Detection of the Human Anti-ActRII Antibody Bimagrumab in Serum by Means of Affinity Purification, Tryptic Digestion, and LC-HRMS
Abstract
Purpose
Inhibitors of the ActRII signaling pathways represent promising therapeutics for the treatment of muscular diseases, but also pose risks as performance-enhancing agents in sports. Bimagrumab is a human anti-ActRII antibody which was found to increase muscle mass and function by blocking ActRII signaling. As it has considerable potential for being misused as doping agent in sports, the aim of this study was to develop a mass spectrometric detection assay for doping control serum samples.
Experimental design
Within this study, a detection method for Bimagrumab in human serum was developed, which combines ammonium sulfate precipitation and affinity purification with proteolytic digestion and LC-HRMS. To facilitate the unambiguous identification of the diagnostic peptides, an orthogonal IM separation was additionally performed.
Results
The assay was successfully validated and the analysis of clinical samples demonstrated its fitness for purpose for an application in routine doping control analysis.
Conclusions and clinical relevance
Although no myostatin inhibitors have obtained clinical approval yet, the proactive development of detection methods for emerging doping agents represents a key aspect of preventive doping research. The presented approach will expand the range of available tests for novel protein therapeutics and can readily be modified to include further target analytes.
1 Introduction
Skeletal muscle mass is negatively regulated by myostatin (GDF-8/growth differentiation factor-8) and other members of the transforming growth factor beta (TGF-β) superfamily.1-7 The cytokines exert their biological functions through binding to activin type II serine/threonine kinase receptors (ActRII), with myostatin having a slightly higher affinity for ActRIIB than ActRIIA.5-8 Following activation, ActRIIA or ActRIIB form a complex with an activin type I receptor (activin like kinase 4 or 5), which results in receptor autophosphorylation and the recruitment and phosphorylation of the transcription factors SMAD2 and SMAD3.3, 6, 7, 9 Phosphorylated SMAD2 and SMAD3 then form a complex with SMAD4, translocate into the nucleus and modulate cellular gene expression (e.g., repression of the myogenic regulator MyoD6, 7, 10). The phosphorylation of SMAD2 and SMAD3 additionally leads to the inhibition of the AKT/mTOR pathway (AKT = protein kinase B, mTOR = protein kinase “mammalian target of rapamycin”) involved in muscle differentiation and hypertrophy.9, 11, 12
Specific blockade of the ActRII signaling pathways may provide important therapeutic approaches for the treatment of muscle disorders. Drug candidates specifically targeting myostatin along with candidates with multi-targeting properties affecting various growth factors are currently under clinical investigation.4, 13 The first treatment strategies focused on myostatin inhibitors such as anti-myostatin antibodies or a modified version of the myostatin propeptide, which specifically neutralize the circulating cytokine.4, 7, 13 However, the inhibition of myostatin alone in adult humans was found to be rather ineffective, suggesting that myostatin predominantly regulates skeletal muscle mass during embryonic development.9, 14, 15 Consequently, approaches targeting multiple ligands of the TGF-β superfamily might be more beneficial for the therapy of muscular diseases. Current strategies include, for example, Fc fusion proteins acting as receptor competitors (e.g., ACE-031/ActRIIB-Fc) and neutralizing therapeutic antibodies.7, 9
Clinical Relevance
Therapeutic antibodies represent an emerging and continuously growing class of pharmaceuticals and comprise several drug candidates with potential performance-enhancing properties. Consequently, mass spectrometric assays for their detection in biological samples are of great value for both the pharmaceutical industry and preventive doping research. Bimagrumab is a human ActRII inhibitory antibody which has already undergone advanced clinical trials in the context of treating different muscle wasting disorders. Due to its effects on muscle mass and function, Bimagrumab has generated concerns regarding its potential for being misused in amateur and professional sport. As a consequence, the antibody has been classified as prohibited in sports, necessitating appropriate test methods that provide both specificity and sensitivity and allow for utmost retrospectivity. Within this study, affinity purification, tryptic digestion, and LC-IM-HRMS were employed for the unambiguous detection of Bimagrumab in doping control serum samples. A comprehensive characterization of the procedure was conducted and its applicability to authentic biological samples was successfully demonstrated by analyzing a set of clinical samples. The presented assay expands the range of available tests for novel protein drugs and all steps of the protocol can readily be modified to include other protein-based therapeutics.
Therapeutic antibodies are an emerging class of pharmaceuticals developed for the treatment of cancer, autoimmune diseases, and infections.16, 17 They are characterized by a high specificity and affinity for their antigen as well as a long half-life,16, 17 and principal mechanisms of action include cytokine and growth factor inhibition, receptor blockade and modulation, cellular depletion, and modulation of signaling pathways.17, 18 Bimagrumab, developed by Novartis, is a human monoclonal antibody competitively binding to ActRIIA and B, thus preventing interaction with endogenous ligands such as myostatin, activin A, and GDF-11.2, 4, 9 Inhibition of ActRII downstream signaling was found to significantly reduce SMAD2/3 phosphorylation, thus increasing both differentiation of myoblasts and hypertrophy of myofibers.9 For that reason, Bimagrumab could be a promising therapeutic for the treatment of muscle wasting disorders.2, 3, 9, 13, 19
Due to their impact on muscle mass and function, inhibitors of the ActRII signaling pathways represent compounds potentially misused as performance-enhancing agents in sports.7 In the current version of the Prohibited List published annually by the World Anti-Doping Agency (WADA), agents modifying myostatin function are included in section S4 “Hormone and metabolic modulators.”20 Hence, test methods are required to enforce these regulations and within this study, affinity purification, proteolytic digestion, and LC-HRMS were employed to develop a detection method for Bimagrumab in human serum. The assay was successfully validated and can readily be modified to include further protein-/antibody-based drugs.
2 Experimental Section
The aim of this study was to develop a detection method for the human anti-ActRII antibody Bimagrumab in doping control serum samples. An overview of the assay's principle is given in Figure 1. First, antibodies are enriched from 200 μL of serum by ammonium sulfate precipitation. To further extract the target analyte from the precipitate, affinity purification with ActRIIB-Fc conjugated magnetic beads is used. Purified proteins are then subjected to tryptic digestion and characteristic peptides of Bimagrumab finally detected by LC-HRMS.
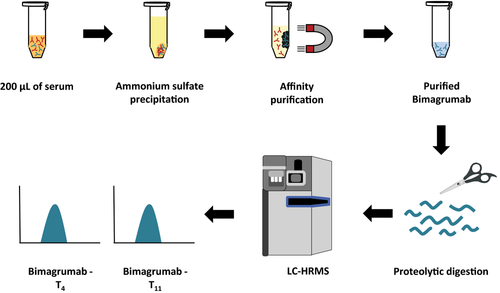
2.1 Reference Material
Bimagrumab is a human monoclonal antibody of the immunoglobulin G1 (IgG1)/λ isotype subclass, which is expressed in Chinese hamster ovary (CHO) cells and directed against human ActRII.
A solution for infusion or injection containing 150 mg mL−1 of Bimagrumab was provided by Novartis (Basel, Switzerland) and stored at 2–8 °C.
2.2 Internal Standard and Quality Controls
Stable isotope-labeled [13C-Lys, 13C-Arg] Bimagrumab was produced by Novartis and used as internal standard (ISTD) in order to monitor sample preparation and analysis. Each serum sample (volume: 200 μL) was fortified with 100 ng of ISTD (final concentration 0.5 μg mL−1) and subjected to antibody extraction as described below.
In order to prove adequate performance of the assay in routine doping control analysis, both a positive and a negative external control sample will be prepared with each analytical batch.
2.3 Serum Samples
Human serum obtained from male AB plasma was purchased from Sigma-Aldrich (St. Louis, MI, USA) and used for method development and validation. To determine the specificity and identification capability of the assay, serum samples were collected from ten healthy volunteers (five male and five female). Ethical approval from the local ethical committee (#137/2015) and written informed consent provided by the participants were obtained.
Authentic clinical samples were kindly provided by Novartis and analyzed as described below to serve as proof-of-concept for the applicability of the presented approach. Elderly healthy male subjects (average age: 73 years) received either intravenous or subcutaneous injections of Bimagrumab or placebo and serum samples were drawn both before and after drug administration. Samples that were found to contain analyte concentrations above the upper limit of the working range (1 μg mL−1) were re-assayed after dilution with commercial human serum prior to sample preparation. Bimagrumab concentrations were estimated by using a calibration curve ranging from 0.025 to 1 μg mL−1, and a positive quality control fortified with 0.1 μg mL−1 of the analyte was analyzed with each sample batch. As each batch included at least one pre-administration sample, no additional negative control was analyzed.
2.4 Isolation of Bimagrumab from Human Serum
2.4.1 Binding Properties of Bimagrumab
As the specificity of Protein A was found inappropriate for the isolation of Bimagrumab from human serum (data not shown), both ActRIIA and ActRIIB were considered as potential ligands for affinity purification. Recombinant fusion proteins of the extracellular domains of ActRIIA and B and the Fc fragment of human IgG1 were obtained from R&D Systems (Minneapolis, MN, Canada) and the binding preference of Bimagrumab for the receptor domains was investigated by means of SDS-PAGE and Western blotting.
First, 100 ng of ActRIIA-Fc or ActRIIB-Fc was mixed with 5 μL of lithium dodecyl sulfate (LDS) sample buffer (Thermo Fisher Scientific, Waltham, MA) and 2 μL of DTT (1 M, Sigma-Aldrich). To achieve protein denaturation and reduction of disulfide bonds, samples were heated for 10 min at 70 °C. Each fusion protein was additionally prepared without DTT and kept at 4 °C until analysis. Proteins were separated on a 12% Bis-Tris mini gel (10-Well, 8 × 8 × 0.1 cm, Thermo Fisher Scientific) for approximately 90 min at a constant voltage of 125 V (XCell SureLock Mini-Cell and MOPS running buffer, Thermo Fisher Scientific; power supply SE260, GE Healthcare, Little Chalfont, Buckinghamshire, UK). No antioxidant was added to the cathode buffer. Subsequently, proteins were transferred to a PVDF membrane (Merck Millipore, Darmstadt, Germany) by using a semi-dry transfer unit (1 mA cm−2, 45 min, GE Healthcare) in combination with a transfer buffer containing 39 mm glycine, 48 mm Tris, 0.0375% SDS, and 20% ethanol.21 The membrane was blocked for 1 h at RT with 5% w/v skim milk in PBST (Phosphate Buffered Saline Tween 20: 0.01 m phosphate buffer, 0.0027 m KCl, 0.137 m NaCl, and 0.02% Tween 20, pH 7.4) in order to prevent unspecific protein binding. Bimagrumab was diluted to a concentration of 1 μg mL−1 (in 1% w/v skim milk in PBST) and added to the membrane, which was incubated with the antibody overnight at 4 °C. For the detection of Bimagrumab, a goat anti-human secondary antibody coupled to horseradish peroxidase (Sigma-Aldrich) was diluted in 1% skim milk (1:10000) and membranes were incubated for ≈45 min at RT. Peroxidase activity was detected using SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific, 5 min, RT) and images were recorded with an ImageQuant LAS 4000 CCD camera system (GE Healthcare).
2.4.2 Magnetic Beads Preparation
As Bimagrumab was found to bind with higher affinity to ActRIIB than ActRIIA, ActRIIB-Fc was used as ligand for affinity purification. ActRIIB-Fc conjugated magnetic beads were prepared by using NHS (N-hydroxysuccinimide) Sepharose (GE Healthcare) according to the manufacturer's instructions. Dimeric ActRIIB-Fc was diluted in coupling buffer (0.2 m NaHCO3, 0.5 m NaCl, pH 8.3) to a final concentration of 100 μg mL−1. A total of 25 μL of the medium slurry was transferred to an Eppendorf tube and placed in a magnetic rack in order to remove the storage solution. Following equilibration with 500 μL of ice cold 1 mm HCl, 50 μL (≡ 5 μg) of the solution containing ActRIIB-Fc were added and the mixture was incubated for 30 min at 1200 rpm and RT. Afterwards, the supernatant was removed and magnetic beads were consecutively washed with 500 μL of blocking buffer A (50 mm Tris-HCl, 1 m NaCl, pH 8.0) and B (50 mm glycine, 1 m NaCl, pH 3.0) for a total of three times in order block residual active groups. The second washing step with blocking buffer A was carried out for 15 min on a rotating sample mixer. Finally, beads were equilibrated twice in 500 μL and 300 μL of PBST.
For method development, optimization, and validation, 15 batches of magnetic beads were prepared in parallel, pooled, and stored at 4 °C until usage.
2.4.3 Affinity Purification Protocol
In order to remove abundant proteins and concentrate serum antibodies, ammonium sulfate precipitation was performed as published by Smits et al.22 In brief, 200 μL of serum were mixed with 600 μL of PBS (Phosphate Buffered Saline: 0.01 m phosphate buffer, 0.0027 m KCl, and 0.137 m NaCl, pH 7.4) and 800 μL of saturated ammonium sulfate, vortexed, and incubated for 30 min at RT. Following centrifugation for 10 min at 10 000 × g, the supernatant was discarded and the pellet dissolved in 200 μL of PBST. The pre-purified sample was mixed with 300 μL of ActRIIB-Fc conjugated magnetic beads and incubated for at least 60 min at RT on a rotating sample mixer. Subsequently, magnetic beads were washed with 500 μL of PBST (1×) and 500 μL of PBS (2×), and the target analyte bound to ActRIIB-Fc was eluted by incubation in 50 μL of 2% acetic acid for 15 min at RT and 1200 rpm. The elution fraction was transferred to a fresh tube and subjected to proteolytic digestion and LC-HRMS.
For re-utilization, magnetic beads were washed with 100 μL of 2% acetic acid (2×) and 500 μL of PBST (3×), re-suspended in 300 μL of PBST and re-added to the stock solution stored at 4 °C.
2.5 Tryptic Digestion
Prior to tryptic digestion, reduction of disulfide bonds was accomplished with 500 nmol of TCEP for 15 min at 60 °C and 900 rpm. Samples were neutralized with 15 μL of 2 m NH4HCO3, and cysteine residues alkylated with 1625 nmol of iodoacetamide for 30 min at RT in the dark. Following alkylation, 7 μL of acetonitrile and 10 μL of a trypsin solution (40 μg mL−1 in 50 mm NH4HCO3, Promega, Madison, WI) were added and proteins digested overnight at 37 °C and 500 rpm. The next day, samples were mixed with 2 μL of glacial acetic acid in order to stop proteolytic cleavage.
2.6 Detection of Bimagrumab by Means of LC-HRMS
For the unambiguous mass spectrometric identification of Bimagrumab, two tryptic signature peptides originating from the heavy chain of the antibody were used: T4 (aa 24–38) at m/z 876.42 ([M + 2H]2+) and T11 (aa 99–119) at m/z 749.70 ([M + 3H]3+) (see also Table 1). The corresponding peptides of the isotope labeled ISTD have mass-to-charge ratios of 879.42 ([M + 2H]2+) and 751.70 ([M + 3H]3+). To ensure utmost specificity, the amino acid sequences of both peptides were subjected to a protein database search by using the Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI), which yielded only matches with a sequence homology of less than 100%.
| Tryptic peptide # | From–To | Amino acid sequence | Charge state | m/z | |
|---|---|---|---|---|---|
| Bimagrumab | 4 | 24–38 | ASGYTFTSSYINWVR | +2 | 876.42 |
| 11 | 99–119 | GGWFDYWGQGTLVTVSSASTK | +3 | 749.70 | |
| ISTD | 4 | 24–38 | ASGYTFTSSYINWVRa | +2 | 879.42 |
| 11 | 99–119 | GGWFDYWGQGTLVTVSSASTKa | +3 | 751.70 |
- a Stable isotope-labeled [13C-Lys, 13C-Arg].
For the detection of the target peptides, two different LC-MS systems were used: A Waters SYNAPT G2S mass spectrometer (Waters, Eschborn, Germany) coupled to a Waters Acquity UPLC, and a Thermo Q Exactive (Thermo Fisher, Bremen, Germany) connected to a Waters nano-Acquity UPLC.
2.6.1 LC-(IM)-HRMS: Waters Acquity UPLC/SYNAPT G2S
The LC-HRMS method for the detection of Bimagrumab in human serum was validated on a Waters SYNAPT G2S mass spectrometer coupled to an Acquity UPLC liquid chromatograph.
The LC system was equipped with an Accucore Phenyl-Hexyl trapping column (Thermo Fisher, 3 × 10 mm, particle size: 2.7 μm) and a Poroshell 120A analytical column (Agilent Technologies, 3 × 50 mm, particle size: 2.7 μm). Samples were loaded with injection volumes of 5 or 10 μL and a gradient program using 0.1% formic acid in water as solvent A and 0.1% formic acid in acetonitrile as solvent B was set as follows: Starting conditions 99% A, 0.00–2.00 min trapping at 99% A (flow rate: 600 μL min−1), 2.00–15.00 min 45% A, 15.00–15.50 min 10% A, 15.51 99% A, 15.51–18.00 min 99% A, 18.01 50% A, 18.01–19.00 min 50% A, 19.00–19.50 min 10% A, 19.51 99% A, 19.51–22.00 min 99% A (analytical flow rate: 300 μL min−1).
The SYNAPT G2S was interfaced with a Z-Spray ESI source and operated in high-sensitivity ion mobility (IM) mode with positive ionization using a capillary voltage of 4.5 kV. The cone voltage was set to 40 V and the desolvation gas was heated to 400 °C. Two different full MS experiments were performed: (I) m/z 300–1500 (target enhancement at m/z 876), 0.5 s; (II) m/z 400–1500 (target enhancement at m/z 749), 0.5 s. IM parameters were manually adjusted to a T-wave velocity of 500 m s−1 and a T-wave height of 28 V. For the pre-mobility cell, helium was used as damping gas, nitrogen was employed as source gas and for the IM cell, and argon as collision gas. To enable accurate measurements, lock spray mass correction was conducted every 30 s by intermittent infusion of glu-fibrinogen (m/z 785.8427) during the chromatographic run. According to the manufacturer's instructions, sodium iodide was utilized for the external calibration of the instrument.
To avoid analyte carryover, a short wash gradient was run between two sample injections. For the first 2 min, the flow was adjusted to 600 μL min−1 and directed to the trapping column only. The percentage of the aqueous solvent A was raised from 1 to 99%. Subsequently, the flow was reduced to 300 μL min−1 and switched to the analytical column. Within 1 min, the percentage of solvent A was lowered to 10%. Finally, both columns were re-equilibrated for 2.5 min with 99% of solvent A at a flow rate of 300 μL min−1.
MS data were evaluated using MassLynx (Version 4.1, Waters, 2013) and DriftScope Software (Version 2.8, Waters).
2.6.2 LC-HRMS: Waters nano-Acquity UPLC/Thermo Q Exactive
Samples containing low analyte concentrations (0.01–0.1 μg mL−1) were additionally measured on a Thermo Q Exactive mass spectrometer connected to a Waters nano-Acquity UPLC.
LC was conducted by using a Waters Symmetry C18 trapping column (180 μm × 20 mm, particle size: 5 μm) and a PicoFrit Reprosil-pur nanospray column (New Objective, Woburn, MA; 75 μm × 100 mm, particle size: 3 μm). The aqueous solvent A was 0.1% of formic acid in water, and the organic solvent B 0.1% of formic acid in acetonitrile. Samples were loaded with an injection volume of 1 μL and trapping was performed for 3 min with 99% of solvent A at a flow rate of 8 L min−1. Then, a gradient was run as follows: Starting conditions 99% A, 0–20 min 30% A, 20–22 min 10% A, 22.01 min 99% A, 8 min equilibration 99% A (analytical flow rate: 350 nL min−1).
The Q Exactive was interfaced with a PicoChip nanospray ion source (New Objective) and operated in positive mode with an ionization voltage of 2.0 kV. The transfer capillary was heated to 350 °C. High-resolution full scan mass spectra from m/z 400–2000 were recorded with a resolution of 35 000 FWHM (at m/z 200). Additionally, targeted SIM (tSIM) experiments with data-dependent MS² were performed by using an inclusion list with the accurate mass-to-charge ratios for the most abundant charge states of the target peptides (m/z 150–2000, resolution of 35 000 FWHM and 17 000 FWHM at m/z 200). Isolation windows of m/z 4 and m/z 3 were applied for the tSIM and ddMS2 experiments. External calibration of the instrument was conducted according to the manufacturer's recommendations employing a mixture of caffeine, the tetrapeptide MRFA, and Ultramark. Moreover, a lock spray mass correction at m/z 445.12003 ([(C2H6SiO)6 + H]+) was performed.23 Nitrogen was utilized as collision gas (N2-generator, CMC, Eschborn, Germany) and the normalized collision energy was set to 25%.
MS data evaluation was conducted by using Thermo Xcalibur Software (Version 3.0.63, 2013).
2.7 Characterization/Validation of the Assay
-
Specificity: Ten different blank serum samples were collected from healthy volunteers and tested for the presence of interfering signals.
-
Linearity: A total of nine serum samples were fortified with 0.01, 0.025, 0.05, 0.075, 0.1, 0.25, 0.5, 0.75, and 1 μg mL−1 of Bimagrumab and analyzed as described above. A calibration curve was constructed on the basis of the normalized peak areas and linearity was determined by regression analysis. The samples containing low amounts of Bimagrumab (0.01–0.1 μg mL−1) were additionally measured on a Q Exactive mass spectrometer interfaced with a nano-Acquity UPLC.
-
Identification capability: To evaluate the identification capability of the method, ten different serum samples were fortified with 0.05 μg mL−1 of Bimagrumab and analyzed as described above. The coefficients of variation (CVs) were calculated on the basis of the ISTD normalized peak areas.
-
Limit of detection (LOD): The LOD of the method was estimated as recommended in the WADA Technical Document TD2015MRPL25: The signal-to-noise (S/N) ratio was obtained by comparing the signals measured in ten different serum samples containing a low analyte concentration of 0.05 μg mL−1 (see iii) with the noise at the respective retention time in the corresponding blank specimens (see i). In general, an S/N ratio of three can be considered acceptable. In order to verify the estimated LOD, both the intra- and interday precision were determined at the respective analyte concentration (see below).
-
Intraday precision: The intraday precision of the method was specified at three analyte concentrations (0.5, 0.1, and 0.02 μg mL−1) by analyzing six replicates for each concentration level. ISTD normalized peak areas were used to determine the CVs.
-
Interday precision: Six replicates for three concentration levels (0.5, 0.1, and 0.02 μg mL−1) were prepared and measured on three consecutive days in order to determine the interday precision of the method. Again, CVs were calculated on the basis of ISTD normalized peak areas.
-
Recovery: Six serum samples were fortified with 0.1 μg mL−1 of Bimagrumab and analyzed as described above. Additionally, six blank specimens were prepared and the respective amount of Bimagrumab was added after affinity purification. Finally, the ratios of the peak areas of the samples spiked before and after analyte extraction were compared to determine the recovery of the assay.
-
Matrix effects: A total of 1 μg of Bimagrumab was mixed with deionized water and centrifuged in an Amicon Ultra-0.5 centrifugal filter unit (cut-off: 3 kDa, Merck Millipore) for 20 min at 14 000 × g and RT. The retentate was washed with 300 μL of deionized water (15 min, 14 000 × g, RT), removed from the centrifugal filter device, and adjusted to a final volume of ≈50 μL with deionized water. Reduction and alkylation of disulfide bonds were performed as described above and the reference material digested overnight at 37 °C and 500 rpm. At the same time, three different blank specimens were prepared according to the presented protocol, and 50 μL of the resulting eluate were fortified with 2 μL of the digest (containing approximately 20 ng of Bimagrumab). The same amount of digested standard solution was mixed with 50 μL of 2% acetic acid and all samples were subjected to LC-HRMS analysis. By comparing the peak areas of the different samples, the effect of the serum matrix on ion suppression and ion enhancement was determined.
-
Carryover (magnetic beads): As the reutilization of magnetic beads involves the risk of sample carryover, the efficiency of the elution/washing protocol was determined. For that purpose, three serum samples were fortified with 0.5 μg mL−1 of Bimagrumab and prepared as described above. Following affinity purification, target analytes were eluted three times with 50 μL of 2% acetic acid (15 min, 1200 rpm, RT). The different eluates were subjected to proteolytic digestion and LC-HRMS and the elution efficiency was finally estimated by comparing the peak areas of the samples.
3 Results and Discussion
The misuse of therapeutic proteins in sports is restricted under the terms of the World Anti-Doping Code (WADC). The latest version of the WADA Prohibited List includes a variety of protein drugs both in sections S2 “Peptide hormones, growth factors, related substances, and mimetics” and S4 “Hormone and metabolic modulators.”20 Immunological as well as mass spectrometry–based proteomic techniques are currently used for the detection of “classical” performance-enhancing protein drugs such as erythropoietin (EPO), growth hormone (GH), and human chorionic gonadotropin (hCG).26, 27 However, there are only a few validated tests for new protein therapeutics such as myostatin inhibitors, Fc fusion proteins, and therapeutic antibodies.
In pharmacokinetic studies, immunoassays such as ELISAs are usually employed for the quantitative detection of therapeutic proteins in biological samples.28 For instance, a competitive ELISA employing anti-human ActRIIA antibodies was used to determine the serum concentrations of Sotatercept, a recombinant fusion protein composed of the extracellular domain of ActRIIA and the Fc fragment of human IgG1.29 In 2016, two alternative, non-targeted immunological assays for the detection of anti-myostatin antibodies and ActRIIA-Fc fusion proteins in human serum/plasma were published, which combine affinity purification and Western blotting to generically detect the protein drugs on the basis of both their ligand specificity (myostatin, activin A) and structure (presence of a human IgG1 Fc domain).30, 31 Additionally, affinity purification, proteolytic digestion, and LC-HRMS were employed for a direct and more sensitive detection of Sotatercept and related ActRIIA-Fc fusion proteins in human serum.31 Similarly, Etanercept, a recombinant fusion protein composed of the extracellular domain of tumor necrosis factor receptor 2 (TNF-R2) and the Fc region of human IgG1, could be detected in equine plasma by using immunoaffinity purification, tryptic digestion, and LC-MS.32
Within this study, a related strategy was used to enable the detection of Bimagrumab, a novel therapeutic antibody targeting both ActRIIA and ActRIIB.
3.1 Aspects of Assay Development
For the isolation of antibodies from biological matrices, crude, general, or/and specific purification methods are usually employed.33, 34 A crude enrichment of serum immunoglobulins can be achieved on the basis of their physicochemical properties (e.g., structure and solubility) by using fractionation techniques such as ion exchange chromatography, ammonium sulfate precipitation, and thiophilic adsorption.34 By contrast, Protein A, G, and L can be applied for the general purification of certain antibody classes.33, 34 Protein A, G, and L are antibody-binding proteins of bacterial origin and highly specific for conserved antibody domains such as the heavy chains of the Fc fragment (Protein A and G) or the kappa light chains of the Fab fragment (Protein L). But as the resulting extracts are still composed of many different immunoglobulins, an antigen-specific affinity purification of particular antibodies can be preferable, especially when a high purity is required for downstream analysis. For that purpose, the respective ligand (or ligand domain) is immobilized to a support, which is subsequently used to purify all antibodies specifically binding the antigen.
For the mass spectrometric detection of Bimagrumab in human serum, two purification methods were combined: First, ammonium sulfate precipitation was employed for a preliminary enrichment of serum antibodies. The resulting extracts were then subjected to affinity purification using antigen-coated magnetic beads. As Bimagrumab binds to the extracellular domains of both ActRIIA and B, either can theoretically be used as ligand for affinity extraction. During method development, the binding preference of Bimagrumab was tested by means of SDS-PAGE and Western blotting. As shown in Supporting Information, Figure 1, the antibody was found to bind much stronger to ActRIIB than ActRIIA. These findings are in accordance with the literature describing a more than 200-fold higher binding preference for ActRIIB over ActRIIA.9 Consequently, ActRIIB-Fc conjugated NHS magnetic Sepharose beads were prepared to specifically isolate Bimagrumab from the biological matrix.
An LC-HRMS method including two proteotypic tryptic peptides of the antibody's heavy chain was set up for the subsequent detection of Bimagrumab: While the peptide T4 comprises amino acids 24–38 and has a mass-to-charge ratio of 876.42 ([M + 2H]2+), the second diagnostic peptide T11 includes amino acids 99–119 and has a mass-to-charge ratio of 749.70 ([M + 3H]3+).
During method development, two different LC-HRMS systems were used for the detection of the target peptides: A Waters SYNAPT G2S interfaced with a Waters Acquity UPLC and a Thermo Q Exactive coupled to a Waters nano-Acquity UPLC. As the UPLC operated under normal flow conditions was found to be less susceptible to sample carryover and the instrument offers an additional IM separation, the Waters SYNAPT G2S was finally chosen for method validation. Exemplary extracted ion chromatograms of a blank and a “positive” serum sample fortified with 0.1 μg mL−1 of Bimagrumab are shown in Figure 2. The signature peptides of the isotope-labeled ISTD and Bimagrumab were unambiguously detected and no interferences were observed in the blank specimen.
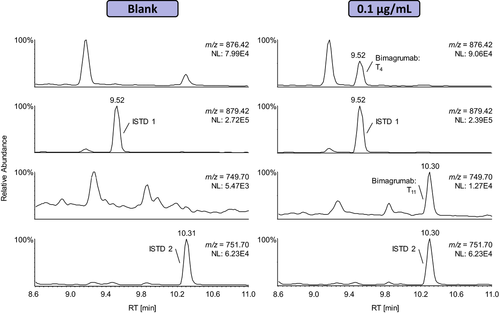
Nevertheless, the higher sensitivity of the Thermo Q Exactive can be beneficial for the confirmation of samples containing low analyte concentrations. Additionally, the instrument was used to record product ion mass spectra of both diagnostic peptides, which are depicted in Supporting Information, Figures 2 and 3.
3.2 Characterization/Validation of the Assay
-
Specificity: In nine out of ten blank serum samples, no interfering signals were detected at the expected retention times of the diagnostic peptides T4 and T11. However, in one sample, a potentially interfering signal was observed close to the retention time of the peptide T11 (m/z = 749.70 ([M + 3H]3+)). To further characterize this sample and facilitate the identification of both target peptides, LC-IM-HRMS was employed (see below).
-
Linearity: On the SYNAPT G2S, the method was linear in the range of 0.025 to 1 μg mL−1 and yielded good correlation coefficients for both diagnostic peptides (T4: R² = 0.9901; T11: R² = 0.9962). Samples containing low amounts of Bimagrumab were additionally analyzed on a Q Exactive interfaced with a nano-Acquity UPLC. Here, the method was found to be linear from 0.01 to 0.1 μg mL−1 with correlation coefficients of R² = 0.9911 (T4) and R² = 0.9894 (T11).
-
Identification capability: For nine out of ten samples, the identification capability of the presented approach was successfully demonstrated at a concentration of 0.05 μg mL−1. However, in one sample, the signal of the diagnostic peptide T11 was overlaid by the abundant isotopic signal of a co-eluting peptide. This sample was exemplarily used to demonstrate the added value of IM as orthogonal separation technique in addition to LC-HRMS (see below). CVs for the diagnostic peptides were 10% (T4) and 12% (T11).
-
LOD: In order to determine the LOD on the SYNAPT G2S, the signals detected in ten different serum samples fortified with 0.05 μg mL−1 of Bimagrumab were compared with the noise at the respective retention time in the corresponding blank samples. At an S/N ratio ≥3, the LOD was estimated as 0.02 μg mL−1 and verified by determining the intra- and interday precision at this analyte concentration (see below). On the Q Exactive, only a visual evaluation of the linearity samples was conducted to roughly estimate the LOD. As the S/N ratio in the sample fortified with 0.01 μg mL−1 of the antibody was clearly above 3 (data not shown), it can be assumed that the LOD is <0.01 μg mL−1.
With regard to the expected pharmacokinetic properties of the drug, this sensitivity should be more than sufficient: Typically, therapeutic human IgGs are administered at high doses of several milligrams per kilogram,35 resulting in maximum serum concentrations ranging from 10 to 100 μg mL−1.17
-
Intraday precision: The intraday precision was determined at three different concentration levels (0.5, 0.1, and 0.02 μg mL−1) and ranged from 3 to 12%.
-
Interday precision: The interday precision was also specified at concentrations of 0.5, 0.1, and 0.02 μg mL−1 and varied from 3 to 18%
-
Recovery: The recovery of the antibody extraction by using ammonium sulfate precipitation and affinity purification was 76.7% at a medium concentration of 0.1 μg mL−1.
-
Matrix effects: No significant matrix effects (ion suppression or enhancement) were observed.
-
Carryover (magnetic beads): The reutilization of magnetic beads can potentially cause sample carryover. For that reason, the efficiency of the elution/washing protocol was determined by comparing the amount of analyte in the first eluate to the two elution/washing fractions collected afterward. Each washing step with 2% acetic acid was found to remove approximately 90% of Bimagrumab from the magnetic beads, resulting in an elution/washing efficiency of more than 99%. Consequently, the risk of sample carryover is negligible.
| Validation parameter | Concentration(s) [μg mL−1] | Results | ||
|---|---|---|---|---|
| T4 (876.42) | T11 (749.70) | |||
| Specificity | — | ✓ | ✓ | |
| Linearity | 0.025–1(SYNAPT) | R² = 0.9901 | R² = 0.9962 | |
| 0.01–0.1 (Q Exactive) | R² = 0.9911 | R² = 0.9894 | ||
| Identification capability | 0.05 | ✓ | ✓ | |
| LOD | — | ≈0.02 μg mL−1 | ||
| Intraday precision | 0.5 | CV = 2.9% | CV = 5.4% | |
| 0.1 | CV = 3.5% | CV = 8.5% | ||
| 0.02 | CV = 11.5% | CV = 9.1% | ||
| Interday precision | 0.5 | CV = 2.6% | CV = 6.4% | |
| 0.1 | CV = 6.0% | CV = 10.4% | ||
| 0.02 | CV = 11.7% | CV = 18.0% | ||
| Recovery | 0.1 | ≈76.7% | ||
| Matrix effects | — | — | — | |
| Carryover | 0.5 | — | — | |
3.3 LC-IM-HRMS of Bimagrumab
In the serum samples of one healthy volunteer, interfering signals were detected at the retention time of the peptide T11. The extracted ion chromatograms of a blank and a sample fortified with 0.05 μg mL−1 of Bimagrumab are displayed in Figure 3. In the “positive” sample, the signal of the tryptic peptide T11 is overlaid by the signal of a nearly co-eluting peptide, which is also present in the corresponding (non-fortified) blank specimen and has a mass-to-charge ratio of 749.01 ([M + 3H]3+).

IM represents an orthogonal separation technique which can be used in combination with LC-(HR)MS to differentiate analytes with similar masses that cannot be separated by LC alone. In IM, ions are separated by both their size and shape in a drift tube which contains a neutral drift gas such as helium and is under the influence of a weak electric field.28 Consequently, this technique can perfectly be used to differentiate the overlapping signals of the diagnostic and interfering peptide in the abovementioned serum sample. Figure 4 shows the drift time versus retention time plots of the diagnostic peptide T11 at m/z = 749.70 ([M + 3H]3+) of both samples. In the sample fortified with Bimagrumab, three intense co-eluting spots are visible, which can clearly be separated by their drift times and retention times. As only two of these spots are present in the blank specimen, the signal corresponding to the signature peptide can be unambiguously identified and the interference eliminated by a drift-time aligned extraction of the mass spectrum.
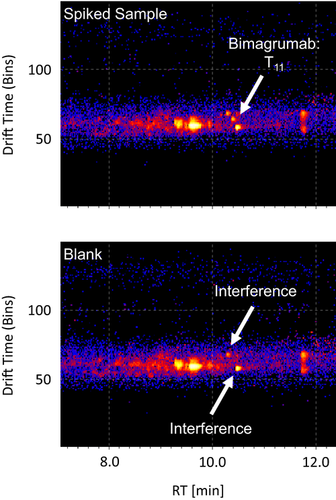
These findings demonstrate the potential of IM as an orthogonal separation technique in addition to LC-HRMS, which can support and facilitate the identification of target analytes sharing similar retention times and molecular masses.
3.4 Clinical Samples
To demonstrate the method's applicability to authentic serum specimens, a set of clinical samples was analyzed as described above. No Bimagrumab was detected in the samples collected prior to the administration of the antibody as well as the placebo controls. In all post-administration samples provided by Novartis, the anti-ActRII antibody was unambiguously identified.
Figure 5 shows the extracted ion chromatograms of both a pre- and post-administration sample collected from one subject who received subcutaneous Bimagrumab injections. In both samples, the signature peptides of the isotope-labeled ISTD were readily detected. In the pre-administration sample collected at study day 0, no interfering signals were observed at the respective retention times of the diagnostic peptides for Bimagrumab. In contrast, the Bimagrumab signature peptides T4 and T11 were unambiguously identified in the post-administration sample collected more than 4 weeks after the last injection of the antibody. Consequently, the successful analysis of the clinical samples demonstrates that the presented approach is fit for purpose and can be applied to routine doping control samples.
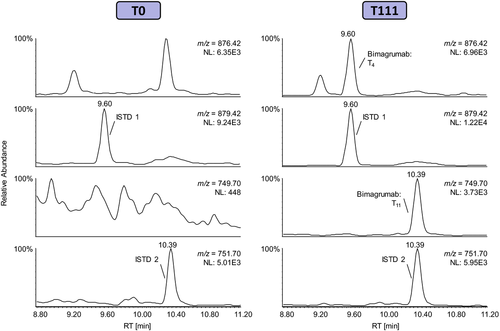
4 Conclusion
Signaling pathways involving the ActRIIs were found to play a central role in the regulation of skeletal muscle mass and their blockade by new therapeutic means can provide treatment strategies for various muscular diseases. Bimagrumab is a human ActRII inhibitory antibody which prevents the interaction of the receptors with endogenous ligands such as myostatin and activin A, thus increasing muscle mass and function. Therefore, the antibody possesses the potential for being misused as doping agent in sports. Within this study, a targeted detection assay for Bimagrumab in doping control serum samples was developed employing ammonium sulfate precipitation, affinity purification, tryptic digestion, and LC-HRMS. To facilitate the unambiguous identification of the target peptides, IM was used as orthogonal separation technique in addition to LC-HRMS. The assay was comprehensively characterized and found to be fit for purpose for an application in routine doping control analysis. It can readily be modified to include other antibody-based drugs and will complement existing assays for emerging protein therapeutics.
Abbreviations
-
- ActRII
-
- activin type II receptor
-
- AKT
-
- protein kinase B
-
- CHO
-
- Chinese hamster ovary
-
- EPO
-
- erythropoietin
-
- GDF-11
-
- growth differentiation factor-11
-
- GDF-8
-
- growth differentiation factor-8
-
- GH
-
- growth hormone
-
- hCG
-
- human chorionic gonadotropin
-
- IgG1
-
- immunoglobulin G1
-
- IM
-
- ion mobility
-
- ISTD
-
- internal standard
-
- LDS
-
- lithium dodecyl sulfate
-
- mTOR
-
- mammalian target of rapamycin
-
- NHS
-
- N-hydroxysuccinimide
-
- PBST
-
- phosphate buffered saline tween 20
-
- RT
-
- room temperature
-
- TGF-β
-
- transforming growth factor-β
-
- TNF-R2
-
- tumor necrosis factor receptor 2
-
- WADA
-
- World Anti-Doping Agency
-
- WADC
-
- World Anti-Doping Code
Acknowledgements
The presented work was conducted with support of Olivier Petricoul from Novartis, the Federal Ministry of the Interior of the Federal Republic of Germany, and the Manfred-Donike Institute for Doping Analysis (Cologne, Germany).
Conflict of Interest
The authors have declared no conflict of interest.




