A Lipidomics Approach to Identifying Key Lipid Species Involved in VEGF-Inhibitor Mediated Attenuation of Bleomycin-Induced Pulmonary Fibrosis
Abstract
Purpose
Poor molecular characterization of idiopathic pulmonary fibrosis (IPF) has led to insufficient understanding of the pathogenesis of the disease, resulting in lack of effective therapies and poor prognosis. Particularly, the role of lipid imbalance due to impaired lipid metabolism in the pathogenesis of IPF has been poorly studied.
Experimental design
The authors have used shotgun lipidomics in a bleomycin (BLM) mouse model of pulmonary fibrosis with vascular endothelial growth factor (VEGF)-inhibitor CBO-P11 as a therapeutic measure, to identify a comprehensive set of lipids that contribute to the pathogenesis of pulmonary fibrosis.
Results
The authors report that attenuation of BLM-induced fibrotic response with CBO-P11 cotreatment is accompanied by a decrease in total lipid content and specific downregulation of lipids, which are upregulated in response to BLM treatment.
Conclusion and clinical relevance
Dysregulated lipids identified in this study hold the potential of being future biomarkers for IPF.
1 Introduction
Idiopathic pulmonary fibrosis (IPF), the most common form of pulmonary fibrosis, is a chronic and lethal lung disease characterized by excessive fibroblast proliferation leading to lung scarring and functionality loss.1-3 IPF occurs primarily in elderly patients and the median life expectancy is usually 5 years.4 Although a few molecular targets that contribute to disease pathology including the initial inflammation and excessive proliferation have been identified, specific molecular mechanisms for disease pathology remain unidentified and poorly understood. Improvements in diagnosis have been made over the years, but the vast majority of clinical trials aimed at improving lung function and reversing fibrosis have proven ineffective thus far.5
Unlike the other –omic techniques, genomics and proteomics, lipidomics—a lipid targeted metabolomics approach—does not measure biological entities that are genetically encoded, but instead measures downstream products of biochemical processes, known as lipids, that are influenced by the biological environment.6 Lipids can broadly be classified in three classes according to their cellular function. First are the energy storage lipids such as triacylglycerols, which are considered to be concentrated energy stores that readily contribute energy required for membrane biogenesis. Second are the structural amphipathic lipids consisting of a hydrophobic and hydrophilic region, directly involved in structural assembly of cellular membranes. Third are the signal transducing amphipathic lipids that can separate into hydrophobic and hydrophilic portions to transmit signals through the membrane and cytosol respectively.7 Lipid imbalance resulting from impaired lipid metabolism has historically been associated with obesity, diabetes, and cardiovascular disease; although emerging evidence shows a role for lipid dysregulation in inflammation, cancer, and cystic fibrosis.8-14 Recent advances have demonstrated role for dysregulated fatty acid synthesis in the pathogenesis of pulmonary fibrosis.15, 16 In light of the recent evidence of lipid laden cells being observed in the lungs of patients with fibrotic lung disease, it has become increasingly critical to identify the lipid axis that drives pulmonary fibrosis.17
Clinical Relevance
Idiopathic pulmonary fibrosis is a chronic, irreversible, and progressively fatal disease beset by limited therapeutic options due to inadequate understanding of the pathogenesis of the disease. Lipidomics is an attractive tool for molecular characterization as it generates a lipid profile, which can be used to compare lipid species in health and disease. We have identified a comprehensive set of lipids, which are upregulated in Bleomycin (BLM)-induced pulmonary fibrosis and are downregulated with VEGF-inhibitor treatment, which was used as a therapeutic measure. The significance of this study lies in the potential utility of these identified lipids as biomarkers in the future that can aid in the development of potential therapeutic and preventive strategies against this fatal disease.
Herein, we aim to study the poorly characterized lipid profile of otherwise well studied murine model of bleomycin (BLM) induced pulmonary fibrosis which has been shown to be useful in understanding the pathogenesis of IPF. The use of BLM as an effective antineoplastic agent was restricted after it was discovered that BLM can cause pulmonary fibrosis as a result of its cytostatic action.18 This side effect has led to BLM being utilized as a pulmonary fibrosis inducer in animal models that is very efficient in mimicking the lung injury, scarring, and fibrotic damage seen in IPF.19, 20 Shotgun lipidomics revealed several lipids that were upregulated with BLM treatment in the murine model of IPF. We have previously shown that BLM induces lung fibroblast proliferation and collagen deposition in a phosphatidylinositol-3-kinase/Akt dependent manner, which in turn leads to upregulation of the central angiogenic mediator vascular endothelial growth factor (VEGF).21 Furthermore, VEGF inhibitor CBO-P11 exerted protective effect in BLM-induced pulmonary fibrosis both in vitro and in vivo.22, 23 Treatment of injured mice with VEGF-inhibitor (CBO-P11), not only showed recovery of lung tissue as observed by histopathology, but also showed downregulation of proteins and pathways driving fibrosis.23 Here, we report that correction of fibrotic tissue and protein dysregulation with CBO-P11 cotreatment was accompanied by a decrease in total lipid content and specific downregulation of lipids, which was reversed in response to BLM treatment. Dysregulated lipids identified in this study hold the potential of being future biomarkers for IPF. More importantly, this study broadens the treatment options for a disease beset by limited options by identifying potential therapeutic targets in the form of metabolic and biochemical processes which leave behind these lipids as a cellular fingerprint.
2 Experimental Section
2.1 Materials
VEGF-inhibitor CBO-P11 was obtained from Calbiochem (San Diego, CA). BLM sulfate was obtained from Sigma–Aldrich (St. Louis, MO). Antibodies against Acetyl-CoA carboxylase (ACC), phospho-Acetyl-CoA carboxylase (Ser79) (pACC), Fatty acid synthase (FASN), AMP-activated protein kinase (AMPK), phosphor-AMP-activated protein kinase, alpha-smooth muscle actin (α-SMA), HRP-conjugated anti-rabbit IgG antibody, and HRP-conjugated anti-mouse IgG antibody were obtained from Cell Signaling Technology (Danvers, MA). Collagen III (COL3A1) was obtained from Santa Cruz Biotechnology (Dallas, TX). Sterol regulatory element binding protein–1c (SREBP-1c) and β-actin antibodies were obtained from Sigma–Aldrich (St. Louis, MO).
2.2 Animal Maintenance and Study Design
For the animal studies, 6–8 weeks old C57BL/6 mice were used (Jackson Laboratories, Bar Harbor, ME). Mice were housed in a barrier facility with specific pathogen-free conditions, and all experiments were performed using protocols approved by the Old Dominion University (ODU) animal facility. Briefly, mice were anesthetized with isoflurane. In the first set of experiments, either BLM sulfate or equal volumes of saline as control was administered intranasally. In a separate set, BLM-treated mice were cotreated every other day by intraperitoneal injection of CBO-P11 (0.3 mg kg–1) starting at day 0 and continued until the mice were euthanized at day 28. Bronchoalveolar lavage (BAL) fluid was collected after the trachea was exposed and cannulated with a 20-gauge catheter. After instillation of 1 mL of cold sterile PBS three times through the trachea into the lung, BAL fluid was recovered at 90% of the original volume. The BAL fluid was centrifuged for 10 min at 1500 rpm and the cell-free supernatant was stored at −80 °C. The lungs were perfused with 5 mL of cold saline through the left ventricle and surgically removed. The left lungs were used to evaluate the fibrotic score by histological examination, and the right lungs were homogenized to analyze protein and lipid levels.
2.3 Preparation of Lipid Extract from Lung Samples
The dissected lung tissue was cut into 2 mm2 and used for lipid/cholesterol extraction. Tissue was suspended with 610 μL M-PER mammalian protein extraction reagent with Halt proteases inhibitors (Thermo Scientific) to make tissue lysates. Tissue lysates were then transferred to Matrix A fast-prep tubes (MP Biomedical) and ruptured 20 s at a speed of (4.0 m s–1) twice without break. Tissue lysates were later centrifuged at 10 000 × g for 10 min at 4 °C and then the supernatant (500 μL) was transferred from each tube to labeled glass tubes on ice. An additional 600 μL M-PER reagent was added to original fast-prep tube and tissue lysates were ruptured, centrifuged, and the supernatant was collected as in previous steps. Chloroform (1000 μL) was added in fast-prep tubes with the remaining tissue lysate and the tubes were vortexed briefly. Lysate/chloroform mixtures were centrifuged at 10 000 × g for 10 min at 4 °C and 900 μL of mixtures was collected and transferred to cold glass tubes containing collected solution from previous steps. Chloroform (1000 μL) and methanol (500 μL) (HPLC grade) were added to glass tubes and the final volume of extraction at this point was 2500 μL. An internal standard triglyceride (TG, catalog #1787, Sigma–Aldrich, Mn) was added to each sample at a final concentration of 3 μm. Samples were vortexed briefly and centrifuged at 3000 rpm for 15 min after a 5-min incubation on ice. Organic phase (800 μL) was collected and transferred to new glass tubes. Chloroform/methanol-based extraction was repeated twice to generate a total volume of 2400 μL, which was used for lipid analysis. Chloroform/methanol in each sample was evaporated under nitrogen gas to form lipid pellets. Lipid pellets were resuspended with 800 μL of LC/MS injection solvent. Injection solvent in our case constituted of 250 μL of 65% acetonitrile, 35% isopropanol, and 5% water.
2.4 MS Analysis of Lipids
Lipid extracts from one lung tissue sample per treatment group were analyzed on the Q-Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, San Jose, CA). We used data dependent acquisition strategy incorporating Full MS data acquisition at 70K resolution and MS2 data acquisition at 35K resolution for the five most abundant peaks resulting from the Full MS data. Our sequence setup for the lipid analysis in Qual Browser included two blanks and a TG mix as quality control within intermediate runs to check for sample carryover, which could skew the lipid quantitation profiles and cause bias in the final results. Electrospray settings for the LC–MS/MS run was set at 4.0 kV in positive ion mode with the S-lens set at 30 volts; capillary temperature was set at 250 °C for efficient desolvation and ionization of the positively charged ions. The mass range used for the study was 200–1800 m/z. The AGC target settings for the Full MS and MS2 ions were at 1E6 and 5E4 respectively. The Orbitrap Fill times were set at 120 ms. HCD energy values were set at 35 eV and used as normalized collision energy for all analytes.
LC was set at high flow mode on a Surveyor 600 (Thermo Fisher Scientific, San Jose, CA); the flow rate was maintained at 200 μL min–1. The column used in this case is a modified C18 column (Supelco, 2.1 × 150 mm, 2.7 μm) from (Sigma–Aldrich, Mn). Solvent A (60% Acetonitrile/40% water with 0.1% formic acid) included 10 mm ammonium formate buffer with reference to the final solution. Solvent B (90% isopropanol/10% acetonitrile with 0.1% formic acid) with 10 mm formic acid added to the mixture made up the final solution. The 75 min gradient consisted of a linear phase from 32% B to 66% B over 60 min and an isocratic phase at 32% B for 15 min.
2.5 Data Processing
Lipid ion identification was performed using Lipid Search algorithm (Mitsubishi Knowledge Industry) that performs peak detection taking into account accurate masses from the lipids; MSn fragmentation (in our case normalized HCD collision energy). The reference fragment database for Lipid Search encompassed 106 lipid molecules. The experimental data was matched against the theoretical database and scored depending on the combination of the precursor ion mass and product ion mass by mass tolerance, presence of adducts, isotopes of dimers, and retention times across all chromatograms. Lipid molecules that were related were grouped together in the component table and represented by the largest adduct peak. For quantification, the deisotoped peaks from the precursor ion, their adducts were aligned taking into account the accurate masses and retention times across all chromatograms and their combined isotope intensity areas were accounted for as the area under the curve. The background ion intensities were taken into account when calculating the area under the curve for the lipid analytes. The data was finally manually checked for any anomalies. Data is displayed as the average of three technical replicates per sample.
2.6 Ingenuity Pathway Analysis
Differentially regulated proteins identified by MS were further analyzed using Ingenuity Pathway Analysis (IPA; Ingenuity Systems, Mountain View, CA). IPA was used to interpret the differentially expressed proteins in terms of an interaction network and predominant canonical pathways.23 The Ingenuity Pathways Knowledge Base (IKB) is a frequently updated curated database that consists of interactions between different proteins culled from scientific literature. IPA uses this database to construct protein interaction clusters that involve direct and indirect interactions. The networks are displayed graphically as nodes (proteins) and edges (the biological relationship between the proteins). A protein interaction network was generated as follows. A dataset containing the up- and downregulated proteins, called the focus proteins, for a particular comparison was uploaded into the IPA. These focus proteins were overlaid onto a global molecular network developed from the information in the IKB. Networks of these focus proteins were then algorithmically generated by including as many focus proteins as possible and other nonfocus proteins from the IKB that are needed to generate the network based on connectivity. Canonical pathways are identified from the IPA library based on their significance to the dataset. The significance of the association between the dataset and canonical pathway is measured in two ways: (i) a ratio of the number of proteins in the dataset that map to the pathway divided by the total number of proteins that exist in the canonical pathway and (ii) a p-value that is obtained by comparing the number of genes/proteins of interest relative (i.e., focus genes) to the total number of genes/proteins in all functional/pathway annotations stored in the IKB (i.e., a right-tailed Fisher's exact test with the Benjamini–Hochberg correction for multiple hypothesis testing).
2.7 Phospholipid Assay
Choline-containing phospholipids were measured using a Phospholipid Assay Kit (Sigma–Aldrich) according to the manufacturer's instructions. Briefly, CRL-1490 cell lysates or mice lung homogenates were seeded in 96-well black plates (20 μL). Eighty microliters of Reaction Mix was added to each well and incubated for 30 min. The absorbance of each sample was measured at 570 nm for the colorimetric assay using a Gen 5 2.0 All-In-One Microplate Reader (BioTek Instruments Inc.).
2.8 Immunoblotting
Mouse lung homogenates were resolved on a 10% SDS-PAGE and transferred onto a nitrocellulose membrane. The protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL), and equal amount of protein was loaded per sample. The membrane was blocked with TBS-T (0.1% Tween-20 in TBS) containing 5% dry milk, and incubated with primary antibody overnight at 4 °C. After three washes with TBS-T, the membrane was incubated with HRP-conjugated secondary antibody for 1 h and then washed with TBS-T. Immunoreactive proteins were detected by chemiluminescence (Supersignal West Femto, Pierce, Rockford, IL) using MyECL Imager (Thermo Scientific).
3 Results and Discussion
3.1 BLM-Induced Phospholipid Upregulation is Inhibited by VEGF Inhibitor
The effect of BLM and VEGF inhibitor CBO-P11 on phospholipid levels was analyzed both in vitro and in vivo using ELISA (Figure 1). BLM treatment induced a dose-dependent increase in phospholipid levels in CRL-1490 cells and pretreatment with the VEGF inhibitor CBO-P11 significantly decreased BLM-induced phospholipid upregulation (Figure 1A). BLM-treated mice lung tissue homogenates showed a significant increase in phospholipid levels as compared to saline-treated control mice. Lung tissue homogenates from mice cotreated with BLM and CBO-P11 showed a significant decrease in BLM-induced phospholipid levels (Figure 1B).
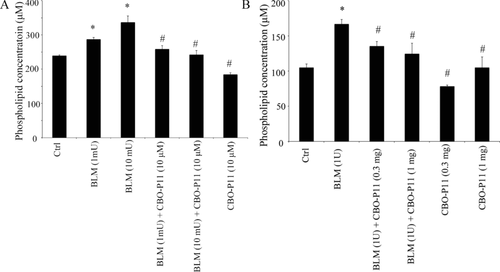
3.2 Altered Lipid Content in the Lungs of BLM and VEGF-Inhibitor Treated Mice
Lungs of mice treated with BLM showed considerable elevation of total lipid content (Figure 2). Multivariate analysis showed perturbations in the lipid profile of BLM treated mice and compared to controls (Supporting Information, Figure S1). The total lipid content in the CBO-P11 and BLM-cotreated group was reduced and restored to levels comparable to that of the control group (Figure 2A). Diacylglycerol (DAG) and lysophosphatidylcholine (LPC) were the lipid classes that showed overall downregulation with CBO-P11 and BLM cotreatment (Figure 2B) as compared to BLM treatment. Although several species of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) showed downregulation with CBOP11 and BLM cotreatment, overall there was no significant reduction in PC and PE. Sphingomyelins were unaffected with BLM and CBO-P11 treatment. Monoacylglycerol and TG, although remained unchanged with BLM treatment, showed upregulation with CBO-P11 treatment. PC was the most abundant lipid species across each of the treatment groups, accounting for more than 50% of the total lipid species in each group.
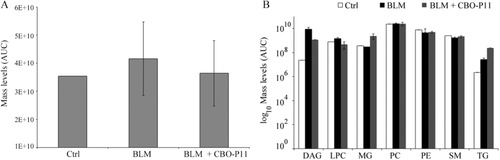
3.3 Effect of CBO-P11 on Lipid Species Most Dysregulated with BLM Treatment
Lipid species upregulated by at least twofold with BLM treatment were further analyzed to study the inhibitory effect of CBO-P11 in an unbiased manner. Out of the lipids that were common to the three treatment groups, 35 lipids showed at least twofold upregulation with BLM treatment, of which 26 comprised of PCs. CBO-P11 downregulated a significant number of these lipids that were upregulated with BLM treatment.
DAG, an intermediate in lipid metabolism, is involved in lipid mediated signaling and is an important component of cell membranes. DAG is not only an important source of free fatty acids, but it's simple structure makes it a precursor for the synthesis of more complex lipids.24 The conversion of phosphatidic acid into DAG is an important committed step in the synthesis of PC, PE, and phosphatidylserine. Impaired regulation of enzymes involved in DAG synthesis has been implicated in several diseases such as cancer, diabetes, and immune system disorders.25-27 While there has been no direct implication of DAG in pulmonary fibrosis, it was recently shown that diacylglycerol kinase alpha (DGKA) influenced profibrotic fibroblast activation.28 Decreased DNA methylation at the enhancer region of DGKA recruited profibrotic transcription factor early growth response 1, whereas inhibition of DGKA had pronounced effect on DAG mediated lipid homeostasis and reduced profibrotic fibroblast activation.28 We demonstrate that BLM treatment led to a fourfold upregulation of 18:1/24:2 species of DAG (Figure 3).
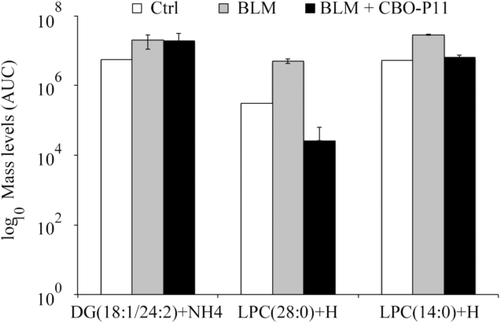
LPC regulates a variety of cellular functions such as cellular proliferation and migration.29, 30 LPC can either undergo deacylation to form DAG or reacylation to form PC.31 LPC is known to alter the expression of a number of genes directly involved in pulmonary fibrosis such as nitric oxide (NO) synthase, growth factors such epidermal growth factor, platelet derived growth factor, and adhesion molecules.32-35 Recently Tager et al. showed that inhibition of autotaxin, an enzyme involved in the generation of lysophosphatidic acid through the conversion of PC into LPC by phospholipase A2 (PLA2) resulted in reduction of BLM-induced pulmonary fibrosis.36 We observed that 14:0 and 28:0 LPC species showed a five- and 16-fold upregulation with BLM treatment and four- and 190-fold downregulation with CBO-P11 treatment indicating that inhibition of LPC might have a suppressive effect on BLM-induced fibrosis (Figure 3).
PCs are lipids that incorporate choline as head group and form the most abundant component in the outer leaflet of the eukaryotic cell membrane. Phospholipase A2 (PLA2) catalyzes the hydrolysis of phospholipids into lysophosphatidic acid and arachidonic acid, which are then modified into eicosanoids that include proinflammatory mediators such as prostaglandins and leukotrienes.37 BLM induced overproduction of proinflammatory cytokines such as thromboxane and leukotrienes is significantly reduced in PLA2 null mice implicating a direct role for PLA2, and a putative role for PC, in the pathogenesis of pulmonary fibrosis.38 Although one study has shown that PC administration in primates prevented alcohol induced hepatic fibrosis by promoting collagen breakdown,39 several other studies have established the link between elevation of cell membrane phospholipids in tumorigenicity and malignancy, suggesting the levels of total cellular PC be used as a predictive biomarker for monitoring tumor response.40-42 Several elevated PC species such as 16:0/16:1 in prostate cancer and 36:1 in breast cancer have been identified as diagnostic markers.43, 44 Several PC species such as 12:0/20:4, 18:2p/15:1, 16:1p/15:1, 15:1/20:4, 18:0e/20:4, 4:0/19:0, 24:6/24:7, 16:0e/20:4, 16:1/20:4, 16:1p/13:0, 15:0/20:4, 18:0/19:1, 16:0p/18:2, 16:1/18:2, 18:1/18:2, 18:0p/18:2, 22:6/24:7, 16:0e/18:2, and 18:0/18:1 showed an increase with BLM treatment and decrease with CBO-P11 treatment, suggesting that these species in particular and PC in general have an important role in the pathogenesis of BLM-induced pulmonary fibrosis (Figure 4). Overall, out of a total of 26 PC species that were upregulated by at least twofold with BLM, 19 species showed downregulation with CBO-P11 treatment. PC species have been grouped in separate panels (A–D) according to their intensities.
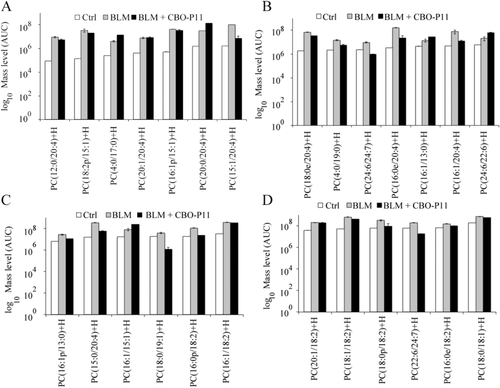
PE is the second most abundant phospholipid in biological membranes and along with PC forms the backbone of most eukaryotic cell membranes. PE is crucial for cell division as PE deficient cells show abnormal mitochondrial morphology and are unable to complete cell division and separate.45-47 From a disease perspective, little is known about the aberrations of PE levels in disease. However, recently it has been shown that increased cellular PE increases its ability to get oxidized and an increase in lipid oxidation is a common hallmark of neurological disorders.48, 49 A recent study showed that increased serum PE levels can be used as a diagnostic marker for lung cancer.50 Figure 5 below shows the PE species 16:0/16:0, 18:0e/23:1, 18:0e/19:1, and 18:0e/19:0 were upregulated by at least twofold with BLM treatment and downregulated with CBO-P11 treatment.
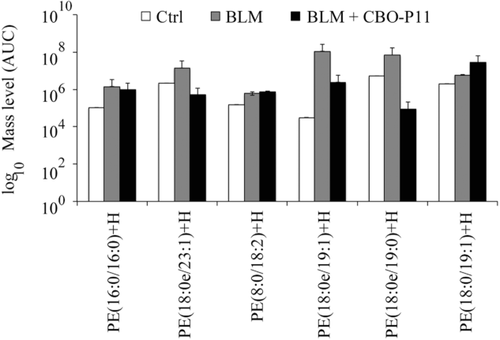
3.4 Validation of Lipidomic Data by High-Throughput Proteomics
The findings of this lipidomics study are supported by data from a high-throughput proteomics approach, a study in which we identified a comprehensive set of proteins and signaling pathways that contribute to the pathogenesis and amelioration of pulmonary fibrosis.23 Proteins that were identified to be significantly upregulated with BLM and downregulated with CBO-P11 treatment by at least 1.5-fold (p < 0.05) in mice lung homogenates were subjected to Ingenuity Pathway Analysis to determine the enriched pathways (Table 1).
| Pathway | Upregulation with BLM treatment | Downregulation with CBO-P11 treatment | ||||
|---|---|---|---|---|---|---|
| p-Value | Ratio | Upregulated Proteins | p-Value | Ratio | Downregulated Proteins | |
| Phospholipase C signaling | 0.05 | 0.05 | ARHGEF5,ITGA3,ARHGEF12,RRAS2,ARHGEF15,GPLD1,SOS2,GNB2,MYL4,NFATC4,ADCY10,RALGDS | 0.17 | 0.04 | TGM2,PLCB4,MPRIP,PLCE1,RRAS2,AHNAK,HDAC11,ARHGEF18,NFKB2,ITGA4 |
| Phospholipase signaling | 0.08 | 0.07 | PLB1,GPLD1,PLA2R1,PLCL2 | 0.08 | 0.07 | PLCB4,PLCE1,PDIA3,PLCL2 |
| AMPK signaling | 0.38 | 0.038 | MTOR,FASN,TSC2,RPTOR,NOS3 | 0.05 | 0.06 | MTOR,ACACB,CKM,FASN,PRKAR2A,RPTOR,PRKAG2,AKT3 |
| Fatty Acid Biosynthesis Initiation II | 0.06 | 0.5 | FASN | 0.06 | 0.5 | FASN |
| Fatty Acid α-oxidation | 0.37 | 0.067 | ALDH1A1 | 0.07 | 0.13 | ALDH2,ALDH1A1 |
We identified a number of proteins and pathways involved in lipid synthesis to be upregulated in response to BLM treatment. Phospholipase C (PLC), similar to PLA, is a phospholipid-hydrolyzing enzyme, which generates several lipid mediators that are capable of regulating several processes such as cellular growth and proliferation. Aberrant PLC signaling has been implicated in cell invasion, metastasis, and angiogenesis.51, 52 We observed several proteins involved in PLC signaling to be upregulated with BLM treatment suggesting an increased flux (p = 0.05) contributing to pathogenesis of pulmonary fibrosis, and downregulated with CBO-P11 treatment indicating decreased flux (p = 0.17) associated with reduced tissue fibrosis.
FASN, an enzyme that catalyzes the last step in fatty acid synthesis, was upregulated with BLM and downregulated with CBO-P11 treatment. Dysregulation of fatty acid synthesis homeostasis has been shown to be involved in pathogenesis and exacerbation of pulmonary fibrosis in mice.15 Aldehyde Dehydrogenase 1 Family, Member A1 was upregulated in mice lung tissue treated with BLM alone and downregulated in mice lung tissue cotreated with BLM and CBO-P11. Aldehyde Dehydrogenase 1 Family, Member A1 is involved in oxidation of fatty acids, and has a strong correlation with increased PE synthesis.48
The pathways enriched using proteomics data were not found to be statistically significant in part due to the low number of differentially expressed focus genes on the related pathways identified using unbiased proteomic profiling. Nevertheless, these enriched pathways and proteins lend significant support to our lipidomics findings. A network map integrating the pathways enriched in the lipidomics and proteomics data, glycerophospholipid metabolism, and fatty acid synthesis respectively, is shown in Supporting Information, Figure S2.
3.5 BLM-Induced Fatty Acid Synthesis is Negatively Affected by VEGF Inhibition
Next, the effect of BLM on various proteins involved in lipogenesis was analyzed. CRL-1490 cells were treated with BLM for 24 hours before Western blot analysis of fatty acid synthesis-related proteins. Upregulation of ACC, FASN, and SREBP-1c confirmed a BLM-induced upregulation in fatty acid synthesis (Figure 6A). Increased fatty acid synthesis can affect cellular energy and homeostasis levels, which can trigger AMPK activation. Analysis of AMPK protein levels revealed upregulation with BLM treatment. When cells were pretreated with CBO-P11; data reveals a downregulation of pro-lipogenesis proteins. Western blot data on 28-day mice lung homogenates revealed similar trend, consistently showing upregulation of prolipogenesis proteins in BLM-treated samples with the reverse observed in mice samples treated with CBO-P11 (Figure 6B). To confirm that BLM is indeed inducing fibrotic response in both our in vitro and in vivo models; we probed cell lysates and lung homogenates for Collagen type III and α-SMA, two common markers used for collagen accumulation and myofibroblast formation in pulmonary fibrosis. We observed a clear upregulation of both Collagen and α-SMA protein levels after BLM treatment and downregulation after CBO-P11 treatment in both cell (Figure 6A, B) and lung tissue lysates (Figure 6C, D).
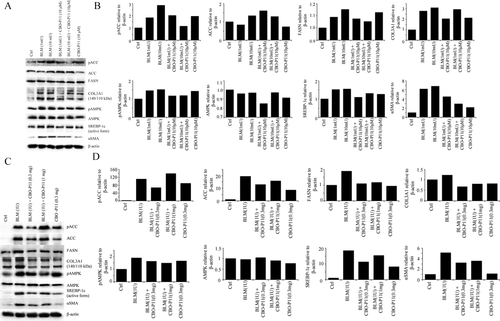
4 Conclusion
Lipids, because of their ability to serve a wide range of functions, are emerging as key biomarkers in various diseases. While the role of lipid dysregulation in various disorders such as diabetes, obesity, and Alzheimer's is well recognized, the role of deregulated lipogenesis in the pathogenesis of IPF has been understudied. The deregulated lipid profile reported in this study with a pulmonary fibrosis mouse model will help broaden the focus of therapeutic strategies in pulmonary fibrosis.
Abbreviations
-
- α-SMA
-
- alpha-smooth muscle actin
-
- AMPK
-
- AMP-activated protein kinase
-
- BLM
-
- bleomycin
-
- DAG
-
- diacylglycerol
-
- DGKA
-
- diacylglycerol kinase alpha
-
- FASN
-
- fatty acid synthase
-
- IKB
-
- Ingenuity Pathways Knowledge Base
-
- IPA
-
- Ingenuity Pathway Analysis
-
- IPF
-
- idiopathic pulmonary fibrosis
-
- LPC
-
- lysophosphatidylcholine
-
- PC
-
- phosphatidylcholine
-
- PE
-
- phosphatidylethanolamine
-
- PLA2
-
- phospholipase A2
-
- PLC
-
- phospholipase C
-
- TG
-
- triglycerides
-
- VEGF
-
- vascular endothelial growth factor
Acknowledgements
Y.M.K. and S.D. contributed equally to this work. We thank the Animal facility staff at ODU for maintaining the animals. None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. This work was supported by National Institutes of Health grants HL112630 and CA173069.
Conflict of Interest
The authors have declared no conflict of interest.




