Gambogic acid inhibits epithelial–mesenchymal transition in breast cancer cells through upregulation of SIRT1 expression in vitro
Shi-ye Jiang and Jun Yu contributed equally to this study.
Funding information: This work was supported by Jiangsu Province Health and Family Planning Commission (Project No. ZD2021012) and Jiangsu Provincial Science and Technology Department (Social Development Project No. BE2017759).
Abstract
Gambogic acid (GA) is a natural product that selectively induces apoptosis of cancer cells in vitro with high efficiency and low toxicity. Our previous data have shown inhibitory effects of GA on breast cancer cells. However, the detailed mechanisms for GA remain largely unknown. This study aimed to investigate the effect of GA on TGF-β1-induced tumor invasion and EMT in MDA-MB-231 cells, and contribution of elevated SIRT1 to antitumor effects of GA. Human breast cell MDA-MB-231 and MDA-MB-231 were incubated with TGF-β1 (100 ng/ml) and GA. Cell viability was determined by MTT assay, while tumor invasion was determined by Boyden chamber invasion assay. The mRNA levels of SIRT1 and TGF-β1 were measured by quantitative real-time polymerase chain reaction (qRT-PCR). The protein expressions of SIRT1 and EMT-associated mesenchymal marker Vimentin were measured by Western blotting. Interleukin-6 (IL-6) protein content was measured by enzyme linked immunosorbent assays (ELISAs). Our results showed that, GA showed decreased proliferation in MDA-MB-231 cells, especially in MDA-MB-231 cells with TGF-β1 incubation. GA inhibited tumor invasion and EMT in TGF-β1-treated MDA-MB-231 cells. GA increased mRNA and protein expression of SIRT1 in MDA-MB-231 cells with and without TGF-β1 treatment. SIRT1 mRNA level in MDA-MB-231 cells was increased by TGF-β1 incubation and decreased by GA treatment. In addition, the production of IL-6 was increased by TGF-β1, and decreased by GA. In conclusion, GA inhibits tumor invasion and EMT in breast cancer, potentially through upregulating SIRT1 expression. This study provides a novel antitumor effect of GA in breast cancer.
1 INTRODUCTION
Gambogic acid (C16H12O5, Figure 1) is a flavonoid isolated from the root of Scutellaria baicalensis Georgi. It is a traditional herbal medicine generally regarded as an analgesic, antipyretic, antitumor, and anti-inflammatory agent, which has been proved to possess multiple pharmacological effects in several types of cancer both in vitro and in vivo, including apoptosis and metastasis inhibition.1, 2 GA has been found to inhibit cell proliferation, induce apoptosis and decrease NF-κB levels, thereby reducing in vivo tumor mass.3 Our previous study found that GA inhibited cell proliferation and cell cycle progression, induced apoptosis and inhibited tumor invasion and epithelial–mesenchymal transition (EMT) process in TGF-β1-treated MDA-MB-231 cells.4, 5 However, the mechanisms underlying invasion and EMT inhibition by GA remain largely unknown.
Sirtuin-1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase, with regulatory function on cell's stress adaption, cell metabolism and apoptosis and DNA damage response.6 SIRT1 has been shown to involve aging-related diseases, such as inflammation, diabetes and neurodegenerative diseases.7 SIRT1 participates in tumor initiation, progression and drug resistance thorough regulating critical carcinogenesis process, such as cell growth, apoptosis, senescence, and angiogenesis.8 However, the roles of SIRT1 in breast cancer are controversial. SIRT1 overexpression is associated with poor prognosis of breast cancer,9 while SIRT1 represses transcriptional response to estrogen receptor and inhibits proliferation in breast cancer cells.10 The roles of SIRT1 in breast cancer are still unclear and need further investigation on different regulating system and pathways.
In this study, we aimed to investigate regulation of SIRT1 by GA in TGF-β1-induced breast cancer cells and its related mechanisms. We incubated breast cells with TGF-β1 to induce tumor invasion and EMT, and analyzed cell viability, invasion, EMT mRNA and protein expressions of SIRT1 by GA. The effect of SIRT1 on invasion and EMT by GA was verified by nicotinamide (NAM), a specific SIRT1 inhibitor. We also detected the mRNA expression and culture supernatant cytokine interleukin-6 (IL-6) to explore potential mechanisms SIRT1 in breast cancer.
2 MATERIALS AND METHODS
2.1 Materials
Gambogic acid (C16H12O5) was isolated from the root of S. baicalensis according to a previously reported method and dissolved in DMSO. Samples containing 99% or higher in GA were used in all experiments. GA was dissolved in DMSO to 200 mM and stored at −20°C. Before every experiment, the stock solution of GA was diluted with basal medium to various working concentrations.
2.2 Cell culture
Human breast cancer cell line (MDA-MB-231) was provided by the cell bank of Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Cells were grown in high glucose DMEM media (Invitrogen-GIBCO, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS) (Sijichun Bioengineering Materials Inc., Hangzhou, Zhejiang, China), 100 U/ml penicillin and 100 mg/ml streptomycin, at 37°C under a humidified atmosphere of 5% CO2 and 95% air. The cells were digested by trypsinization when reaching confluence, and then they were sub cultured in new culture flasks at lower numbers.
2.3 Cell viability assay
The cell viability of MDA-MB-231/MDA-MB-231 cells was determined by MTT (Sigma Chemical Co., St. Louis, MO) assay. Briefly, cells at the logarithmic growth phase were seeded to each well of 96-well culture plates (cell density: 1 × 103 cell/ml; each well 100 μl cell suspension). After 24 h incubation at 37°C in a humidified atmosphere with 5% CO2, cells were incubated with recombinant human TGF-β1 (100 ng/ml) (R&D Systems Inc., Minneapolis, MN). The cells with or without TGF-β1 incubation were then treated with different concentrations of GA (0, 100, 200, 500 μmol/L). After treatment for 72 h, 10 μl MTT solution (concentration: 5 mg/ml) was added to each well, followed by incubation at 37°C for 4 h. After centrifugation at 1000g for 10 min, the supernatant was discarded to obtain formazan pellet, and then it was dissolved completely with 100 μl DMSO after being shaken for 10 min. An enzyme linked immunosorbent assay (ELISA) plate reader was applied to measure the optical density (OD) at 570 nm wavelength to determine the amount of pellet.
2.4 Cell migration assay
Boyden chamber invasion assay was performed to measure the in vitro invasive capability of MDA-MB-231/MDA-MB-231 cells. In 24-well tissue culture plate, each well was separated by Transwell filter membrane. The lower side of filters was coated with type I collagen (0.5 mg/ml), with low-serum media in the lower part of Transwell plate. In each well, MDA-MB-231 cells were resuspended in 100 μl DMEM media (cell density 5 × 104 cells/ml) and seeded in the upper part of Transwell plate, and were then incubated with TGF-β (100 ng/ml) and (or) GA (200 μmol/L) for 72 h. The cells on the upper surface of the filter were removed, and the cells that had migrated to the lower part were stained with hematoxylin and eosin (Sigma), and they were counted and regarded as migrated cells (invasion index). Each sample was experimented in triplicate.
2.5 Quantitative real-time polymerase chain reaction (qRT-PCR)
MDA-MB-231 cells were incubated with different concentrations of GA (0, 100, 200, 500 μmol/L), or TGF-β1 (100 ng/ml) and (or) GA (200 μmol/L) for 24 h, to determine mRNA level of SIRT1. In another experiment, the MDA-MB-231 cells were incubated with TGF-β1 (100 ng/ml), TGF-β1 + GA (200 μmol/L), TGF-β1 + GA+ nicotinamide (NAM, 100 μmol/L) for 24 h, to determine mRNA level of TGF-β1. Total RNA was extracted from MDA-MB-231cells using Trizol® reagent according to the manufacturer's protocol (Life Technologies, Carlsbad, CA). Total RNA (2 μg) was treated with DNase (Life Technologies), and was then reverse-transcribed into cDNA using the Superscript III enzyme (Life Technologies). The qRT-PCR assay was performed based on resulting cDNA as a template using specific SIRT1 or TGF-β1 primer and SYBR Green reagent (TaKaRa, Japan) in the StepOne Plus Real-time PCR System (Applied Biosystems). The PCR conditions were as follows: initial denaturation at 95°C for 3 min, followed by 35 amplification cycles of denaturation at 95°C for 5 s, annealing at 60°C for 30 s and elongation at 72°C for 30 s. Housekeeping gene GAPDH served as an internal control and SIRT1 or TGF-β1 mRNA expression was calculated. The primer sequences used in our study were as follows: SIRT1: forward: 5′-GCCTCATCTGCATTTTGATG-3′; reverse: 5′-TCTGGCATGTCCCACTATCA-3′; TGF-β1: forward: 5′-GAGATGCAATGCTATTCCT-3′; reverse: 5′-CGACGTCACAGCGCACTT-3′; GAPDH: forward: 5′-GGAGTCCACTGGCGTCTTC-3′; reverse: 5′-GCTGATGATCTTGAGGCTGTTG-3′. All reactions were performed in triplicate. The relative SIRT1 or TGF-β1 expressions of four groups were all normalized to control group.
2.6 Western blotting
MDA-MB-231cells were incubated with TGF-β1 (100 ng/ml), TGF-β1 + GA (200 μmol/L), TGF-β1 + GA + nicotinamide (NAM, 100 μmol/L) for 24 h to determine protein level of Vimentin. In another experiment, MDA-MB-231/MDA-MB-231 cells were incubated with different concentrations of GA (0, 100, 200, and 500 μmol/L), or incubated with TGF-β1 (100 ng/ml) and (or) GA (200 μmol/L) for 24 h to determine protein level of SIRT1. The cells were harvested and washed twice with ice-cold PBS, and then were lysed with RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS 50 mM Tris-Cl, pH 7.4) containing 20% (V/V) cocktail protease inhibitors (Sigma-Aldrich). Sonication was applied in the cell lysates and then incubated on ice for 30 min. In order to remove insoluble debris from cell lysates, centrifugation was performed at 12 000g for 30 min at 4°C. Protein concentrations were measured by bicinchoninic acid protein concentration assay kit (Beijing Biosea Biotechnology Co. Ltd., China). Proteins (50 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels (polyacrylamide concentration 12%) and then electrophoretically transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 3% BSA in TBS-T buffer (3 g/L Trisbase, 8 g/L NaCl, 0.2 g/L KCl, 0.1% Tween-20, pH 7.4) at 4°C. After that, the membranes were incubated with specific mouse monoclonal antibodies against human Vimentin or SIRT1 (both 1:1000 dilutions), followed then by incubation with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse secondary antibodies (IgG) (1:1000 dilutions) at room temperature for 1 h. The density of protein bands was visualized using enhanced chemical luminescence (ECL, Pierce® ECL Plus Western Blotting Substrate, Pierce Biotechnology, Inc., Rockford, IL), and was detected by Bio-Rad ChemiDoc XRS (Bio-Rad).
2.7 Enzyme linked immunosorbent assays
MDA-MB-231 cells were plated into 6-well plates (cell density: 2 × 105 cells/well). After grown to confluence, the cells were serum starved overnight and treated with TGF-β1, TGF-β1 (100 ng/ml) + GA (200 μmol/L), or TGF-β1 + GA + nicotinamide (NAM, 100 μmol/L) for a period of 24 h. The cytokines level of IL-6 was measured by ELISAs (R&D Systems, Minneapolis, MN) using 50 μl of the cell culture media.
2.8 Statistical analysis
All quantitative data were presented as mean ± SD. Statistical analysis was performed using the commercially available software (SPSS version 19.0). Student t-test (unpaired, two tailed) was used to compare the difference between two groups. A probability value of <.05 (p < .05) was considered as statistically significant difference.
3 RESULTS
3.1 GA inhibited MDA-MB-231 cells growth, invasion and EMT process induced by TGF-β1
MDA-MB-231 cells were incubated with TGF-β1 (100 ng/ml), and then the cells with or without TGF-β1 incubation were simultaneously treated with various concentrations of GA (0, 100, 200, or 500 μmol/L) for 72 h. The MTT assay showed that, compared with normal control, TGF-β treatment promoted cell proliferation and increased cell viability significantly (P < .05) (Figure 2A). GA inhibited cell viability in a concentration-dependent manner in MDA-MB-231 cells with or without TGF-β1. Compared with cells without TGF-β1 incubation, GA demonstrated greater inhibitory effect on cell viability in cells with TGF-β1 incubation. GA significantly decreased cell viability at 200 μmol/L concentration in cells with TGF-β1 incubation, but not in the cells without TGF-β1 incubation. Therefore, we selected this concentration of GA for further study on invasion and EMT.
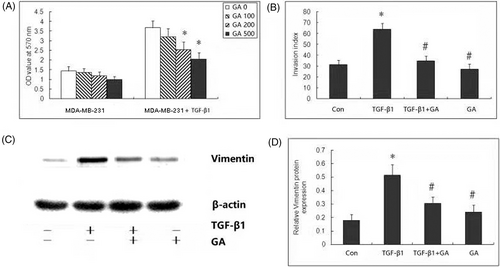
Boyden chamber invasion assay was performed to investigate the effect of GA on migration capability of breast cancer cells. Our results showed that TGF-β1 can significantly increase invasion index of MDA-MB-231 cells. Compared with cells treated with TGF-β, GA significantly decreased invasion index (Figure 2B). However, GA treatment alone showed no significant difference in invasion index.
To investigate whether the EMT process participates in the anti-invasive effect of GA, the expression of EMT marker and Vimentin protein was measured by Western blot analysis. TGF-β1 treatment significantly induced EMT in MDA-MB-231 cells, as evidenced by increased expression of Vimentin protein. After GA treatment, TGF-β1 induced Vimentin protein was significantly decreased. However, compared with control MDA-MB-231 cells, Vimentin protein remained unchanged after GA treatment alone (Figure 2C,D).
3.2 GA enhanced mRNA and protein expressions of SIRT1 in MDA-MB-231 cells induced by TGF-β1
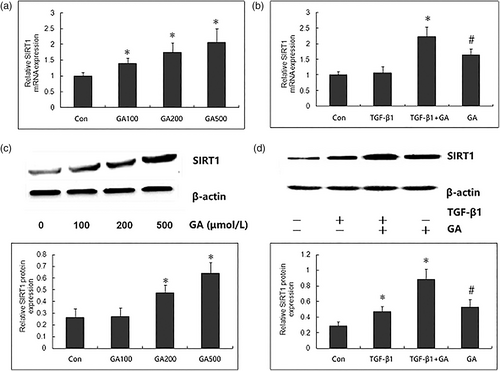
To explore whether SIRT1 mediates the mechanism underlying the inhibitory effect of GA against TGF-β1-induced invasion and EMT, we firstly determined whether GA regulates the expressions of SIRT1 in MDA-MB-231 cells by qRT-PCR. After treatment with different concentrations of GA (100, 200, and 400 μmol/L) for 24 h, the mRNA expression of SIRT1 in MDA-MB-231 cells was significantly increased in a concentration dependent manner (Figure 3A). Furthermore, the SIRT1 mRNA expression remained unchanged by exposure to TGF-β1 (100 ng/ml, 24 h), but was significantly increased by treatment with GA (200 μmol/L, 24 h) (Figure 2B). Compared with MDA-MB-231 cells, the SIRT1 mRNA expression was significantly higher by GA treatment in MDA-MB-231 cells with TGF-β1 incubation. Furthermore, Western blot confirmed the results from the qRT-PCR, as demonstrated by concentration-dependently increased expression of SIRT1 protein by GA, and higher SIRT1 protein in TGF-β1-induced MDA-MB-231 cells than cells without TGF-β1 incubation (Figure 3AC,D). These data indicate the upregulatory role in SIRT1 expression in breast cancer cells with decreased invasion and EMT by GA.
3.3 Effect of specific SIRT-1 inhibitor on GA-exerted inhibition on invasion and EMT induced by TGF-β1
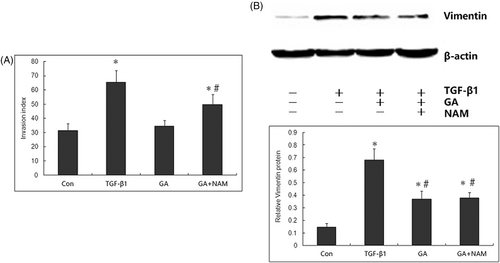
To determine whether SIRT1 mediated the inhibitory effect of GA on TGF-β1-induced tumor invasion and EMT in MDA-MB-231 cells, we further explored whether nicotinamide, a specific inhibitor of SIRT1, reverse this inhibitory effect of GA against invasion and EMT. We pretreated MDA-MB-231 cells with nicotinamide (100 μmol/L) 1 h before the administration of TGF-β1 (100 ng/ml) and GA (200 mM). Compared with MDA-MB-231 cells treated with TGF-β1 and GA, nicotinamide significantly increased the invasion index (Figure 4A). Furthermore, nicotinamide reversed the expression levels of Vimentin which was suppressed by GA (Figure 4B), indicating that the inhibition of SIRT1 reversed the protective role of GA against FA-induced ER stress. Taken together, these data suggest that SIRT1 mediates the protective action of GA against FA-induced ER stress.
3.4 GA decreased TGF-β1 mRNA expression and cytokines level of IL-6 in culture media
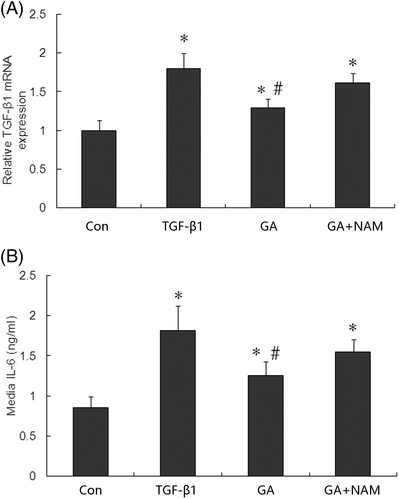
To explore the detailed mechanisms of SIRT1 underlying the inhibitory effect against GA in tumor invasion and EMT, we analyzed the expression of TGF-β1 and cytokine IL-6. We performed qRT-PCR to measure the mRNA expression of TGF-β1, and found TGF-β1-induced cells showed higher TGF-β1 mRNA expression compared with control cells (Figure 5A). GA treatment significantly decreased TGF-β1 mRNA expression in TGF-β1-induced cells, and this decrease could be abolished by nicotinamide. This indicates that SIRT1 might lie in upstream of TGF-β1 and regulate its expression. We further analyzed the cytokines IL-6 in culture media from breast cancer cells by ELISA over a period of 24 h of TGF-β1 and GA incubation. The levels of IL-6 in culture media were significantly increased by TGF-β1, and were significantly decreased by GA in TGF-β1-induced cells (Figure 5B). The decrease in IL-6 levels by GA was abolished by nicotinamide.
4 DISCUSSION
Natural products and their structural analogues have historically made a major contribution to pharmacotherapy, especially for cancer, as well as cardiovascular and neurodegenerative disorders. In this study, we found that the GA showed anticancer effects on TGF-β1-breast cancer cells, as demonstrated by inhibition of cell proliferation, tumor invasion and EMT by GA, a bioactive compound releasing GA. Furthermore, GA upregulated mRNA and protein expressions of SIRT1 in TGF-β1-breast cancer cells, and the role of SIRT1 was confirmed by a specific SIRT1 inhibitor nicotinamide, which suppressed the inhibitory effect of GA on invasion and EMT. The downstream pathway for SIRT1 in breast cancer might include TGF-β1 and IL-6, and these two cytokines were decreased by GA and increased by nicotinamide. Herein, we have demonstrated that the SIRT1 participates in anticancer effects on progressive phase of breast cancer.
We have previously reported the anticancer effect of GA against breast cancer, as demonstrated by inhibition of cell proliferation, cell cycle progression, invasion and EMT, and apoptosis induction.11 We in this study investigated the possible mechanisms for the inhibitory effect of GA against TGF-β1-induced breast cancer. SIRT1 regulates aging and longevity and is involved in cell apoptosis, differentiation, metabolism, and stress adaption. SIRT1 overexpression is associated with distant metastatic relapse and shorter survival in breast cancer patients. SIRT1 is essential for oncogenic signaling and cell growth induced by estrogen and estrogen receptor α in breast cancer. Therefore, SIRT1 might be a therapeutic target in breast cancer and SIRT1 inhibitor can inhibit proliferation and induce apoptosis in breast cancer cells by downregulating Bcl-2.12, 13 However, some reports also showed anticancer effect of SIRT1 in breast cancer. In triple-negative breast cancer cells with mutant p53, SIRT1 activator YK-3-237 inhibited cell proliferation, induced cell cycle arrest at G2/M phase, and promoted PARP-dependent apoptotic cell death.14 Therefore, we focused on whether SIRT1 mediates the promoter or inhibitory role of GA against TGF-β1-induced invasion and EMT. We found that the GA not only upregulated the mRNA and protein expressions of SIRT1 in MDA-MB-231 cells in a concentration dependent manner, but also it increased SIRT1 expression more significantly in TGF-β1-induced cells compared with cells without TGF-β1. Indeed, SIRT1 activation by GA has been found in many physiopathological processes of age-associated diseases, including endothelial cell senescence and oxidative damage of cardiomyocytes.15-17 Our study has firstly found upregulation of SIRT1 by GA and indicates that the SIRT1 might demonstrate higher sensitivity to GA regulation in breast cancer cells.
To explore whether SIRT1 mediate the inhibition of invasion and EMT by GA, we further pretreated MDA-MB-231 cells with nicotinamide, a specific inhibitor of SIRT1, and analyzed the effect on invasion and EMT by GA. Our present result demonstrated that this SIRT1 inhibitor reversed the inhibitory effect of GA on tumor invasion and EMT in MDA-MB-231 cells treated with TGF-β1. This suggests that GA-exerted inhibition of TGF-β1-induced invasion and EMT is involved in upregulation of SIRT1. The epithelial-to-mesenchymal transition (EMT) is a process in epithelial cells with lots of cell-adhesive properties and increased mesenchymal properties and cell mobility.18 EMT can enable epithelial cancer cells to acquire high-grade malignancy and metastasis potential.19, 20 EMT is also associated with aging in cancers,21, 22 which is an aging disease with nearly 65% of cancer patients ≥65 years old.23 As a protein regulating the pathology for aging, SIRT1 might participate EMT process in cancer cells. Indeed, the regulatory effect of SIRT1 on EMT is controversial. SIRT1 could induce EMT in gastric cancer cells and osteosarcoma cells.24, 25 However, decreased SIRT1 expression promotes invasion and metastases via EMT in breast cancer and lung cancer,26, 27 which is in accordance with our results. The discrepancy on EMT by SIRT1 may be caused by different cancer types and various dependences of cancer cells on aging.
We further explored the detailed mechanisms of SIRT1 underlying the invasion and EMT by GA in breast cancer cells. We analyzed the mRNA expression of TGF-β1 and cultured supernatant from cytokine IL-6 level. Because MDA-MB-231 cells were induced with TGF-β1, the culture supernatant TGF-β1 level and TGF-β1 protein expression in MDA-MB-231 cells were not appropriate for measurement. Therefore, we performed qRT-PCR to measure mRNA expression. Our results showed TGF-β1 treatment increased TGF-β1 mRNA expression and culture supernatant IL-6 levels. GA significantly decreased TGF-β1 mRNA expression and culture supernatant IL-6 levels in TGF-β1-induced cells, and this decrease could be abolished by nicotinamide. This suggests SIRT1 might lie in upstream of TGF-β1 and regulate its expression. Inhibition TGF-β pathway by SIRT1 has been reported in renal fibrosis,28 tissue fibrosis,29 and AGEs-induced rat glomerular mesangial cells.30 However, this regulation has not been reported in breast cancer cells, and was confirmed in our study by the result that decreased TGF-β1 expression can be reversed by SIRT1 inhibitor. In our study, culture supernatant IL-6 level was also increased by TGF-β1 and decreased by GA. In mouse embryonic fibroblasts oxidative stress-induced inhibition of SIRT1 can stimulates the secretion of IL-6.31 Whether IL-6 production was enhanced directly by SIRT1 in breast cancer cells need further study. Cytokines, including IL-6, IL-8 and TGF-β, are frequently elevated in breast cancers and constitute tumor microenvironment for the expansion of cancer stem cells (CSCs).32 Furthermore, in breast cancer cells, activation of IL-6 leads to expansion of CSCs population, and highly enriched CSCs which display an EMT phenotype secreting over 100-fold more IL-6 than parental cells, thus making an IL-6 inflammatory positive feedback loop.33 Therefore, GA and SIRT1 may block this positive feedback loop and reverse EMT phenotype, and make a therapeutic strategy for advanced stage breast cancer, which deserve further investigation.
In conclusion, our present study demonstrated that GA can inhibit TGF-β1-induced tumor invasion and EMT phenotype in MDA-MB-231 cells through upregulating SIRT1 expression. GA also decreased TGF-β1 mRNA expression and culture supernatant IL-6 level in MDA-MB-231 cells. Moreover, inhibition of SIRT1 reversed GA-elicited inhibition of invasion, EMT, TGF-β1 mRNA expression and culture supernatant IL-6 level in MDA-MB-231 cells induced by TGF-β1. These findings suggest that the inhibition of invasion, EMT, TGF-β1 mRNA and IL-6 secretion by GA is mediated by SIRT1. Our results provide SIRT1-TGF-β1-IL-6 as a novel molecular mechanism underlying GA-mediated anticancer effect in breast cancer.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
AUTHOR CONTRIBUTION
Qing Hu and Min Lv designed the study; Xiaomei Zhang and Ming Zhu collected the data; Yuanying Zhang analyzed the data; Shiye Jiang and Jun Yu drafted the pape;Qin Zhang reviewed the manuscript. All authors interpreted the data, edited or commented, and approved the final manuscript.
ETHICS STATEMENT
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was exempt by Institutional Review Board (IRB) approval because the original data were from a public database.




