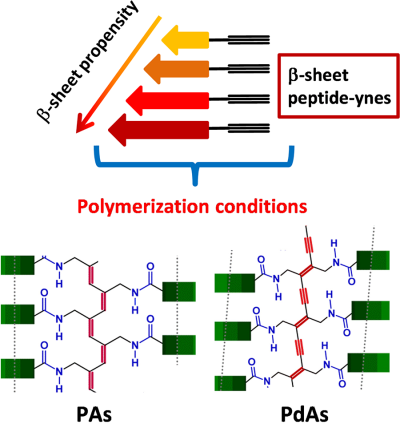
From self-assembled peptide-ynes to peptide polyacetylenes and polydiacetylenes
Funding information: Fondazione CARIPARO and MIUR (Italian Ministry of University and Research)
Abstract
A set of four organogelators from an α-amino acid derivative to a tetrapeptide, covalently linked to an acetylenic moiety, was studied in terms of polymerization efficiencies to afford peptide polyacetylenes and polydiacetylenes. Peptides were designed to improve the organogelator behavior via formation of intermolecular H-bonding-mediated β-sheet networks as a function of their main-chain length. The polymerization experiments were run under appropriate conditions for the various monomers with the aim at elucidating how the monomer self-assembly process might influence polymer formation. Starting compounds and their corresponding polymers were characterized by a variety of spectroscopic and microscopic techniques.
Graphical Abstract
1 INTRODUCTION
Amino acids, sugars, lipids, and nucleic acids are naturally occurring bioactive and biocompatible compounds. These properties make them excellent building blocks for the development of novel materials. In recent years, due to these characteristics, peptide-based compounds became a topic of intensive research1 mainly because peptides exhibit great potential as bio-based alternatives to other types of currently used synthetic materials. When rationally designed, peptide-based materials were shown to offer unprecedented advantages as those to provide modular and generalizable platforms with tunable mechanical, chemical, and biological properties.2 Whether peptides are made up exclusively of amino acids or are hybridized with other functional moieties (i.e., polymerizable sites), they are known to adopt well specific conformations and interesting self-assembly morphologies, thus providing useful platforms for the development of nanoscale materials.3-5 Over the last decade, this class of hybrid compounds was extensively investigated by combining their routinely easy peptide synthesis and the possibility to conveniently design a specific secondary structure which may possess a precise function.6-16
In this work, we focused our attention on the chemical polymerizations of triple-bond functionalized peptides that can produce colored (optical absorption above 380 nm) polymers with repeated peptide units joined together by conjugated double or alternating double–triple bonds.17-25 As a result, such peptide-based polyacetylenes (PAs) or polydiacetylenes (PdAs) are expected to be electronically active and may be good candidates for the preparation of novel materials. Therefore, we rationally varied the polymerizability of the peptide–acetylene monomers by changing the number and types of α-amino acids within the monomers with the aim at exploring the monomer templation effect on the polymerization efficiency and the properties of the resulting materials.
2 EXPERIMENTAL
2.1 Materials
The 1-hydroxy-7-aza-1,2,3-benzotriazole (HOAt) was purchased from GL Biochem (Shanghai). Tert-butyloxycarbonyl (Boc)-protected α-amino acids and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) hydrochloride were obtained from Iris Biotech. Propargylamine, triethylamine (TEA), N,N,N′,N′-tetramethylethylenediamine (TMEDA), copper(I) iodide and nickel(II) chloride hydrate were obtained from Sigma–Aldrich. The deuterated solvent dimethylsulfoxide (DMSO-d6), acetonitrile (CH3CN,d3) and chloroform (CDCl3) were purchased from Euriso-Top, while the anhydrous solvents for peptide coupling reactions were purchased from Sigma–Aldrich. All other chemicals and solvents are Sigma–Aldrich, Fluka or Acros products and used as provided without further purifications.
2.2 Nuclear magnetic resonance
1H and 13C NMR spectra were recorded at room temperature on a Bruker AC-500 instrument using TMS (tetramethylsilane) as the internal reference. The multiplicity of a signal is indicated as s, singlet; d, doublet; t, triplet; m, multiplet; and br, broad. Chemical shifts (δ) are expressed in ppm.
2.3 Fourier-transform infrared absorption
FT-IR absorption spectra in KBr disk were recorded using a Perkin-Elmer 1720X spectrophotometer. The frequency maxima values for the main absorption bands are given.
2.4 Mass spectrometry
Mass spectra were obtained by electrospray ionization (ESI) on a Perceptive Biosystem Mariner ESI-ToF 5220 spectrometer. Data were collected in the positive mode.
2.5 Ultraviolet–visible absorption
UV–vis absorption spectra were recorded using a Shimadzu model UV-2501 PC spectrophotometer. A 1-cm path length quartz cell was used.
2.6 Electronic circular dichroism
ECD measurements were carried out at room temperature using a Jasco J-715 spectropolarimeter. A fused quartz cell of 1-mm path length (Hellma) was used.
2.7 Transmission electron microscopy
Samples were analyzed on a Jeol 300 PX TEM instrument. A glow discharged carbon coated grid was floated on a small drop of the nanosphere suspension and excess was removed by #50 hardened Whatman filterpaper. All TEM samples were negatively stained with a 2% (w/v) uranyl acetate aqueous solution.
2.8 Scanning electron microscopy
A Carl Zeiss Merlin field emission SEM instrument operating at 5 kV accelerating voltage was used. A small drop of the nanosphere suspension was placed on a microscope glass coverslip and allowed to dry overnight.
2.9 Size-exclusion chromatography
SEC analyses were performed on an Agilent 1260 Infinity system equipped with 1260 isopump, 1260 TCC, 1260 VWD VL, 1260 RID, Phenogel 5-μm linear/mixed guard column (30 × 4.6 mm2), followed by a Phenomenex Phenogel 5-μm 104 Å (300 × 4.6 mm2) column working at 60°C. DMF (N,N-dimethylformamide) was used as eluant at a flow rate of 1 mL min−1. Before SEC analysis, the samples were filtered through a 0.2-μm PTFE filter (15 mm, Phenomenex). Molecular weights were calculated according to a calibration using polystyrene standards.
2.10 Synthesis of compounds
2.10.1 Boc-L-Val-NH-CH2-C≡CH (1)
Boc-L-Val-OH (3.53 g, 16.2 mmol) was dissolved in 20 mL of anhydrous DCM (dichloromethane) and C-activated with HOAt (2.19 g, 16.1 mmol) and EDC·HCl (3.09 g, 16.1 mmol). Propargylamine (1.5 mL, 23.4 mmol) and TEA (2.3 mL, 16.1 mmol) were added to the solution of the active ester to pH 8–9. The mixture was stirred at room temperature for 4 days. The solvent was removed under reduced pressure and the residue was dissolved in ethyl acetate (EtOAc). The organic phase was washed with 5% KHSO4(aq) (4v), water (1v), 5% NaHCO3(aq) (3v), and brine. Then, the organic layer was dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was dissolved in EtOAc and precipitated by addition of petroleum ether. After filtration and drying, the product was obtained as a colorless solid (3.35 g, 82% yield).
MS (ESI+): m/z [M + H]+ calcd. for C13H22N2O3 255.3334, found 255.1760. FT-IR absorption: ῡ 3296 (m), 1657 (s), 1538 (m) cm−1. 1H NMR (400 MHz, CDCl3) δ 6.52 (br, 1H, NH propargyl), 5.10 (br, 1H, NH urethane), 4.06-4.02 (m, 2H, CH2 propargyl), 3.94-3.86 (m, 1H, αCH Val), 2.20 (t, 1H, CCH), 2.14-2.07 (m, 1H, βCH Val), 1.43 (s, 9H, 3 x CH3 Boc), 0.93 (t, 6H, 2 x CH3 Val). 1H NMR (500 MHz, CH3CN-d3) δ 6.83 (br, 1H, NH amide), 5.38 (br, 1H, NH urethane), 4.01–3.92 (m, 2H, CH2 propargyl), 3–86-3.83 (m, 1H, αCH Val), 2.43 (t, 1H, CCH), 2.07-2.01 (m, 1H, βCH Val), 1.45 (s, 9H, 3 x CH3 Boc), 0.93 (dd, 6H, 2 x CH3 Val). 13C NMR (50 MHz, CDCl3) δ 172.83, 157.32, 81.52, 75.16, 70.94, 56.27, 30.28, 29.91, 27.13, 17.03.
2.10.2 Boc-L-Phe-L-Val-NH-CH2-C≡CH (2)
TFA− +H2-L-Val-NH-CH2-C≡CH [obtained after TFA (trifluoroacetic acid)/DCM treatment of 1] was dissolved in 10 mL of anhydrous DCM with addition of TEA (1.2 mL, 8.61 mmol). This solution was added to a solution of Boc-L-Phe-OH (1.15 g, 4.34 mmol) dissolved in 10 mL of anhydrous DCM and C-activated with HOAt (593 mg, 4.36 mmol) and EDC·HCl (826 mg, 4.31 mmol). A few mL of TEA was added to pH 8–9. The mixture was stirred at room temperature for 24 h. The solvent was removed under reduced pressure and the yellowish residue was acidified with 5% KHSO4(aq) and diluted with EtOAc. The organic phase was washed with 5% KHSO4(aq) (3v), water (1v), 5% NaHCO3(aq) (3v), and brine. The organic layer was dried over Na2SO4, filtered, and evaporated under reduced pressure. The product was obtained as a colorless solid (1.39 g, 88% yield).
MS (ESI+): m/z [M + H]+ calcd. for C22H31N3O4 402.2395, found 402.2359. FT-IR absorption: ῡ 3275 (br), 2128 (w), 1684 (m), 1643 (s) cm−1. 1H NMR (500 MHz, CH3CN-d3) δ 7.35-7.25 (m, 5H, Ar Phe), 6.78 (br, 2H, 2 x NH amide), 5.54 (br, 1H, NH urethane), 4.34-4.30 (m, 1H, αCH Phe), 4.19-4.16 (m, 1H, αCH Val), 3.95-3.94 (m, 2H, CH2 propargyl), 3.16-2.87 (m, 2H, βCH2 Phe), 2.44 (t, 1H, CCH propargyl), 2.15-2.10 (m, 1H, βCH Val), 1.40 (s, 9H, 3 x CH3 Boc), 0.92 (dd, 6H, 2 x CH3 Val). 13C NMR (50 MHz, CDCl3) δ 172.59, 171.01, 157.15, 135.68, 127.15, 126.95, 125.11, 79.62, 74.20, 71.23, 56.03, 55.09, 37.03, 30.59, 27.14, 18.40.
2.10.3 Boc-L-Cys(Me)-L-Phe-L-Val-NH-CH2-C≡CH (3)
Boc-L-Cys(Me)-OH (492 mg, 2.09 mmol) was dissolved in 10 mL of anhydrous DCM and C-activated with addition of HOAt (284 mg, 2.09 mmol) and EDC·HCl (400 mg, 2.09 mmol). TFA− +H2-L-Phe-L-Val-NH-CH2-C≡CH (obtained after TFA/DCM treatment of 2) was added to the solution of the active ester with TEA (584 µL, 4.18 mmol), monitoring the basicity of the resulting mixture (pH 8–9). Because a gel was formed, a few mL of CH3CN were added to promote the dissolution of the compound. The mixture was stirred at room temperature for 2 days. The solvent was removed under reduced pressure. The residue was acidified with 5% KHSO4(aq) and dissolved in EtOAc. The organic phase, where the product formed a gel, was washed with 5% KHSO4(aq) (4v), 5% NaHCO3(aq) (1v), and brine (2v). The organic layer, after dissolution of the gel under thermal treatment, was evaporated under reduced pressure. The product was obtained as a colorless solid (640 mg, 65% yield).
MS (ESI+): m/z [M + H]+ calcd. for C26H38N4O5S 519.6767, found 519.2637. FT-IR absorption: ῡ 3281 (s), 2126 (w), 1693 (s), 1638 (s) cm−1. 1H NMR (500 MHz, CH3CN-d3) δ 7.33-7.25 (m, 5H, Ar Phe), 7.10 (d, 1H, NH amide), 6.86-6.81 (m, 2H, 2 x NH amide), 5.57 (br, 1H, NH urethane), 4.65-4.61 (m, 1H, αCH Cys), 4.20-4.15 (m, 2H, 2 x αCH Phe and αCH Val), 3.96-3.94 (m, 2H, CH2 propargyl), 3.21-2.96 (m, 2H, βCH2), 2.86-2.61 (m, 2H, βCH2), 2.44 (t, 1H, C≡CH), 2.15-2.09 (m, 4H, SCH3 Cys and βCH Val), 1.44 (s, 9H, 3 x CH3 Boc), 0.92-0.88 (m, 6H, 2 x CH3 Val). 13C NMR (50 MHz, CDCl3) δ 171.39, 170.11, 169.72, 157.13, 135.96, 127.93, 127.50, 125.51, 78.56, 74.03, 69.58, 56.30, 53.56, 52.11, 36.01, 31.73, 29.21, 27.14, 17.40, 13.77.
2.10.4 Boc-L-Leu-L-Cys(Me)-L-Phe-L-Val-NH-CH2-C≡CH (4)
Boc-L-Leu-OH (123 mg, 0.53 mmol) was dissolved in 6 mL of anhydrous CH3CN and C-activated with addition of HOAt (72 mg, 0.53 mmol) and EDC·HCl (102 mg, 0.53 mmol). TFA− +H2-L-Cys(Me)-L-Phe-L-Val-NH-CH2-C≡CH (obtained after TFA/DCM treatment of 3) was added to the active ester with DIPEA (diisopropylethylamine) (93 μL, 0.53 mmol), monitoring the basicity of the resulting mixture (pH 8–9). An organogel was formed within a few seconds in the reaction mixture, which was stirred at room temperature for 20 h. The resulting suspension was filtered and the colorless solid was washed with diethyl ether (245 mg, 69% yield).
MS (ESI+): m/z [M + H]+ calcd. for C32H49N5O6S 632.3484, found 632.3520. FT-IR absorption: ῡ 3270 (s), 2958 (m), 1698 (s), 1660 (s), 1634 (s), 1540 (s) cm−1. 1H NMR (500 MHz, CH3CN-d3) δ 7.33-7.25 (m, 6H, 5H Ar Phe and 1H NH amide), 7.15 (br, 1H, NH amide), 6.94 (br, 2H, 2 x NH amide), 5.64 (br, 1H, NH amide), 4.61-4.57 (m, 1H, αCH), 4.37-4.36 (m, 1H, αCH), 4.16-4.13 (m, 1H, αCH), 4.05-4.01 (m, 1H, αCH), 3.95-3.94 (m, 2H, CH2 propargyl), 3.24-2.98 (m, 2H, βCH2), 2.87-2.74 (m, 2H, βCH2), 2.43 (br, 1H, CCH), 2.13-2.10 (m, 4H, SCH3 Cys and βCH Val), 1.74-1.68 (m, 1H, γCH Leu), 1.54-1.53 (m, 2H, βCH2 Leu), 1.46 (s, 9H, 3 x CH3 Boc), 0.98-0.91 (m, 12H, 2 x δCH3 Leu and 2 x γCH3 Val). 13C NMR (50 MHz, CDCl3) δ 172.97, 171.48, 171.03, 169.97, 157.25, 135.96, 127.53, 127.01, 125.37, 79.54, 74.25, 70.91, 57.03, 54.23, 53.82, 51.39, 39.66, 37.53, 32.59, 30.74, 29.31, 26.89, 24.10, 22.93, 17.31, 14.09.
2.11 Determination of critical self-assembly concentration
Peptides were dissolved in THF solutions thermostatted at 50°C in a range of concentrations from 1 to 20 mM. The solutions were allowed to equilibrate at room temperature. As critical self-assembly concentration, we assumed those peptide concentrations able to form stable organogel via the tube inversion test. All of the organogel processes were found to be thermoreversible.
2.12 Rh-catalyzed polymerization (reported example for 1)
Polymerization was carried out in a dry three-neck flask under dry nitrogen atmosphere. Compound 1 (500 mg, 1.97 mmol) was placed in the dry flask, which was then evacuated on a vacuum line and flushed with dry N2. The evacuation-flush procedure was repeated three times. Then, THF (20 mL) and TEA (345 µL, 2.46 mmol), prepared in N2 atmosphere, were added with a syringe. Finally, a solution of catalyst [Rh(nbd)Cl]2 (nbd, 2,5-norbornadiene) (5 mg, 0.011 mmol), prepared under a dry N2 atmosphere in 0.5 mL of THF, was added at room temperature. The polymerization proceeded rapidly: the solution changed color from yellow to brown and the polymer appeared as a viscous mixture after few seconds. The polymer was stirred at 50°C for 5 or 24 h. The resulting polymer was precipitated into a large amount of methanol and the yellow solid was collected by centrifugation, washed twice, and dried.
2.13 Direct topochemical polymerization (reported example for 1)
CuI (5 mol%) and NiCl2·H2O (5 mol%) were dissolved in 500 μL of THF (tetrahydrofuran). Then, TMEDA (20 mol%) was added. The resulting solution was stirred at room temperature under air for 2 min. Compound 1 (500 mg, 1.97 mmol) was dissolved in 4 mL of THF. The mixture was stirred at 50°C under air for 8 h giving a yellow, gel like, solution. The resulting polymer was precipitated into a large amount of methanol (MeOH) and the orange solid was collected by centrifugation, washed twice, and dried.
3 RESULTS AND DISCUSSION
The first part of this work was devoted to the preparation of peptide-yne monomers with a strong propensity to adopt β-sheet conformations. On the basis of the extensive literature existing on oligo- and polypeptides,26-28 we decided to evaluate the combination of four strongly β-sheet promoter α-amino acids, namely Val, Leu, Phe, and Cys(Me) (S-methyl cysteine).29-33 In the self-assembly process, both non-covalent side-chain interactions and CO···HN intermolecular H-bonds are known to play fundamental roles in molecular recognition and formation of regular microstructures. Importantly, as we worked in organic media, intermolecular CO···HN H-bonds force the molecules to pack each other, while hydrophobic side chains may result to be, at least partially, solvated. Thus, in the sequences we selected for our study the dominant force driving self-assembly is related to the number of amide units present in the monomeric peptides. Amino acid side-chain interactions may help in the formation of microstructures at a later stage. Therefore, we designed and synthesized the following four peptide-ynes: Boc-L-Val-NH-prop (1), Boc-L-Phe-L-Val-NH-prop (2), Boc-L-Cys(Me)-L-Phe-L-Val-NH-prop (3), Boc-L-Leu-L-[Cys(Me)]-L-Phe-L-Val-NH-prop (4) (prop, propargyl) by following a step-by-step solution protocol. All of them are characterized by a propargyl amine moiety covalently linked to their C-termini (Figure 1). It is worth noting here that the effect of the Boc-NH-urethane group on peptide self-association is not easy to be established. This is related to the ambiguous (partly hydrophobic, the tert-butyl moiety, and partly hydrophilic, the OCONH function) nature of this type of Nα-protection.34
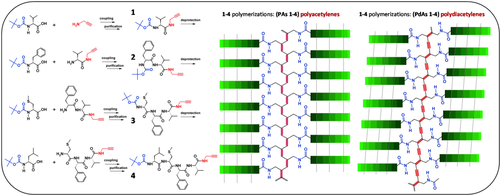
Left: Syntheses and chemical structures of monomers 1–4. Right: Schematic representations of the polyacetylenes (PAs) and polydiacetylenes (PdAs) polymers. Highlighted in red are the novel, unsaturated and conjugated, polymeric backbones
For details on the coupling conditions and chemical characterizations, see the Experimental section. All of the monomers were treated under appropriate experimental conditions to produce the corresponding PAs or PdAs (Figure 1) as discussed in the next sections.
3.1 Characterization of peptide-yne monomers
In Figure 2A the 1H NMR spectra of peptide-yne monomers 1-4 are reported. As a function of the peptide main-chain length, the related NMR spectra showed different signals, which belong to the different α-amino acid constituents. It is worth noting that the NMR spectra were recorded in CH3CN-d3. The use of this solvent of low polarity emphasized the already strong, intrinsic tendency to self-aggregation of these compounds, especially evident for 3 and 4 (as suggested by the presence of broad signals).35 We also measured the solid-state FT-IR absorption spectra for all of the samples. As it can be seen from Figure 2B, in the amide NH stretching range (amide A, 3400-3200 cm−1) and in the amide CO stretching range (amides I-II, 1700-1500 cm−1) all of the peptide bonds of 1-4 are involved in intermolecular H-bonds typical of β-sheet-like 3D-structures.36-38 In particular, all of the amide A and I-II bands of 1 to 4 move progressively to higher energies (amide A: 3303 to 3268 cm−1; amide I: 1660 to 1625 cm−1; amide II: 1540 to 1522 cm−1). This overall feature is particularly pronounced for 2-4. This observation suggested that a high supramolecular organization took place in the solid phase for the longest compounds.
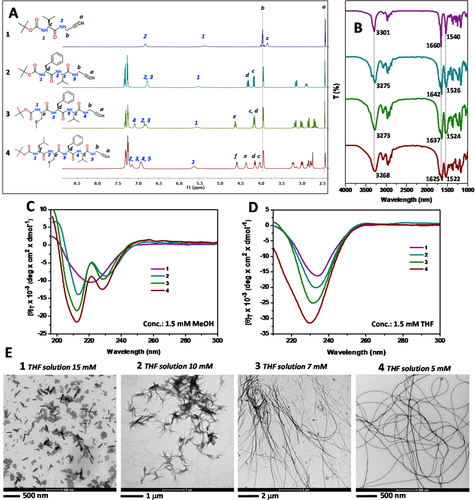
A-D, 1H NMR (recorded in CH3CN, d3), solid-state FT-IR absorption and CD (recorded in MeOH and THF) spectra, respectively, of compounds 1 (purple), 2 (blue), 3 (green), and 4 (red). E, TEM images of compounds 1–4
To prove that this organization may occur in solution as well, we performed CD experiments in different solvents. More specifically, we made use of MeOH (a competitor for H-bond formation) to avoid aggregation and detect the conformation of the isolated, solvated molecule, and THF to promote the onset of intermolecular H-bonds and observe the overall supramolecular organization. The results are illustrated in Figure 2C. In the CD experiments carried out in MeOH (at 1.5 mM concentration for all of the four compounds) we obtained different profiles for 1–4. These distinct features are due to the different amino acid sequences (intrinsic chirality of each residue) and peptide lengths (formation of chiral secondary structures) characteristic of peptides 1–4 accompanied by the presence in 2–4 of the Phe benzyl chromophores, the electronic transitions of which in the far-UV region are known to overlap those of the peptide group.33, 39 In THF, however, all of the four compounds exhibited similar CD curves with an intense negative maximum at 230 nm which we attributed to a β-sheet-like conformation (Figure 2D).40, 41 This information suggested that 1-4 may self-aggregate in this less polar solvent following a similar molecular recognition pathways. Moreover, not surprisingly, it also indicates that the peptide tendency for β-sheet formation is enhanced in THF relative to MeOH. On the basis of the observations obtained from the CD spectroscopy, we decided to investigate the morphological organization of 1-4 in THF. To explore this phenomenon, we prepared a set of THF solutions at different concentrations with the aim at finding the corresponding critical concentration for each compound needed to obtain organogels. In the organogel state, the overall non-covalent interactions (especially those of the H-bonding type) may cooperatively induce formation of ordered nanostructures able to entrap the solvent. After several attempts, we found stable organogels for each compound at the following critical concentrations (in THF solution): 1, 15 mM; 2, 10 mM; 3, 7 mM; 4, 5 mM.
These values for the critical concentration gave a direct information on the intrinsic tendency of each compound to self-aggregate and, in particular, highlighted that 4 displayed the optimal conformational geometry to cooperatively induce gelation of the mixture at the lowest concentration with respect to those of the other compounds. To prove the formation of organized self-assembled structures, we run transmission electron microscopy (TEM) experiments. The results, reported in Figure 2E for the four compounds, were recorded for their specific critical concentration values. The analyses indicated the occurrence of different self-assembled morphologies. The TEM image of compound 1 revealed formation of a flake-like structure, while compounds 2-4 are characterized by fiber-like structures. In particular, long, but not well defined, fibers were obtained for 3, while 4 afforded accurately defined, regular and long fibers.
The overall results obtained from the chemical, physical, and microscopic characterizations performed for the 1-4 monomers may be summarized as follows: (i) according to their increased main-chain length, similar strands of H-bonded networks, typical of β-sheet-like structures, are formed in organic solvents for all of the monomers. Interestingly, comparable typologies of intermolecular interactions are found for all of the monomers at both low and high monomer concentrations; (ii) despite the occurrence of similar β-sheet-like H-bonding interactions, the longest peptides (3 and 4) afforded more ordered microstructures under their critical self-assembly conditions. This finding may suggest the onset of a templating effect that is progressively induced by the presence of more units (from 1 to 4) of β-sheet supporting amino acids in the peptide backbone. As a consequence, we now have in our hands a system where the alkyne units are strictly self-assembled with a supramolecular geometry imposed by the corresponding conjugated peptide. Under these conditions, we may consider that polymerization of the triple bonds is controlled by a template effect generated by the molecular preorganization of the 1-4 monomers.
3.2 Peptide polyacetylenes
We applied the well established acetylene polymerization via rhodium catalysis42-50 to monomers 1-4 following the schematic representation illustrated in Figure 3A. As it is known from this type of polymerization, depending on the catalyst involved, four unsaturated chains of different conformers may be obtained: cis-transoid, trans-cisoid, trans-transoid, and cis-cisoid forms (Figure 3B).44 The Rh (I) catalyst was suggested as being able to induce a stereospecific polymerization for acetylenes to afford selectively cis-transoid polyacetylene isomers.51
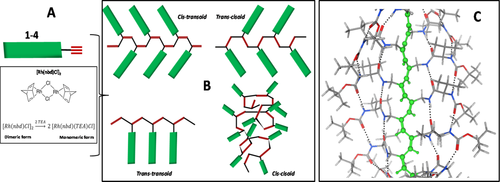
A, Chemical structure of the catalyst and its activation by means of the co-catalyst. B, Schematic representation of the possible conformations adopted by the polyacetylene backbone. C, Energy minimized structures for the cis-transoid PA 1, highlighting the unsaturated backbone and all peptide molecules aligned in the parallel β-sheet conformation
Thus, what we expected from the β-sheet peptide-yne polymerizations induced by the Rh catalyst was formation of cis-transoid polyacetylenes with peptides precisely aligned laterally, with respect to the unsaturated backbone, along their parallel β-sheet axis (Figure 3C, example for polymer PA 1). With this geometry in mind, we also believed that longer peptides should generate more stable structures extra-stabilized by formation of multiple H-bonded interactions between all of the amide groups present in the peptides. Clearly, one of the chemical polymerization pre-requisites is to run the reaction at high monomer concentration. Under these conditions, we already demonstrated that monomers 1-4 are susceptible to formation of organized packed structures (organogels). To explore the role of a molecular pre-organization induced by the supramolecular self-assembly of the monomers, we carried out all polymerizations for peptide-ynes (1-4) at the concentration 0.1 M in THF at 55°C. Under these conditions, all monomers are above their minimum critical concentration, but at temperature 50°C their organogel organization are in the molten state, resulting in a viscous solution or in a solid mixture. After the addition of the catalyst and cocatalyst to the molten organogel solutions, polymerizations proceeded rapidly for monomers 1-3 (very dense mixtures were obtained after few minutes of reactions). Conversely, monomer 4 did not undergo this polymerization process. The polymers were collected after precipitation upon MeOH addition and centrifugation.
After drying the polymers, we took optical microscope images of the solid materials. As can be seen from Figure 4, PA 1–3 displayed a yellow/orange color21 and are present in the form of flakes. We proceeded with the morphological characterization of the polymers by using TEM. The three polymers were dissolved at low concentration in a mixture of DMF/THF, deposited on a TEM grid, and let stand to dryness. The results are displayed in Figure 4B, which shows formation of linear assemblies of fibers for all samples. In particular, for PA 1 small fibers were detected prior to their aggregation into larger assemblies, while PAs 2 and 3 displayed a tendency to aggregate in wide, linear assemblies. We may assume that the solid flakes of polymers obtained after centrifugation would be formed by highly packed and organized polymeric chains kept together in fibrillar-like aggregates. Interestingly, when the organic mixtures were dialyzed against water, the polymeric materials re-assembled in nanoparticle-like structures with the diameter decreasing from PAs 1 to 3 (Figure 4C). We succeeded in processing the polymers by moulding them into appropriate shapes. As an example, by dissolving PA 3 at high concentration in DMF (dense homogeneous solution), placing this solution in an appropriate Teflon-covered state, and subsequently waiting for an aging time under a high-vacuum oven at 45°C, an orange-transparent and macroscopic material was obtained (Figure 4D). The SEM analysis highlighted formation of ordered aligned microscopic fibers extended over all of the material surface (Figure 4D). To our knowledge, this is the first example of a processable peptide based polymer, which results in large scale fabrication of a bioinspired optical material. We are planning to study its potentialities as material in a future work.
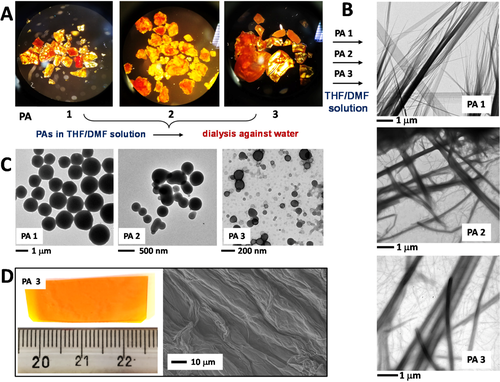
A, Optical microscope images of PA 1–3. B, TEM images of PA 1–3 in diluted DMF/THF mixtures. C, TEM images of aqueous solutions of self-assembled PA 1–3. D, Macroscopic material from processed PA 3 and its SEM characterization
PAs 1-3 were characterized on the basis of their molecular weights and spectroscopic features. In Figure 5, we illustrate the results of the SEC analyses after polymerization. They highlighted the polymer molecular weight decrease as a function of the elongation of the corresponding monomeric peptide-ynes. A longer time of polymerization did not affect significantly the polymer lengths. In all cases, polymerization is almost complete after 5 h of reaction. The recovered material is quantitative for PA 1 (1 000 monomer units for polymer chain), while the yield decreases for PAs 2 (400 monomer units for polymer chain) and 3 (80 monomer units for polymer chain). These results, together with the absence of reactivity for 4, suggested that steric hindrance, combined with an efficient molecular packing, may decrease the polymerization efficiency. It is worth noting that Boc-Ala-NH-prop (similar to our 1 monomer) was used by Masuda and coworkers50 to promote PA formation. In their work, the authors reported molecular weights similar to those found by us for monomer 1. To our knowledge, studies on any β-sheet forming peptide, directly conjugated to a unsaturated polymer backbone, was not reported in the literature.
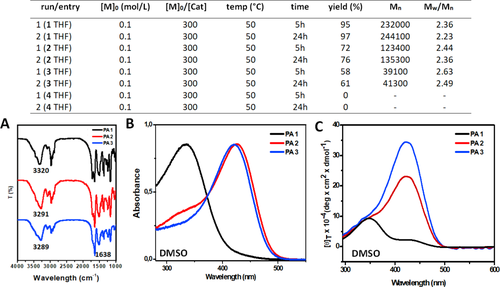
Top, Results of the SEC analyses after polymerization under the reported experimental conditions. A, Solid-state FT-IR absorption spectra for PAs 1–3. B, UV–vis absorption spectra in DMSO for PAs 1–3. C, CD spectra in DMSO for PAs 1–3
PAs 1-3 were characterized by solid-state FT-IR absorption (Figure 5A). The results of the NH and CO stretching regions indicated increasing β-sheet-like H-bonding interactions from PA 1 to PA 3 (both frequency maxima shift to lower wavelengths). The UV-Vis absorption spectra of PAs 1-3 were recorded in DMSO to minimize the backbone···backbone interactions between the polymeric chains. The results reported in Figure 5B indicated that PA 1 is characterized by an absorption maximum at 325 nm, while PA 2 by a shoulder at the same wavelength and a maximum at 430 nm. Finally, PA 3 displayed only the absorption maximum at 430 nm. The presence of a maximum at 325 nm suggested a less efficient conjugation in the unsaturated polymer backbone, while that at 430 nm indicated a more favorable conjugation between the double bonds of the polymer backbone. Thus, we may assume that the peptide segments contained in PA 1 are randomly interacting with each other along their unsaturated backbone, probably due to the good solvation effect of DMSO that competes with all of the intra- and intermolecular H-bonding networks of PA 1. This solvation effect may be the reason for the poor conjugation in the corresponding polyacetylene that will tend to adopt many different conformations. On the other hand, in PA 2 and 3 (PA 3 more efficiently than PA 2) the corresponding peptide chains, even under a remarkable solvation, were able to efficiently (intramolecularly) interact along their parallel β-sheet axis thus to drive the unsaturated backbone in the preferred all-trans conformation. These results were confirmed by the CD analysis which showed that, despite their higher length with respect to that of PA 1, polymers PAs 2 and 3 displayed a strong chiroptical positive signal with a maximum at 430 nm.
These results highlight a steric-hindrance effect on the polymerization efficiency (shortest monomers produced longest polymers). In contrast, we observe that a positive stabilizing effect to the transoid PA conformation (with high electronic conjugation) is induced by the longest monomers. On the basis of these data, we conclude that 2 and especially 3, once covalently connected to the unsaturated-conjugated system, are parallelly aligned along the PA backbone. This disposition efficiently templates them along all their amino acid sequence in two strands of an intramolecular, parallel β-sheet (one for each side of the unsaturated PA backbone). We believe that the overall alignment of the H-bonding interactions would represent the driving force for the stabilization of PAs 2 and 3 in their transoid isomeric form.
3.3 Peptide polydiacetylenes
Our next step was to apply to our peptide-acetylene hybrids the alkyne-alkyne cross-coupling reaction52 which is expected to lead to formation of 1′-4′ diacetylene homo-dimers (Figure 6A). Traditionally, a molecular alignment with only minimal packing rearrangements of the diacetylenic (DA) monomer, satisfies the requisite to undergo a (thermal- or photochemical-induced) topochemical polymerization. Recently, we demonstrated that not only single crystals of monomers can be transformed into highly conjugated polydiacetylenes,22-25 but also DAs efficiently packed as organogels.53 Thus, according with the alkyne-alkyne cross-coupling reaction, all of the 1–4 compounds were dissolved in THF at concentration 0.5 M and allowed to react (as indicated in the Experimental section) with the aim at obtaining homo-diacetylene peptides 1′-4′. Quite surprisingly, the reactions involving the 1-3 compounds turned rapidly (from 5 to 8 h) into the corresponding PdAs 1-3 polymers providing well colored solutions with a gel-like consistence (Figure 6B). In the case of the reaction involving 4, it did afford neither 4′ nor the corresponding PdA 4 (4 was recovered quantitatively from the reaction mixture). Addition of MeOH to the crude PdA 1-3 mixtures afforded the PdA 1-3 series as solid compounds (from the crude mixtures we did not recover any 1′-3′ homo-diacetylene peptides). In Figure 6C we show the UV–vis diffuse reflectance spectra of the PdA 1-3 polymers, which confirmed that the topochemical polymerizations did indeed take place. A careful spectral comparison provided information on the degrees of their polymerization. On the basis of the wavelength absorption maxima (that is related to the double-triple bond repetition along the polymer backbone), we were able to establish formation of longer polymeric chains for PdAs 2 and 3. We characterized morphologically PdAs 1-3 by dissolving small amounts of each polymer in DMSO. The TEM analyses of these samples gave information on the polymer molecular organizations and lengths. In more detail, after drying a drop of DMSO solution of each PdA over the TEM grid, we observed: (i) for PdA 1, formation of short strip-like structures with a length of 20–200 nm (Figure 6D); (ii) for PdA 2, a large distribution in size (100 nm to 2 μm) of similar strip-like structures as seen for PdA 1 (Figure 6E); (iii) for PdA 3, compact, large rod-like structures (Figure 6F). Considering the size of the structures obtained for PdA 1, we assume that the topochemical condensation of four homo-diacetylenic monomers would result in an end-to-end linear distance of 2 nm. Thus, each of the average 100 nm-long PdA 1 molecules contains as many as 100 diacetylenic monomers.54 We extended a similar consideration to PdA 2, where apparently the large distribution of size affords from 1- to 2-μm-long strips each containing up to 2 000 homo-diacetylenic monomers. As for PdA 3, the TEM analysis showed very large aggregates with crystalline features (Figure 6 F, inset).
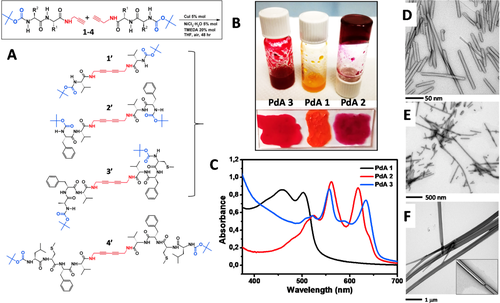
A, Schematic representations of the alkyne-alkyne cross-coupling reaction and the expected homo-diacetylene products. B, PdAs obtained from the alkyne-alkyne cross-coupling reaction. C, UV–Vis diffuse reflectance spectra of PdAs 1–3. D-F, TEM images of PdAs obtained from diluted solutions
Taken together, the results obtained in the case of formation of the PdAs showed an intriguing templation effect for compounds 1-3. While compound 4 was not able to afford the corresponding homo-diacetylene dimer 4′ (probably due to its strong tendency to self-associate), monomers 1-3 were rapidly converted into their homo-acetylene dimers (1′-3′) and polymerized (PdAs 1-3). In particular, we observed that the polymerization degree increases, while the polymerization yield decreases, from 1 to 3. In conclusion, we assume that in the course of the PdA polymerizations, once the homo-acetylene dimers (1′-3′) formed, they self-assembled in such a way to adopt the appropriate disposition to undergo a moderately thermally (50°C) induced topochemical polymerization.
4 CONCLUSIONS
To summarize our work, we found that Boc-L-Val-NH-prop (1), Boc-L-Phe-L-Val-NH-prop (2), Boc-L-Cys(Me)-L-Val-NH-prop (3), and Boc-L-Leu-L-[Cys(Me)]-L-Val-NH-prop (4) are able to hierarchically self-assemble in well-defined organogels. These structures are build-up through networks of intermolecular β-sheet interactions and show critical concentration values (for 1-4 in THF) that decrease with main-chain elongation. The presence of a propargyl unit at the C-terminus of 2-4 allowed us to investigate for the first time formation of peptide-based polyacetylene (PAs) and polydiacetylene (PdAs) organogels. According with the schematic representation reported in Figure 1, both PA and PdA backbones are able to template formation of parallelly oriented β-sheet structures within their respective polymeric systems, thanks to the onset of geometrically perfect repetitions of highly conjugated double and double-triple bonds, respectively.55-57 We are currently exploring the limits (dictated by the negative steric hindrance) and potentialities (governed by the positive template effect of well-ordered networks) of the organogel-forming peptide-acetylene hybrid compounds for a further development of this fascinating opto-electronic-active, stimuli-responsive, peptide-based, polymer field.17-19