Deciphering RGDechi peptide-α5β1 integrin interaction mode in isolated cell membranes
Funding information: Ministero dell'Istruzione, dell'Università e della Ricerca, PRIN 2010-2011, Grant Numbers: 2010C4R8M8, PRIN 2015, 20157WZM8A, FIRB 2012-2017, RBFR124FEN; Programma Operativo Nazionale Ricerca e Competitività 2007–2013, Grant Number: PON 01_ 02388; Programma Operativo Nazionale Ricerca e Competitività 2007–2013, Grant Number: PON 01_01078
Luigi Russo, Biancamaria Farina, and Annarita Del Gatto equally contributed to this work.
Abstract
Integrins are a large family of heterodimeric receptors critically engaged in pathological processes such as tumor progression and metastasis. Although they are validated therapeutic targets, the molecular determinants governing integrin-ligand interactions are not yet fully understood, leading to a scarcity of integrin sub-type exclusive antagonists. In the past decade, we have investigated the biological behavior of the RGDechi, a chimeric peptide able to specifically bind αvβ3 integrin without cross reacting with αvβ5 and αIIbβ3 integrins. Here we have investigated the capability of the peptide to bind α5β1 integrin and characterized the molecular determinants governing this interaction through a combined experimental and computational approach. The detailed comparison of RGDechi-α5β1 structural model with that previously determined of RGDechi in complex with αvβ3 shows how the bifunctional nature of the peptide renders the molecule an important tool to recognize integrins with different recognition modalities, providing novel insight on the structural requirements needed to their specific recognition.
Graphical Abstract
1 INTRODUCTION
Integrins are αβ heterodimeric transmembrane receptors responsible for cell–cell and cell–extracellular matrix adhesion, regulating multiple physiological processes such as cell migration, proliferation, survival, and apoptosis.1 By controlling the cross talk between cells and their surrounding microenvironment, integrins play relevant roles in different diseases such as tumor progression, inflammation, adenovirus infection mediation, and multiple sclerosis.2-6
One third of the 24 known integrin subtypes including all αv integrins, the integrin α5β1, and the blood platelet integrin αIIbβ3 are reported to bind the Arg-Gly-Asp (RGD) motif present within extracellular matrix (ECM) proteins such as vitronectin, fibronectin and fibrinogen. The structural differences between the subtypes are commonly small because most of them share different RGD-containing ECM proteins; however, they exhibit extremely different binding affinities.
In the past two decades the scientific community has focused the attention mainly on αvβ3 and α5β1 integrins due to their relevance in tumor vascularization and metastasis,7 in wound healing acceleration, drug resistance and tumor recurrence in different types of solid cancers8-10 including breast, lung, prostate, ovary, pancreatic and colon cancers, as well as in hematological malignancies.11 However, the precise roles in pathological angiogenesis and in tumor progression of αvβ3 and α5β1 integrins, alone or combined with other integrins, are not yet fully understood and thus the availability of integrin sub-type-exclusive antagonists is highly desirable.12, 13
To date, several αvβ3 ligands or combined α5β1/αvβ3 antagonists have been designed, mainly based on the crystal structure of αvβ3 integrin extracellular domain, free and bound to a peptide ligand.14, 15 The high sequence similarity between αvβ3 and α5β1 receptors (αv:α5 53% identity; β3:β1 55% identity in the integrin's headgroup) has limited the obtainment of specific antagonists for α5β1 integrin,16 though some of them were initially designed on the base of the homology model of the receptor.17
Over the time, also peptides not based on the RGD epitope have been developed. One example is the pentapeptide Ac-PHSCN-NH2 which has been clinically developed, under the trade name ATN161,18 for the treatment of several solid tumors and proved to be highly active for the α5β1 integrin with reduced affinity also for αvβ3 and αvβ5.12 Recently, cyclic peptides containing the isoDGR motif and capable of achieving high binding affinity and selectivity between αvβ3 and α5β1 receptors have been also reported. In particular, c(phgisoDGRk) has shown a good activity for α5β1 and selectivity over αvβ3, though it recognizes also other types of integrins.19
The determination of the X-ray structure of the α5β1 ectodomain in absence and in presence of a ligand peptide containing the RGD motif20 has shed light on the binding modes of RGD sequence to α5 subunit and on the structural differences between αv and α5, contributing to the design of new and more selective ligands.
Over the past decade, we have successfully designed and developed RGDechi (Figure 1), a chimeric peptide able to selectively target αvβ3 receptor without cross reacting with αvβ5 and αIIbβ3 integrins. RGDechi encompasses a cyclic portion, containing the RGD triad for integrin binding, and a linear sequence derived from the C-terminal fragment of the echistatin protein conferring specificity for β3 subunit,21 as demonstrated by mutation and photoaffinity cross linking experiments and NMR conformational analysis combined with docking studies.22, 23 In vitro and in vivo studies21, 24-28 proved the selective binding of this peptide to αvβ3 integrin and a recent structural analysis performed in presence of isolated cell membranes has detailed the key structural determinants of this interaction.29 Noteworthy, the structural data confirmed that the selectivity of the peptide derives from its combined nature: while the RGD motif recognizes the extracellular domain of αvβ3 (but also other integrins), the C-terminal fragment provides specificity for αvβ3. In particular, the C-terminal sequence preserves the contacts present in the native sequence of echistatin gaining a fundamental interaction with the Specificity Determining Loop (SDL) of the β3 chain. As matter of fact, by mutating a single residue of the C-terminal tail we have been able to convert RDGechi specific binding from αvβ3 to αVβ5, obtaining a novel αvβ5 selective antagonist.29 All these evidences indicate that RGDechi peptide, because of its chimeric nature, could represent an efficient molecular tool for designing new ligands selective for α5β1 integrins as well. Therefore, herein we have evaluated the binding capability of RGDechi to α5β1 by means of biological assays and NMR experiments. Moreover, we have described, using NMR data in combination with computational binding studies, the molecular details of the interaction between α5β1 and RGDechi peptide with the aim to provide novel structural hints to help the design of new and more selective ligands for α5β1.

Schematic representation of the chemical structure of RGDechi peptide
2 EXPERIMENTAL SECTION
2.1 Cell lines and culture conditions
Chronic myelogenous leukemia cells line (K562) (ATCC U.S.) were grown in RPMI added with heat-inactivated 10% FBS (fetal bovine serum), 2 mM glutamine, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (Euroclone, Italy). Human adenocarcinoma cells line (HeLa), kindly provided by Dr. Annalisa Tito (Arterra Bioscience), were grown in DMEM added with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. Cells were maintained in humidified air which contained 5% CO2 at 37°C.
2.2 FACS analysis for α5β1 integrin
Cell aliquots (2.5 × 105 cells, suspended in cold 1x PBS containing 0.5% BSA) were treated with primary monoclonal antibody anti-α5β1, anti-αvβ3 or anti-αvβ5 (Millipore MA), or isotype control (Santa Cruz Biotechnology, Germany), at a final concentration of 14 µg mL−1, in a whole reaction volume of 50 µL for 45 min at 4°C. After washing, the cells were incubated with secondary antibody PE-conjugated (Thermofisher, MA), washed and analyzed. For the analysis, a flow cytometer equipped with a 488 nm argon laser (FACScan, Becton Dickinson, USA) was used. The fluorescence intensity values were attained from histogram statistic of CellQuest software.30
2.3 Cell adhesion and detachment assays
NUNC MaxiSorp 96 well plates (Dasit Sciences, Italy) were covered at 4°C overnight with fibronectin (Millipore, USA) diluted at 10 μg mL−1 in PBS, pH 7.4; alternatively, coating was carried out with 10 μg mL−1 anti-α5β1. Successively, a blocking step of 1 h at room temperature for nonspecific binding was done with 1.5% BSA in PBS. K562 cells were suspended and mixed for 30 min at 4°C to prevent receptor internalization, in Hank's balanced salt solution24 with peptides (50 μM) or anti-α5β1 antibody (10 μg mL−1). Afterward, cells were plated on precoated plates (1.5 × 104 cells/well). Nonadherent cells were delicately removed by a series of washings. After 1 h of incubation, the adherent cells were counted by crystal violet (Sigma Aldrich, Italy) assay, which correlates optical density with cell number.31 Statistical differences were calculated using the Student's test, unpaired, two-sided. All experiments were done in triplicate and repeated three times at least; a P value <0.05 was evaluated as significant.
2.4 Membrane preparation for NMR studies
K562 cellular membranes were used for NMR experiments. Cells were washed two times using PBS, then were moved into homogenization buffer, homogenized and centrifuged at 4°C for 1 h at 28 000 rpm. The homogenization buffer consisted in 20 mM Tris-HCl pH 7.4 containing 10% glycerol and protease inhibitor cocktail (Roche). The pellet was then washed and centrifuged at 4°C for 30 min at 28 000 rpm. The membranes were resuspended with 180 μL of HBSS.29
2.5 Nuclear magnetic resonance spectroscopy
NMR measurements were performed utilizing an Inova 600 MHz spectrometer (Varian Inc., Palo Alto, CA) at 298 K equipped with a cryogenic probe optimized for 1H detection. Samples were prepared as above reported.
NMR binding studies of RGDechi with isolated K562 cell membranes were performed, according to the methods reported in Farina et al.29 Specifically, isolated K562 cell membranes from 80 × 106 cells were resuspended in 180 μL of HBSS buffer (pH 7.4) and 10% 2H2O in 3 mm tube. 1H standard and Saturation Transfer Difference (STD) NMR spectra were acquired as reference. A 300 nmol of peptide was initially dissolved in 20 μL of HBSS buffer and then added to the membrane suspension. STD spectra were acquired with 10 000 scans with on-resonance irradiation at 0.2 ppm to selectively saturate protein resonances and off-resonance irradiation at 30 ppm for reference spectra. A total saturation time of 2 s has been obtained using a train of 40 Gaussian shaped pulses of 50 ms with 1 ms delay between pulses. STD spectra were achieved after internal subtraction of the saturated spectrum from the reference spectrum by phase cycling with a 7191.66 Hz spectral width, 1.0 s relaxation delay, 8 k data points for acquisition, and 16k for transformation. The STD effect was defined as the percentage of the STD factor relative to the maximum, set to 100%. In the case of the HN Arg2 and Arg11 sidechains, considering the solvent exchange contributions, the STD values were calculated by taking as reference the STD profile of the HN Lys1 backbone. 2D [1H-1H] TOCSY (TOtal Correlation SpectroscopY) and NOESY (Nuclear Overhauser SpectroscopY) spectra of RGDechi in the presence of isolated membranes were acquired similarly to those of the peptide alone with a mixing time of 70 and 250 ms, respectively.29 To test for the specific interaction between α5β1 integrin and RGDechi, the same experiments were performed in the presence of anti-α5β1 antibody (10-fold excess to the α5β1 integrin). Moreover, since for STD experiments using living cells it was suggested to place the off-resonance pulse at ±300 ppm,32 we performed as control a STD experiment using two irradiation frequencies at 30 and 300 ppm. The spectrum difference did not show signals from membranes, but only from low molecular weight impurities (i.e., glycerol, acetonitrile), confirming that 30 ppm represents a suitable off-resonance frequency for the membrane preparations (Figure S1, Supporting Information).
Data were processed by means of the software VNMRJ 1.1.D (Varian Inc.). Analysis of 1D spectra were performed utilizing ACD/NMR Processor 12.0 (www.acdlabs.com). 2D TOCSY and NOESY spectra were examined by means of CARA (Computer Aided Resonance Assignment) software.33
2.6 Molecular dynamics simulations
Molecular dynamics (MD) simulations were carried out using GROMACS34 software. The Amber99SB35 force field was utilized with explicit TIP3P water.36 The theoretical model of the αvβ3/RGDechi complex reported in a previous manuscript21 was used to obtain the starting 3D structure for the RGDechi peptide. Energy minimization was performed using 100 steps of steepest descent to avoid any kind of bad contacts generated while solvating the system. All simulations utilized a 2 fs inner step and were completed in NVT ensemble utilizing a Nose-Hoover thermostat after equilibration to constant box volume in the NPT ensemble. All simulations were run at 298 K. Electrostatic and van der Waals interactions were measured by means of partial mesh Ewald algorithm; the short-range neighbor list cut-off, short-range electrostatic cut-off and short-range van der Waals cut-off were fixed at 1 nm. The time-averaged NOE distance restraints were imposed with a force constant of 6 000 kJ mol−1 nm−2 and was utilized a 20 ps memory relaxation time.37, 38 Numerous simulations were run and the ensemble attained from the 10 ns MD simulation was considered to get the reference structure. In this case, the snapshots were saved every 100 ps in order to have 100 conformers total in the conformational ensemble. The obtained structures were clustered by using the tools implemented in UCSF Chimera39 and for each cluster the representative structure has been selected. The comparison of the representative structures has been performed by using UCSF Chimera.39 All molecular dynamics simulation models were evaluated by using the PyMOL40 and MolMol41 software.
2.7 Molecular docking protocol
The molecular docking studies were carried out using the software AutoDock 4.0.42 The optimized Molecular docking protocol involved several steps: (1) Preparation of α5β1 receptor and RGDechi peptide structures: ligand and integrin coordinates files were initially prepared to include the required information for the docking calculation (atom types, spatial charges, polar hydrogen atoms and torsional degrees of freedom). In particular, for the receptor the X-ray structure of the complex between the α5β1 integrin and the RGD peptide (PDB ID code: 3VI4) was utilized as reference structure; whereas the representative structure of the main cluster selected from the 10 ns MD ensemble was used for RGDechi. Polar hydrogens were added to the reference structure by means of the software REDUCE.43 and the RGDechi rotatable bonds were selected automatically. (2) Performing the Autogrid calculation: the tool Autodock needs pre-calculated grid maps obtained by AutoGrid. The grid map is defined as a 3D lattice of regularly spaced points, surrounding (either entirely or partly) and centered on some region of interest of the receptor. The grid size was chosen to be 64 × 64 × 64 with grid space of 0.658 Å. (3) Performing the docking calculation by using Autodock: a docking file containing the input parameters for the docking calculation was generated by the AutoDockTools. In our molecular docking protocol the Lamarckian genetic algorithm (LGA) search method was chosen and the minimized ligand was randomly located within the grid box. The docking process was started with the following parameters: torsion step 58, torsional degrees of freedom 3, number of energy evaluations 2 500 000 and run number of 100. Finally, the final representative structure of each cluster was minimized by using the UCSF Chimera39 tools and validated by using PROCHECK44and MolProbity45 (Supporting Information Table S1).
The relative STD factors (relative NOE) were calculated for each RGDechi residue of the analyzed cluster pose as a sum of intermolecular peptide-receptor 1/r6 distances. In particular, as previously described,44 we determined Ʃ(1/r6) for each residue of the RGDechi peptide, where r is the inter-proton distance between each RGDechi nonexchangeable proton and surrounding receptor protons within 6.5 Å. The structures were clustered evaluating the binding free energy of the complex at the end of the docking simulation. The structures were displayed and analyzed by means of the software PyMOL40 and MolMol.41
3 RESULTS AND DISCUSSION
3.1 Expression of α5β1 integrin on K562 and HeLa cells
The expression levels of α5β1 integrin on membrane surface of K562 and HeLa cells were evaluated by means of flow cytometric analysis utilizing anti-α5β1 monoclonal antibody. K562 cells resulted to express α5β1 at high levels, and αvβ3 or αvβ5 at very low levels (Figure S2, Supporting Information), and HeLa cells did not express α5β1 (Figure S3, Supporting Information). These results indicate that K562 cells represent a good model to study α5β1 interaction.
3.2 RGDechi effect on cell adhesion
The effect of RGDechi (synthesized as previously reported24) on the adhesion of K562 cells seeded onto fibronectin (extracellular matrix recognizing a range of different integrins, including αvβ3 and α5β1 receptors) or, alternatively, on α5β1 antibody was studied in the specific cell adhesion buffer as reported in a previous manuscript.21
As shown in Figure 2A, RGDechi decreases the α5β1 mediated adhesion of K562 cells plated onto fibronectin having similar effect of cRGDfK, a αvβ3/αvβ5/α5β1 antagonist used as positive control12, 46, 47 and the adhesion inhibition by RGDechi occurs in a concentration-dependent mode (Figure 2B). Incubation with scrambled peptide (Ac-KPGRGHNDPDPGhCitDeMHAT-OH),24 utilized as negative control, does not decrease K562 adhesion onto matrix protein or specific antibody. To highlight the binding to α5β1 integrin, a further adhesion assay was carried out onto anti-α5β1 antibody coated plate (Figure 2C). Data obtained indicate that RGDechi decreases the adhesion of K562 with an activity slightly but significantly lower than cRGDfK. More, the adhesion assay performed on HeLa cells not expressing α5β1 (Supporting Information Figure S3) and αvβ3 and likely expressing other fibronectin binding integrins clearly confirms the binding of peptide to α5β1 (Supporting Information Figure S4).
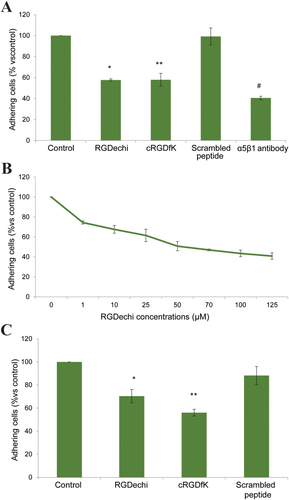
Effect on K562 adhesion. A, Cells were pre-incubated with peptides (50 μM) or anti-α5β1 antibody (10 mg mL−1) for 30 min at 4°C and then seeded on fibronectin precoated plates at 37°C. Cell adhesion was evaluated after 1 h of incubation using crystal violet reagent. B, Dose-effect curve of RGDechi on K562 adhering on fibronectin coated plates. Cells were pre-incubated with increasing concentrations of RGDechi for 30 min at 4°C and then seeded on fibronectin. C, Effect on K562 adhesion on α5β1 antibody coated plates. K562 were preincubated with peptides (50 μM) for 30 min at 4°C then seeded on anti-α5β1 precoated plates. The results are presented as the percentage of adherent cells respect to the control (untreated cells) and are expressed as means ± SE of three independent experiments performed in triplicate. Statistical significance was analyzed using Student's t test, unpaired, two-sided (*P = 0.012; **P = 0.008; #P = 0.02)
Very recently, Kapp et al. reported the IC50 values, calculated by competitive ELISA assay, of cRGDfK peptide for different integrins subtypes including αvβ3 (IC50 = 2.25 ± 0.34 nM) and α5β1 (IC50 = 141 ± 15 nM).12 On the basis of these data and considering that RGDechi inhibits the adhesion of cell seeded onto αvβ3 antibody plates similarly to cRGDfK (Figure 7C in Farina et al.29), we can reasonably conclude that RGDechi significantly binds to α5β1 but with an affinity lower than that to αvβ3 integrin.
3.3 NMR binding studies of RGDechi to α5β1 integrin using K562 membrane preparations
In the last years, we have characterized the structures and functions of peptides recognizing membrane receptors either in vitro and on cellular membranes.29, 48, 49 Recently, we have also investigated the use of isolated cellular membranes and provided significant advantages in the structural study of the RGDechi interaction with αvβ3 integrin.29 On this basis, to gain structural insights into the binding of RGDechi to α5β1 integrin, STD and trNOESY experiments were performed using K562 membrane preparations.
Interestingly, STD spectrum indicates that in presence of isolated K562 membranes RGDechi receives saturation transfer (Figure 3A). This effect, negligible in the absence of cell membranes (Figure S5, Supporting Information), indicates the binding of the peptide to the K562 membrane cells and, thus, to the α5β1 integrin. To further confirm that the STD effects observed are ascribed to the specific binding between RGDechi and α5β1, STD experiments were repeated in the presence of the anti-α5β1 antibody. Interestingly, a strong intensity reduction in the STD spectrum was observed, according to the presence of a 10 folds excess of the antibody with high affinity for α5β1 and a large excess of the peptide with lower affinity (Figure S6, Supporting Information). This result confirms the specificity of the effects observed above. Relative STD values were calculated for amide, aromatic and aliphatic protons and pictured as a function of the amino-acid residue (Figure 3B). STD effects from K562 membranes higher than average and higher than average plus one standard deviation (SD) were classified, respectively, as medium or strong. In the amide/aromatic region, the Lys1, Gly3, Asp4, Gly16 backbone amides and Arg2 and Arg11 side chain amide protons show strong STD effects (Figure 3B, top). Medium STD signals were assigned to Arg2 overlapped to Gly10, Asp8, Asn12, His14, and hCit15 HNs. Strong STD effects in the aliphatic region were ascribed to the Arg2 Hβ2 and Hβ3, DGlu5 Hα and Hβ3, and Pro13 and Pro17 Hβ protons (Figure 3B, bottom). Medium STD effects were observed for Arg2 Hγ, Asp4 Hβ2 and Hβ3, Arg11 Hα and Hγ, hCit15 Hγ, Hδ and Hɛ, and Pro17 Hγ. These data outline a RGDechi surface interacting with α5β1 that shares a certain similarity to that of RGDechi with αvβ3 previously reported.29 Indeed, the side chains of Arg2, Asp4, and DGlu5 of the RGD cycle and that of Arg11, Pro13, hCit15, and Pro17 of the C-terminal echistatin moiety seem to directly contact both integrins, though with different modality. The most significant difference was observed for the HN Lys1, which was not influenced by αvβ3 binding, while it appears here affected by α5β1 binding. To obtain information on the α5β1-bound conformation of RGDechi, trNOESY spectrum of the peptide in the presence of K562 cell membranes was acquired and compared to that obtained in presence of HeLa membranes.29 Interestingly, several new peaks appear and the intensities of many others increase in the presence of K562 cell membranes (Figure 4A,B). trNOEs were identified and mapped on the primary sequence of the peptide (Figure 4C). In particular, Lys1, Arg2 and Asp4 of the cyclic portion, together with the Arg11, Asn12, His14, and hCit15 of the C-terminal segment exhibited the highest number of trNOEs. Moreover, similarly to WM266 membranes, strong crosspeaks connecting Asp8, Asn12, and Gly16 Hα to the δ protons of Pro9, Pro13, and Pro17, respectively, were observed, indicating a trans-arrangement for all three bonds in the presence of K562 membranes.
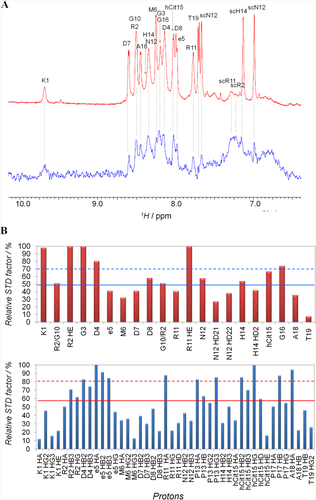
STD binding studies of RGDechi to K562 membranes. A, Expansion of the HN/aromatic region of the reference 1H (red) and STD (blue) spectra of RGDechi in the presence of K562 cell membranes. B, Bar graphs of the percentage of the STD factor relative to the maximum, set to 100%, for HN/aromatic protons (top) and the aliphatic protons (bottom). The mean value is shown as a continuous line; the mean value plus one standard deviation is indicated as a broken line
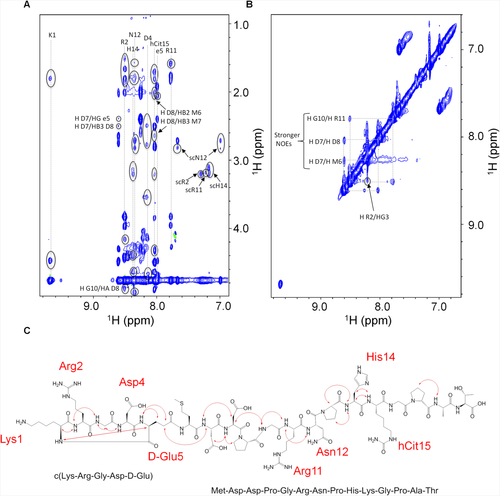
trNOEs investigation of RGDechi to K562 membranes. A, HN-aliphatic and B, HN-HN correlation regions of the 2D [1H, 1H] NOESY spectrum of RGDechi in the presence of K562 cell membranes. Negative and positive cross-peaks are shown in blue and green, respectively. trNOEs are indicated by circles. C, Mapping of the trNOE network on the primary structure of the RGDechi peptide. trNOEs involving the HN backbone amide are shown by red arrows. Residues showing intraresidue trNOEs involving side chains are indicated in red
3.4 Sampling RGDechi conformational flexibility by NMR and MD simulations
To elucidate the details of the α5β1 integrin recognition mechanism by the RGDechi peptide, we performed a series of Molecular Dynamics simulations (MD) studies. To achieve a rigorous structural investigation of this interaction we applied a combined approach29, 49, 50 that includes in the Molecular Docking protocol data obtained by MD and Nuclear Magnetic Resonance (NMR) techniques. This strategy allows to take into account in the description of the binding mechanism not only the structural properties, but also the conformational heterogeneity of the RGDechi peptide. In fact, on one side NMR and MD are two complementary tools providing a detailed description on the atomic level of the structural features, of the dynamic events that occur in proteins/peptides on the picosecond and millisecond timescale51-54 and of the protein folding mechanisms.55 On the other side, the Molecular Docking represents a powerful computational tool in structural biology and computer-aided drug design to predict at the atomic level the preferred orientation of a molecule to a second one in protein–protein and protein–ligand complexes.56 At first, we performed a series of MD simulations of varied length, as reported in the experimental section, using as structural restraints the intra-molecular NOEs observed in the trNOESY NMR experiment for the RGDechi peptide in the presence of the K562 cell membranes. Successively, in order to describe the conformational peculiarities (i.e., backbone flexibility) of the RGDechi, we analyzed the trajectories of the time-averaged restrained 10 ns MD ensemble. The root mean square fluctuations (RMSF) profile of the 10 ns MD simulation (Supporting Information Figure S7A), in agreement with the absence of long range intra-molecule NOEs (Figure 4A,B), indicates that the RGDechi does not adopt in solution any well-defined secondary structure with high backbone conformational flexibility on the picoseconds time-scale (Supporting Information Figure S7A,B). Conformational families are believed to be a more realistic representation of the structure when dynamics or motions are extensive.57-60 For this reason, to detect the structural diversity induced by the conformational heterogeneity of the RGDechi peptide, we extracted the conformationally related subfamilies of structures using the program NMRCLUST.57 Therefore, we clustered the 10ns MD ensemble on the backbone heavy atoms of residues 1–19 (Supporting Information Figure S7B) obtaining 17 subfamilies of structures (Supporting Information Figures S7B, S8A). Among these, the first four clusters contain more than 70 of the 100 structures, whereas each of the other classes is poorly populated. Then, we restricted the conformational analysis on the more populated clusters (1–4) (Supporting Information Figure S8B) by evaluating the structural differences between the representative structures of each cluster. Interestingly, despite to the high conformational flexibility, the representative structure of cluster 1 shows a similar contacts map to the other representative clusters (Supporting Information Figure S8B). In particular, as reflected by the root mean square distributions (RMSDbb1-19= 0.767 Å) (Supporting Information Figure S7B), the representative structures of the two most populated MD clusters (cluster 1 and cluster 2) are characterized by similar conformational proprieties. Altogether the data indicate that the representative structure of the MD cluster 1 represents a reasonable description of the RGDechi conformational heterogeneity observed within and between the four investigated MD clusters. Therefore, in order to explore the structural details of the RGDechi-α5β1 interaction, considering the contribution of the molecular flexibility in the recognition mechanism, we used as reference structure the representative conformer of the most populated MD cluster (cluster 1) to perform a molecular docking investigation.
3.5 Molecular docking studies of the α5β1/RGDechi complex
Molecular docking calculations were performed, as reported in the experimental section, by using as reference structure for the RGDechi peptide the representative conformation of the most populated cluster (cluster 1) of the 10ns MD ensemble. We used the coordinates of the α5β1 integrin in complex with the RGD motif (PDB ID: 3VI4) for the receptor. Following the optimized docking protocol,29 100 solutions were generated that, based on the binding energy values, were successively sorted into clusters (Supporting Information Figure S9). This procedure selected four most populated clusters, characterized by different binding energy and showing substantial structural differences in terms of RGD motif position and orientation with respect to the α5β1 active site (Figure 5A–D). On one hand, in the first two clusters (cluster 3 and 7) composed of fourteen and nine conformers, respectively, the recognition mechanism of the α5β1 integrin by the RGDechi peptide is principally modulated by the RGD motif, though not completely embedded inside the RGD binding cavity, with the C-terminal echistatin residues that participate in the complex formation (Figure 5A,B). On the other hand, in the clusters 29 (5 conformers) and 17 (8 conformers) the RGD region is inserted into the binding pocket delimited by the two integrin subunits with the C-terminal part that strongly contributes in the stabilization of the interaction (Figure 5C,D). In both cases the RGD motif, despite being located in the conventional binding site, presents significant structural differences in terms of position and orientation with respect to that observed in the αvβ3/RGDechi complex suggesting that the recognition of the α5β1 integrin may occur with a different mechanism. Then, in order to correctly identify the model able to describe the structural details of the α5β1 recognition mechanism by RGDechi peptide, the obtained docking models were investigated by calculating, as reported in the experimental section, relative STD factors for each RGDechi residue of the analyzed representative structure as a sum of peptide-receptor intermolecular proton distances (relative NOE) and comparing the results with the experimental STD effects. (Figure 6A–D). In the reference model of cluster 3 (Figure 6A), although the RGD motif is particularly exposed to the solvent, the amide backbone of the Lys1 (strong effect) is directly involved in the interaction whereas in disagreement with the experimental STD data the relative intermolecular NOE profile indicates that side chains of the Arg2, Asp4, DGlu5 as well as the residues (Pro13, hCit15) located in the C-terminal region appear to be less involved in the binding. Instead, for the model of the cluster 7 (Figure 6B), the back-calculated relative NOE profile, results to be more in line with the experimental STD data with respect to the representative model of the cluster 3 but it still presents significant differences. In particular, the Lys1 and the residues of the RGD motif as well as the C-terminal echistatin moiety appear to have a marginal role in the α5β1/RGDechi complex stabilization. On the other hand, the representative models of the cluster 29 (Figure 6C) and cluster 17 (Figure 6D), as mentioned above, suggest that the recognition process of the α5β1 is principally mediated by the RGD motif that is situated inside the conventional binding pocket. In the case of the cluster 29, the calculated relative NOEs are in good agreement with the observed STD data. In particular, the data indicate that the interaction is mainly modulated by the residues surrounding the RGD region (Lys1, Arg2, Gly3, Asp4) and it is further stabilized by the residues of the C-terminal part (Arg11, hCit15, Pro17). Notably, in the case of the glycine the calculation of the relative NOE profile is strongly influenced by the nature of the side-chain that has a single hydrogen atom . On the contrary, the back-calculated relative NOE profile for the model of the cluster 17, contradicting the experimental STD data, indicate a lower contribution of the RGD region than the C-terminal part in the recognition process of the integrin. Overall, the data analysis indicates that the representative model of cluster 29 agrees more with the experimental data with respect to the other representative conformers and it has been therefore chosen as the reference structure of the α5β1/RGDechi complex (see the Supporting Information for the coordinate file a5b1RGDechicomplex.pdb).
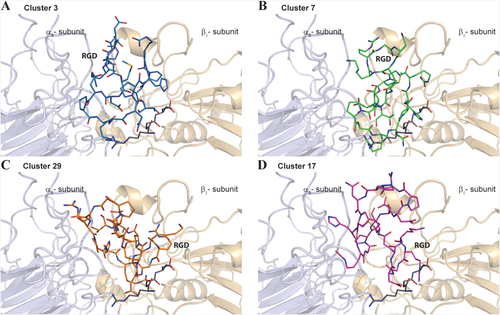
Molecular Docking studies of the RGDechi-α5β1 complex. The representative structure from cluster 3 (blue) A, cluster 7 (green) B, cluster 29 (orange) and cluster 17 (magenta) are reported as stick. The α5 (light blue) and β1 (light orange) integrin subunits are shown as ribbon drawing representation. The RGD motif reported in the X-ray structure of the complex α5β1/RGD peptide (PDB ID code: 3VI4) is also illustrated (dark grey)
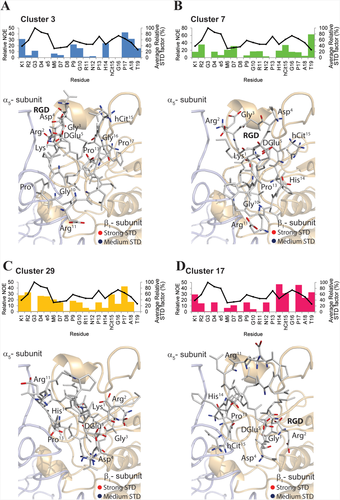
RGDechi-α5β1 models analysis by using NMR data. Evaluation of the representative conformer of the four most populated clusters by using STD and NOEs NMR data. Mapping of the STD effects [strong STD (red); medium STD (blue)] onto the representative models. Relative STD factors of the four representative RGDechi/α5β1 complex structure. The values are reported as 1 000 × Σ(1/r6) for all nonexchangeable RGDechi-integrin proton pairs within r ∼6.5 A of each RGDechi residue. The average per residue experimental STD effects are also reported (black line)
3.6 The α5β1 integrin recognition mechanism of RGDechi
The structural model of the α5β1/RGDechi complex, as obtained by using experimental and computational data, indicates that the recognition mechanism of the α5β1 integrin by the RGDechi peptide is mainly modulated by the Lys1, by the residues of the RGD cycle (Arg2, Gly3, Asp4, D-Glu5) interacting with the α5 subunit and it is stabilized by the C-terminal region of the peptide (Figure 7A–D) (Table 1). Notably, all residues of the RGDechi showing STD and trNOEs effects are involved in the α5β1 integrin binding. In particular, the residue Lys1 mainly interacts with the β1 subunit (Gln191, Tyr133) (Figure 7A); whereas the Arg2 (Figure 7B), makes contact with the β1-Pro186 and Cys187. The other residues within the RGD region (Gly3, Asp4, and D-Glu5) contribute to stabilize the interaction (Figure 7B). As reported in Figure 7B, the side chain carbonyl oxygen of the Asp7 (Figure 7C) (Table 1) interacts with the residues β1-Lys182 (Figure 7C). The interaction between RGDechi and α5β1 integrin, as observed in the αvβ3/RGDechi complex, is further stabilized by the residues of the C-terminal echistatin moiety (Arg11-Thr19) that contact both the α5 and β1 subunits. In particular, the Arg11 and the side chain carbonyl oxygen of the α5-Glu126 are hydrogen bonded (Figure 7C) whereas the hCit15, located in the proximity of the Asp4 of the RGD cycle is found to establish interactions only with the α5 subunit (Figure 7D). Instead, the Pro13 (Figure 7C) is buried into a hydrophobic pocket situated on the surface of the α5 subunit and formed by the residues Ala159, Trp157. Additionally, the His14 interacts with the residue Trp157 of the α5 subunit. (Figure 7D). The proline 17 is deeply embedded into a hydrophobic cleft principally formed by the residues β1–Pro186 β1-Leu225 (Figure 7D). Finally, the residue Thr19 slightly contributes to stabilize this interaction making contacts with the α5-Ser129 and α5-Asp130 (Figure 7D).
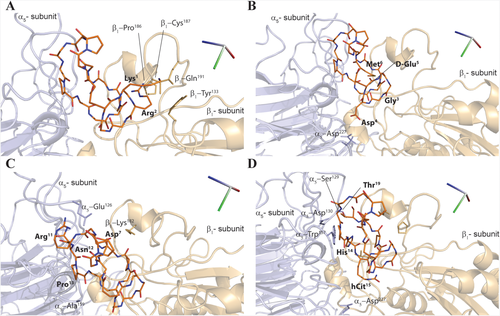
Structural model of the RGDechi-α5β1 complex. A–D, Close-view up of the representative model of the RGDechi-α5β1 complex obtained by molecular docking and selected by using experimental NMR data. Side chains of the residues of the α5-subunit, β1-subunit involved in the RGDechi-α5β1 interaction are shown as stick and are depicted in light blue and light orange, respectively. The RGDechi peptide is also reported as stick (orange)
| RGDechi | α5 | αva | β1 | β3a |
|---|---|---|---|---|
| Lys1 | D150 | Q191,Y133 | ||
| Arg2 | D150,Y178 | P186, C187 | ||
| Gly3 | ||||
| Asp4 | D227 | A149, D150,Y178 | Y166, R214, R216 | |
| D-Glu5 | D148 | |||
| Met6 | ||||
| Asp7 | K182 | |||
| Asp8 | Q145, D146, I147 | |||
| Pro9 | ||||
| Gly10 | ||||
| Arg11 | E126 | Q145 | ||
| Asn12 | E126 | |||
| Pro13 | A159 | Met118, I147 | ||
| His14 | W157 | |||
| hCit15 | D227 | E,121 D148 | D179, R214, R216 | |
| Gly16 | ||||
| Pro17 | W157 | K182, P186, L225 | ||
| Ala18 | ||||
| Thr19 | S129, D130 |
- a Determined by using the model of the RGDechi/ αvβ3 complex reported in a previous publication (Ref. 29).
3.7 Molecular basis driving the αvβ3 and α5β1 integrins recognition by RGDechi
The availability of selective subtype integrin ligands is crucial for developing of effective targeted systems in drug delivery, diagnosis and tumor imaging applications.
We have thus compared the here obtained structural model of RGDechi in complex with α5β1 with that previously determined for αvβ3/RGDechi complex29 to derive the molecular determinants of the two interactions. As shown in Figure 8A,B, the RGDechi orientation and position in the α5β1 binding site is rather different with respect to that observed in αvβ3. In particular, the RGD region of the RGDechi peptide, despite being located in the conventional RGD binding site, presents a substantial displacement of ∼10 Å in the direction of the β1-subunit and a rotation of ∼90° around the X-axis with respect to that observed in the case of αvβ3 (Figure 8A,B). This profound structural difference clearly depends on the amino acid composition of the two integrins subunits. In fact, the two negative charged Asp residues (αv-Asp148, αv-Asp150) (Figure 8C) (Table 1) that have a crucial role in the stabilization of the αvβ3/RGDechi complex by interacting with the residues of the RGD motif are replaced by two hydrophobic residues (α5-Trp157 and α5-Ala159) (Figure 8C) in the α5-subunit. Because of these significant structural differences within the recognition site, when RGDechi binds to α5β1 integrin Arg2 interacts with the residues of the β1-subunit (Table 1) driving the RGD motif in a different position. Moreover, α5β1 recognition mechanism of RGDechi is also influenced by the structural differences of the β1-SDL with respect to β3-SDL (Figure 8D). Indeed, as previously described, the interaction between αvβ3 and RGDechi is primarily modulated by hCit15, which is projected toward the β3-Arg216 and the β3-Asp179 (Table 1) (located within the SDL loop) and by Asp4 of the RGD portion that recognizes the β3-Arg214, Arg216 (Table 1) and SDL Tyr166 (Figure 8D). In the case of α5β1 integrin, β3-Tyr166, Asp179, Arg214, and Arg216, are replaced by β1-Lys182, Pro186, Gln191, and Leu225, thus driving the C-terminal echistatin moiety to bind α5β1 in a different way to that observed for the αvβ3 integrin. Particularly, h-Cit15, which solely contacts with the residues α5-Asp227 (Table 1), appears to play a reduced role in stabilizing the binding, whereas His14, Pro17, and Thr19 are involved in an extended number of interactions with the both subunits (Table 1). Some of them are located in the β1-SDL loop (Lys182 and Pro186) and others at the α5 β1 interface, giving rise to a recognition mechanism which, though preserving a degree of similarity with the one used to bind αvβ3, nonetheless contains a significant level of peculiarity.
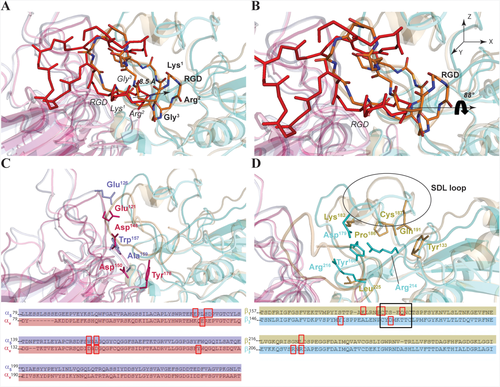
Structural features of the α5β1 and αvβ3 selectivity. Comparison of the representative model obtained by Molecular Docking studies for the α5β1 and αvβ3 integrin in complex with the RGD peptide. The RGDechi in complex with α5β1 A, (orange) and αvβ3 B, (red) integrin is reported. The residues having a crucial role in the RGDechi complex stabilization of the α5 (light blue), αv (rose pink) C, β1 (light orange) and β3 (cyan) D, are depicted. The sequence alignment is also reported in which the red boxes indicate the portions involved in the interaction while the black box indicates the SDL loop
4 CONCLUSIONS
In the last decade, in vitro and in vivo studies have shown the capability of the RGDechi peptide to selectively recognize αvβ3 integrin without cross reacting with αvβ5 and αIIbβ3 integrins.
Here, we studied the ability of the RGDechi peptide to bind α5β1 integrin and characterized, through a combined experimental and computational approach, the molecular determinants at the basis of this interaction. RGDechi is able to bind α5β1 with a lower affinity than αvβ3 and with a clearly different recognition modality. In particular, the cyclic RGD moiety contacts the α5β1 interface through a reduced number of ionic interactions, allowing a merely partial insertion of the peptide within the protein cleft. On the other side, peptide C-terminal moiety, comprising an echistatin portion, interacts with a large surface of α5β1, including the SDL loop and the subunit interface. This interaction is largely mediated by hydrophobic interactions which involve many residues of RGD linear sequence. The detailed comparison of the two recognition mechanisms provides important structural clues to design RGDechi derivatives with improved selectivity for α5β1. Particularly, RGD cycle should reduce its cationic character to better insert into the interface cleft, whereas the C-terminal part should somehow increase its hydrophobic properties to better contact the β5 subunit. Overall, this study further shows how its bifunctional nature renders RDGechi an important molecular tool to recognize integrins with structurally different modalities and to derive the structural requirements needed to their specific recognition.
ACKNOWLEDGMENTS
The authors thank Leopoldo Zona, Luca De Luca, Maurizio Amendola, and Florinda Pignatiello for the excellent technical/administrative support and the Consorzio Interuniversitario di Ricerca in Chimica dei Metalli nei Sistemi Biologici.
AUTHORS’ CONTRIBUTIONS
LR, RF, and LZ conceived and designed the experiments. SDG, DC, and AL collected the biological data and performed the analysis. ADG and DC performed the synthesis and the characterization of the molecules. BF, LR, and GM collected and analyzed the NMR data. LR carried out the molecular docking studies and the computational analysis. LR, RF, and LZ wrote the paper. LZ, RF, and MS supervised the findings of this work. All authors read and approved the final manuscript.