Metabolic Signature of Microvesicles from Umbilical Cord Mesenchymal Stem Cells of Preterm and Term Infants
Abstract
Purpose
Microvesicles (MVs), 200–1000 nm bodies budding from the cell plasma membrane, are a promising source of biomarkers. This study aimed at comparing the proteome of MVs collected by ultracentrifugation from cultured Mesenchymal Stem Cells (MSCs) from Human Umbilical Cord of Preterm newborns (<34-weeks gestational age) in comparison to infants at Term (≥37 weeks). This discovery study was designed to establish the signature of prematurity.
Experimental design
Orbitrap MS, statistical, bioinformatics and biochemical analyses were employed.
Results
A total of 3253 proteins were identified, 78.3% matching among Preterm and Term. Principal component dimensional analyses showed that the two proteomes cluster separately. Cytoscape analysis showed that the top gene signatures cluster around inflammation and oxidative metabolism. Both Preterm and Term MVs consumed oxygen, and express ATP synthase and cytochrome oxidase, but only Preterm MVs synthesized ATP. The gene signature of Preterm condition mainly clusters around inflammation and metabolism.
Conclusion and clinical relevance
MVs from MSCs conduct aerobic metabolism similarly to exosomes from the same cells, with interesting differences related to their biogenesis and function. The clinical relevance of the study lays in the perspective to utilize MVs as promising sensor of the inflammatory and metabolic state of the preterm newborn, to help in preventing the complications of prematurity.
1 Introduction
Vesicles released from cells [1,2] have extended the list of intracellular signaling players. Extracellular vesicles (EVs) have been detected in biological fluids and in cell culture mediums3 also from mesenchymal stem cells (MSC), stromal self-renewing cells that can be also isolated from umbilical cord.4 Microvesicles (MVs), also referred to as ectosomes (100–1000 nm diameter), bud outwardly from the plasma membrane, in a process involving contractile proteins.1 Due to their soluble and membrane-bound protein and RNA content, once internalized, EVs act as “translational” organelles, altering the expression of target cells.5 Exosomes released from cultured Mesenchymal Stem Cells from Human Umbilical Cord (HUCMSC)of both Term and Preterm newborns consume oxygen and synthesize ATP.6The same applies to urinary exosomes.7 This is consistent with many reports showing that oxidative phosphorylation (OXPHOS) can be carried out ectopically with respect to the mitochondria.8 MVs also appear a promising source of biomarkers.9, 10
Clinical Relevance
The clinical relevance of the study lays in the perspective to utilize Microvesicles (MVs) derived from the Human Umbilical Cord Mesenchymal Stem Cells (HUCMSc), as sensors of the inflammatory and metabolic state of the Preterm newborn, to help preventing their complications. There is the clinical need for an early identification of preterm at risk for developing clinical complications, as most of them undergo hyperoxic challenge. The goal of this study was to show that MVs from both Term and Preterm HUCMSC conduct aerobic metabolism, as their proteome is enriched with oxidative metabolism proteins. However, aerobic ATP synthesis was exclusively detectable in Preterm MVs. Upon premature exposure to ambient O2, Preterms and their HUCMSC, not ready to cope with normoxia, become involved in oxidative metabolism. This would promote autophagy and MVs production, to discharge the mitochondrial machinery detrimental for the cell. Term HUCMSC, would not undergo this process, even in normoxia. The present study moves the field forward as it opens the perspective to use the MVs from HUCMSC as a model of prematurity and moreover as biomarkers of the general inflammatory and metabolic status of the Preterm infant, without the need for invasive procedures.
Approximately 10% of newborns are born prematurely. Complications of premature birth are inversely proportional to gestational age and can impact on the infant's short- and long-term outcome.11 Morbidity of survivors, especially the very low birth weight (VLBW) premature infants primarily includes bronco-pulmonary dysplasia, retinopathy of prematurity, and periventricular white matter lesions, causing immediate or long term disability. It is currently supposed that premature oxygen exposure contributes to the pathogenesis of the observed injuries.12 Our preliminary data showed that the blood of VLBW infants contains high levels of adenosine, as a function of body weight.13 The oxygen challenge may promote the observed adenosine increase. It has been assessed that initial resuscitation in air is preferable to 100% oxygen, as oxidative stress is among the causes of periventricular white matter lesions.14
Here, in search for biomarkers of prematurity, we compared MVs from HUCMSC cultured from umbilical cord of Preterm in comparison to term newborns, by proteomic statistical, bioinformatical, and biochemical analyses.
2 Experimental Sections
2.1 Isolation and Expansion of HUCMSCs
After written informed consent, umbilical cords from full-Term (gestational age ≥ 37 weeks, n = 3, Term) and Preterm (gestational age <34 weeks, n = 3 in the range 28–32 weeks) deliveries between February 2015 and July 2015 were collected following elective cesarean section to avoid the influence of mode of delivery that can affect cellular functional state.15 Study was approved by the Institutional Review Board of the IRCCS G. Gaslini, Genoa–Italy. The MSCs were isolated from umbilical cord using a non-enzymatic digestion protocol.16 To identify the isolated cells as MSC flow cytometric analysis was performed at P4. All cultured medium was pre-centrifuged at 100 000 × g to avoid any EVs contamination.
2.2 Sample Collection and Micro Vesicle Separation
MVs were collected by centrifugation-based protocol, a simple and fast system to isolate EVs.17 In particular, the supernatant of umbilical cord MSC culture media was collected and centrifuged at 16 000 × g for 15 min to avoid any cross-contamination with cells, debris, and organelle such as mitochondria. Then, the supernatant was removed and centrifuged at 22 000 × g for 75 min. The pellet containing the MVs fraction were washed three times with PBS by means of additional centrifugation at 22 000 × g for 75 min. and resuspended in PBS.
2.3 Micro Vesicle Preparation for MS
Samples were solubilized in 0.1 mL of 4% w/v SDS, 50 mM DTT, and 0.1 M Tris/HCl, pH 7.6, at 90 °C for 5 min, sonicated and processed by FASP procedure using 30k Vivacom filtration devices. Procedure is detailed in Supporting information.
2.4 Mass Spectrometry
A linear Trap Quadropole Orbitrap Velos Pro MS was used for analysis of all samples. The MS setup is detailed in Supporting Information.
2.5 Electrophoresis and Western Blot
SDS–PAGE on a gradient 8–16% acrylamide gels and western immunoblotting analysis were done for β subunit of FoF1-ATP synthase (Sigma Aldrich, 1:500) and COX5A (Sigma Aldrich, 1:500). The ChemiDoc Touch Imaging System (Bio-Rad, Hercules, CA) was used for detection and analyzed using Image Lab software. Procedure is detailed in Supporting information.
2.6 Oxygen Consumption and ATP Synthesis Measurements
O2 consumption was assayed on Term or Preterm HUCMSC MVs with a thermostatically controlled oxygraph (Unisense Microrespiration, Unisense A/S, Denmark) as reported18 in:137 mm NaCl, 5 mm KCl, 0.7 mm KH2PO4, 25 mm Tris–HCl, pH 7.4, and 25 mg ml–1 ampicillin (0.015 mg protein in 1.7 mL). ATP synthesis was assayed on Preterm or Term HUCMSC MVs by the luciferin/luciferase chemiluminescent method (Roche Applied Science).
2.7 Complex III Activity Assay
Complex III activity was evaluated spectrophotometrically, following the reduction of cytochrome c at 550 nm (extinction molar coefficient: 1 mm/l/cm). Assay medium contained:10 μg of protein, 100 mm Tris-HCl pH 7,4, and 0,03% cytochrome c. Reaction was started by adding of 0.7 mm NADH.
2.8 Statistical and Bioinformatics Analysis
Protein identity and quantitative data were exported for further statistical and bioinformatics analysis. Each label-free quantification data was normalized. After quantile normalization, unsupervised hierarchical clustering analyses, such as multidimensional scaling (MDS) with k-means and Spearman's Correlation, were used to identify outlier and samples dissimilarity. Differentially expressed proteins between Preterm and Term were detected using a non-parametric U-Mann Whitney test. p-values were adjusted for multiple-testing using the Benjamini–Hochberg false discovery rate (FDR). Proteins were considered to be significantly differentially expressed between the two conditions with an adjusted p-value ≤ 0.05 and 80% power. Moreover, the proteins had to be identified in all biological replicates of one of the two groups. To determine the lowest statistically significant fold change expression value between Preterm and Term samples, a power analysis was used considering the number of samples and their biological variability (fold change ≤–8 or ≥ 8). Volcano plot was used to quickly visualize the statistical differences. The cutoff lines were established using the function y = c/(x – x0). Finally, a non-linear support vector machine was used to develop a ranked list of significant proteins. Cross-validation approach (fourfold) was applied to estimate the accuracy of this classification.
Cytoscape software, with ClueGO app, was used for gene ontology and pathway analysis. The list of all identified proteins was submitted, to identify the enrichment of the biological process and the cellular component classification of the two cohort of samples, according to their GO annotation extracted from the UniProt, KEGG, Reactome, and ClueGOpedia database. The biological processes of the two groups were considered significant with at least 20% of associated genes and p ≤ 0.05 value after FDR correction. The results of GO analysis were exported for further statistical analysis. To quickly visualize the ability of each biological process to distinguish Preterm form Term subjects and establish the top gene signatures of Preterm condition the MDS analysis, volcano plot and enrichment analysis was used. For western blot, oxygen consumption, and ATP synthesis data analysis, U-Mann-Whitney test was used to assess differences between the Term and Preterm condition. All statistical analyses were performed using the last version of software package R, available at the time of experiments.
3 Results
MVs for proteome profiling were isolated from culture mediums of HUCMSC from ≥37-week-old or 28- to 30-week-old newborns, i.e. Term or Preterm newborns respectively. A proteomic analysis was conducted by MS. MS analysis identified a total of 3253 proteins (detailed results are in Supporting Information, Table S1). Good separation between the two cohort of samples was evidenced by MDS of the proteome profiles (Figure 1A). Spearman's correlogram is in Supporting Information, Figure S1. Average Spearman's coefficient (R2) was high in the two groups, confirming that Preterm (0.76 ± 0.04) and Term (0.74 ± 0.05) samples represent two separate clusters. The limited number of biological samples may reduce the significance of our results; for this reason we applied a power analysis.19 Considering the number and the mean of the biological variation coefficient (CV = 0.47), we identified as a lower limit for quantitative protein variation an eightfold change for a significance level of 0.05 and a power of 80% (see Supporting Information, Figure S2 for detail).
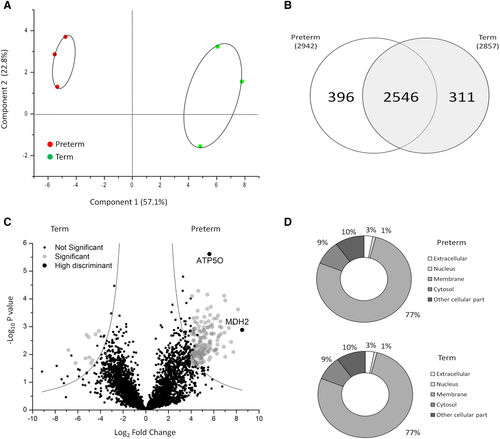
Out of the 3253 proteins identified by MS, 2546 (78.3%) matched when Preterm were compared to Term samples. The remaining 396 proteins (12.2%) in Preterm MVs and 311 proteins (9.5%) in Term MVs, were exclusive of one of the two groups (Figure 1B).To better describe the differences between the two cohorts of MVs, univariate statistical analysis was performed. In addition, to increase the protein ability to discriminate the two conditions using a limited number of samples, we considered only the statistically significant proteins identified in all biological samples of one of two groups. A total of 173 proteins significantly changed between Preterm and Term conditions are evidenced, 163 of which are upregulated in Preterm (Figure 1C). Among these, eight proteins are involved in oxidative phosphorylation (OXPHOS). To establish a rank list of significant proteins and select the highly discriminant proteins between Preterm and Term conditions, a support vector machine analysis was used. Interestingly, two proteins of the OXPHOS (MDH2 and ATP5O) are the proteins possessing the highest power of discrimination between Preterm and Term condition.
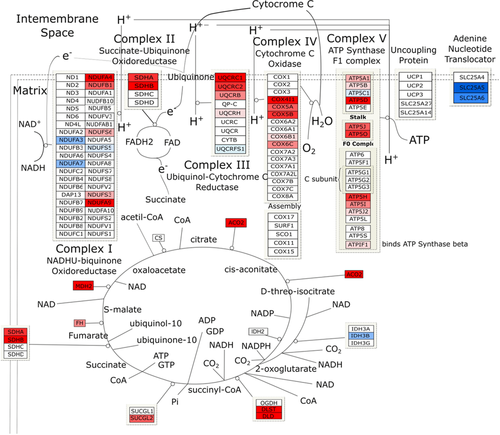
The specific diversity of the proteins identified in the Preterm/Term could imply a different origin and/or function of these proteins. To assess this, we performed GO analysis based on cellular component and biological processes annotation, using cytoscape software. As shown in Figure 1D, most of the MVs proteins were annotated as membrane (77%); in contrast, 9% of the proteins annotated as cytosol, 3% as extracellular, 1% as nuclear, and 10% as other cellular component. The membrane proteins were significantly enriched (77%) in MVs, consistently with their biogenesis. A total of 328 significant top gene signatures were identified through GO analysis. The results of GO analysis are shown using MDS scatter plot. This shows three different clusters, characterized according to biological processes, common or exclusive of Preterm or Term MVs (Figure 2A). The major percent difference between biological process annotation of Preterm and Term can be associated to metabolism and inflammatory responses (Figure 2B).To test whether the tendency towards a specific biochemical pathway in Preterm or Term was related to this diversity, we estimated the relative functional GO enrichment using volcano plot. Figure 2C shows the mean fold change of proteins associated with each biological process, plotted against their probability to be active in Preterm or Term MVs. A 2D bubble diagram summarizes the above results and shows the top gene signatures identified (Figure 2D). The circle size is proportional to the number of proteins associated with each statistically significant biological process. Accordingly, the main biological processes present in HUCMSC MVs are: transport, immune system process, cell differentiation, cell motility, cell adhesion, cytoskeleton organization, vesicular trafficking, apoptosis regulation, proteolysis, tissue-related miRNA content, and notably, metabolism. A remarkable enrichment in metabolic pathways was found: most of gene named signature was associated with glucose metabolism, oxidative metabolism, including tricarboxylic acid (TCA) cycle and Electron Transport Chain (ETC). In particular, the TCA cycle enzymes (with the exception of CS and IDH2), and 31 out of 103 proteins of respiratory chain proteins were identified. These are anyway representative of each of the five respiratory chain complexes, as shown in Figure 3. Such results confirm the protein statistical analysis that highlighted MDH2 and ATP5O, as well as our previous findings of the capacity of exosomes from both Preterm and Term HUCMSC to conduct an aerobic metabolism, so that expression of proteins involved in aerobic metabolism is characteristic of EVs.6 In light of these results, we decided to assess whether these proteins were active in the process of OXPHOS also in MVs.
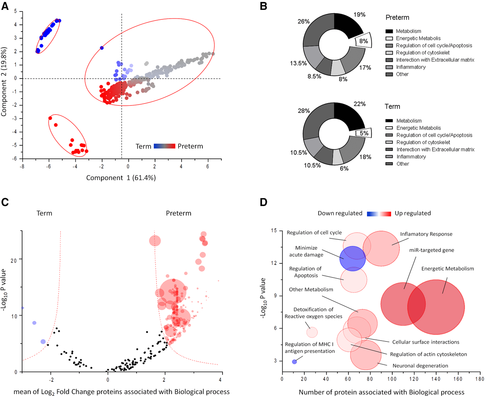
O2 consumption and ATP synthesis were assayed on MVs, in the absence of detergents. Results highlight interesting differences. Figure 4A and B shows the respiration in Preterm or Term MVs, after addition of NADH or of succinate, to induce the pathways composed by Complexes I, III, and IV, or Complexes II, III, and IV, respectively. MVs from mature (Term) HUCMSC consume less O2 in the presence of both respiring substrates, as compared to Preterm conditions. NADH was chosen as a respiring substrate because it is useful to distinguish the extra-mitochondrial from mitochondrial respiration, as we have demonstrated previously,20, 21 being impermeant to isolated mitochondria. By contrast, NADH appears a good respiratory substrate for MVs (Figure 4A). It donates its electrons directly to Complex I, as suggested by the assay of the activity of complex III (through complex I, see Methods) with NADH, which was higher in Preterm MVs (0.46 0.44–048IU/min/mg) than in Term MVs (for 0.27 0.25–0.29 IU/min/mg), in line with the results form O2 consumption assay. ATP synthase activity was also assayed (Figure 4B). Preterm MVs only produce ATP, indicating that the O2 consumption observed in these is coupled to ATP synthase. Considering that the P/O ratio was about 2.5 ± 0.4, a value similar to that reported in literature for mitochondria, it is concluded that Preterm O2 consumption is coupled with the ATP synthesis. Consistently, Figure 4C and D shows that the beta subunit of ATP synthase (ATP5B) and the 5A subunit of Cytochrome c oxidase (COX5A) are expressed in the MVs, relatively enriched in the Preterm ones.
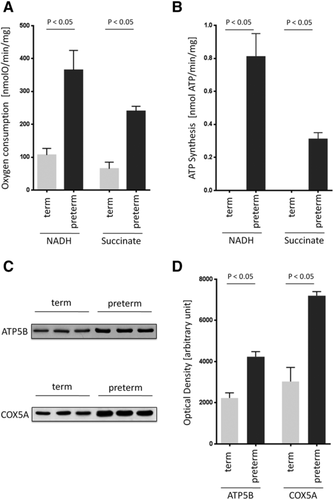
Oxygen consumption, ATP synthesis, and western blot analysis of MVs samples. A) Oxygen consumption in Preterm and Term MVs (dark and light grey columns, respectively), as nmol O consumed/min/mg; B) ATP synthesis in MVs as nmol ATP synthesized/min/mg; C) western blot of ATP5B (subunit of ATP synthase) and COX5A (subunit of Cytochrome c oxidase); and D) their analysis in MV samples. Oxygen consumption in Term is lower than in Preterm MVs, whereas the ATP production from Term MVs is not detectable. Also the expression of ATP5B and COX5A in Term is lower than in Preterm MVs. All experiments show that preterm conditions display a hyperactivation of ETC pathway. Data reported are expressed as median and interquartile range. The statistically significant difference among Preterm and Term MVs is indicated in the graph.
4 Discussions
The proteomes of the MVs from Preterm or Term HUCMSC cluster separately, in face of identical percent composition: both MVs display an enrichment (77%) in membrane proteins. Such plasma membrane protein enrichment is consistent with MVs biogenesis. In Preterm MVs, some processes were up-regulated, with respect to Term ones: cell surface interactions, inflammatory responses, and oxidative metabolism (Figure 4C). An up-regulation was found in the Preterm MVs of some proteins of the ETC and of the TCA cycle (Figure 3) especially of ETC Complex II and Aconitase. Aconitase is a key enzyme of the TCA cycle, therefore its up-regulation in Preterm MVs is in line with their increased oxidative metabolism. MVs from both Term and Preterm HUCMSCs express ATP5B and COX5A subunits (Figure 4C and D), and display an O2 consumption ability (Figure 4A). However, aerobic ATP synthesis was exclusively detectable in Preterm MVs. By contrast, Term MVs displayed lower expression of ATP5B and COX5A and O2 consumption. Here, aiming at mimicking the conditions of the premature infant, the HUCMSC were grown in normoxia for 1 week. Indeed, recently it was shown that once in normoxia, MSC undergo a shift towards the aerobic metabolism, accelerating O2 consumption.22
A main issue is why an oxidative metabolism should be present in the MVs membranes. It is tempting to presume that the OXPHOS proteins are expressed on the plasma membrane of the HUCMSC from which they derive. This would imply a transfer of the ETC from the mitochondrial inner membranes to the ER and to the plasma membrane, consistently with previous data on the hepatocyte,23 HUVEC,24C6 glioma cells,18 rod disks,25 and myelin.26 Conceivably, preterm HUCMSC are unable, due to immaturity or dysregulation, to limit the expression of the OXPHOS proteins on the plasma membranes, and possibly to the ER.27 Therefore, upon exposure to ambient O2, they undergo an oxidative burst. This may rise autophagy and in turn promote MVs production, to discharge the mitochondrial machinery, expanded to the detriment of the cell. By contrast, Term HUCMSC, equipped for birth, would be able to prevent massive OXPHOS transfer to the ER and plasma membranes, even in normoxia. This idea is consistent with the results obtained on the parental Term and Preterm HUCMS cells, whose energy metabolism was found to undergo a switch from a prevalently anaerobic to aerobic, around the 34th week of gestational age, associated to the organization of mitochondrial reticulum.28 Embryonic Stem cells rely more on glycolysis,29 and express low levels of OXPHOS, the more Preterm they are. Hypoxia is a major player in the maintenance of an undifferentiated state for the MSC.29 A switch to the OXPHOS is a cause of oxidative stress and senescence,22 by mechanism, possibly involving the hypoxia inducing factor 1 (HIF-1) promoting the efficiency of the OXPHOS increasing the number of mitochondria was reported.30, 31
The metabolic signature of HUCMSC MVs is consistent with previous data on exosomes from the same cells.6 HUCMSC exosomes conduct an aerobic metabolism, but remarkably behave the opposite way with respect to MVs, in that an ATP synthetic ability was exclusive of Preterm exosomes, being undetectable in Term ones. As discussed previously, the presence in the exosome membranes of CD39 and CD73, two ecto-enzymes that convert ATP to adenosine, may be the reason for missed detection of ATP.6 This would also justify the elevated blood adenosine content of the premature infants.13 Interestingly, MVs express P2Y1 and P2 × 1 purinergic receptors (see Supporting Information, Table 1). Therefore, Preterm MVs have the potential to increase the extracellular ATP concentration, contributing to triggering inflammation.32 Interestingly, VLWB Preterm (below 1.500 g) display a generalized inflammatory status.33
Different composition of exososmes and MVs would reflect their biogenesis and function. Exosomes mostly act as messengers: they bud inside endosomes, retaining the characteristics of the ER while displaying a secretive destiny. Exosomes may not be representative of the cell of origin. By contrast, MVs shed from the plasma membrane,1, 34 randomly retaining its composition and the functional status of the interior/exterior interface.
Shedding vesicles play important physiological and pathological roles. MVs from HUCMSC were shown to promote human renal cancer cell proliferation and malignance10, 35, 36 and angiogenesis.3 This is consistent with the enrichment of the GO related to miR-targeted gene (Figure 2C).
Preterm HUCMSC apparently become actively involved in oxidative metabolism, after premature birth, not being equipped to cope with ambient O2 concentration. Notably, the premature newborn faces the same challenge: following exposure to atmospheric O2, these undergo an oxidative stress.37, 38 This opens the perspective to use HUCMSC MVs as a model of prematurity. MVs may become biomarkers of the general inflammatory and metabolic status of the Preterm infant, without the need for invasive procedures. In fact, MVs can also be collected from blood and/or urine.
Abbreviations
-
- COX5A
-
- cytochrome c oxidase subunit 5A
-
- ETC
-
- electron transport chain
-
- EV
-
- extracellular vesicle
-
- FDR
-
- false discovery rate
-
- GO
-
- gene ontology
-
- HUCMSC
-
- Human Umbilical Cord Mesenchymal Stem Cell
-
- MDS
-
- multidimensional scaling
-
- MSC
-
- mesenchymal stem cell
-
- MVs
-
- microvesicles
-
- NADH
-
- reduced β-nicotinamide adenine dinucleotide
-
- OXPHOS
-
- oxydative phosphorylation
-
- TCA
-
- tricarboxylic acid cycle
-
- VLBW
-
- very low birth weight
-
- WB
-
- western blot
Acknowledgements
G. C. and I. P. contributed equally to this work. This work was supported by Cinque per mille e Ricerca Corrente Ministero della Salute, to Istituto Giannina Gaslini; Compagnia di San Paolo, 2016.AAI1017.U1260/2015.0408/1039 grant to M.Bruschi: “Esosomi Circolanti quali Sensori dello stato Metabolico del nato Pre-Termine: Ipotesi di Validazione di una supplementazione dietetica nella Gestante”; and funds from Renal Child Foundation.
Conflict of Interest
No financial/commercial conflicts of interest to declare for all authors.




