Establishment of a CRISPR/Cas9-Mediated Cysltr1 Knockout Mouse Model and iTRAQ-Based Proteomic Analysis
Abstract
Purpose
To clarify the role of Cysteinyl leukotrienes receptor type 1 (CYSLTR1) and find the potential predictors of CYSLTR1 antagonists (leukotriene receptor antagonists [LTRAs]) responsiveness in vivo.
Experimental Design
Cysltr1 knockout (KO) mouse model is established by the CRISPR/Cas9 system. The phenotype of Cysltr1 KO mice are tested by western blotting (WB), histological examinations, and experiment of zymosan A-induced peritoneal inflammation. The differentially expressed proteins (DEPs) between the Cysltr1 KO and the wild type (WT) mice lung tissues are analyzed by the iTRAQ-based proteomic technology. WB is used to validate a subset of DEPs. The total nitric oxide (NO) concentration in lung tissues are measured.
Results
The Cysltr1 KO mice show the decrease of vascular permeability in comparison with the WT mice. Our quantitative proteomic analysis identified 239 DEPs in total. WB confirms an increased expression of protein kinase C-δ (PKC-δ), while N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (DDAH1) and β-Catenin expression are reduced. The total NO concentrations are significantly reduced in lungs from Cysltr1 KO mice.
Conclusions and Clinical Relevance
This study not only provides a comprehensive dataset on overall protein changes in Cysltr1 KO mice lung tissues, but also sheds light on interpreting the description of lower vascular permeability in Cysltr1 KO mice.
1 Introduction
The Cysteinyl leukotrienes (CysLTs), including leukotriene C4, D4, and E4, are proinflammatory lipid mediators that mediate many diseases by binding to and activating specific receptors. Two subtypes of the CysLTs receptor, designated CYSLTR1 and CYSLTR2, have been determined by pharmacological studies. CYSLTR1 is sensitive to the classical antagonists including montelukast, zafirlukast, pranlukast, pobilukast, and MK571, while no specific antagonist is available yet to CYSLTR2.1, 2
CYSLTR1 antagonists, also termed leukotriene receptor antagonists (LTRAs), have been validated to be effective in preventing many inflammatory disorders in animal models, such as noise-induced cochlear injury,3 portal hypertension in rat liver cirrhosis,4 pulmonary fibrotic changes in eosinophil-mediated respiratory inflammation,5 and so on. The therapeutic effect of LTRAs had been confirmed by large-scale randomized, double-blind, placebo-controlled clinical trials in allergic diseases such as asthma or allergic rhinitis.6 Currently, LTRAs are an acceptable alternative first-line drugs to inhaled corticosteroids (ICS) for patients with mild persistent asthma, and as add-on therapy to ICS in moderate persistent asthma.7
Among patients with asthma, due to differences in leukotriene biology, genotype, or clinical characteristics, a varying response to LTRAs was observed. In a multicenter, randomized controlled trial with montelukast, the lung function was improved in only 35–50% of patients with asthma.8 Up to now, the most prevalent method to predict the LTRAs responsiveness was to test the level of urinary leukotrienes E4 (LTE4) in clinical practice, but the results were controversial.9, 10 It is urgent to get an ideal predictor illuminating CYSLTR1 activation to evaluate the therapeutic effect of LTRAs.
Clinical Relevance
Cysteinyl leukotrienes (CysLTs) are proinflammatory lipid mediators involving in asthma. The CysLTs receptor type 1 (CYSLTR1) antagonists (leukotriene receptor antagonists [LTRAs]) are an acceptable drugs for patients with asthma. Due to differences among patients with asthma in leukotriene biology, genotype, or clinical characteristics, the response to LTRAs is various, while an ideal method to predict the LTRAs responsiveness is lacked. Furthermore, CYSLTR1 activation has complex signaling pathways and could lead to the release of various mediators in a tissue-specific manner. So we sought to clarify the role of CYSLTR1 and find the potential predictors of LTRAs responsiveness in vivo. We generated mice carrying Cysltr1 gene disruption and performed iTRAQ-based proteomic technology to analyze the differentially expressed proteins (DEPs) between the Cysltr1 knockout (KO) and the wild type (WT) mice lung tissues. The Cysltr1 KO mice showed the decrease of vascular permeability. A total of 172 and 67 proteins were obviously upregulated and downregulated in the Cysltr1 KO mice lung tissues, respectively, which is instrumental in exploring predictors of CYSLTR1-related diseases. Furthermore, increased expression of protein kinase C-δ (PKC-δ) and reduction of N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (DDAH1) and β-Catenin helped interpreting the description of lower vascular permeability in Cysltr1 KO mice.
CYSLTR1 has complex signaling pathways in different cells. Furthermore, CYSLTR1 activation could lead to the release of various mediators, which is implicated in a variety of pathophysiological conditions in a tissue-specific manner.11 So we try to clarify the role of CYSLTR1 and find the potential predictors of CysLTs-CYSLTR1 signaling pathway in vivo. Owing to the high expression of CTSLTR1 in the lung, we generated Cysltr1 knockout (KO) mice by clustered regularly interspaced short palindromic repeat-associated system (CRISPR/Cas9) and performed isobaric tags for relative and absolute quantitation (iTRAQ)-based proteomic analysis of lung tissues in Cysltr1 KO mice.
2 Experimental Section
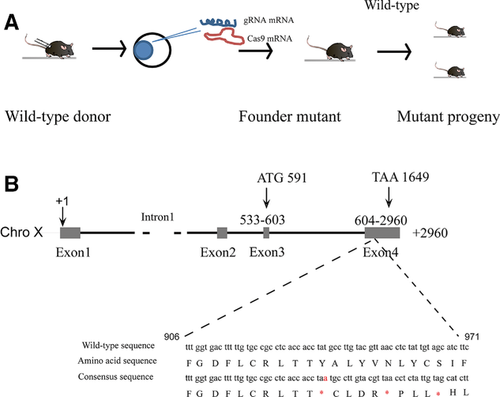
2.1 Generation of KO Mice Using the CRISPR/Cas9 System
All the animals were raised and bred in an SPF animal room accredited by Assessment and Accreditation of Laboratory Animal Care. Handling procedures were in accordance with the Chinese Guidelines for Animal Welfare and were approved by the Sun Yat-sen University Animal Care and Use Committee (Guangzhou, China). The CRISPR online design tool (http://tools.genome-engineering.org) was used to build the guide RNA (gRNA) oligomeric nucleotide sequences targeting Cysltr1 gene (Supporting Information, Table S1), which were cloned into pDR274 vector (Addgene) and in vitro transcribed using the MEGA short script T7 kit (Life Technologies). pT7-3xFlag-hSpCas9 vector donated by Dr. Liang (Zhongshan Ophthalmic Center, Sun Yat-sen University) was linearized with Pme I and in vitro transcripted using the mMESSAGE mMACHINE T7 ULTRA kit (Life Technologies). The Cas9 mRNA and the gRNAs were microinjected into the 0.5-day zygotesof C57BL/6J mice. The injected zygotes were transplanted into the oviduct of pseudopregnant mothers. Genomic DNA was extracted from the tail of pups using lysis reagent (Tiangen Biotech Co.) and amplified by PCR using the primers shown in Supporting Information, Table S1. The PCR products were TA cloned into the pGEM-T vector (Promega) and the mouse genotype were determined by Sanger sequencing (Life Technologies). The Cysltr1 knockout allele was bred onto C57BL/6J background and the animals were maintained and crossed using standard procedures (Model animal research center of Nanjing university) (Figure 1A). Potential off-target sites were excluded by deep sequencing according to the previous report.12
2.2 Zymosan A-Induced Peritoneal Inflammation
Experiment of zymosan A-induced peritoneal inflammation was carried out according to previous literature.13 In brief, 0.5% Evans blue (10 mL kg–1) was injected through mouse tail vein immediately before intraperitoneal injection of 1 mL zymosan A (1 mg mL–1, Sigma). Subsequently, mice were underwent peritoneal lavage with 3 mL of cold PBS at 30 and 120 min after the injection of zymosan A. The lavage fluid was collected for evaluation of Evans blue extravasation by light spectrophotometry of the supernatants at 610 nm and neutrophil counts. LTB4 or LTC4 (10 mg kg–1, Cayman Chemical) was administered to mice (i.p.) treated with zymosan A. Exudates were collected at indicated time and analyzed as described above. The amounts of cysLTs and LTB4 in the peritoneal lavage fluid were measured by enzyme immunoassay kits (Cayman Chemical) according to the manufacturer's instructions.
2.3 Tissues Preparation for Histological Examination
Animals were anesthetized and transcardially perfused with sterile saline solution until the venous effluent was free of blood. The tissues including lung, liver, spleen, kidney, myocardium, and skin were removed and immediately immersed in 4% formalin for 24 h and embedded in paraffin. Hematoxylin and eosin staining and immunohistochemistry were performed on 5-μm-thick sections according to standard procedure using the anti-CYSLTR1 antibody (Cayman Chemical).
2.4 Protein Extraction from Lung Tissues
The lung tissues were isolated from three pairs of male Cysltr1 KO and wild type (WT) mice at 4 weeks as mentioned above. After removal, the lung tissue samples were weighed and ground to dry powder with liquid nitrogen. The dry powder was dissolved with 200 μL triethyl ammonium bicarbonate dissolution buffer (TEAB, Sigma) and broken by the ultrasonic wave for 15 min. And then after centrifugation at 12 000 rpm for 20 min, the supernatant was subsided by adding fourfold volume cold acetone containing 10 mm Dithiothreitol (Sigma) for about 2 h. The precipitate was collected by centrifugation at 12 000 rpm for 20 min at 4 °C and mixed with 800 μL cold acetone at 56 °C to break disulfide bonds in protein. Again centrifugation at 12 000 rpm for 20 min at 4 °C, the dried precipitate was collected and dissolved with 100 μl triethyl ammonium bicarbonate dissolution buffer. After measuring the concentration using the Bradford method (Sigma), the total proteins were stored at –80 °C for later use.
2.5 Protein Digestion and iTRAQ Labeling
A total of 50 μg protein was digested as previously described14 and the resultant peptide mixture was labeled with iTRAQ Reagent-8 plex Multiplex Kit (AB Sciex U.K. Limited) according to the manufacturer's instructions. Briefly, six iTRAQ labels, 115/118, 116/119, and 117/121 were used for the three pairs of Cysltrl KO and WT mice lung tissues.
2.6 Fractionation and LC–MS/MS Analysis
All of the labeled samples were mixed with equal amount and fractionated using HPLC system (Thermo DIONEX Ultimate 3000 BioRS) using a Durashell C18 (5 μm, 100 Å, 4.6 × 250 mm). A total of 12 fractions were collected. Each collected fraction was analyzed on an AB SCIEX nano LC–MS/MS (Triple TOF 5600 plus) system. Samples were chromatographed using a 90-min gradient from 2 to 30% (buffer 0.1% (v/v) formic acid, 5% (v/v) acetonitrile, buffer 0.1% (v/v) formic acid, 95% (v/v) acetonitrile) after direct injection onto a 20-cm PicoFrit emitter (New Objective) packed to 12 cm with Magic C18 AQ 3 μm 120 Å stationary phase. Mass survey spectra (MS1) were collected in the range 350–1500 m/z for 250 min. The 20 most intense precursors with charge state 2–5 were selected for fragmentation, and MS2 spectra were collected in the range 50–2000 m/z for 100 min, precursor ions were excluded from reselection for 15 s.
2.7 Data Analysis
The original MS/MS file data were submitted to Protein Pilot Software v4.5 for data analysis as previously described.14 Only proteins with at least one unique peptide and unused value more than 1.3 were considered for further analysis. Among the identified peptides, some of them were excluded from the quantitative analysis for one of the following reasons: (i) the peaks corresponding to the iTRAQ labels were not detected. (ii) The peptides were identified with a peptide ID confidence < 15%. (iii) The peptides were claimed by more than one protein. (iv) The peptides with an S/N < 9. (v) Peptides with a combined feature probability < 30%. For output of our quantitative iTRAQ results, all protein ratios were expressed as KO over WT to present relative protein quantification ratios. Statistical significance of the difference in the levels of expression of proteins between samples to be compared was determined by Student's t-test (two-tailed and unpaired). The minimum among the replicate experiments was displayed, which was treated as the final p-value. We took a 1.5-fold change (upregulate ≥ 1.5, downregulate ≤ 0.67) and p-value < 0.05 as the threshold to identify significant changes.
2.8 Western Blot Analysis
Western blot analysis (WB) of the lung samples of 4-week-old mice was conducted. Primary antibodies against CYSLTR1 (Cayman Chemical), protein kinase C-δ (PKC-δ), vascular endothelial (VE)-cadherin (Cell Signaling Technology), N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (DDAH1) and β-Catenin (Santa Cruz Biotechnology) were used. Blots were stripped and reprobed with anti-β-actin antibody (Cell Signaling Technology) to demonstrate equal loading.
2.9 Measurement of the Total Nitric Oxide
The lung tissue samples were weighed and homogenized with ninefold volume ice-cold lysis solution. The homogenates were centrifuged at 3000 rpm for 20 min at 4 °C. The supernatants were estimated for the total nitric oxide (NO) concentration in lung tissues using the Total Nitric Oxide assay kit (Beyotime, China) in accordance with the manufacturer's instructions.
3 Results
3.1 Generation of Cysltr1 KO Mice and Phenotype Analysis
We successfully generated 23 founder mice using CRISPR/Cas9 system after the reconstructed zygotes were transferred into pseudopregnant surrogate mothers. We backcrossed seven of the 23 founders with C57BL/6J WT mice and obtained 17 F1 pups. The Cysltr1 genotypes were verified by PCR combined with TA clone and DNA sequencing analysis in eight F1 mice. Different mutations included fragment deletion (c.225_230delATACAT, NM 021476.5; c.222_229delAGTATACA, NM 021476.5) (Supporting Information, Figures S1 and S2) and a ‘A’ insertation in the fourth exon of the Cysltr1 which formed stop coden ‘TAA’ (c.347_348 insA, NM 021476.5) resulting in the loss of expression of the protein CYSLTR1 (Figure 1A, B; Supporting Information, Figure S3). We performed the iTRAQ-based proteomic analysis using the mice harboring a homozygous ‘A’ insertional mutation.
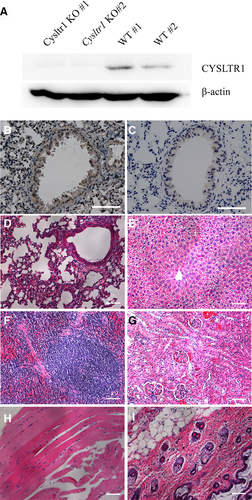
WB showed that CYSLTR1 protein was not detectable in lung tissues of Cysltr1 KO mice (Figure 2A). Immunohistochemical analysis showed that bronchial muscle layer was stained positively in WT mice but not in Cysltr1 KO mice (Figure 2B, C). In addition, Hematoxylin and eosin staining exhibited no significant pathological change in the lung, liver, spleen, kidney, myocardium, and skin of Cysltr1 KO mice (Figure 2D–I). Zymosan A challenge to the peritoneum caused the concentrations of CysLTs and LTB4 increase to the same degree in WT and KO mice (Figure 3A, B). However, the lower level of Evans blue extravasation was observed in the Cysltr1 KO mice than WT after zymosan A-induced inflammation. Administration of LTC4 seemed to enhance the extravasation of Evans blue and cause the higher vascular permeability in WT mice (Figure 3C, D). There was no significant difference in neutrophil infiltration between Cysltr1 KO and WT mice after zymosan A-induced inflammation. Intraperitoneal injections of LTB4 rather than LTC4 resulted in higher peritoneal neutrophil infiltration in magnitude (Figure 3E, F). Taken together, these data suggested that peritoneal CysLTs concentration had obvious correlation with plasma protein extravasation, and Cysltr1 KO would result in decreased protein extravasation.
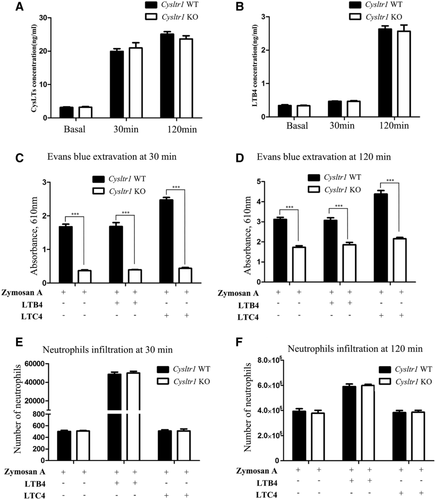
3.2 Identification of Differentially Expressed Proteins in the Lung Tissues between the Cysltr1 KO and WT Mice
A total of 366 219 spectra were generated from iTRAQ experiments. Based on the Protein Pilot search, 14 613 peptides, which matched to 3652 proteins, were identified (Supporting Information, Table S2). Overall, 3363, 1917, and 2242 proteins were sub-categorized into 52 gene ontologies, 24 clusters of orthologous groups of protein systems, and 256 Kyoto Encyclopedia of Genes and Genomes (KEGG) classifications (Supporting Information, Figure S4). Among the identified proteins, 172 and 67 proteins exhibited increased (KO:WT ≥ 1.5, p < 0.05) and decreased (KO:WT ≤ 0.67, p < 0.05) levels, respectively (Supporting Information, Table S3). On the basis of gene ontology analysis, the 172 upregulated proteins in Cysltr1 KO mice were assigned to 25 biological process, nine cellular components, and 13 molecular functions. In contrast, the 67 downregulated proteins fell into 25 biological process, nine cellular components, and eight molecular functions (Supporting Information, Figure S5). CYSLTR1 could recognize activation signals and activate downstream protein kinases. Thus, we searched all the DEPs that belonged to the protein kinase families. There was an increase in expression of PKC-δ (Q53YN4), serine/threonine-protein kinase PAK 2 (Q8CIN4), and STE20-like serine/threonine-protein kinase (O54988) in Cysltr1 KO samples. Conversely, the expression of A-kinase anchor protein 2 (A2API8) were decreased. The proteins involved in the endothelial cell adhesion junction and angiogenesis were searched in the DEPs to explain the decrease of plasma protein extravasation of vasculature in Cysltr1 KO mice. Strikingly, the endothelial cell adhesion junction proteins, including catenin alpha-1 (P26231), catenin beta-1 (β-catenin) (Q02248), and catenin delta-1 (P30999), were decreased. A total of 11 angiogenesis-related proteins were found in the DEPs of our proteomic data. Chitinase-3-like protein 1 (Q61362), integrin beta (D3YXH8), tryptophan–tRNA ligase, cytoplasmic (P32921), signal transducer and activator of transcription (Q8C8M3), and integrin beta-2 (P11835) were increased and the other proteins, including plasminogen (P20918), DDAH1 (Q9CWS0), complement C5 (P06684), cell surface glycoprotein MUC18 (Q8R2Y2), neuropilin-1 (P97333), and heat shock 70 kDa protein 12B (Q9CZJ2), were decreased in Cysltr1 KO samples (Table 1A).
| Accession number | Protein namea | KO:WTb | p-Valuec |
|---|---|---|---|
| Protein kinase | |||
| tr|Q53YN4 | Protein kinase C delta type | 1.70 | 0.0148 |
| sp|Q8CIN4 | Serine/threonine-protein kinase PAK 2 | 1.56 | 0.0088 |
| sp|O54988 | STE20-like serine/threonine-protein kinase | 1.52 | 0.0336 |
| tr|A2API8 | A-kinase anchor protein 2 | 0.62 | 0.0006 |
| Cell adhesion | |||
| sp|P26231 | Catenin alpha-1 | 0.62 | 0.0002 |
| sp|Q02248 | Catenin beta-1 | 0.62 | 0.0011 |
| sp|P30999 | Catenin delta-1 | 0.65 | 0.0332 |
| Angiogenesis | |||
| sp|P20918 | Plasminogen | 0.44 | 0.0005 |
| sp|Q9CWS0 | DDAH1 | 0.55 | 0.0048 |
| sp|P06684 | Complement C5 | 0.62 | 0.033 |
| sp|Q8R2Y2 | Cell surface glycoprotein MUC18 | 0.66 | 0.0196 |
| sp|P97333 | Neuropilin-1 | 0.67 | 0.0048 |
| sp|Q9CZJ2 | Heat shock 70 kDa protein 12B | 0.67 | 0.0106 |
| sp|Q61362 | Chitinase-3-like protein 1 | 1.51 | 0.0030 |
| tr|D3YXH8 | Integrin beta | 1.62 | 0.0287 |
| sp|P32921 | Tryptophan–tRNA ligase, cytoplasmic | 1.72 | 0.0210 |
| tr|Q8C8M3 | Signal transducer and activator of transcription | 2.31 | 0.00004 |
| sp|P11835 | Integrin beta-2 | 2.55 | 0.0004 |
- aName of the protein identified by MS/MS.
- bThe ratio of protein levels between Cysltr1 Knockout (KO) and wild type (WT) mice lung.
- cStudent's t-test p-value.
3.3 WB and NO Concentration Measurement in the Lung Tissues
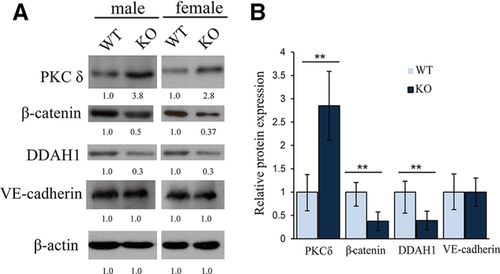
WB of three DEPs including PKC-δ, DDAH1, and β-catenin were conducted to validate iTRAQ results. We demonstrated the upregulated expression of PKC-δ and downregulated expression of β-catenin and DDAH1 in the lung of both the male and female Cysltr1 KO mice compared with WT, which were coincided with the findings from the iTRAQ analysis. Given the important role of the transmembrane adhesion protein VE-cadherinin mediating the opening and closing of the endothelial barrier, the abundance of VE-cadherin was detected. WB analysis showed the same level of VE-cadherinin expression in WT and Cysltr1 KO mice lung tissues (Figure 4). NO is one of the major endothelium-derived vasoactive mediators, which is modulated by DDAH1, so we further compared NO concentration in the lung tissues of WT and KO mice. The concentration of NO metabolites was significantly reduced in lungs of Cysltr1 KO mice compared with WT mice (30.9 ± 2.8 vs 44.4 ± 5.08 μmol L–1) (Supporting Information, Figure S6).
4 Discussion
The mouse CYSLTR1 shares 87.3% homology with humans, which is located in the seven transmembrane domain, making it an ideal model for studying the human CYSLTR1 signaling pathway.15 In this study, we have successfully used the CRISPR/Cas9 technology to disrupt the Cysltr1 gene in mice and the resultant phenotype demonstrated that the Cysltr1 KO mice could reproduce normally and exhibit no glaring abnormalities in histological examination. The decreased peritoneal serous effusion in zymosan A-induced abdominal cavity inflammation indicated decreased vascular permeability in Cysltr1 KO mice. Furthermore, we used iTRAQ-based quantitative proteomics approach to obtain valuable evidence to understand the mechanisms of the decreased vasopermeability in Cysltr1 KO mice.
CYSLTR1 signaling pathways are dependent on the cell type and the availability of trimeric G protein, which activate downstream protein kinases, culminate in myriad cellular and tissue responses including bronchoconstriction, mucus secretion, edema, and so on.16 Our proteomic data revealed a number of DEPs related to protein kinase, cell adhesion junction, and angiogenesis. Furthermore, WB analysis confirmed an increased expression of PKC-δ in Cysltr1 KO mice, while DDAH1 and β-Catenin were reduced.
PKC, a family of protein kinases, can phosphorylate serine and threonine residues of various substrates and therefore regulate a number of cellular responses.17 The activation of various PKC isozymes had been found in lung tissue under CysLTs stimuli.18 Three PKC isozymes including PKC-α, -δ and -λ/ι were identified in our proteomic data, but only different expression of PKC-δ was detected. PKC-δ was reported to involve in stress-fibre formation induced by LTD4 in intestinal epithelial cells.19 And PKC-δ played an important role in the maintenance of the lung vascular barrier by stabilizing stress fibers and focal adhesions, increasing cytoskeletal stiffness in lung endothelial cells under unstimulated conditions.20 So, we speculated that PKCδ was an important downstream protein kinase of CYSLTR1 and implicated to the decrease of vasopermeability in Cysltr1 KO mice.
CYSLTR1 activation could result in increase of NO production in the lung airways.21 NO has emerged as an important factor involving in endothelial permeability.22 We found DDAH1 in DEPs, which is related to angiogenesis and NO biosynthetic process. DDAH1 modulates NO signaling by hydrolyzing the majority of asymmetric dimethylarginine in mammals, an endogenous inhibitor of NO synthase.23 We further confirmed the NO concentration decreased in lungs from Cysltr1 KO mice, which was probably associated with DDAH1 reduction.
Being central components of the cell adherens junction complex, VE-cadherin and β-catenin play important role in the vascular integrity. β-Catenin also plays an essential role in the Wnt signaling pathway. And abolishment of the pathway could suppress the vascular hyperpermeability.24 WB showed the unchanged expression of VE-cadherin and reduction of β-catenin, implying the Wnt signaling pathway was involved in the decreased vascular permeability in Cysltr1 KO mice.
PKC-δ, β-catenin could be used as a potential prognostic and predictive marker of various cancers.25, 26 Furthermore, DDAH1 had been regarded as a potential therapeutic target for treating cardiovascular diseases or portal hypertension.27, 28 Whether they could be selected as predictors of CYSLTR1 activation also remained to be tested by further biological experiment. In addition, many DEPs in the Cysltr1 KO samples such as chitinase-3-like protein 129 and Cell surface glycoprotein MUC1830 also have been used as predictor, but the change of these proteins remained to be verified by western blot analysis. Further investigation by applying more sensitive proteomic approaches or focusing on protein posttranslational modifications would gain further insight into the changes of proteins related to CYSLTR1 pathway. Collectively, these findings may have profound implication for improvement in CYSLTR1-related diseases.
5 Conclusions
Using iTRAQ and bioinformatics analysis, we described the proteomic variation of the lung tissue in Cysltr1 KO mice and subsequently validated the results that the PKC-δ was increased and DDAH1 and β-Catenin were reduced which maybe explained why vascular permeability was reduced in Cysltr1 KO mice. The DEPs were instrumental in exploring new predictors for improvement in CYSLTR1-related diseases.
Abbreviations
-
- CysLTs
-
- Cysteinyl leukotrienes
-
- CYSLTR1
-
- cysteinyl leukotrienes receptortype 1
-
- CRISPR/Cas9
-
- clustered regularly interspaced short palindromic repeat- associated system
-
- iTRAQ
-
- isobaric tags for relative and absolute quantitation
-
- PKCδ
-
- protein kinase C δ
-
- DDAH1
-
- N(G),N(G)-dimethylarginine dimethylaminohydrolase 1
Acknowledgements
M.J. and H.X. contributed equally to this work. This work was supported by the National Natural Science Foundation of China (Grant Nos. 81430041, 81501561, 81620108017), Natural Science Foundation of Guangdong Province (2014A030310043), the Science and Technology Planning Project of Guangzhou (201604020098, 201610010006), the Science and Technology Planning Project of Zhuhai (20171009E030008).
Conflict of Interest
The authors declare no conflict of interest.




