guidelines for the prevention and treatment of radiation-induced esophagitis
Abstract
Acute radiation-induced esophagitis is a common complication of radiotherapy for esophageal, lung, and other malignancies. Therefore, understanding the diagnosis, grading, risk factors, prevention, and treatment of radiation-induced esophagitis is essential. Currently, there are few consensuses and guidelines on radiation-induced esophagitis worldwide, mainly the American College of Gastroenterology (ACG) clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) and the Digestive Endoscopy Society of Chinese Medical Association's “Guidelines for the Diagnosis and Treatment of Reflux Esophagitis.” However, no consensus or guidelines specifically addressing radiation-induced esophagitis have been established. Efforts have been made to organize experts to draft Chinese consensus or guidelines, but the recommendations in these guidelines also vary owing to differences in expert backgrounds. The clinical practice guidelines presented herein were developed for the first time with the joint participation of Chinese radiotherapy experts. Drugs and methods with clinical significance were selected by reviewing and summarizing the prevention and treatment of radiation-induced esophagitis and combining them with China's national conditions. After multiple rounds of discussion and revision, clinical practice guidelines were established in line with the needs of Chinese clinicians, providing useful clinical guidance for the prevention and treatment of radiation-induced esophagitis.
1 OVERVIEW
1.1 Definition
Radiation-induced esophagitis (RE) is a nonspecific inflammatory response caused by radiation damage to the esophagus, and is often observed in patients receiving radiotherapy for thoracic and mediastinal malignant tumors. Generally, esophagitis occurring within 90 days of initiating radiotherapy is defined as acute radiation-induced esophagitis (ARE), whereas reactions occurring after 90 days are called late radiation-induced esophagitis (LRE).1 ARE usually develops rapidly with noticeable clinical manifestations and is easy to detect. Most patients recover from tissue damage given proactive prevention or treatment. In contrast, LRE often leads to irreversible tissue fibrosis because of delayed treatment, affecting the structure or function of tissues and organs to varying degrees.
1.2 Pathogenesis
During or after radiotherapy for esophageal cancer, lung cancer, mediastinal malignant tumors, lymphoma, and other malignancies, normal esophageal epithelial cells can be damaged and the esophageal mucosa can become congested and edematous. In addition, radiotherapy can suppress bone marrow, reduce immunity, and increase the risk of infectious esophagitis. A radiotherapy dose of 30 Gy can damage the esophageal nerves and muscles, leading to weakened esophageal peristalsis.
Because radiation causes a large number of water molecules in the esophageal tissue to disintegrate into oxygen-free radicals, the most likely time for ARE to occur is approximately two to three weeks following the beginning of radiotherapy treatment. Excessive amounts of oxygen free radicals can damage cell membranes by attacking fatty acids, proteins, and nucleic acids. This can result in decreased membrane fluidity, increased permeability, mitochondrial swelling, lysosomal damage, and the release of lysosomal enzymes, all of which can contribute to tissue damage and inflammatory reactions.
In LRE, delayed changes in blood vessels and connective tissues occur, resulting in esophageal tissue fibrosis, local scar formation, and esophageal mucosal atrophy. Esophageal nerve damage can also occur, leading to motility disorders, esophageal wall stiffness, and irreversible changes.
2 CLINICAL MANIFESTATIONS AND GRADING CRITERIA
2.1 Clinical manifestations
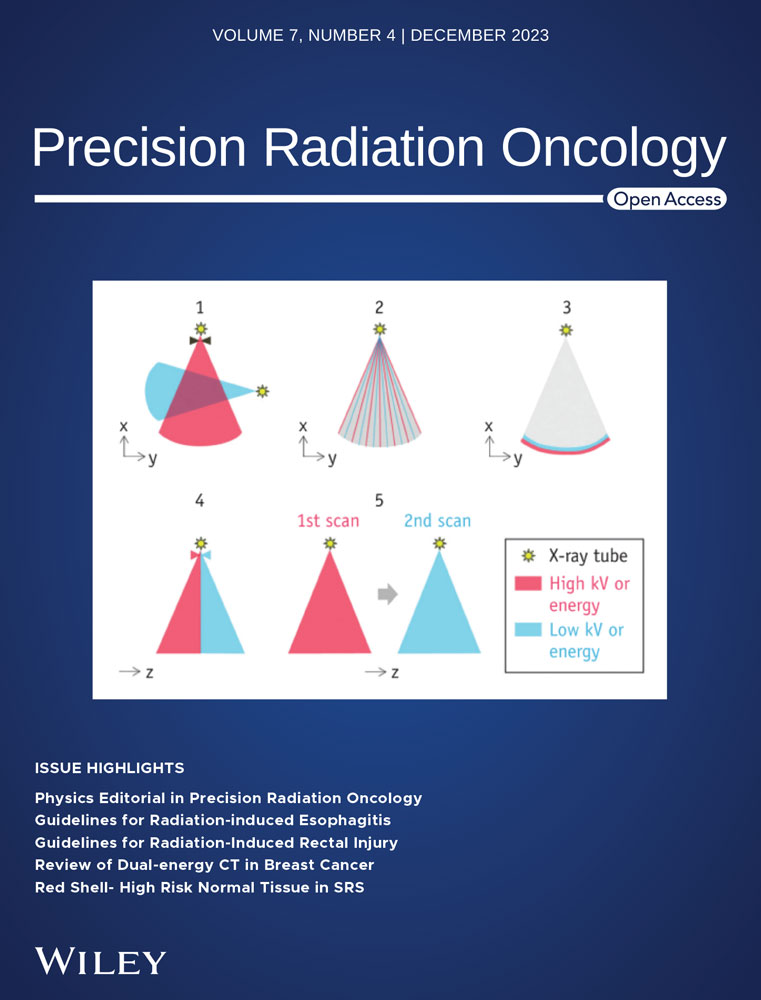
The initial clinical manifestations of ARE include a foreign body sensation when swallowing, followed by pain when eating or swallowing saliva and gradual progression to persistent retrosternal pain unrelated to swallowing. Severe cases may present with chest pain, choking, difficulty in breathing, nausea, and vomiting, warranting vigilance for esophageal perforation, esophagotracheal fistula, and esophagoaortic fistula. Early fistula signs include severe chest and back pain, fever, and an elevated white blood cell count, with a barium swallow test revealing signs of perforation (Figure 1).
Patients with LRE often exhibit fibrosis, muscle layer, or nerve damage, leading to esophageal strictures or motility changes and subsequent difficulty in swallowing or pain caused by chronic ulcers. This is more common in patients 3 months after the end of radiotherapy, although some patients may experience symptoms 1 year after treatment completion.2
2.2 Grading criteria
Different evaluation scales can be used to grade the severity of RE. A perfect RE assessment tool would have the following qualities: objective, sensitive, validated, reliable, and relevant in all clinical settings. The most frequently used scales are the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, published by the National Cancer Institute (NCI) in 2017, the toxicity criteria published by the Radiation Therapy Oncology Group (RTOG) in 1995, and the toxicity criteria published by the European Organization for Research and Treatment of Cancer (EORTC).3 There is no clear superiority among the scales, and the assessment scale is selected based on the actual situation (Table 1).
| Assessment Criteria | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| NCI CTCAE V5.0 | Asymptomatic | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; altered eating/swallowing; oral supplements indicated | Severely altered eating/swallowing; tube feeding, TPN, or hospitalization indicated | Life-threatening consequences; urgent operative intervention indicated | Death |
|
RTOG Acute Radiation Injury (Pharynx & esophagus) |
No change over baseline |
Mild dysphagia or odynophagia may require topical anesthetic or non-narcotic analgesics/may require soft diet |
Moderate dysphagia or odynophagia may require narcotic analgesics/may require puree or liquid diet |
Severe dysphagia or odynophagia with dehydration or weight loss >15% from pre-treatment baseline) requiring N-G feeding tube, iv. fluids or hyperalimentation | Complete obstruction, ulceration, perforation, fistula | / |
|
RTOG Chronic Radiation Injury (Pharynx & esophagus) |
None |
Mild fibrosis; slight difficulty in swallowing solids; no pain on Swallowing |
Unable to take solid food normally; swallowing semisolid food; dilatation may be indicated |
Severe fibrosis; able to swallow only liquids; may have pain on swallowing; dilatation required |
Necrosis/Perforation Fistula |
- Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; NCI, National Cancer Institute; RTOG, Radiation Therapy Oncology Group.
3 EXAMINATIONS
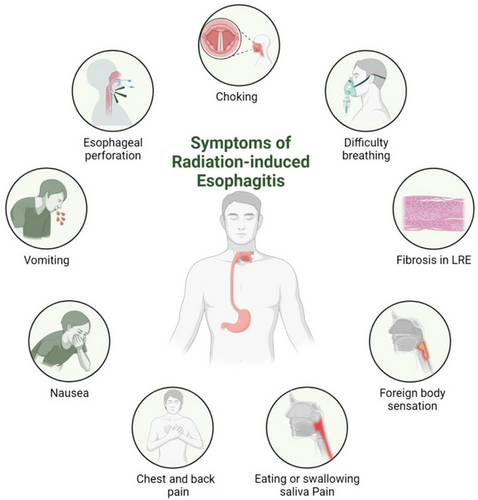
3.1 Laboratory tests
Routine blood tests often show normal or decreased white blood cell counts (see Figure 2).
3.2 Esophagogram
The most common esophagographic findings are abnormal esophageal motility and bird-beak-like strictures. In early symptomatic cases, weakened peristaltic waves and esophageal ulcers can be observed, and esophageal strictures can be observed in later stages.
3.3 Esophagoscopy and pathological biopsy
3.3.1 Esophagoscopy
Esophagoscopy helps confirm the diagnosis and exclude other causes. Endoscopic examination should be considered for patients with symptoms that progress after symptomatic treatment to confirm the diagnosis and exclude other reasons, such as infectious esophagitis. Esophagoscopy allows visualization of esophagitis at different stages, and the extent of esophagitis can be assessed using esophageal tissue samples for biopsy.
3.3.2 Pathological biopsy
In the acute phase, inflammatory cell infiltration is observed in the submucosal and muscular layers of the esophagus. The following pathological stages of RE can be observed through pathological examination: (1) Necrotic phase: After the esophagus is exposed to radiation, basal cells stop dividing, degeneration and necrosis occur, submucosal edema develops, blood vessels dilate, and the epithelium detaches. At this stage, the esophageal mucosa presents with congestion, edema, erosion, and ulcers. (2) Atrophic phase: Several weeks after radiotherapy, necrotic tissue sloughs off, the esophageal wall becomes thinner, and the mucosa becomes smoother. Some patients exhibit significant esophageal smooth muscle abnormalities. At this stage, esophageal bleeding and perforation can easily occur. (3) Regenerative phase: Several months after radiotherapy, the remaining cells in the basal layer begin to regenerate, gradually extend and migrate upward, and the surface is covered with newly formed epithelial cells. At this stage, fibrosis gradually develops owing to radiation-induced vascular and tissue damage. As the esophagus becomes thinner and narrower, esophageal motility disorders worsen.
4 DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
4.1 Diagnosis
Diagnosis can be made based on the history of radiotherapy, clinical manifestations, auxiliary examinations, and the exclusion of other factors and diseases through comprehensive analysis.
4.2 Differential diagnosis
4.2.1 Suppurative esophagitis
The ailment is most frequently caused by mechanical damage from foreign materials. Bacteria multiply within the esophageal wall, resulting in local inflammation, different degrees of tissue necrosis, and pus development. It can also manifest as extensive cellulitis.
4.2.2 Esophageal tuberculosis
Esophageal tuberculosis typically exhibit antecedent symptoms of TB in other organs, particularly pulmonary tuberculosis, prior to its development. It is difficult to detect esophageal cancer in a timely manner because the signs of the disease are sometimes mistaken for or concealed by the symptoms of diseases affecting other organs. In the early infiltrative progression stage of tuberculosis, symptoms such as weariness, low-grade fever, and an increased erythrocyte sedimentation rate may be present. This information was based on the pathological process of tuberculosis. However, there may be circumstances where there are no noticeable symptoms. Consequently, patients experience discomfort while swallowing as well as progressive dysphagia. It is frequently accompanied by ongoing pain in the pharyngeal and retrosternal regions, which worsens when the patient is trying to swallow. Patients with ulcerative lesions frequently experience pain in the lower pharynx. The presence of dysphagia suggests the development of an esophageal stricture due to fibrosis produced by the lesion.
4.2.3 Fungal esophagitis
Clinical symptoms of fungal esophagitis are sometimes unusual, and some individuals may not exhibit any clinical indicators. Pain during swallowing, also known as dysphagia, discomfort in the upper abdomen, retrosternal pain, and a burning sensation are common symptoms. Patients who do not receive treatment are at risk of developing complications, such as esophageal epithelial shedding, perforation, and widespread candidiasis. Checking for disseminated acute candidiasis in the skin, liver, spleen, and lungs of patients who have continuous high fever and diminished granulocytes is necessary. This condition can affect any organ of the body.
4.2.4 Viral esophagitis
Herpes simplex viral infection of the esophagus frequently develops in conjunction with herpes infections of the nose and mouth. The most prominent symptom is discomfort during swallowing, which is frequently severe when food is consumed. The esophagus is a long tube through which food travels. Some individuals can experience difficulty in swallowing as their primary symptom, while others, particularly those with less severe infections, may not exhibit any symptoms.
5 RISK FACTORS
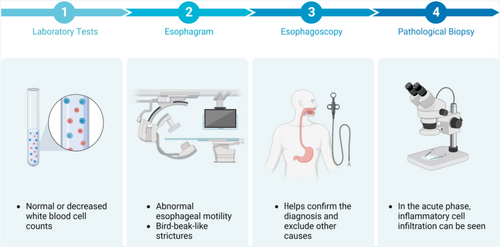
The risk factors for RE are not fully understood; however, previous data suggest that they are closely related to tumor treatment. Risk factors can be divided into treatment- and patient-related (Figure 3).
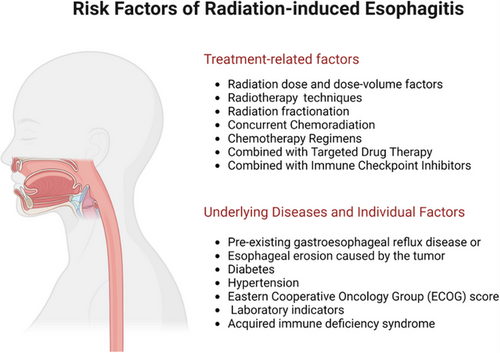
5.1 Treatment-related factors
5.1.1 Radiation dose and dose-volume factors
RE is a common adverse reaction and a dose-limiting toxicity associated with thoracic tumor radiotherapy. RE usually occurs after conventional fractionated irradiation at 20–30 Gy, and symptoms generally appear after a minimum dose of 15 Gy. A radiotherapy dose of 30 Gy can damage the esophageal nerves and muscles, leading to weakened esophageal peristalsis. As the irradiation dose increases, esophageal damage worsens. Currently, the prescribed radiotherapy doses for esophageal and lung cancers are between 50–70 Gy, and most patients experience varying degrees of RE. Numerous studies have shown that many parameters, including the length of the esophagus exposed to radiation, irradiation dose, and dose-volume factors, are closely related to the occurrence of RE.
The RTOG 0617 study showed that during radiotherapy for locally advanced non-small cell lung cancer (NSCLC), the incidence of Grade≥3 esophagitis in the 74 Gy group was three times that of the 60 Gy group (21% vs. 7%).4, 5 Gu et al.5 studied 106 NSCLC radiotherapy patients and showed that tumor radiosensitivity, target area esophageal length, average esophageal irradiation dose, and esophageal V50 were independent predictive factors for RE occurrence, with V50 being the most important factor. Maguire et al.6 also considered esophageal V50 as a valuable predictive factor for the development of ARE in patients with lung cancer receiving thoracic irradiation. Caglar et al.7 reported that in patients with NSCLC receiving concurrent chemoradiotherapy, the average dose and V45-V60 of the esophagus in the target area were related to grade 2 RE. Rosenman et al.8 found that the incidence of > grade 3 ARE was associated with the length of the esophagus treated with 40 or 60 Gy. Palma et al.9 conducted a meta-analysis of the predictive factors of esophagitis after NSCLC chemoradiotherapy and reported that only V60 was the best predictor of grade 2 or 3 RE. Rose et al.10 conducted a comprehensive analysis of 18 studies on the factors influencing RE occurrence and concluded that 5 dosimetric parameters (average esophageal irradiation dose, V20, V30, V40, and V45) might have predictive value for the occurrence of various forms of RE in patients receiving thoracic radiotherapy. The above studies suggest that medium- and high-dose-volume factors are related to the occurrence of ARE. However, multiple studies have shown that low and medium doses are associated with the occurrence of SCLC-related AREs. According to Watkins JM,11 for SCLC patients receiving accelerated hyperfractionated radiotherapy combined with chemotherapy, the dosimetric factor most closely related to grade 3 ARE is V15 (the incidence of grade 3 ARE with V15<60% and ≥ 60% is 15% and 64%, respectively). Grant et al.12 found that the proportion of the esophageal volume receiving medium and low doses (V5-40) had a predictive effect on the occurrence of ARE.
These indicators can guide clinicians in establishing radiotherapy target areas and evaluating treatment plans. However, more accurate, dynamic, and individualized evaluation indicators require further in-depth exploration through high-quality research, particularly regarding different drug combinations and radiotherapy modalities.
5.1.2 Radiotherapy techniques
In locally advanced cases of non-small cell lung cancer (NSCLC) and esophageal cancer, concomitant chemoradiation therapy with intensity-modulated radiotherapy (IMRT) has been shown to be more effective than three-dimensional conformal radiotherapy (3DCRT). In contrast to 3DCRT, however, studies have indicated that IMRT does not lower the risk of disease recurrence. For instance, Gomez et al.13 employed the Lyman (Kutcher–) model to examine the prevalence of RE in patients with NSCLC who underwent 3DCRT, IMRT, and proton radiation therapy (PRT). These patients were treated with radiation in one of three ways: proton, electron, or ion. They discovered that patients who underwent IMRT had a higher probability of developing grade 3 esophagitis than those who received 3DCRT or PRT. The corresponding risks were 28%, 8%, and 6%, respectively.
Possible reasons for this may include the following. (1) The inherent dose distribution characteristics of IMRT make patients more prone to higher-grade esophageal toxicity. IMRT involves the use of multiple fields to achieve uniformity, exposing most of the esophagus to lower doses of radiation, whereas 3DCRT and PBT can partially spare the esophagus. (2) The spatial distribution of esophageal doses in the IMRT group may be different, mainly referring to the radiation areas in the anterior/posterior and/or superior/inferior positions of the cross-section, indicating that the risk of esophagitis in the irradiated esophageal anatomical areas may differ among the three treatment groups.
Compared to conventional fractionated radiotherapy (CFRT), stereotactic body radiation therapy (SBRT) has higher single doses, fewer irradiations, less impact on the surrounding normal tissues, and a strong immune activation capacity. It has demonstrated robust antitumor effects in the treatment of patients with early stage NSCLC and late-stage oligometastasis. According to the findings of a meta-analysis conducted by Li et al.14 of patients with stage I NSCLC treated with either CRT or SBRT, the incidence of RE was considerably lower in the SBRT group than in the CRT group.
However, it is important to keep in mind that since the introduction of SBRT for the treatment of lung tumors, the incidence of toxicity in tumors less than 2 cm away from the proximal bronchial tree has been significantly higher than that in peripheral tumors, even when utilizing the same dose and fractionation.15, 16 These include esophagus-related adverse reactions. Esophageal toxicity is a particular concern when using SBRT to treat central lesions. The lung parenchyma is a parallel tissue organ, while the esophagus is a serial tissue organ, and even small-volume esophageal radiation injuries can lead to severe consequences.17, 18
5.1.3 Radiation fractionation
Hyperfractionation involves administering smaller doses per fraction than conventional fractionation, usually at a rate of ≥2 times per day. The aim is to reduce late toxicity reactions; however, acute toxicity reactions may increase significantly. A single dose is reduced when hyperfractionated or accelerated hyperfractionated radiation is used for early responsive tissues (including most tumors). To maintain tumor control rates, the total radiation dose often needs to be increased or the total treatment time shortened; this is known as accelerated hyperfractionation. Ideally, hyperfractionated or accelerated hyperfractionated radiation can improve tumor control rates; however, acute toxicity reactions are significantly increased, and late toxicity reactions are similar.
Gomez et al.13 and Bar-Ad et al.19 found that an increased risk of grade 2 RE may be related to higher single-fraction radiation doses. Both studies suggest that there may be a correlation between fractionation doses and RE severity. Most current research in this area reflects the situation of patients undergoing conventional fractionation chest radiotherapy, and the extent to which these data can be applied to hyperfractionated concurrent chemoradiation.
The RTOG 9410 trial reported that 45% of patients with NSCLC undergoing concurrent hyperfractionated chemoradiation developed grade 3 acute radiation esophagitis, revealing a statistically significant association between severe acute esophagitis and concurrent hyperfractionated chemoradiation.20 Ball et al.21 reported that the incidence of ARE increased from 21% with conventional radiotherapy to 42% with hyperfractionated concurrent chemoradiation. Manapov et al.22 reported that in NSCLC treated with hyperfractionated concurrent chemoradiation, the absolute esophageal volume receiving >42.8 Gy (within a 95% equivalent dose) was a dose-volume predictor for the severity of ARE, and the increase in irradiated volume was closely related to the severity of esophagitis. These three studies indicate that the risk of developing RE is higher with hyperfractionated concurrent chemoradiation than with conventional chest RT.
Oral et al.23 reported that in a group of accelerated hyperfractionated radiotherapy cases, 86% (65 patients) developed grade 1–3 ARE, with three unable to complete the radiotherapy plan due to grade 3 injuries, a 42% higher rate than that in the conventional group. The incidence of ARE within the irradiation field (small field) of the esophagus was 100%, which was significantly higher than that in the esophageal regions outside the radiation field.
5.1.4 Concurrent chemoradiation
Several studies have demonstrated that concurrent chemoradiation significantly increases the incidence of Grade≥3 RE (18% to 37%).24-29 Werner-Wasik et al.29 reported that concurrent chemoradiation significantly reduced esophageal tolerance to radiotherapy compared with radiotherapy alone, increased the incidence of ARE, exacerbated injury severity, and prolonged the duration of ARE, particularly in cases of hyperfractionated radiotherapy with concurrent chemotherapy. According to the findings of the RTOG 9204 trial,26 the incidence of RE was considerably higher in the group that received contemporaneous chemoradiotherapy than in the group that received neoadjuvant chemotherapy. In some instances, it has been suggested that chemotherapy administered in cycles does not significantly increase the risk of RE. In contrast, the risk of RE with concurrent chemoradiation is nearly five times higher than that with sequential chemoradiation (18% vs. 4%).30
5.1.5 Chemotherapy regimens
Although gemcitabine, paclitaxel, and vinorelbine have radiosensitizing effects, various clinical trials have shown that chemotherapeutic regimens affect ARE differently. In concurrent chemoradiation regimens for NSCLC, gemcitabine and paclitaxel (or docetaxel) have shown higher ARE rates, whereas vinorelbine has a lower ARE.31-33
5.1.6 Radiotherapy combined with targeted drug therapy
Studies have shown that combining cetuximab with chemoradiation for esophageal cancer increases skin toxicity and hypersensitivity reactions, but does not increase esophagitis or other radiation toxicities.34
5.1.7 Radiotherapy combined with immune checkpoint inhibitors
To date, there have been limited reports on RE's incidence of RE and the factors influencing the combination of radiotherapy and immune checkpoint inhibitors. A case report showed that the incidence of Grade≥2 ARE in thoracic radiotherapy combined with immunotherapy was as expected and acceptable, with no statistically significant difference in the incidence of ARE between concurrent and sequential immunotherapy.35
Zhang et al.36 found that first-line concurrent chemoradiation combined with camrelizumab for esophageal squamous cell carcinoma resulted in a controllable safety profile, with a >3 grade RE incidence rate of 20%. Diamond et al.37 found that consolidative thoracic radiotherapy followed by first-line chemotherapy combined with immunotherapy for extensive-stage small resulted in a grade 2 esophagitis incidence rate of 5%, which is safe and reliable. There are no reports on whether immunotherapy combined with radiotherapy increases the incidence of RE compared with radiotherapy alone.
5.2 Underlying diseases and individual factors
Even with similar irradiation volumes and doses, the risk of RE varies, suggesting that RE may be related to underlying diseases, individual factors, and dosimetric factors. A retrospective study of 91 patients receiving high-dose (64.2-85.6 Gy) conformal radiotherapy showed that the risk of RE increased in patients with pre-existing gastroesophageal reflux disease or esophageal erosion caused by the tumor.6 Other studies have shown that diabetes and hypertension can induce vascular inflammatory responses by inducing the production of inflammatory factors in the body, leading to immune dysfunction and an increased risk of esophagitis. Diabetes and hypertension can also reduce the local tissue repair capacity and delay the tissue healing process after injury, exacerbating the inflammatory response.38, 39
Additionally, the Eastern Cooperative Oncology Group (ECOG) score before radiotherapy may also be a factor affecting the occurrence of RE, with patients with an ECOG score of ≥1 having a higher risk of ≥3 grade RE than those with a score of 0.40 Laboratory indicators may also serve as risk assessment factors for RE, with the lowest neutrophil count during radiotherapy closely related to the occurrence and severity of RE.41, 42 Patients with higher pretreatment platelet counts and lower hemoglobin levels have a higher incidence of RE.43 Case reports have shown that patients with acquired immune deficiency syndrome can develop severe RE.44, 45 Furthermore, the baseline nutritional status of patients is closely related to the occurrence of ≥2 grade RE in esophageal cancer patients receiving radiotherapy, with 41% of severely malnourished patients experiencing ≥2 grade RE.46
[Expert Recommendation 1] Assess the risk of RE occurrence in patients before radiotherapy from treatment-related and patient factors. Treatment-related risk factors include radiotherapy techniques, fractionation patterns, dose-volume factors, and whether the treatment is combined with chemotherapy, targeted therapy, or immune checkpoint inhibitors (Level I evidence, Grade A recommendation). Individual risk factors mainly include the presence of esophageal erosion, hypertension, diabetes, patient performance status, nutritional status, levels of white blood cells, red blood cells, or platelets, or the presence of immune deficiency diseases (Level III-IV evidence, Grade C recommendation).
6 TREATMENT
RE treatment should be managed by a team with relevant professional experience, preferably attending physicians, nurses, nutritionists, and pharmacists. Good patient communication and education facilitate smooth treatment. Regular assessment and monitoring of patients should be performed during treatment.
6.1 Treatment of ARE
6.1.1 General management
Smoking, alcohol consumption, coffee consumption, consumption of spicy and irritating foods or extremely cold or hot foods may cause esophageal mucosal inflammation. A bland, soft, or semi-liquid diet can reduce irritation of the esophageal mucosa. Patients with RE should avoid spicy, coarse, icy, hot, or hard foods and consume a high-calorie, high-quality protein, high-vitamin, low-fat bland, soft, or semi-liquid diet. Oral nutritional supplements (ONS) are recommended for eligible patients. After eating, a sitting or semi-recumbent position for 1–2 hours to minimize reflux esophagitis due to the body position.
6.1.2 Nutritional support treatment
Patients with severe RE may experience painful swallowing, difficulty eating, and other symptoms, be only able to consume semi-liquids or liquid foods, or even have trouble with water intake. Nutritional support can be provided along with dietary guidance. Specific methods can be found in the “ Expert consensus of enteral nutrition for esophageal cancer patients with radiotherapy ”.47
6.1.3 Pharmacotherapy
Drug treatment of RE mainly focuses on symptomatic therapies, such as pain relief, anti-inflammation, esophageal mucosa protection, and promotion of mucosal healing. These treatments can significantly improve symptoms and enhance the quality of life of patients. Treatment drugs include analgesics, topical anesthetics, mucosal surface protectants, antibiotics, vitamins, hormones, and acid-suppressing drugs (Figure 4).
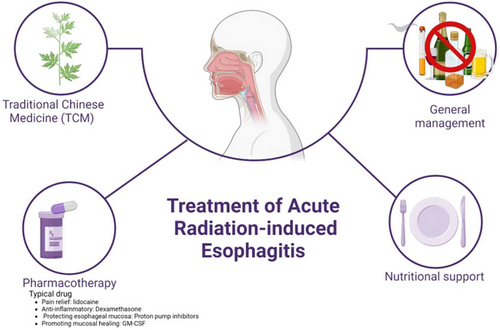
- (1)
Pain relief: For pain during swallowing, topical anesthetics such as lidocaine, sodium bicarbonate, gentamicin, or homemade oral solutions containing vitamin B12 can be used along with oral analgesic medications. Lidocaine is an anti-inflammatory and antibacterial anesthetic that alleviates local pain. Gentamicin, an aminoglycoside antibiotic, suppresses inflammation and reduces edema. Vitamin B12 promotes the growth and repair of the gastrointestinal mucosal epithelial and vascular endothelial cells, thereby accelerating wound healing.
- (2)
Anti-inflammatory: Dexamethasone is a glucocorticoid that, in the early stages, can reduce congestion at the site of inflammation, inhibit the production and release of inflammatory mediators, and promote the repair of damaged tissues.
- (3)
Protecting the esophageal mucosa: Mucosal surface protectants can form a colloidal thin film over an ulcer or inflammation site, shielding it from gastric acid attacks. They also promote ulcer healing, adsorb epidermal growth factor in saliva, stimulate the synthesis of prostaglandin E, and stimulate the secretion of carbonate from surface epithelial cells to exert protective effects such as those of montmorillonite powder, recombinant human epidermal growth factor, and sucralfate suspension. Proton pump inhibitors or H2 receptor antagonists are acid-suppressing drugs that inhibit gastric acid secretion, prevent acid reflux into the esophagus, and reduce damage to the esophageal mucosa. Drug treatment can reduce the severity of RE and the incidence of LRE; however, clinical attention should be paid to adverse drug reactions. Moreover, as their long-term effects are unsatisfactory, they are rarely used in clinical practice to prevent RE.
- (4)
Promoting mucosal healing: Some cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) may promote mucosal healing in patients with RE. In a cohort study, after continuous use of GM-CSF 400 μg/d for 5–10 days, 10 out of 23 patients with grade 3 RE were cured, 8 were reduced to grade 1, and 3 were reduced to grade 2. In total, 21 patients completed the entire radiotherapy plan, with an overall relief rate of 91%.48 Chinese scholars treated 31 patients with grade 3 RE with oral GM-CSF for five consecutive days, with RE reduced from grade 3 to 0–1 in 13 cases (41.94%), reduced to grade 2 in 15 cases (48.39%), and remaining at grade 3 in three cases (9.68%). The total effectiveness rate was 90.32%,49 which is similar to the above findings.48
6.1.4 Traditional Chinese Medicine (TCM)
Using TCM Syndrome Differentiation and Treatment, integrated Chinese and Western medicine, specialized treatments, Chinese medicine injections, and acupoint application methods to prevent and treat RE can significantly reduce the incidence and severity of RE, improve radiotherapy completion rates, and enhance patients' clinical symptoms and quality of life with fewer side effects.38 Some Chinese medicines can suppress the expression of proteins such as cyclooxygenase-2, matrix metalloproteinases, interleukin 8 (IL-8), and transforming growth factor β1, thereby alleviating the pathological changes in esophageal injury caused by radiation. For example, the oral liquid of Baimudan root can repair damaged cellular and humoral immunity by regulating CD3+, CD4+, and CD8+ T lymphocyte counts and IgG and complement C3 levels, thus reducing the severity of RE.50 The modified Zhuye Shigao decoction can prevent and treat RE by decreasing the production and release of inflammatory factors such as tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-8.49
6.2 Treatment of LRE
When a patient develops an LRE, the esophageal mucosa undergoes irreversible changes, and esophageal dilation or stent implantation is necessary to alleviate patient discomfort. For patients with esophageal stenosis, esophageal dilation is required, whereas esophageal perforation and tracheoesophageal fistulae require esophageal stent implantation. Nickel-titanium alloy mesh stents and silicone membrane-covered stents are commonly used to treat symptoms such as dysphagia. Watkins et al.51 reported that for benign esophageal stenosis, the Nitinol stent is a safe, effective, and rapid palliative treatment for dysphagia.
[Expert Recommendation 2] The treatment of RE is divided into the treatment of ARE and LRE. The treatment of ARE is a comprehensive process that includes: (1) General measures: providing dietary and positional guidance to patients. Patients with RE should avoid spicy, coarse, icy, hot, or hard foods and consume high-calorie, high-quality protein, high-vitamin, and low-fat bland soft or semi-liquid diets. ONS can be recommended for patients with the means. After eating, patients should maintain a sitting or semi-recumbent position for 1–2 hours (Level II evidence, Grade A recommendation). (2) Nutritional support therapy: refer to the “Expert Consensus on Enteral Nutrition for Esophageal Cancer Radiotherapy Patients” for specific methods (Level I evidence, Grade A recommendation). (3) Pharmacotherapy: For mild to moderate dysphagia pain, surface anesthetics such as lidocaine and sodium bicarbonate, or gentamicin, or a homemade oral solution with vitamin B12 as the main ingredient can be given, combined with oral analgesics (Level II evidence, Grade B recommendation). Antibiotics can inhibit inflammation and reduce edema (Level II evidence, Grade B recommendation). Vitamin B12 can promote the growth and repair of digestive tract mucosal epithelial cells and vascular endothelial cells, accelerating wound healing (Level II evidence, Grade B recommendation). Early glucocorticoids can reduce congestion at the site of inflammation, inhibit the production and release of inflammatory mediators, and promote the repair of damaged tissues (Level I evidence, Grade A recommendation). Mucosal surface protectants can form a thin film in a colloidal form to cover ulcers or inflamed areas, resist gastric acid attacks, and also provide mucosal protection (Level I evidence, Grade A recommendation). Acid-suppressing drugs can inhibit gastric acid secretion, prevent gastric acid reflux into the esophagus, and thereby reduce gastric acid-induced esophageal mucosal damage (Level II evidence, Grade A recommendation). Some cytokines, such as GM-CSF, can promote mucosal healing in patients with ARE (Level II evidence, Grade B recommendation). (4) Traditional Chinese medicine treatment: using syndrome differentiation, the integration of Chinese and Western medicine, specialized prescription, Chinese medicine injections, and acupoint application methods to treat ARE can improve the completion rate of radiotherapy, clinical symptoms, and patient quality of life (Level III-IV evidence, Grade B recommendation). LRE treatment: there is insufficient clinical evidence for drug treatment; non-drug treatments include esophageal dilation for patients with esophageal stenosis and esophageal stent implantation for patients with esophageal perforation and tracheoesophageal fistula (Level II evidence, Grade B recommendation).
7 PREVENTION
Ideal intervention measures include reducing the incidence of RE, improving quality of life, and preventing malnutrition. Severe RE can lead to treatment interruption, thus affecting local tumor control and survival rates. The active prevention of RE alleviates patient symptoms, improves treatment safety, and contributes to tumor control.
7.1 Radiotherapy dose and mode
Selecting appropriate radiotherapy fractionation patterns and optimal radiotherapy doses based on the patient's condition, and using more precise radiotherapy techniques can help reduce the occurrence of RE. Therefore, mechanical and chemical irritations should be avoided during and after radiotherapy.
7.2 Drug prevention
7.2.1 Amifostine
Amifostine is an organic thiophosphate. Its active metabolite wr-1065 can scavenge free oxygen radicals, reducing the occurrence of RE without affecting tumor control.52 However, side effects, such as nausea and vomiting, should be considered.
7.2.2 GM-CSF
GM-CSF is a multilineage cytokine that promotes immune cell function. It shortens the mucosal healing time and alleviates pain in patients with esophagitis caused by chemoradiotherapy.53
7.2.3 Glutamine
Glutamine is one of the most abundant essential amino acids found in humans. Glutamine can inhibit disease-related toxic factors, prevent or delay the occurrence of RE, reduce the grade of RE, and improve malnutrition.54
7.3 Others
It has been reported that the effects of cytokines such as IL-1, TNF-α, and interferon gamma (IFN-γ) are related to esophageal mucosal basal cell apoptosis, DNA damage, micro-ulceration, and esophagitis.55 Corresponding drugs are yet to be developed. Superoxide dismutase inhibits esophagitis and apoptosis. The inhibition of active oxygen in the esophagus during radiotherapy may prevent RE.
[Expert Recommendation 3] The ideal intervention measures aim to reduce the incidence of RE and improve the quality of life and prevent malnutrition. For patients with risk factors, RE prevention measures can be implemented at the start of radiotherapy, with the recommendation to combine various prevention modes and methods. (1) Radiotherapy dose and mode: Based on the patient's condition and staging, select the appropriate radiotherapy technique, dose, and fractionation pattern (Level I evidence, Grade A recommendation). (2) Drug prevention: It is recommended that amifostine be used concurrently with radiotherapy to prevent RE in patients with high-risk factors (Level II evidence, Grade B recommendation). Cytokines such as GM-CSF, glutamine, IL-1, TNF-α, and IFN-γ can also be used for RE prevention (Level II-III evidence, Grade B recommendation).
8 ASSESSMENT AND HEALTH EDUCATION
8.1 Risk assessment
For patients receiving radiotherapy for esophageal cancer and similar conditions, the responsible nurse should inquire in advance about the patient's age, underlying diseases, and pre-radiotherapy diet. The risk of RE should be comprehensively assessed based on factors such as radiotherapy dose, need for chemotherapy, and patient's combined risk factors. Low-risk patients regularly examine their esophagus, actively manage internal medical conditions, receive antidiabetic or antihypertensive medications in advance to reduce the impact of underlying diseases on post-radiotherapy complications, and actively control infections and bleeding. For medium-to high-risk patients, drug prevention can be achieved by increasing the frequency of esophageal examinations, timely detection of RE, proper assessment, and arrangement of corresponding nursing measures to reduce the incidence of RE.
8.2 Nutritional assessment
Good nutrition is crucial for resistance to infection, maintenance of mucosal integrity, enhancement of mucosal tissue repair, and slowing the worsening of mucositis. During radiotherapy, patients are prone to malnutrition due to the disease and increased energy consumption caused by cancer treatment. Therefore, all patients should undergo routine nutritional status assessments and comprehensive measurements before, during, and after treatment. Nutritional status assessments use scales, and comprehensive nutritional measurements include stress levels, inflammatory responses, energy expenditure levels, metabolic status, organ function, body composition, and psychological state. Consultations with a nutritionist may be necessary.
8.3 Health education
Patients should be provided with professional education on diet and nutrition, including education on potential RE complications so that they can identify and report them to their primary care physicians early. As part of the prevention and treatment of RE, all patients should receive written and verbal dietary education to increase their awareness of nutrition and ensure an adequate nutritional supply during radiotherapy for better treatment tolerance. Patient education is an essential part of this clinical practice guideline for clinical and patient-standardized treatment and should be carried out regularly before, during, and after treatment. The format should be diverse and engaging, and include mini-lectures, slides, videos, WeChat pushes, and educational brochures.
Diet and nutrition education suggestions are as follows. (1) Ensure a balanced diet with high protein, high calories, high vitamins, and low fat, such as lean meat, seafood, and fresh fruits and vegetables, without avoiding certain foods. (2) Avoid smoking, alcohol consumption, and extremely cold, hard, hot, greasy, and spicy foods. Focuses on a light, soft, and easily digestible diet, consuming cooked, stewed, and steamed foods. (3) Consume iron-rich foods for blood production and supplementation, such as animal liver, chicken, duck, fish, lean meat, and dates. (4) Patients should adjust their diet according to the radiotherapy response. Consumes smaller meals without reducing the overall calorie intake. When symptoms such as dry mouth, altered taste, and sore throat occur, opt for a bland, non-irritating, easy-to-chew semi-liquid, and soft food diet with high water content to facilitate swallowing, reduce damage, and maintain oral mucosal integrity. Drink plenty of water, eat foods that promote saliva production, relieve thirst, nourish yin, clear heat, and increase vitamin supply. Herbal teas include Sterculia lychnophora, chrysanthemum, Ophiopogon japonicus, and American ginseng slices. Patients undergoing oral, pharyngeal, and esophageal radiotherapy should drink a small amount of warm water before meals to lubricate the mouth, pharynx, and esophagus; chew slowly and swallow carefully; and avoid sticky foods such as glutinous rice balls to prevent obstruction. (5) For patients with severe oral, hypopharyngeal, and esophageal mucosal reactions, it is recommended to use enteral nutrition, such as nasogastric feeding, as early as possible and, if necessary, perform gastrostomy surgery to maintain nutrition and physical strength, ensure the continuity of treatment, and achieve the desired treatment effect.
9 CONCLUSION
RE is a common complication of radiotherapy for thoracic tumors, directly affecting treatment efficacy and reducing patients' quality of life. The prevention and treatment of RE requires multidisciplinary experts. The purpose of developing these clinical practice guidelines was to summarize the current clinical experience and feasible diagnostic, preventive, and treatment approaches for preventative and active treatment.
The treatment needs for RE should not be neglected, and the primary goal of treatment is to alleviate patient symptoms and reduce the occurrence of grades III-IV RE. The existing clinical research on radiotherapy-related mucositis lacks large-scale randomized controlled clinical studies, and prevention strategies should focus on patients' individualized treatment needs. The principles proposed in this guideline are intended to support and reference, and should not replace clinical decisions related to specific patients and clinical situations.
ACKNOWLEDGMENTS
Guiding experts: Jinming Yu (Shandong Cancer Hospital).
Writing experts: Congrong Yang, The Fourth Hospital of Hebei Medical University
Jun Wang, The Fourth Hospital of Hebei Medical University
Editorial Board Members: (Alphabetize by Last Name)
Feng Cao, The Fourth Hospital of Hebei Medical University
Weiqing Chen, Chongqing University Cancer Hospital
Xiaozhong Chen, Zhejiang Cancer Hospital
Yan Cheng, First Affiliated Hospital of Zhengzhou University
Mei Feng, Sichuan Third People's Hospital
LiYing Gao, Gansu Provincial Cancer Hospital
Xianshu Gao, Peking University First Hospital
Yuanhong Gao, Sun Yat-sen University Cancer Center
Xia He, Jiangsu Cancer Hospital
Man Hu, Shandong Cancer Hospital
Xiaobo Huang, Sun Yat-Sen Memorial Hospital
Baosheng Li, Shandong Cancer Hospital
Guiling Li, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology
Jie Li, Shanxi Provincial Cancer Hospital
Jingao Li, Jiangxi Provincial Cancer Hospital
Qin Lin, The First Affiliated Hospital of Xiamen University
Fang Liu, The People's Liberation Army General Hospital
Qing Liu, The Third Affiliated Hospital of Air Force Medical University
Zi Liu, The First Affiliated Hospital of Xi'an Jiaotong University
Tenghui Ma, The Sixth Affiliated Hospital of Sun Yat-sen University
Shuhuai Niu, The Fourth Hospital of Hebei Medical University
Qiao Qiao, The First Affiliated Hospital of China Medical University
Jianguang Qiu, The Sixth Affiliated Hospital of Sun Yat-sen University
Mei Shi, Xijing Hospital of Air Force Military Medical University
Hui Wang, Hunan Cancer Hospital
Jun Wang, The Fourth Hospital of Hebei Medical University
Qifeng Wang, Sichuan Cancer Hospital
Rensheng Wang, The First Affiliated Hospital of Guangxi Medical University
Ruozheng Wang, Cancer Hospital Affiliated to Xinjiang Medical University
Weiping Wang, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College
Sangang Wu, The First Affiliated Hospital of Xiamen University
Qin Xu, Fujian Provincial Cancer Hospital
Junlin Yi, The Cancer Hospital of Chinese Academy of Medical Sciences
Shuanghu Yuan, Shandong Cancer Hospital
Xianglin Yuan, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology
Daxin Zhang, the First Affiliated Hospital of Harbin Medical University
Fuquan Zhang, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College
Liyuan Zhang, The Second Affiliated Hospital of Soochow University
Yunyan Zhang, Harbin Medical University Cancer Hospital
Zhen Zhang, Fudan University Shanghai Cancer Center
Anping Zheng, Anyang Tumor Hospital
Li Zhu, Tianjin Medical University Cancer Institute and Hospital
Hongqing Zhuang, Peking University Third Hospital
Dongling Zou, Chongqing University Cancer Hospital
CONFLICT OF INTEREST STATEMENT
Jinming Yu is the Editor-in-Chief of the journal and co-author of this article. He was excluded from the peer review process and from all editorial decisions related to the acceptance and publication of this article. Peer reviews were handled independently by another editor to minimize bias.
ETHICS STATEMENT
The authors are accountable for all aspects of this work and ensure that questions related to the accuracy or integrity of any part are appropriately investigated and resolved.