Advances in understanding Kawasaki disease-related immuno-inflammatory response and vascular endothelial dysfunction
ABSTRACT
Kawasaki disease (KD) is a systemic vasculitis of unknown etiology, which tends to involve coronary arteries and can lead to acquired heart disease in children. The immuno-inflammatory response and vascular endothelial dysfunction are important causes of coronary artery disease in patients with KD. Multisystem inflammatory syndrome in children (MIS-C) is a rare inflammatory disease in children identified in recent years, which is caused by severe acute respiratory syndrome coronavirus 2 infection; this disease overlaps with KD. This review examines research progress concerning the immuno-inflammatory response and vascular endothelial dysfunction associated with KD, as well as differences between KD and MIS-C.
INTRODUCTION
Kawasaki disease (KD) is a systemic vasculitis of unknown etiology that can lead to coronary artery disease (CAD), myocarditis, and myocardial infarction. It is the main cause of acquired cardiovascular disease in children. Although no specific connections have been identified between KD and specific pathogens or pathogen-related structural substances, KD is presumably caused by an infection-related abnormal inflammatory response in genetically susceptible individuals.1, 2 Infection causes amplification of immune and inflammatory responses in vivo, which result in systemic vascular injury.3 The immuno-inflammatory response and vascular endothelial dysfunction are important causes of CAD in patients with KD4; they are also risk factors for advanced atherosclerosis in such patients.5
Multisystem inflammatory syndrome in children (MIS-C) is a newly discovered syndrome that involves an intense inflammatory response. It occurs approximately 3–4 weeks after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents. As of March 28, 2022, the Centers for Disease Control and Prevention reported a total of 7880 MIS-C cases in the United States; 66 resulted in death.6 Although MIS-C is clearly caused by SARS-CoV-2 infection, its pathogenesis is unknown. Antibody-dependent enhancement of coronavirus and superantigenic behavior of SARS-CoV-2 are reportedly involved in MIS-C pathogenesis.7 Rare inherited immunity disorders have been suggested as the basis of MIS-C pathogenesis.8 Because many patients with MIS-C have KD-like clinical manifestations, the diseases were initially presumed to have a close relationship. However, MIS-C and KD have since been clearly distinguished, although they have many similarities (e.g., immune system activation and vascular endothelial inflammation) and share aspects of clinical manifestations, laboratory tests, and treatment.
This review focus on research advances in understanding KD-related immuno-inflammatory response and vascular endothelial dysfunction, as well as similarities and differences between KD and MIS-C.
IMMUNO-INFLAMMATORY RESPONSE
In patients with KD, the innate immune response is activated and the acquired immune system exhibits abnormal function. The rate of KD recurrence is 1%–3%; recurrence is most common within 12 months after the initial onset of the disease.9 Moreover, patients with recurrent disease have an increased risk of coronary artery abnormalities, suggesting that innate immune memory enhances the immune response to heterologous reinfection signals or endogenous risk signals.3 Immuno-inflammatory mechanism in KD is shown in Figure 1.
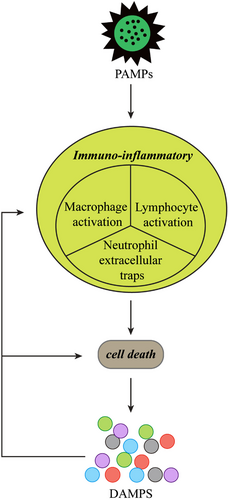
Pathogen-associated molecular patterns
Pathogen-associated molecular patterns constitute pathogens and molecular substances on pathogens, both of which can directly induce damage to organs, tissues, and cells. Small outbreaks of KD have occurred in a manner consistent with the spread of infectious diseases.10 The clinical manifestations of some infectious diseases are similar to KD; for example, group A β-hemolytic streptococcal infection can cause scarlet fever, as well as strawberry tongue, cervical adenopathy, rash, and convalescent skin desquamation.11 The KD-associated pathogen may be a ubiquitous source of infection, presumably a single virus or group of closely related viruses, which causes KD only in a small number of genetically susceptible children.12 Other candidate causative factors include water-soluble components of Candida albicans, cell wall components of Lactobacillus casei, or fk565 in Streptomyces olivaceus; these components all act as “pathogen-associated molecular patterns”. Notably, the immuno-inflammatory response and medium-sized arterial dilatation that these components induce in mice are similar to the manifestations in patients with KD,3 which supports a relationship between “pathogen-associated molecular patterns” and KD pathogenesis.
Damage-associated molecular patterns
In the classic immunological concept, “risk theory”, pathogens, toxins, and mechanical injury are presumed to cause stress- or injury-damaged cells to release or activate endogenous “danger signals”; this contributes to the further spread of an inflammatory response, which includes antigen-presenting cell-mediated activation of the adaptive immune response.13 In contrast to the components released by healthy cells or cells undergoing normal physiological death, the components released by necrotic cells may serve as danger signals.14 The “risk theory” can be used to explain the amplified inflammatory and immune response in the early stage of KD. Some exogenous substances or physical injury can cause damage to the body's cells; damaged cells then release endogenous signal molecules, thereby activating a series of immuno-inflammatory responses throughout the body.
High mobility group protein-1 (HMGB1) is an endogenous damage-associated molecular pattern protein; it can activate the natural immune system, promote the host inflammatory response, and participate in the onset of CAD in patients with KD.15 Importantly, the release of interleukin (IL)-1α and HMGB1 may mediate immunoglobulin resistance in patients with KD.16 Pentraxin 3 is a soluble recognition molecule that is rapidly produced in response to inflammation and pathogenic microorganisms; it is important in the innate immune response.17 Neutrophil-derived Pentraxin 3 may be related to vascular dysfunction in KD.18 Additionally, S100 proteins may contribute to the onset of CAD in children with KD.19 The combined detection of neuron-specific enolase and S100 levels in cerebrospinal fluid can be used in the differential diagnosis of KD when alternatives include aseptic meningitis and purulent meningitis.20
Lymphocyte activation
Activated lymphocytes are key drivers of vascular inflammation in KD. Histopathological studies of vasculitis lesions in patients with KD have demonstrated infiltration by CD8+ T cells, CD3+ T cells, granzyme B cells, TIA-1 cells, and cytotoxic T cells.21 Analysis of infiltrating immune cells revealed that, compared with samples from healthy controls, the numbers of effector memory CD4+ T cells, monocytes, and neutrophils were increased in samples from patients with KD, while the numbers of naive B cells, CD8+ T cells, and natural killer cells were decreased.21 Sun et al.22 found that, compared with controls, patients with KD had stronger Th1, Th17, and Th22 responses, along with a weaker Treg response. In the same study, IL-35 stimulation suppressed Th1, Th17, and Th22 responses; it enhanced the Treg response and CD8+ T cell-induced cytotoxicity while inhibiting CD8+ T cell-induced target cell death. These effects were mediated by the decreased expression of interferon (IFN)-γ and secretion of perforin/granzyme B, as well as the increased expression of PD-1, CTLA-4, and LAG-3.22 The findings suggested that IL-35 has a key immunosuppressive role in T-cell function; it may help to protect against KD-related inflammation.22 Furthermore, a subset of CD8+ T cells expressing CXC-chemokine receptor 5—termed follicular cytotoxic T cells—have recently been reported in association with coronary artery aneurysm formation in patients with KD.23
Neutrophil extracellular traps
Neutrophil extracellular traps constitute a secondary bactericidal mechanism in neutrophils. They are produced in large numbers at sites of inflammation, where they provide high local concentrations of antibacterial molecules that trap and kill various pathogens, rapidly control bacterial infections, and generally support immune and antibacterial activity.24 Neutrophil extracellular traps reportedly promote pro-inflammatory cytokine production, NF-κB activation, vascular endothelial growth factor and hypoxia-inducible factor-1α upregulation, and PI3K/Akt activation; their effects in KD include vascular injury, mediated by altered biological responses in peripheral blood monocytes.25
Macrophage activation
Macrophage activation plays an important role in the pathologies of acute vasculitis and (in later stages of KD) chronic vasculitis.21, 26, 27 In acute KD-related vasculitis, activated macrophages are mainly M2 type; in chronic KD-related vasculitis (complicated by CAD), activated macrophages are mainly M1 type.28, 29 Dectin-2 (secreted by cardiac resident macrophages) has been shown to promote aortic root inflammation and coronary arteritis in KD model mice by inducing caspase-1 activation and IL-1 β production in bone marrow prototype dendritic cells.30 KCa3.1 is a calcium-activated potassium channel that has been reported to reduce inflammation in KD by blocking macrophage-activated inflammatory signaling pathways (e.g., NF-κB and STAT3), thereby alleviating KD-related coronary artery endothelial cell injury.31
VASCULAR ENDOTHELIAL CELL DYSFUNCTION
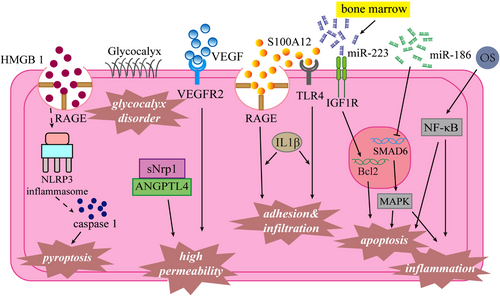
The strong immuno-inflammatory response in acute KD and persistent low-grade inflammation in chronic KD cause substantial and persistent damage to vascular endothelial cells (VECs).32 The production of pro-inflammatory mediators (e.g., IL-1, IL-6, tumor necrosis factor, and vascular endothelial growth factor [VEGF]) promotes inflammation and tissue destruction, which are important causes of coronary artery endothelial injury in patients with KD.33 The number of early fever days in patients with KD is positively correlated with the risk of VEC dysfunction.34 CAD is not a determinant of endothelial dysfunction in patients with KD or with a history of KD. In acute KD, endothelial dysfunction may be present despite the absence of obvious coronary abnormalities.35
Oxidative stress
Oxidative stress (OS) is reportedly involved in various cardiovascular disease-related pathophysiological events, such as endothelial dysfunction, vascular inflammation, and atherosclerosis. In active sites of inflammation, inflammatory cells, VECs, and vascular smooth muscle cells can release reactive oxygen species, enzymes, and chemical mediators, all of which contribute to OS. OS-related molecules (e.g., ox-phospholipids, oxidized low-density lipoprotein, and peroxynitrite) are also involved in regulating NF-kB activation, inflammation, thrombosis, angiogenesis, and endothelial function, and immune tolerance. Ox-phospholipids have been shown to participate in KD-related coronary artery injury by enhancing the expression of E-selectin, an endothelial adhesion molecule.36 The concentration of oxidized low-density lipoprotein may be a predictor of coronary artery injury in acute KD.37
Changes in VEC permeability
Changes in vascular endothelial permeability are important in the pathogenesis of KD-related vasculitis; such changes contribute to increased vascular leakage while promoting the infiltration of inflammatory cells and secretion of inflammatory factors. Huang et al.38 found that VECs from patients with KD exhibit high expression of neurocapillary protein-1and VEGF receptor 2, which interact with angiopoietin-like protein-4 and VEGF respectively to enhance endothelial permeability.
VEC death
Abnormal programmed death in VECs is an important research focus in terms of KD-related vascular endothelial injury. In an in vitro model of KD, miR-186 and bone marrow-derived miR-223 were able to induce VEC apoptosis. MiR-186 activated the MAPK pathway by inhibiting SMAD6 gene expression, while miR-223 regulated the expression of insulin-like growth factor-1 receptor.39, 40 Pyroptosis (also known as cell inflammatory necrosis) is a mechanism of programmed cell death. VEC pyroptosis (mediated by the HMGB1/RAGE/cathepsin B/NLRP3 inflammasome pathway) is reportedly involved in the onset of KD-related CAD.41
Endothelial adhesion and infiltration
Endothelial adhesion and infiltration are important aspects of vascular endothelial injury. S100A12 is a neutrophil-derived RAGE and TLR-4 receptor agonist; it can stimulate VECs to express inflammatory factors and adhesion molecules with the help of monocyte secretion of IL-1β.42 Additionally, miR-182-5p overexpression can significantly enhance neutrophil infiltration around human coronary artery endothelial cells. S100A12 and miR-182-5p are potential therapeutic targets for KD treatment.43, 44 Platelet endothelial cell adhesion molecule-1 (PECAM-1) is a key factor in platelet adhesion and aggregation; abnormal expression of the PECAM-1 gene can induce atherosclerosis, inflammation, and other diseases. Lu et al.45 reported that the Leu-Ser-Arg haplotype of PECAM-1 was associated with an increased risk of CAD in patients with chronic KD.
Endothelial glycocalyx disorder
The vascular endothelial glycocalyx is a type of omental glycoprotein that covers almost all of the vascular endothelium and has an important role in the vascular pathophysiological activity. There is some evidence that acute KD-related vasculitis can lead to changes in endothelial glycocalyx and endothelial dysfunction46, 47; long-term endothelial glycocalyx dysfunction can also cause atherosclerosis.46
Assessment of endothelial function
Because KD-related vasculitis is systemic, endothelial dysfunction is expected to be systemic in affected patients. Additionally, in patients with KD who experience coronary artery aneurysms, intima-media thickness of the carotid artery is increased and arteriosclerosis is present; these manifestations are associated with increased risks of adverse cardiovascular events (e.g., atherosclerosis, coronary artery embolism, myocardial ischemia, and sudden cardiac death) in the late stage of KD.48, 49
Many clinical methods are available to assess endothelial function, such as invasive detection, cuff compression, and Endo-PAT. Endo-PAT is the only FDA-certified device for the non-invasive detection of vascular endothelial function; it is suitable for evaluating endothelial function in patients with KD.
In summary, OS, altered vascular endothelial permeability, pathological death of endothelial cells, adhesion and infiltration of the vascular endothelium, and imbalance of the endothelial glycocalyx are all involved in KD-related vascular endothelial injury, which may eventually lead to endothelial dysfunction. Additionally, endothelial dysfunction may be chronic in patients with KD, thus increasing their risks of other age-related vascular diseases. Therefore, endothelial function assessment and long-term follow-up monitoring are needed in patients with KD. VEC dysfunction pathologies in KD are shown in Figure 2.
COMPARISONS BETWEEN KD AND MIS-C
MIS-C is a rare inflammatory disease in children; it is caused by SARS-CoV-2 infection and is characterized by multisystem inflammation, fever, gastrointestinal symptoms, hypotension, and cardiac complications. Because MIS-C and KD overlap in many respects, there has been considerable controversy concerning the relationship between the two diseases. First, in terms of clinical manifestations, patients with MIS-C may have prolonged fever, rash, strawberry tongue, systemic vasculitis, and coronary aneurysm formation; these are similar to symptoms in patients with KD. However, patients with MIS-C have a shorter duration of fever and are more likely to develop myalgia, gastrointestinal symptoms (e.g., abdominal pain and diarrhea), nervous system damage, and signs of shock.50, 51 Additionally, patients with MIS-C have greater risks of ventricular dysfunction and decreased cardiac output.52 Second, similar to patients with KD, patients with MIS-C have significantly increased levels of inflammatory markers (e.g., white blood cell count, erythrocyte sedimentation rate, C-reactive protein, and procalcitonin), along with coagulation abnormalities (e.g., prolonged prothrombin time and elevated d-dimer level), liver function abnormalities, and other laboratory findings indicative of disease. Compared to patients with KD, patients with MIS-C had lower lymphocyte and platelet counts, as well as higher levels of ferritin, BNP, and pro-BNP.52-54 Moreover, lymphocytopenia may be independently predictive of MIS-C and enable differentiation from KD.53 Third, intravenous immunoglobulin and glucocorticoids can be administered for anti-inflammation in both MIS-C and KD patients and biological agents also can be used in both refractory cases. However, patients with MIS-C are more likely to require intensive care unit admission and respiratory support, compared to patients with KD.55 Here, we highlight the similarities and differences between MIS-C and KD in terms of the immuno-inflammatory response and endothelial dysfunction.
Both MIS-C and KD are characterized by cytokine storms, but the inflammatory response in MIS-C is more extensive and exhibits greater severity.56 Although increased levels of canonical and noncanonical inflammasome-related mRNAs were detected in whole blood from patients with MIS-C and patients with KD, activation of the caspase-4/5-dependent noncanonical inflammasome was unique to patients with MIS-C.57 Moreover, the activation of atypical inflammatory bodies in neutrophils may contribute to greater inflammation intensity in patients with MIS-C.57 In one study of patients with MIS-C, high-dimensional cytokine analysis revealed increased expression of inflammation signals (IL-18 and IL-6), lymphocyte and myeloid chemotaxis activators (CCL3, CCL4, and CDCP1), and mucosal immune dysregulation markers (IL-17A, CCL20, and CCL28).58 Additionally, the upregulation of ICAM1 and FcR1 in neutrophils and non-classical monocytes suggested that myeloid function was activated in patients with MIS-C.58 Cytokine storms in MIS-C also involve macrophage activation syndrome.59 Patients with MIS-C reportedly exhibited prominent type II IFN-dependent and NF-κB-dependent signatures, matrisome activation, and increased levels of circulating spike protein; these findings were not associated with SARS-CoV-2 PCR status at admission.60 Compared to patients with KD, patients with MIS-C have higher INF-γ levels, lower initial CD4+ T cell numbers, and greater proportions of activated memory T cells.55 Additionally, an elevated CXCL9 level may help to distinguish patients with MIS-C from patients with KD.61 Another pathophysiological feature similar to KD is the presence of strong innate and acquired immune responses in patients with MIS-C.55 The transient expansion of the TRBV11-2 T-cell clonotypes in MIS-C is potentially related to inflammation and T-cell activation characteristics; patients with MIS-C exhibited a higher B-cell mutational load compared to children with coronavirus disease 2019 or healthy children.60 During autopsy analysis of a previously healthy patient who died of MIS-C, SARS-CoV-2 spike protein was found in intestinal cells, indicating that SARS-CoV-2 in the intestine may contribute to the MIS-C-related immune response.62 Activation of type 1 dendritic cells and imbalance of NK cells, coordinated by complex cytokine signaling, have been suggested as key pathophysiological features of MIS-C; these may promote antigenic cross-talk and macrophage activation syndrome, respectively.63 Most autoreactive peptides in patients with MIS-C are characterized by enrichment in central organs, which differs from such peptides in patients with KD.58
In summary, both MIS-C and KD are characterized by cytokine storms that have extensive effects on the activation of myeloid cells and lymphoid cells; these effects promote the onset and maintenance of immune-related inflammation during the course of the disease. However, differences in the secretion levels of some factors (CXCL9 and caspase-4/5-dependent inflammasome) may help to distinguish the two diseases. Additionally, both MIS-C and KD are characterized by immune dysfunction, such as the activation of T, B, dendritic, and NK cells, as well as increased numbers of CD4+ memory T cells and increased production of autoreactive antibodies. However, while the immune response in KD mostly causes mild or moderate vascular injury, the immune response in MIS-C more often causes multisystem organ injury to the heart, lung, gastrointestinal tract, and other critical tissues.
Endothelial dysfunction has been reported to occur in patients MIS-C, although the extent of such dysfunction is limited. Furthermore, the evidence of increased local tissue exudation and thrombosis in patients with MIS-C,7 indicates that such patients may have concurrent vascular endothelial dysfunction. SARS-CoV-2 invades the vascular endothelium directly or indirectly (i.e., through a viral component); it may also induce vascular endothelial inflammation through endothelial angiotensin-converting enzyme 2 receptors, eventually leading to vascular endothelial dysfunction.64 Moreover, children with MIS-C exhibit signs of endothelial glycocalyx injury with elevated levels of syndecan-1 and heparan sulfate, which may participate in MIS-C-related endothelial dysfunction.65 Additionally, OS may be involved in the onset of vascular endothelial inflammation and endothelial dysfunction in patients with MIS-C.66 The latter two mechanisms are similar to KD.
CONCLUSION
KD has been studied for more than 50 years. The disease is currently presumed to result from one or more of the following: excessive activation of the immuno-inflammatory response, endothelial cell dysfunction, respiratory infection, increased intestinal permeability, and genetic susceptibility. However, the specific etiology and pathogenesis have not been elucidated. Furthermore, there remain controversies concerning whether CAD is self-limiting and whether endothelial dysfunction leads to cardiovascular complications in patients with KD. For all patients with KD, long-term and standardized follow-up approaches are needed to assess vascular endothelial function and evaluate the complications of coronary artery dilatation. There is also a need to achieve accurate prognostic prediction and individualized interventions for patients with concurrent cardiovascular disease during the later stages of KD. Finally, additional studies are needed to characterize the pathophysiological mechanisms of MIS-C and KD.
CONFLICT OF INTEREST
None.