The Impact of the Glomerular Filtration Rate on the Human Plasma Proteome
Abstract
Purpose
The application of proteomics in chronic kidney disease (CKD) can potentially uncover biomarkers and pathways that are predictive of disease.
Experimental design
Within this context, this study examines the relationship between the human plasma proteome and glomerular filtration rate (GFR) as measured by iohexol clearance in a cohort from Sweden (n = 389; GFR range: 8–100 mL min–1/1.73 m2). A total of 2893 proteins are quantified using a modified aptamer assay.
Results
A large proportion of the proteome is associated with GFR, reinforcing the concept that CKD affects multiple physiological systems (individual protein–GFR correlations listed here). Of these, cystatin C shows the most significant correlation with GFR (rho = –0.85, p = 1.2 × 10−97), establishing strong validation for the use of this biomarker in CKD diagnostics. Among the other highly significant protein markers are insulin-like growth factor-binding protein 6, neuroblastoma suppressor of tumorigenicity 1, follistatin-related protein 3, trefoil factor 3, and beta-2 microglobulin. These proteins may indicate an imbalance in homeostasis across a variety of cellular processes, which may be underlying renal dysfunction.
Conclusions and clinical relevance
Overall, this study represents the most extensive characterization of the plasma proteome and its relation to GFR to date, and suggests the diagnostic and prognostic value of proteomics for CKD across all stages.
1 Introduction
Chronic kidney disease (CKD) is characterized by a progressive loss of renal function as measured by glomerular filtration rate (GFR).1 The International Society of Nephrology has established clinical guidelines for diagnosing CKD severity starting with stage 1, defined as normal kidney function of GFR >90 (mL min–1/1.73 m2) and marker of kidney damage, to stage 5 defined as severe kidney injury with a GFR less than 15 mL min–1/1.73 m2.1 GFR <60 mL min–1/1.73 m2 is classified as CKD regardless of kidney damage marker. The natural history of CKD often progresses slowly with several comorbid pathophysiological changes that occur in parallel with decreasing renal function such as increased risk for cardiovascular disease.2 The high prevalence of CKD, which tops 10% in the US,3 highlights the need for more effective methods of early detection to avoid or slow the progression to terminal renal failure.
One of the early effects from renal decline is the accumulation of molecules in the blood, including polypeptides and proteins due to changes in the glomerular barrier of the kidneys. The transport of proteins across the glomerular barrier depends on the charge, shape, and size of the molecule, as well as the charge and pore-size of the glomerular capillary wall.4 Plasma proteins with a molecular mass <20 kDa are freely filtered at the glomeruli while there is reduced permeability related to the pore size of the glomerular membrane for proteins up to about 50 kDa. As renal function declines, the filtration of water-soluble molecules with a molecular mass of <50 kDa decreases, leading to subsequent accumulation of proteins in the blood. Simultaneously, impairment of kidney function may be associated with altered protein synthesis, with diminished erythropoietin as a well-known example.5 Thus, CKD progression may lead to both an increase and decrease in plasma proteins levels.
The gold standard for measured GFR (mGFR) is clearance of a kidney filtration marker such as iohexol, which is a complex, expensive, and time-consuming process. Other methods provide an estimate of GFR based on blood concentrations of creatinine or cystatin C or a combination of both.6 More than a decade of research has now established cystatin C as the most sensitive biomarker for assessment of GFR across all stages of CKD.6, 7 Nevertheless, the application of omics technologies in clinical research and practice opens new possibilities for discovering candidate biomarkers that may be more sensitive in prediction of renal decline at a point when prevention of the disease is still possible.
Clinical Relevance
Chronic kidney disease (CKD) is common and affects about 10% of the general population. Early detection is important and enables preventive measurements to avoid terminal renal failure. There is a need for more accurate biomarkers of glomerular filtration rate (GFR). Knowledge about the association of plasma proteins and renal function, as measured by iohexol clearance, provides an opportunity to discover new GFR markers that detect early stage CKD. Using aptamer-based proteomics, results revealed a significant proportion of the human plasma proteome that is associated with GFR levels, highlighting the impact of CKD on multiple physiological systems. This knowledge facilitates the use of biomarkers in renal insufficiency and points the way to explaining the mechanisms of action underlying CKD.
Proteomics represents a promising analytical tool to identify blood-based biomarkers associated with the full spectrum of GFR. However, the studies using proteomic technologies to date have been limited in their clinical application because they were tested in small, overly homogenous cohorts, and lacked validation from iohexol clearance as an independent standard.8-10 Furthermore, most proteomics methodologies rely upon MS, which has shown limited reproducibility and specificity for biomarker development in CKD.11
In contrast, aptamer-based proteomics offers many unique advantages for unbiased biomarker discovery and diagnostics including highly sensitive and specific measurements of thousands of proteins at a given time across a wide dynamic range of concentrations.12 In fact, this technology was recently used in a cohort of 42 CKD patients, and identified a total of 60 plasma proteins that significantly differentiated early- from late-stage CKD.13
Building upon these promising results, the primary purpose of this study was to use aptamer-based proteomics for large-scale discovery of plasma proteins across the entire range of GFR values as well as restricted ranges corresponding to early CKD.1 Besides cystatin C, there are very few established biomarkers that indicate renal decline. Thus, a secondary purpose of this study was to explore novel proteins suitable as GFR biomarkers to support early diagnosis in the most extensive analysis of proteins to date. To this end, we analyzed plasma samples subjects with GFR values that spanned the full range of kidney function from 8 to 100 mL min–1/1.73m2 as well as restricted ranges of mGFR. Overall, these results provide a new understanding of the plasma proteome and its relation to kidney function, which could lead to the development of more sensitive diagnostic procedures for therapeutic development and prevention of CKD.
2 Experimental Section
2.1 Study Population
Participants were selected from a cohort of patients from Lund, Sweden, to examine the relationship between GFR and the plasma proteome. None of the patients were on dialysis. All participants were of Western European Caucasian origin. The cohort included doctor-referred male and female subjects for determination of iohexol clearance at Skåne University Hospital.14 We randomly selected 389 subjects with GFR values from 8 to 100 mL min–1/1.73 m2 for proteomic analysis. Samples were Li-Heparin plasma.
The study cohort was characterized as 51% male (n = 201), with a median age of 64 years (interquartile range (IQR): 57.5–71 years), and median BMI of 25.3 (IQR: 22.7–28.6). The median GFR value was 62 mL min–1/1.73 m2, with median creatinine levels of 91.3 μmol L–1 (IQR: 71.2–126.7 μmol L–1) and median cystatin C as 1.4 mg L–1 (IQR: 1.1–1.8 mg L–1).
All procedures involving participants and data agreed with the ethical principles for medical research involving human participants established in the Helsinki Declaration of 1975, and revised in 2000. Samples and patient data were blinded in all analyses.
2.2 Determination of GFR by iohexol
Glomerular filtration rate was measured (mGFR) as plasma clearance of iohexol using a single plasma sample technique,15 which is considered as reliable as using multisampling.16 Five microliters of iohexol (Omnipaque 300 mg I mL–1, GE Healthcare, Oslo, Norway) was administered intravenously in an antecubital vein. One plasma sample was drawn at varying times depending on the estimated GFR (eGFR)17 calculated by the Cockcroft–Gault equation (3–4 h at eGFR > 50 mL min–1; 6–8 h at eGFR 20–50 mL min–1; 22–30 h at eGFR < 20 mL–min–1). The exact times of administration and blood sampling were documented. Plasma iohexol concentrations were determined by HPLC.18 The total analytical coefficient of variation (CV) of the iohexol method was 2.2% for a control sample with an assigned value of 32 mg iohexol L–1 and 1.9% for a control sample with an assigned value of 63 mg iohexol L–1. mGFR values were expressed relative to body surface area for each participant (mL min–1/1.73 m2).
Blood was also collected from each participant prior to iohexol exposure; samples were immediately centrifuged, and plasma stored in aliquots at –80 °C until shipment to SomaLogic Inc. (Boulder, Colorado) for proteomic analysis. Laboratory testing of plasma samples also included cystatin C using an automated particle-based immunoassay, adjusted to the international reference preparation ERM-DA 471/IFCC.19 Cystatin C and creatinine assays were run on a Cobas c-system (Roche Diagnostics, Basel, Switzerland).
2.3 Protein Quantification in Plasma
Proteins were quantified using a Slow Off-rate Modified Aptamer (SOMAmer) capture in the SOMAscan assay (SomaLogic, Inc), described in detail elsewhere.13 In brief, this technique uses chemically modified DNA to bind to individual proteins, forming highly specific and stable SOMAmer-protein complexes. After a series of washes that disrupt nonspecific interactions, SOMAmers are released from their target-protein complexes in a denaturing buffer, hybridized to complementary sequences on a microarray chip, and quantified by fluorescence. The resulting signal intensity is a direct reflection of the protein levels in the original sample.
2.4 Proteomic Data Processing
Plasma samples were randomly assigned to assay runs (84 samples per assay run) in addition to a set of calibration and control samples. All technicians and analysts were blinded to sample identification.
Intra-run normalization and inter-run calibration in accordance with QC procedures defined by SomaLogic, Inc. Intra-run normalization controls for “bulk” signal intensity biases that can result from either differential hybridization efficiency or differential sample dilution (or other collection protocol artifacts) that change the total protein concentration in the sample. The former effect is captured by a set of controls used to monitor the hybridization reaction for each sample whereas the latter uses the median of the ratio of median signal levels in each sample to the median signal level for the modified aptamers over all samples within the run. Typical normalization scale factors are close to unity and QC acceptance criteria for normalization scale factors for each sample must fall between 0.4 and 2.5.
Inter-run calibration removes “batch effects” between the successive assay runs by matching the median signal over replicate observations of a pooled plasma calibrator sample included in each assay run to a fixed signal level reference. Typical calibration scale factors are close to unity and acceptance criteria of these factors are set to the median scale factor ±40%.
Hemolyzed samples can artificially inflate protein signals due to cell lysis. Fortunately, we can identify these samples by their distinct pattern of extreme hemoglobin and haptoglobin levels. As part of our QC procedures, we check for hemolysis of samples and remove any with this indication from analysis.
In this study, we measured 2893 unique human proteins in the cohort. The proteins that passed quality control standards had median intra- and inter-assay coefficients of variation less than 5%.
2.5 Statistical Methods
2.5.1 Samples Analyzed
We assayed 2893 proteins in the plasma of 389 male and female subjects, and removed 25 samples due to hemolysis, for a total of 364 samples analyzed.
2.5.2 Analyses
For our primary analysis in the cohort, each protein was log10 transformed and analyzed for its association with mGFR using Spearman's rank correlations, and corrected for 2893 tests (corresponding to the number of unique human proteins analyzed). Protein correlations were examined across the full range of mGFR values and across a restricted range of mGFR values from 45 to 100 mL min–1/1.73m2, and mGFR values from 60 to 100 mL min–1/1.73 m2. Secondary analyses examining the association between age and mGFR were analyzed using Spearman's rank correlation coefficient, while the difference in mGFR between sexes was analyzed using Wilcoxon rank-sums test. Finally, Spearman's rank correlation was used to evaluate the associations between the SOMAmer reagent and an immunoassay measuring cystatin C in the plasma of the same subjects to validate the SOMAmer measurement against a gold-standard. All statistical computing was performed in R (version 3.2.1).
3 Results
3.1 Study Design
Analyses were conducted using a 2893 protein assay across male and female subjects from healthy states to varying stages of CKD (n = 364 analyzed).
3.2 Proteins Associated with GFR
Our primary interest was to explore the relationship between the plasma proteome and mGFR to determine if protein information could improve the specificity of CKD staging at diagnosis, as well as uncover novel biological information related to renal function. We have listed the proteins that are correlated with mGFR values in Supporting Information, Table S1 (negative correlations, FDR < 0.20) and Supporting Information, Table S2, (positive correlations, FDR < 0.20).
3.2.1 Negative Correlations with GFR
Our results confirm many well-established biomarkers in the field that show a negative association with mGFR values. Cystatin C (rho = –0.85, p = 1.2 × 10−97), beta-2 microglobulin (rho = –0.76, p = 1.9 × 10−66), and trefoil factor 3 (rho = –0.76, p = 7.7 × 10−68) were among the known diagnostic biomarkers of CKD and in the top nine markers with the strongest associations to mGFR. Other proteins in this list included insulin-like growth factor binding protein 6 (rho = –0.81, p = 1.0 × 10−82), part of a family of signaling molecules that play critical roles in cellular energy metabolism, growth, and proliferation; matrix-remodeling-associated protein 7 (rho = –0.81, p = 4.7 × 10−82), involved in cell adhesion and matrix remodeling; DnaJ homolog subfamily B member 12 (rho = –0.80, p = 1.1 × 10−80), important for modulation of protein assembly, disassembly, and translocation; neuroblastoma suppressor of tumorigenicity 1 (rho = –0.79, p = 5.7 × 10−76), part of a family of proteins implicated in diabetic kidney neuropathy; the activin-antagonist follistatin-related protein 3 (rho = –0.79, p = 8.6 × 10−76); and transmembrane emp24 domain-containing protein 10 (rho = –0.77, p = 4.1 × 10−69), involved in vesicular protein trafficking. A list of these top nine proteins negatively associated with mGFR are highlighted in Supporting Information, Table S1, and shown in Figure 1.
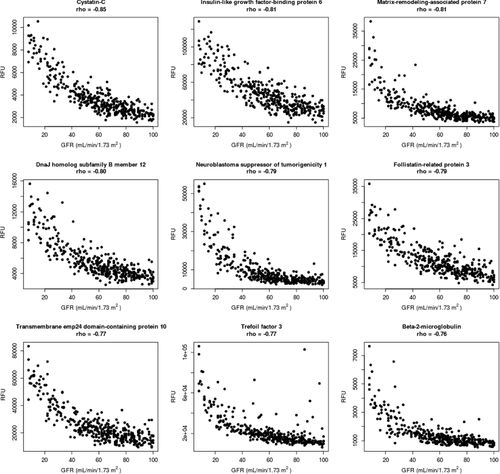
3.2.2 Positive Correlations with GFR
We also identified proteins that were positively correlated with mGFR. Among the markers showing the strongest positive relationship with mGFR included testican 2 (SPOCK2: rho = 0.42, p = 1.3 × 10−15), which plays a role in extracellular protease cascades; glutaminyl-peptide cyclotransferase (QPCT: rho = 0.36, p = 2.0 × 10−11), a modifier of pituitary hormones; protein kinase c-binding protein NELL1 (NELL1: rho = 0.31, p = 2.2 × 10−8), involved in cellular growth and regulation; thyroxine-binding globulin (SERPINA7: rho = 0.28, p = 4.1 × 10−7), important for thyroid hormone transport, protocadherin gamma c3 (PCDHGC3: rho = 0.280, p = 5.7 × 10−7), critical for cell-adhesion; and serine protease 27 (PRSS27: rho = 0.28, p = 9.0 × 10−7), which facilitates a range of functions from digestion to inflammation. Other proteins have been shown to have a relationship with diseases such as cancer and Alzheimer's including the family with sequence similarity 189 member a2 (FAM189A2: rho = 0.49, p = 6.4 × 10−22), and Dickkopf-like protein 1 (DKKL1: rho = 0.27, p = 2.2 × 10−6). These proteins could reflect comorbidities in addition to renal dysfunction, although this is largely speculative at this point. A list of the top nine candidate proteins positively correlated with mGFR are highlighted in Supporting Information, Table S2, and shown in Figure 2.

Interestingly, three proteins illustrated in Figure 2 show a degree of bimodal behavior aross GFR, specifically Protocadherin gamma C3, Serine proteins 27, and Dickkopf-like protein 1. We investigated whether these distributions were related to sex differences, but found no relationship (p > 0.05, data not shown). However, this pattern has been characterized for other proteins and determined to arise from single nucleotide polymorphisms (SNPs) present within the population. While we have not characterized this specific set of proteins, we anticipate this pattern is due to SNP-induced differences in modified aptamer reagent affinities for the target proteins.
3.2.3 Proteins that Signal Early CKD Stages
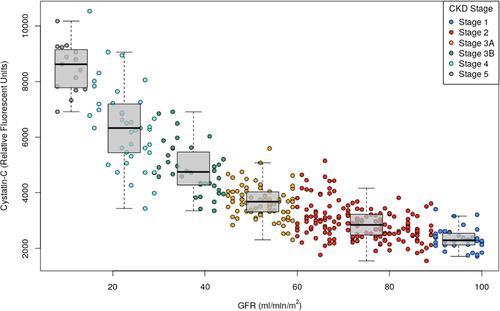
We also investigated whether the plasma proteome was impacted across early stages of CKD. We divided subjects into the following CKD stages established by the International Nephrology Society: Stage 1: mGFR ≥ 90 mL min–1/1.73 m2; Stage 2: mGFR = 60–89 mL min–1/1.73 m2; Stage 3A: mGFR = 45–59 mL min–1/1.73 m2; Stage 3B: mGFR = 30–44 mL min–1/1.73 m2; Stage 4 and 5: mGFR < 30 mL min–1/1.73 m2.1 Importantly, cystatin C was the most significant negatively correlated protein with mGFR regardless of the range of values examined (Correlation with mGFR ≥ 60 mL min–1/1.73 m2: rho = –0.51, p = 1.1 × 10−10; Correlation with mGFR ≥ 45 mL min–1/1.73 m2: rho = –0.67, p = 1.4 × 10−33; Table 1). This close correlation between cystatin C and GFR reinforces the clinical utility of this protein as a diagnostic biomarker for specific CKD staging as shown in Figure 3.
| Parameter | Lund Cohort (N = 389) |
|---|---|
| Sample size (as analyzed) | 364 |
| Male, N (%) | 201 (55%) |
| Age, Years, Median (IQR range) | 64 (57.5–71) |
| BMI, Median (IQR range) | 25.3 (22.7–28.6) |
| Creatinine, μmol/L, Median (IQR range) | 91.3 (71.2–126.7) |
| Cystanin C, mg/L, Median (IQR range) | 1.4 (1.1–1.8) |
| GRF, mL/min/1.73 m2, Median (IQR range) | 62 (44–78) |
Overall, there were a greater number of negatively correlated proteins with mGFR values corresponding to early CKD stages 1–3A (Supporting Information, Table S1) than positively correlated proteins (Supporting Information, Table S2). In fact, only two proteins, Brother of CDO and Tenascin-X, both of which are involved in cell adhesion processes, were positively correlated across these early CKD stages (Supporting Information, Table S2).
3.3 Gender Effects
A Wilcoxon rank sums test revealed a significant sex difference in mGFR values (W = 13944.5, p = 0.01465, n = 364). Specifically, median mGFR values were significantly lower in males than females (59 vs 65 mL min–1/1.73 m2).
3.4 Relationship between GFR and Age
A Spearman's rank correlation coefficient showed a significant negative association between mGFR values and age (rho = –0.377, p = 1.032 × 10−13, n = 364; data not shown).
3.5 Correlation of SOMAscan with Clinical Laboratory Measures
We compared the protein levels of plasma cystatin C, a common biomarker of kidney function, as quantified by the SOMAmer reagent that binds to cystatin C versus automated particle-based immunoassay. A Spearman's rank correlation coefficient revealed a significant relationship between log-transformed values of both the SOMAmer reagent and automated particle-based immunoassay (rho = 0.94, p = 2.2 × 10−16, n = 364; data not shown).
4 Discussion
Biomarkers that can accurately stratify individuals by CKD severity, especially early in the disease process, are critical for prevention, early intervention, and precision-based care. Blood-based proteomics offers one approach to improve the accuracy of GFR assessments and uncover important biology to lead to new therapeutic developments. While various methods have been used to characterize the plasma proteome including 2DE, MS, and antibody arrays, it has been difficult to quantify thousands of proteins simultaneously across a broad range of concentrations, except with the aptamer-based technology used here.
In this study, 2893 human plasma proteins were quantified using modified aptamers as binding reagents to proteins in participants with a broad range of GFR measured by iohexol clearance. To the best of our knowledge, this study is the most extensive description of the human plasma proteome and its relationship to GFR to date. Inverse associations between plasma protein levels and GFR were expected, given the decline in the filtration barrier with advancing kidney disease. Notably, in our large-scale, unbiased investigation of the plasma proteome, our results confirmed cystatin C to be the most significant protein biomarker of renal function, negatively associated across the entire GFR range. This information is important for the nephrological community as determination of renal function is the key point in all renal diseases. The fact that we did not find a superior protein biomarker of GFR among 2893 plasma proteins tested confirms the clinical utility of cystatin C and strengthens its role as a diagnostic tool for CKD staging.20 We also discovered other biomarkers that are well-established in the field for their relationship with GFR including beta-2 microglobulin, trefoil factor 3, and follistatin-related protein 3 reflecting key roles in kidney repair.21-24 Importantly, these results confirm our previous study revealing cystatin C, beta-2 microglobulin, and follistatin-related protein 3 as top proteins that significantly varied as function of GFR.13
The biological mechanisms that explain the observed elevation in protein levels in face of declining GFR may relate to the specific stage of CKD. In advanced CKD, plasma proteins are elevated partly due to a reduced renal clearance but may also be the result of an upregulation of proteins whose production or release is stimulated by uremic toxins.25 Conversely, in earlier stages of renal disease, elevated plasma levels may result from an underlying pathogenic mechanism such as inflammation, in addition to changes in filtration. Accumulation of plasma proteins in CKD may also be attributed to reduced metabolism of circulating proteins by peritubular uptake.26, 27
In contrast, the positive correlations that we observed may be interpreted as physiological markers of kidney disease rather than simple biochemical relationships related to filtration, as exhibited by the negatively correlated proteins. For example, our finding of the positive relationship between the secreted glycoprotein testican 2 and GFR may reflect a protective effect of this protein on renal function and its important role in glomerular remodeling.28
The utility of these biomarkers ultimately depends upon their translation to clinical practice. Additional research is needed to determine optimal combinations of individual proteins that are both negatively and positively correlated with GFR to enhance CKD diagnostics. The top candidate proteins are most likely to be studied for this purpose to improve estimation of GFR. Another approach is to combine biomarkers from both blood and urine. Argiles et.al. validated the first urine proteomic-based classifier of 273 peptides (CKD 273) for prognosis of CKD in an independent cohort.29 The results of our study of plasma biomarkers may complement these urine biomarkers for added precision and sensitivity of CKD staging.
4.1 Strengths
In this study, we measured GFR using iohexol clearance. In contrast, previous studies in this field have relied on estimated values of GFR in small samples.
This was the most extensive proteomics study of GFR to date. The multiplex modified aptamer technology used here has the selectivity and specificity for unbiased biomarker discovery (protein concentrations can be detected across eight logs). Given its strengths, there is considerable utility for validating use of this assay in the clinic, possibly even as a surrogate for iohexol clearance and for identification of early stages of CKD.
4.2 Limitations
This study does not provide any information on the pathogenesis underlying these changes in plasma levels of proteins. Therefore, we cannot rule out mechanisms other than GFR as determinants of plasma concentration of the proteins. We were not able to take albuminuria into consideration for the definition of CKD, as no urine samples were available.
In conclusion, we show a broad range of plasma proteins negatively and positively related to GFR levels and renal physiology. Our findings may assist in identifying new biomarkers for assessing CKD and GFR.
Abbreviations
-
- CKD
-
- chronic kidney disease
-
- eGFR
-
- estimated glomerular filtration rate
-
- GFR
-
- glomerular filtration rate
-
- mGFR
-
- measured GFR
Acknowledgements
A.C. and J.A.A. are the co-first authors of the study. The authors would like to to thank Drs. Tom Parker, Dan Levine, and Barry Smith at the Rogosin Institute, New York, for fruitful discussions. The work was supported by grants from the Fulbright Commission, the Faculty of Medicine at Lund University, Research Funds of Region Skåne, Stiftelsen för njursjuka, Swedish Kidney Association, and the Research Fund of Skåne University Hospital, Sweden.
Conflict of Interest
Drs. Christensson, Lindström and Grubb report no conflict of interest. Drs. Ash, DeLisle, Ostroff, and Williams are all employees of SomaLogic, Inc. No other conflicts of interest to report.




