Precision health in Alzheimer disease: Risk assessment-based strategies
Abstract
Alzheimer disease (AD) is a chronic neurodegenerative condition that affects an individual's cognitive function over an extended period. Treatment and prevention for AD have long been sought after; however, no strategy has yet been successful, as individuals with cognitive impairment usually present to the clinic when they have reached an advanced stage of the disease. The disease is progressive and multifactorial in its pathogenesis. Precision medicine (PM) is the new era of medicine comprising a holistic approach in dealing with diseases. With scientific innovations, PM has improved our disease knowledge, altered diagnoses and therapy approaches resulting in a more precise, predictive, preventative and personalized patient care. PM in AD focuses, among other things, on stratifying individuals according to their risk factors of developing the disease and applying preventative strategies and personalized treatment approaches for a better outcome. In this mini-review, we have focused on a few modifiable and non-modifiable risk factors and presented recommendations for future consideration to implement.
1 INTRODUCTION
Dementia is a syndrome characterized by a gradual deterioration in memory, language, and cognitive skills, and the loss of basic functions such as walking and swallowing, and the support of full-time caretakers.1, 2 The syndrome affects 40 to 50 million people worldwide, according to statistics in 2016.3 Alzheimer disease (AD) is the most common cause of dementia. Specifically, 60% to 80% of dementia cases are due to AD.1 AD is the most common neurodegenerative disease and it is a progressive disorder where neurons degenerate and die. The three most common risk factors for AD are aging, genetics and family history.1 Alzheimer and other dementias were the fifth leading cause of death worldwide according to the World Health Organization (WHO).4 AD imposes a cost of between 159 and 215 billion US dollars and is estimated to reach up to $ 500 billion annually, in 2040 economic burden in the United States.5 The hallmarks of AD are the accumulation of extracellular senile plaques' (amyloid beta fibrils) and intraneuronal neurofibrillary tangles (hyper-phosphorylated tau proteins) aggregation.6, 7 The prodromal phase of AD is 20 to 30 years long. Therefore, a myriad of factors plays a role in the pathogenesis, including genetics, environment, and socio-economic factors. Once an individual present with clinical signs and symptoms, neurons have already started to degenerate. Early diagnosis is shown to provide a better therapeutic outcome and is therefore imperative.1, 8
Precision medicine (PM) identifies an individual's specific pattern of risk factors and the specific underlying pathophysiological processes (ideally before clinical onset, in case of neurodegenerative diseases). PM focuses on prevention of disease based on risk stratification of patients, while the traditional approach focuses on treating the disease.9 Clinical trials have failed to show an effect on the progression or the pathology of the disease because they treat the disease as a single entity.10 Most drugs were withdrawn during phase II, some examples include Dimebon, Sargramostim and Atabecestat (clinicaltrials.gov). AD is a heterogeneous disease state, where in many factors like environment, genetics, socioeconomic status, lifestyle, and their interaction said to play significant roles. The current challenge is to identify neuropathological changes during latent disease, correlate these molecular changes to the risk factors, and target them or the risk factors to halt the progression of AD and maintain the quality of life of the patients. This paper focuses on such specific risk factors in AD, presenting hypotheses about some, and strives to integrate the known information regarding these risk factors into PM paradigms. The available paradigms, most of which are mentioned in this paper, focus on the overall idea of PM in AD. Furthermore, this paper gives specific examples to elaborate on how to delay, prevent, or base the treatment of AD.
2 STATE OF THE ART
Various models have been developed over the years to tackle the utilization of precision medicine for one of the most devastating diseases: including AD. Most models aligned with one another in that they each identify the same underlying risk factors, like genetic variants or environmental exposures, that should be addressed first.11-14 Some even proposed a large-scale approach to screen the whole population for risk factors.12, 75 Other models identify AD patients according to different clinical phenotypes, depending on the combination of the protein causing the pathology, location of this pathology, and the genetic and cellular background,15 or they add additional criteria to the primary stratification like lifestyle, single nucleotide polymorphisms, and demographics.14 According to the model, this would be done through clinical, neuroimaging, biochemical, and genetic testing.15 Focus on early detection of the pathology while in the latent stage was also of great interest in some models.11 The systems biology approach plays a major role in some paradigms.13, 16 It focuses on the complex interactions within biological systems. It is like putting the puzzle pieces together because it adapts a holistic approach. Neuroimaging, in addition to multi-omics methods, should reveal molecular signatures and biomarkers to stratify different groups.13 The combination of early screening, risk factors identification, systems biology, neuroimaging, and genetics informs tailored treatment, prevention, and palliative interventions which are the core of precision medicine.11-15 Prevention or delay of the progression might include lifestyle modification and treatment, ranging from amyloid beta lowering agents to tau mediated or neuro-inflammation based treatment.14 Table 1 summarizes the literature on the different paradigms for AD.
| Study | Screening/risk stratification | Patient clustering | Diagnosis | Treatment | Prevention | Strategy used |
|---|---|---|---|---|---|---|
| 11 | Genetic | Based on genetic makeup (mutation, high risk genes, environment interaction) | Detection of pathophysiologic process | Tailored intervention based on risk and pathophysiologic proof of disease | ||
| 12 | Whole population screening | According to sex especially, clinical, genetic demographic, imaging, and fluid biomarkers | Using big data analysis for prevention, diagnosis, prognosis and treatment | Big data analysis | ||
| 13 | Genomic screening and molecular signatures and biomarkers | According to risk score (high medium or low) | Neuroimaging, multi-omics methods | Targeted interventions | Primary and secondary prevention based on the findings | Big data analysis/systems biology holistic approaches |
| 14 | Demographics, Family History, Genetics, Environment, Lifestyle | Imaging and serum biomarkers | Personalized therapeutic intervention (based on pathologic protein) | Removal of risk if possible and alter lifestyle | ||
| 15 | Protein causing the pathology, location of this pathology and the genetic and cellular background | Based on different clinical phenotypes. | Clinical, neuroimaging, biochemical and genetic testing | According to clinical phenotype | ||
| 16 | Omics tools, imaging tools, clinical records and biomolecular data | Biomarker guided integrative disease model | Big data analysis and systems biology | |||
| 17 | Population screening for contributors of AD | Personalized treatment according to contributors of AD | uMETHOD health's software platform |
Hampel and colleagues developed the Alzheimer Precision Medicine Initiative which is “an international network of leading interdisciplinary clinicians, scientists, and researchers devoted toward the transformation of Neurology, Psychiatry, and Neuroscience embracing Precision Medicine, based on complex systems theory (using systems biology, systems neurophysiology, and systems pharmacology), biomarker guided integrative disease modeling (IDM), and 'big data science' to facilitate health care solutions for brain proteinopathies and neurodegenerative diseases like AD.”16
Tailored intervention should be focused mainly on sex and age. Females were found to experience a more drastic decline in cognitive function compared to men, and higher rates of brain atrophy. APOE4 allele and cardiovascular risk factors showed sex-specific susceptibility. Response to treatment (such as hormone replacement therapy and cholinesterase inhibitors) have also been reported to vary according to sex.12
In the light of using a multi-modal approach, Keine and colleagues used uMETHOD Health's software platform to specifically assess contributors of AD and create personalized combination treatment. They followed 40 people with mild cognitive impairment and subjective cognitive impairment for 8.4 months and the results were mostly statistically insignificant, especially because the sample size was small and not representative of the whole population. Some of the results included a decrease in Mild Cognitive Impairment Self-Administered Gerocognitive Exam (Mild cognitive impairment [MCI] SAGE) scores decreased by 0.6 points over 8.4 months, which is less than the average of 1.91 in a year seen in untreated AD patients. Some data were collected but not shown in the results, like genetics, alcohol intake, menopause, and so on, which would have been useful in the establishment of a tailored plan. The method is potentially applicable in studies with large sample sizes.17
3 PRECISION MEDICINE FROM A POPULATION NEUROSCIENCE POINT OF VIEW
“Population Neuroscience is the study of the full range of brain disease, risk factors, and underlying biological pathways as they present in the population at large.”18 As most elderly people who do not develop dementia will not seek medical attention, we will never know the factors that helped them age normally. Therefore, an approach that would target the population as a whole would be ideal to assess lifestyle factors such as diet and physical activity or education to correlate them with the risk of dementia.18 Ganguli focuses on the fact that most clinical studies recruit samples that are not representative of the whole population, leading to misinterpretation of data. Alternatively, Ganguli et al (2018) propose the integration of social sciences and epidemiologic research to the paradigm of precision medicine, which is focused on omics and systems biology of dementia or AD, to guarantee the translation on a population basis.
4 CLINICAL PRESENTATION AND DIAGNOSIS OF AD
AD patients usually seek help when most of their neurons have already degenerated. Amyloid-beta (Aβ) levels and tau levels increase in preclinical AD reaching their maximum before and at the onset of dementia, respectively. These increases are not accompanied by any dysfunction and therefore people with neurocognitive impairments cannot be diagnosed at this time.19, 20 MCI, which precedes AD most of the time, is sometimes confused with normal aging. This also delays the diagnosis of AD and reduces the efficacy of the current palliative medications, which are hypothesized to work on the early stages of this disease. Nearly 40% of individuals with MCI will develop AD/dementia.21, 22
The diagnosis of AD remains indefinite until a brain autopsy is done after the demise of the subject. The Alzheimers Disease Neuroimaging Initiative (ADNI) aims to foresee the progression of AD by utilizing imaging studies, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), cerebrospinal fluid (CSF) and blood biomarkers, as well as genetics, together with cognitive assessments (http://adni.loni.usc.edu/). The most successful imaging technique for AD is based on detecting Aβ and neurofibrillary tangles. Amyloid PET tracer, Pittsburgh Compound-B (PIB), can detect insoluble amyloid beta by being retained in the brain.23 FDG (18F-fluorodeoxyglucose)-PET, which measures glucose metabolism as a proxy for neuronal activity at rest, decreased with increasing PIB retention in cortical areas, especially the parietal cortex in AD patients.23, 24 Tau protein is found in Parkinson disease (PD) and Frontotemporal dementia, Down syndrome, Pick disease, myotonic dystrophy, in addition to AD, which makes it nonspecific. There are different morphological and ultrastructural conformations of tau isoforms depending on the condition with which it is associated. 3R/4R (in which R represents repeats) isoforms represent type 1 tauopathy and are seen in AD, Down syndrome, as well as other conditions.25 Being intra and also extracellular, it is harder to find a radiotracer for tau. A reliable and tau tracer was developed in 2013 which is a hyper-phosphorylated (PHF)-tau targeting positron emission tomography imaging agent, [F-18]-T807, targeting the 3R/4R isoform.26 Aβ and tau are found in both AD and cognitively normal people.27-29 High levels of Aβ are also found in individuals with normal cognition, especially if they have genetic risk factors like the ApoE4 allele or if they are older. As a result, it should be used as the sole marker for AD.27-30 So, there is a need for a unique, accurate approach to address this problem, and early diagnosis is the key that opens the door to more effective prevention methods. While this is beyond the scope of this paper, the importance is of significant value to the researcher, and will be addressed in the future.
In the next two sections, we will present AD risk factors that represent an increased chance to develop AD. These risk factors embody complex web of interactions of genetic background, environmental surrounding influence, and lifestyle characteristics. The most salient risk factors in the non-modifiable and modifiable categories will be discussed.
5 NON-MODIFIABLE RISK FACTORS OF AD
The strongest known non-modifiable risk factors for dementia and AD are genetic makeup, age, gender, and ethnicity. These risk factors are largely responsible for the most common form of AD that is called sporadic Alzheimer disease, that is, non-familial in nature (not inherited). The combination of environmental factors and lifestyle characteristics (a.k.a. lifestyle medicine) affect risk status.
5.1 Genetics
Familial AD is hereditary and accounts for less than 5% of all cases. It is due to specific genes changes or alterations. The three well-documented genes that are linked to familial AD onset are two presenilin genes (PSEN1 and PSEN2) and an amyloid precursor protein (APP) gene. These three genes together are responsible only for about half of familial cases suggesting the involvement of other undiscovered genes yet.31 Moreover, clinical features and pathology vary depending on the mutation.32 Children of parents that carry these genes have a 50% chance of inheriting AD. At least 21susceptibility genes found that potentially might increase the risk of developing sporadic Alzheimer disease.33 For instance, association for APOE, CLU, CR1, BIN1, PICALM, CD33, ABCA7, CD2AP, EPHA1, MS4, and TREM2. It is noteworthy to mention that the associated genes roughly cluster within three pathways: cholesterol and lipid metabolism; immune system and inflammatory response; and endosomal vesicle cycling. Both environmental factors and lifestyle play an important role in making the person more susceptible to developing AD in their lifetime. However, the susceptibility gene APOE4 is alleged to have the greatest magnitude on an individual's chances of developing sporadic AD. Genetic testing for APOE4 is a controversial matter.
5.2 Aging
Age is a known risk factor for late onset AD (LOAD). Nearly a third of people above the age of 85 have AD.22 With advancements in the medical field, people tend to live longer. This yields a larger proportion of elderly in the whole population, and simultaneously an increase in the incidence of dementia. One known difference between normal aging and AD is the increased atrophy in specific regions of the brain that develops more rapidly in AD patients compared to normal aging phenomena,34 but the reason behind this is still unknown. Despite AD and aging being separate entities, at the molecular and cellular level, both show abnormalities in proteostasis, stem cell activity, and inflammation.35 There is an increased need to understand the process of normal aging to be able to address the gaps in our knowledge regarding AD or dementia.
5.3 Gender
AD is not only known to be more prevalent in women than men. In addition, clinical symptoms, brain atrophy rates (progression is even faster in ApoE4 carriers,36 and response to pharmacological therapy differ according to sex.37 ApoE4 in females has worse implications than in men38 and the main reason behind this bias is not yet well understood. According to the Alzheimer's Association, at the age of 45, women have a 20% risk of developing AD while men have a 10% risk.1 The fact that women live longer than men and the lack of education women received in their 20s may play a part in the higher prevalence of AD among women.1, 39 The ApoE4 genotype in women is also presumed to have an interaction with estrogen, affecting the risk in women.1 After menopause or the age of 60, cardiovascular and cerebrovascular risk factors are the same in men and women, if not higher in women. Some conditions such as depression and sleep disorders are both more common in women and also associated with a higher risk of AD.12
Studies were inconsistent regarding the number of gestations and the risk of Alzheimer's disease. A higher number of pregnancies had a protective effect on the brain in British study groups, while Koreans and Greeks showed the opposite.40, 41 This might be due to genetic makeup and lifestyle differences. An important factor in pregnancy is postpartum depression (PPD), which affects up to 20% of women.42 Depression is a known risk factor for AD, and there is likely a link between PPD and AD,43 however more research is needed. Preeclampsia, which affects up to 10% of pregnant women, is also associated with cognitive decline.44, 45
In regards to menopause, a study showed that when menopause was induced surgically (as a result of ovarian removal, chemotherapy, aromatase inhibitor treatment, or premature ovarian insufficiency) at an earlier age (before the age of 40-45), cognitive decline was faster than controls and neuritic plaques were present in those individuals.12, 46 Deposition of Aβ, brain hypometabolism, and reduced brain white and grey matter in AD regions was seen in perimenopausal and menopausal women.47 Removal of the ovaries in premenopausal women, usually due to cancer, also increased dementia risk.48 Antiestrogen treatment for breast cancer survivors also poses a risk of cognitive decline and should be assessed more deeply as there is a high prevalence of breast cancer (1 in 8 women) as well as a high survival rate.49
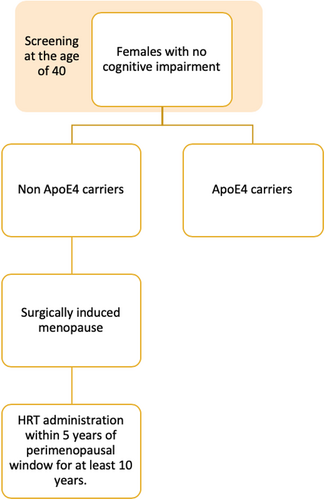
As for the subjects who had menopause induced surgically, hormone replacement therapy (HRT) has shown an improvement in cognition if taken within 5 years of the perimenopausal window, for at least 10 years.46 Other studies showed that older people, or those who are already cognitively impaired, showed elevated thrombotic events and a higher risk of dementia with HRT.50, 51 ApoE4 carriers also show minimal response to HRT compared to non-carriers in reducing cognitive decline.52-54 In another study, it even worsened cognitive decline.55 Other studies showed no interaction at all.56, 57 The studies should focus on the timing of HRT initiation as it is thought to affect the response of the subjects. It is recommended to start HRT as close to the menopausal critical window as possible to maximize the benefit.58 Estrogen has been described as a protective factor for cholinergic neurons which degenerate in LOAD.59, 60 As each female has a different age at menopause depending on their genetics, lifestyle, and medical conditions, HRT should be initiated accordingly to minimize the risk of AD.
Female ApoE4 carriers show higher tau levels (phosphorylated and total) in CSF, higher amyloid beta levels in the brain compared to male carriers.38, 61, 62 Enzyme levels such as beta-secretase and alpha-secretase were also increased in female apoE4 carriers in the cerebral cortex, and gamma secretase was increased in the hippocampus.62 Cholinesterase inhibitor Tacrine is one of the drugs used to alleviate the symptoms in people with AD. It has been shown that ApoE4 female carriers do not respond fully to the treatment as do non-carriers and male carriers.63 Some research suggests that at an older age, both sexes have a similar risk of AD.64
As men age, they also have decreased levels of testosterone, which has been linked to an increased risk of AD65 due to the diminished protective effect of testosterone on the deposition of Aβ.66, 67 This effect was also associated in ApoE4 allele carriers.68 In the treatment process of prostate cancer, men will be deprived of hormones, which will reduce the testosterone level in their body, also exposing them to a higher risk of AD. Nevertheless, hormone supplements, in large doses and in frail men, increased the risk for cardiovascular events.69, 70
5.4 Race
There is a difference in the prevalence of AD among different racial groups. Above the age of 65, AD has been diagnosed in 10.3% of whites, 12.2% of Hispanics/Latinos, and 13.8% of black/African Americans.1 It is hypothesized that this difference is due to the socio-economic status, lifestyle, and medical conditions such as diabetes and cardiovascular diseases.14, 71 Cardiovascular diseases and diabetes are more common in African Americans and Hispanics (Mexican-Americans or Caribbean-Americans). Lower levels of education and higher rates of poverty are also characteristics of the discrimination faced by African Americans and Hispanics.22 Another important issue is the misdiagnosis of African Americans, which is quite common. African Americans and Hispanics are 2 and 1.5 times, respectively, as likely to develop AD compared to non-Hispanic whites.14, 72, 73 Genetics seem to play a rather minor role in the racial/ethnic difference in AD prevalence.14, 22 African-American individuals have a strong association between ABCA7 and APOE4 with late onset AD (LOAD).74 Icelanders and populations of European ancestry have also shown an increased risk of AD with specific mutations in the ABCA7 gene.75 The CLU gene has been identified as a risk factor in Caucasian individuals, especially when it comes to the progression from MCI state to AD.76-79 TREM2 variant is likely not associated with AD in the East Asian population, however there is an association with Caucasian people.80
The Middle East and North Africa (MENA region) are lacking evidence-based research and reporting of numbers, therefore, there is no accurate estimation of AD cases among the races in these regions. Nevertheless, the WHO dementia report estimates that 6% of those above 60 years of age suffer from dementia in these regions. Other Arab regions like Dubai reported a 14% prevalence of dementia and AD.81 More research and focus should target these populations/races as carry valuable information about the disease pathogenesis. The difference in lifestyle and cultural traditions in the aforementioned societies in addition to their genetic make-up will explain possible differences in prevalence rates of AD.
5.5 Traumatic brain injury
Traumatic brain injury (TBI) not only accelerates the onset, but is also a risk factor for AD.82 Adults with a history of moderate or repeated TBI injury had a 2.3 times greater risk of developing Alzheimer than seniors with no history of head injury. Moreover, TBI accelerates the age of onset of cognitive impairment by few years. TBI triggers multiple biochemical cascades that lead to the release of neurotoxic substances that ultimately exacerbate neuronal cell death. The biological underpinnings of TBI and ensued neurometabolic cascade has been reviewed.162 Since we can achieve the reduction of secondary brain injury through a modification of these molecular pathways it represents PM opportunity to limit AD risk.
Research shows there may be a gene-environment interaction between the APOE gene and TBI which leads to an even higher risk of developing AD.14, 82 A proposed mechanism for this injury is direct axonal injury (DAI). With DAI, the cytoskeletal proteins of the neuron, including the APP which is cleaved to amyloid beta, presenilin 1, and the beta-site amyloid precursor protein cleaving enzymes accumulate.82 This neuron is thought to break and release AB into the interstitial space where it accumulates.
Aβ 42 was found in nearly 30% of the brains of TBI patients who died due to this injury, only a few hours after their injury.82-85 These plaques are diffuse, like the ones observed in early-onset AD (EOAD).86 In TBI survivors, studies showed mixed results regarding the increased amyloid beta. The reason for the mixed results was the specimen tested; some tested the ventricular fluid, while others tested the lumbar CSF fluid. Some studies showed an increase in Aβ 42 up to5 days after the injury, which then decreased to be comparable with the elderly controls.82 In addition, the mechanism of travel of amyloid beta from the interstitial fluid to the CSF is debatable. One might have an increased permeability due to the inflammation caused by the TBI. Caspase inhibitors show potential in inhibiting injury-induced caspase-3 activation by reducing Aβ concentrations, which leads to reduced neuron degeneration in TBI patients.82
Epidemiological data suggest that APOE, a lipid transport protein, has been shown to be associated with an increased risk of developing AD after the exposure to TBI. ApoE4 carriers also showed an increased accumulation of Aβ 42 following TBI. Individuals with the ApoE4 allele have also a poor prognosis after TBI compared with controls. Although, this information is still debatable, as there are many contradictory studies.82 In addition to the effect on amyloid beta, CSF tau levels were also 1000 times more elevated in TBI individuals compared to controls.87, 88 Another interesting finding was the interaction between the APOE gene and the neprilysin gene (the enzyme which degrades amyloid-beta, Aβ) polymorphisms in forming Aβ plaques. Together, these genes help to predict which subjects will accumulate Amyloid Beta following TBI. In sports and military, it is suggested to screen for those polymorphisms to identify individuals at high risk of developing Aβ plaques.89 Through autopsy-confirmed cases, TBI was found to hasten the onset of AD and diagnosis by 3.6 years.90 Another retrospective study also showed an earlier onset of AD in patients with TBI and ApoE4 independently.91 A prospective study following veterans from WWII with severe TBI showed an increased risk for AD, regardless of the quantity of APOE4 alleles.92 Mild TBI in Veterans from Iraq and Afghanistan showed a cortical thinning associated with affected episodic memory, both of which are present in AD.93 There was also an environmental-gene interaction between mild TBI and ABCA7 gene, which is a risk gene for EOAD and LOAD.93, 94 Some studies also neglect this association between TBI and AD,95 mostly because of recall-bias of cognitively impaired patients, which is not reliable.
5.6 Post-traumatic stress disorder
There is scarce data about psychosocial determinants of AD and post-traumatic stress disorder (PTSD) is one of those determinants which impose a higher risk of AD.96-98 One of the proposed mechanisms is chronic low-grade neuro-inflammation,99 another is epigenetics.100 PTSD is associated with alterations in the hippocampus (hippocampal volume101), anterior cingulate, and prefrontal structures,102 all of which are anatomical structures involved in AD pathology. A case control study of PTSD veterans compared to healthy veterans showed an increase in serum glial fibrillary/serum glial fibrillary acidic protein, tumor necrosis factor-alpha, interleukin 6, and MMP2 and MMP9 and a decrease in brain-derived neurotrophic factor and nerve growth factor-beta (NGFβ), which are indicators of brain damage. These would not be seen by brain scans such as magnetic resonance imaging103 and therefore are potential early biomarkers for nervous system damage diseases like PTSD before the clinical onset. Because PTSD is a risk factor for AD, these biomarkers could potentially show the early pathogenesis that lead later to AD. Alternatively, these biomarkers can be used to distinguish the etiology of AD, further aiding the treatment. These biomarkers are especially useful because some studies showed that the odds of a PTSD patient testing positive for amyloid is very low.102
The newest study linking PTSD and LOAD suggests that there is genetic pleiotropy between these two disorders. MS4A gene family seemed to play the most significant role. The study suggested a role of the immune system and regulation of cell activation in both LOAD and PTSD. There was a moderate level of polygenic overlap, which can be used in the risk stratification process for AD.104
6 MODIFIABLE RISK FACTORS OF AD
Modifiable risk factors are those that one can change to reduce the chance of developing AD. There are many risk factors to consider that involve physical health, mental health, nutrition etc. For instance, obesity and lack of physical activity, stress and depression, poor diet, disease co-morbidity in particular diabetes and high-blood pressure are all contributing factors. The strongest known modifiable risk factors for dementia and AD are alcohol consumption, smoking and high cholesterol. Therefore, a healthy lifestyle that takes care of both body and brain is effective to mitigate AD risk. This holistic approach of use of “predominantly wholesome diet, regular physical activity, restorative sleep, stress management, avoidance of risky substances and positive social connection is termed lifestyle medicine”.105
6.1 Alcohol consumption
Previous literature showed mixed results regarding alcohol consumption and AD development. Some studies showed that the APOE4 allele and alcohol consumption increased the risk of dementia106 or cognitive decline,107 which also increased with alcohol quantity.106 Regardless of the ApoE allele, binge drinking was shown to increase the risk of HIV associated dementia by a mechanism which is unknown but is thought to include glial cells activation and neuronal death.108 Alternatively, alcohol consumption led to increased hippocampal volume in APOE4 carriers without dementia.109
The amount of alcohol is also a debate point and mild consumption could increase the cognitive function, especially in APOE4 carriers,110 while moderate consumption had benefits on the white matter of the brain.111 Nondrinkers who were carriers for APOE4 showed a quicker decline in memory than controls.112 Contradictory studies showed alcohol consumption of all levels was associated with more atrophy of the hippocampus113 and an earlier age of AD onset.114 A level above 48 g/day was also associated with reduced hippocampal volume.115
Nondrinkers seemed to have reduced cortical thickness compared with drinkers (below 24 g/day). This might suggest that moderate alcohol consumption is associated with better structural brain health than no alcohol.115 People with a high AD 33-SNP polygenic risk score (the higher the score the higher the risk of developing AD) who consumed 12-24 g/day alcohol showed more reduction of the cortical thickness.115
A systematic review of 28 articles published after the year 2000 even suggests that a moderation in alcohol intake might even lower the risk for dementia development and dying from dementia.116-118 Red wine was especially beneficial because of the presence of bioflavonoids, the morin, and resveratrol, which are natural antioxidants.116 Drinking beer was also associated with lower Aβ aggregation in the brain.118 Nevertheless, more than 60 g/day for men and 40 g/day for women or heavy irregular drinking increased the risk of dementia.117 Other studies stated a level of 38 g/day as excessive and increased the risk for AD.118 However, moderate consumption is a vague term. Although118 stated modest alcohol intake as 6 to 12.5 g/day, and should be specified according to specific alcohol types,118 and dependent on the individual's genetic makeup, ethnicity, age, and sex.
Nevertheless, it is difficult to determine the link between alcohol and AD due to additional factors such as survivor bias, recall bias, amount of alcohol, variations of alcoholic drinks, and assessment only at baseline.117 This should be also taken into consideration. More high-quality research should be done in this area, since age, sex, ethnicity; genetics are all factors that affect the amount of alcohol that is safe for each individual. There is no specific level that is safe for everyone.
6.2 Smoking
Smoking does not only increase cardiovascular risk but cigarettes also contain neurotoxins. Both of these factors increase the risk of AD. There has been an increase in smoking in the Eastern Mediterranean and Africa in parallel to a decrease in other countries.119 A study showed that there was a higher risk of AD among current smokers with the APOE-ε4 allele and no increased risk among smokers who had quit.120
6.3 Hyperlipidemia
Cholesterol synthesis in the brain is carried out mainly by oligodendrocytes and astrocytes, which contain hydroxy-methylglutaryl-coenzyme A reductase. Neurons also synthesize cholesterol, but to a lesser extent. It leaves the oligodendrocytes and astrocytes by being transported by ApoE and then secreted to the extracellular matrix via ABCA1 and ABCG1 transporters.121, 122 This cholesterol then binds LDL-R receptors on neurons. Cholesterol in the brain is important for synapse formation, development, and transmission in addition to dendrite differentiation.122 Genetics might be able to explain this phenomenon.123
Peripheral cholesterol does not move freely to and from the brain due to the blood brain barrier.122 This type of cholesterol is either produced in the liver or acquired through diet, and in case of elevated levels of it, it is hypothesized to damage the blood brain barrier.124 The 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) catalyzes the rate-limiting step of cholesterol biosynthesis.6 A SNP at −911 in the HMGCR gene has been associated with both an increased risk of AD and an accelerated clinical progression of the disease.125 The mechanism might be due to an effect on the APP processing and Aβ production, or reduced inhibition by Aβ 40, therefore increasing cholesterol and increasing the risk of disease (as shown in “A-allele” carrier patients in contrast to “C-allele” carriers which have a reduced risk of AD).125, 126 This SNP seems to exert its effect differently depending on race. Swedish people, for example, do not exhibit an increase in AD risk if they have this SNP, regardless of whether they carry the ApoE4 allele or not.127 Another SNP in the HMGCR gene is rs3846662, which was shown to affect MCI in females regardless of ApoE4.128 This same SNP might also be protective like HMGCR's rs3846662 G negative status in French Canadians, which delayed the onset of AD by 3.6 years and so modified the risk of AD and also decreased the conversion rate from MCI to AD.129 Additionally, Northern Han Chinese population was at reduced risk of LOAD when presenting with the minor A allele at rs3846662 of the HMGCR gene in APOE4 non-carriers.130 In a Spanish population, the rs3931914 GG genotype together with the TT genotype of ABCA1 rs1800977, posed a greater risk of developing AD. The ABCA1 rs1800977 TT genotype showed an increased risk of developing AD regardless of ApoE4 variants.121
Hyperlipidemia is a cluster of conditions, which can range from elevation in the cholesterol to triglyceride or low-density lipoprotein (LDL) level in the blood. It has been associated with higher risk of AD.120, 124, 131 Low levels of LDL cholesterol might reduce the risk of AD.132 Lipid lowering agents are thus at the frontline in the strategy of AD prevention. But this information is still controversial.120 It has been found that out of all cardiovascular risk factors, hyperlipidemia has the most genetic commonalities with AD.123, 133 The genetic variants were among these genes: APOA4, ATP-binding cassette transporters, such as ABCA1 and ABCG5, and phospholipases, such as ATP8B4 and LIPG.123, 134 According to the AlzForum (http://www.alzgene.org/default.asp), ABCA1, ABCG5 and APOA4 are found in AD.
Genetic pleiotropy is one very relevant discussion point when assessing two disorders comparing their genetic risk factors. One gene could be common to the pathogenesis of both diseases and allows us to understand more about these diseases. Polymorphisms associated with triglycerides, HDL, LDL and C-reactive protein (CRP) have also been associated with a higher risk of AD. Namely rs13113697 (on chromosome 4; closest gene, HS3ST1) and rs7920721 (on chromosome 10; closest gene, ECHDC3), were genome-wide significant. Also, HS3ST1 and ECHDC3 transcript expression were different in AD brains compared with control brains.133 This information is still inconsistent and therefore warrants further extensive research.
Some of the most popular drugs to treat high cholesterol that are presumed to lower risk of dementia are discussed below and shown in Table 2.
| Chemical class | Target | Drug structure | Reference |
|---|---|---|---|
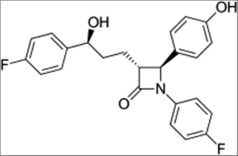
| Statins | HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase) | Representative of the family, Atorvastatin, also known as Lipitor
|
PubChem Comment: Simvastatin (Zocor) is another popular statin |
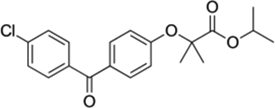
| Fibrates | PPAR (peroxisome proliferator-activated receptors) | Representative of the family, Fenofibrate, also known as Ticor
|
PubChem Comment: contraindicated in combination with statins |
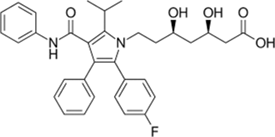
| Ezetimibe | Niemann-Pick C1-like 1 (NPC1L1) protein Also, caveolin 1–annexin A2 complex involved in trafficking cholesterol |
|
PubChem Comment: indicated in the USA as an add-on to dietary measures to reduce levels of certain lipids |
6.3.1 Statins
Statins are HMG-CoA reductase inhibitors, Lovastatin, the first marketed drug was a compound isolated from Aspergillus terreus. Statins have been shown to reduce the risk of AD, and improve cognitive impairment in some cases.163 The response to statins (shown by the lowering of LDL-c level) is determined by phenotypic (smoking, ancestry and age) and genetic factors. Some of the genes responsible for the variable response to statins include regulators of cholesterol metabolism such as HMGCR, APOE, PCSK9, ACE, and LDLR and several SNPs in the LPA and APOE/TOMM40 loci. Alone, these genes and SNPs account only for 4% of the variability to statins. However, gene-gene interactions and a combination of SNPs (polygenic risk) have the potential to predict 15% of the variance.6
Some genes affect the response of subjects to medications such as statins. ApoE-4allele carriers showed poorer response to statin treatment in contrast to the ApoE-2 allele carriers. The latter were also associated with larger cholesterol-lowering effect.135, 136 ApoE3 homozygotes respond to statins based on the sex. Females show better response to treatment compared to males.137 An important gene for statins is the HMGCR gene, which codes for the HMG-CoA reductase enzyme, the target of statins. SNPs rs17244841 and rs17238540, were associated with attenuated LDL cholesterol response to pravastatin and simvastatin.138
AA genotype at rs3846662 is thought to cause differences in HMGCR splicing and lead to higher baseline total and LDL cholesterol, and a lower response to statins in women.138 Atorvastatin response might also depend on the rs17671591 T allele which confers greater LDL reduction and HDL increase.138 The variability in the HMGCR gene in the form of rs17238484 G allele seems to be protective in the normal population by informing lower cholesterol LDL levels.138
The gene-gene effect also plays a role. A combination of ApoE-2 allele and ACE insertion deletion (I/D) has shown more LDL-c level reduction when treated with statins.136 TLR4 299Gly allele in combination with ApoE-2 or ApoE-4 shows a negative effect on LDL-c levels unless treated with statins. On the other hand, TLR4 299 Gly in combination with ACE (I/D), does not benefit from receiving statin therapy, as their LDL-c levels are already low without treatment.136 CYP7A1 C-204C homozygotes with an ApoE-4 allele have minimal effect on LDL cholesterol in response to atorvastatin. In contrast, ApoE-2 allele carriers with at least one CYP7A1 C-204C show a much better response to atorvastatin.135
PCSK9 is a gene, which regulates the number of LDL-receptors on the cell surface. It has a positive correlation with LDL-c level.139 Two loss of function (LOF) PCSK9 variants were identified, and carriers had 40% lower LDL-c compared to controls.140 Statins can upregulate LDL receptors as well as PCSK9 expression. The response to statins is thought to be larger when the individual carries a LOF PCSK9 variant gene compared to controls.139 Rs11591147 is a known LOF variant in the PCSK9 gene. This variant is expressed differently on LDL-c levels depending on the race of the individual and the specific statin and the age used. Rosuvastatin and Atorvastatin (Lipitor) work best, while Pravastatin works in a poorer pattern in the elderly population aged 70-82.141 Caucasians do not seem to be affected by this variant, while European Americans and African Americans LDL-c levels are affected by it. Some studies suggest that PCSK9 inhibitors might be more useful in the prevention of AD compared to statins.142 The authors prefer the personalized treatment approach according to age, genetics, race, and sex instead of a one-drug-fits all approach.
6.3.2 Fibrates
Fibrates are a chemical class of amphipathic carboxylic acids utilized to treat various metabolic disorders. They are another class of medications that act as agonists of peroxisome proliferator-activated receptor (PPAR encoded by PPARA), which leads to an increase in HDL and lowering triglycerides (TG), and a minimal LDL lowering effect138 and in that class Fenofibrate is the most popular one. The level of increase in HDL is dependent on a SNP near APOA1 (rs964184), which encodes a downstream target of PPAR.138 This same SNP also determines the response to fibrates in terms of TG, in addition to another SNP (rs3135506), which is specifically associated with the response to Fenofibrate.138
6.3.3 Ezetimibe
Ezetimibe is a cholesterol absorption inhibitor. Its mechanism of action is through targeting the Niemann-Pick C1-like 1 protein (encoded by NPC1L1), a protein involved in cholesterol absorption. Differences in response to ezetimibe may be due to the sterol regulatory binding protein 1 (SREBP-1c) gene, which, when homozygous for the minor allele of a SNP, may have variable cholesterol absorption. Variants of the NPC1L1 gene itself are also associated with the variable response of LDL cholesterol to ezetimibe.138
7 CONCLUSION-TOWARD A NEW PARADIGM
Current medicine mainly uses a one-size-fits-all approach. PM transforms the way medicine is practiced and delivered. PM has the conviction that the same disease manifestation can have a different cause, course or therapeutic efficacy, depending on the patient. Therefore, an individualized approach to each patient is crucial. PM utilizes clinical, genomic, environmental and lifestyle data that are unique for each patient to prevent, diagnose- including early detection- or decide on disease treatment. Precision medicine from a population neuroscience perspective demands the deployment of AI and machine learning to recognize patterns resulting from superimposing clinical data and genetics information, and environmental and lifestyle risk factors.
Precision medicine paradigms of AD focus on genetic risk stratification at the beginning of each screening. Genetic risks such as single SNPs, or variable gene variants account for only a minority of the population but have a major impact on prevention and treatment. To highlight this, genetic SNPs and variants should not be a single factor in the risk stratification phase of individuals. They should be embedded into the paradigm of precision medicine in AD. Recommendations based on specific gene-environment interaction, gene-gene interaction, or other interactions should be at the forefront.
A polygenic risk score is also a viable strategy to identify individuals at high, moderate, and low risk levels.143 It emphasizes the need to look at population genetics instead of focusing on European studies. This approach requires a great deal of work to determine the different polygenic risk scores according to various ethnicities and races. In the future (maybe 50 years or more), there will be a greater number of individuals with mixed ethnicities, which will allow for a more general polygenic risk score independent of race or ethnicity. We should adopt a holistic approach for each individual and take into consideration patients' individuality; no two AD patients are alike. The combination of risk single nucleotide polymorphisms (SNPs), environmental exposure, education, awareness, lifestyle, and socioeconomic status account for the prevalence of the disease. The risk factors for each subject is their biomarker for dementia, and the subjects with the highest number of risk factors are the ones that are going to benefit from clinical trials with preventative drugs.
7.1 Treatment and prevention
AD therapy and prevention should be tailored to each individual according to the etiology of the disease. Table 3 contains some examples. A new therapeutic target for AD, especially in subjects with PTSD, is to target the mast cell associated inflammation that occurs in the brain.99 We propose using Cromolyn in the prevention of AD of PTSD etiology, in particular. Cromolyn is a mast cell stabilizer, which affects amyloid beta deposition and might potentially be therapeutic in some cases of AD.144 Cromolyn, in combination with ibuprofen, is currently also being tried in a clinical trial for AD patients in general (https://clinicaltrials.gov/ct2/show/NCT02547818?term=ALZT+OP1). Another potential treatment might also be Montelukast (discussed below), which is used for mastocytosis as adjunct therapy.145, 146
| Etiology | Prevention or therapy | Proposed medication | Rationale |
|---|---|---|---|
| PTSD | Prevention or therapy | Cromolyn | Mast cells are associated with inflammation that occurs in the brain. Cromolyn is a mast cell stabilizer which affects amyloid beta deposition. |
| Ischemic stroke | Prevention or therapy | Montelukast | CysLT receptors are upregulated in cerebral ischemic conditions. Montelukast blocks CysLT1 receptor. |
| Hyperlipidemia | Prevention | Choice of antihyperlipidemic agent based on pharmacogenomics | Even though it is still inconclusive whether hyperlipidemia is a cause of AD, studying the response of hyperlipidemic patients to drugs, and choosing the most appropriate drug based on genetics, age, and sex may prevent cardiovascular side effects, and in the far future, AD. This could make a promising longitudinal study. |
| Surgically induced menopause | Prevention | Hormone replacement therapy | Women who are not ApoE4 carriers should receive HRT within 5 years of perimenopausal window for at least 10 years. This should be avoided in already cognitively impaired individuals with old age. |
| Interleukin-1 receptor accessory protein (IL1RAP) rs12053868-G carriers | Treatment | Targeting microglia or interleukin receptors | IL1RAP rs12053868-G carriers are at higher risk of progressing from MCI&$$$; to AD. This SNP is associated with reduced microglial activation and more amyloid deposition. |
- Abbreviations: AD, Alzheimer disease; PTSD, post-traumatic stress disorder.
Montelukast is a leukotriene receptor antagonist, which works by blocking the CysLT1 receptor.147 CysLT receptors are upregulated in cerebral ischemic conditions.148 Polymorphonuclear leukocytes reach the cerebral ischemia site and release free radicals, proteases, and leukotrienes, which exacerbate the injury leading to irreversible damage.149 Montelukast has been shown to be neuroprotective in a dose and time dependent manner in mice with focal cerebral ischemia.150 The antioxidant, anti-inflammatory, and anti-apoptotic effects of this drug were also shown to provide benefits in another study on mice with cerebral ischemia.149 This illuminates the potential use of Montelukast for the prevention of AD in ischemic stroke patients. Montelukast has already been used in some clinical trials.151 Studies injecting mice with amyloid beta, and subsequently treating them with Montelukast, showed positive results.147 Another study demonstrated the positive neuroprotective effects of Montelukast on amyloid beta levels by down-regulating CysLT1R-mediated NF-κB signaling.152 The misconception of the one-drug-fits-all approach should be avoided by specifying the treatment option for a single group of patients, which in the opinion of the authors are ischemic stroke affected patients.
Treatment of hyperlipidemia should be based on pharmacogenomics as a prevention method for AD. Even if it is still inconclusive whether hyperlipidemia is a cause of AD, studying the response of hyperlipidemic patients to drugs, and choosing the most appropriate drug based on genetics, age, and sex, may prevent cardiovascular side effects and, in the future, AD. The authors suggest a relevant longitudinal study, which could widely benefit a great number of patients.
Subjects with LOF variants of the PCSK9 gene should be assessed for dementia later in life to see if the lower baseline LDL-c levels affect cognition and brain physiology. This can further help in assessing the etiology of AD and the role of PCSK9 in AD.
HRT should be administered in women with surgically induced menopause who are not ApoE4 carriers within 5 years of perimenopausal window and for at least 10 years. Individuals who are already cognitively impaired or are older should avoid HRT (Figure 1).
Personalized biomarkers for veterans or PTSD susceptible people include plasma/serum glial fibrillary acidic protein, tumor necrosis factor-alpha, interleukin 6, and MMP2 and MMP9 and a decrease in brain-derived neurotrophic factor and nerve growth factor-beta. These biomarkers aid in the early diagnosis of PTSD that might lead to AD. The data from this group will guide personalized treatment according to this specific etiology (Figure 2).
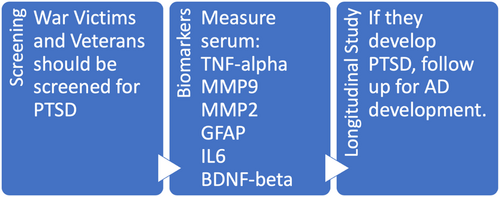
7.2 Risk stratification
Familial AD patients show symptoms at different ages. The age of onset cannot be predicted based solely on familial genetics. Additional genetic risks must either exhibit increased risk or protection from AD.153, 154 We suggest that while doing GWAS, more stratification should be done on people with FAD, dividing them depending on when they showed symptoms, and accordingly identifying SNPs related to increasing the risk, delaying the onset of AD, or modifying the course of the disease.
Interleukin-1 receptor accessory protein (IL1RAP) rs12053868-G carriers are at higher risk of progressing from MCI to AD. This SNP is associated with reduced microglial activation and more amyloid deposition.14 We suggest that this SNP should be added to the risk stratification guideline, since these individuals are at higher risk of AD than controls. When developing a treatment strategy for these individuals, it might also be beneficial to target microglia or interleukin receptors.
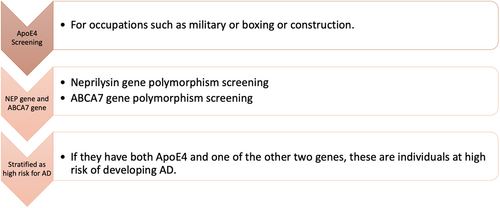
Individuals with occupations such as boxers, construction workers, and soldiers, who are at high risk of developing TBI should be screened for the ApoE4 allele and ABCA7 gene mutations, and placed under the high-risk criteria in the risk stratification phase of AD. Figure 3 shows a proposed strategy for risk stratification according to occupation.
7.3 Diagnosis and prognosis
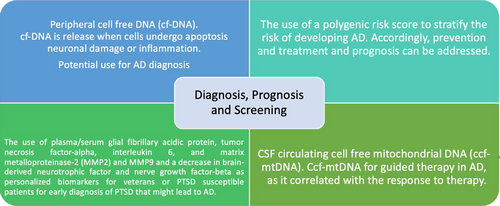
Peripheral cell-free DNA (cf-DNA) is a relatively new diagnostic method that has great potential for the diagnosis of AD.164 In individuals who experienced acute ischemic strokes, higher levels of cf-DNA were associated with worse prognosis and correlated with the severity of stroke. In the future, the type of intervention may be determined according to cf-DNA levels. Research suggests that cf-DNA is released from the cells when they undergo apoptosis, neuronal damage, or inflammation,155 all of which also occur in AD. We propose integrating cf-DNA in the diagnostic procedure of AD to assess its applicability, as it is noninvasive and can be used as routine screening. This can also be specified, as in the diagnosis of AD of ischemic stroke etiology.
CSF circulating cell free mitochondrial DNA (ccf-mtDNA) was shown to correlate negatively with PD and AD.156, 157 The reason is still unknown. The level of plasma ccf-mtDNA was shown to correlate with the un/responsiveness of drugs156 and may potentially guide therapy for AD, as well as lower the expenses of unnecessary treatments and interventions. Figure 4 summarizes the screening, prognosis, and diagnostic recommendations.
8 FUTURE PERSPECTIVES
PM ability to classify individuals into subpopulations that differ in their susceptibility to a particular disease or their response to a specific treatment offers great promise to manage AD. Currently, definite diagnosis of AD can only be achieved by post mortem autopsy. Future research should focus on finding early biomarkers for the disease so that disease-modifying agents can be administered in the early stages and reach their full potential.
The proteomics field has also shown significant potential in explaining disease pathogenesis of AD in terms of biology, finding new therapeutic targets, and potential biomarkers (diagnostic and prognostic). Brain tissue has been used to identify the proteins in the brains of AD patients and controls. The next step is to extrapolate these data to body fluids (CSF and blood). The most promising technique would be ultra-deep serum discovery by TMT-LC/LC-MS/MS, and a validation experiment by TOMAHAQ targeted LC-MS3.158-161 Blood biomarkers should be the focus in the future as it is a minimally invasive technique and routinely done procedure.
Another approach gaining traction is clinical genomics sequencing as nest generation sequencing cost has been lowered tremendously to make it affordable, routine valuable tool. With massive parallel sequencing, it is anticipated that there will be new wave of AD gene discovery. This would have wide-ranging clinical implications from diagnostic and predictive testing to drug development to go beyond the restricted focus to only early insights of the molecular mechanisms and pathways involved in AD that solely followed three AD hypotheses: the cholinergic, amyloid cascade, and tau hypotheses.
PM molecular profiling using integrated 'omics' approaches should lead to considerable progress in a complex disease like AD. This would permit shifting the equation toward prediction and prevention including paving the way to druggable targets for better therapy outcomes.
In the future, identifying high-risk individuals as mentioned in this review, and focusing on the prevention of AD also has tremendous value in the future for slowing the progression of the disease. Diagnosis and screening, like treatment, should be individualized and tailored to specific etiologies. This can be achieved by moving towards a mixed biological diagnosis mode, in which we understand the pathogenesis of the disease on a molecular basis and therefore apply appropriate treatment and prevention strategies.
Abbreviation
-
- ACE
-
- angiotensin converting enzyme
-
- AD
-
- Alzheimer disease
-
- ADNI
-
- The Alzheimer disease neuroimaging initiative
-
- APOE
-
- apolipoprotein E
-
- Aβ
-
- amyloid beta
-
- CDC
-
- Center for Disease Control and Prevention
-
- CSF
-
- cerebrospinal fluid
-
- DALY's
-
- disability adjusted life years
-
- HMGCR
-
- HMG-CoA reductase
-
- HRT
-
- hormone replacement therapy
-
- LDL-c
-
- low density lipoprotein concentration
-
- LDLR
-
- low density lipoprotein receptor
-
- LPA
-
- lipoprotein(a)
-
- MCI SAGE
-
- mild cognitive impairment self-administered gerocognitive exam
-
- MRI
-
- magnetic resonance imaging
-
- PCSK9
-
- proprotein convertase subtilisin/kexin type 9
-
- PD
-
- Parkinson disease
-
- PET
-
- positron emission tomography
-
- PIB
-
- pittsburgh compound-B
-
- PM
-
- precision medicine
-
- PPD
-
- postpartum depression
-
- PTSD
-
- post-traumatic stress disorder
-
- SNP
-
- single nucleotide polymorphism
-
- TBI
-
- traumatic brain injury
-
- WHO
-
- World Health Organization
ACKNOWLEDGEMENTS
The authors want to thank their respective institutions for their continued support. A sincere thank you to Mr. Thomas Litecky and Ms. Tessa Litecky for their diligent scientific proofreading of the paper. Language editing by The Editing Refinery, MD, USA is highly acknowledged.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interests.
AUTHOR CONTRIBUTIONS
Jihan Azar and Mohamed Salama performed literature research, gathered and analyzed information, and generated short preliminary write-ups. Buthaina Al-Balushi and Saravana Babu Chidambaram supported wide ranging aspects of the manuscript development process. Mohamed Salama, Musthafa Mohamed Essa, and M. Walid Qoronfleh conceptual work, framework, draft write-up, critical reading and editing. All authors read and approved the final manuscript.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
This is a review article. All data generated or analyzed during this study are included in this published article.