FAM89A and IFI44L for distinguishing between viral and bacterial infections in children with febrile illness
Funding source
This study was supported by the Special Fund of the National Science and Technology Major Project of China (No. 2018ZX10305409), the Beijing Hospital Authority “Dengfeng” Talent Training Plan (DFL 20181201), and the Sanming Project of Medicine in Shenzhen (SZSM201512030).
ABSTRACT
Importance
The current lack of reliable rapid tests for distinguishing between bacterial and viral infections has contributed to antibiotic misuse.
Objective
This study aimed to develop a novel biomarker assay that integrates FAM89A and IFI44L measurements to assist in differentiating between bacterial and viral infections.
Methods
This prospective study recruited children with febrile illness from two hospitals between July 1, 2018, and June 30, 2019. A panel of three experienced pediatricians performed reference standard diagnoses of all patients (i.e., bacterial or viral infection) using available clinical and laboratory data, including a 28-day follow-up assessment. Assay operators were blinded to the reference standard diagnoses. The expression levels of FAM89A and IFI44L were determined by quantitative real-time polymerase chain reaction assessment.
Results
Of 133 potentially eligible patients with suspected bacterial or viral infection, 35 were excluded after the application of exclusion criteria. The resulting cohort included 98 patients: 59 with viral diagnoses and 39 with bacterial diagnoses. The areas under the curve (AUCs) of diagnoses using FAM89A and IFI44L were 0.694 [95% confidence interval (CI): 0.583–0.804] and 0.751 (95% CI: 0.651–0.851), respectively. The disease risk score (DRS) [log2(FAM89A expression) − log2(IFI44L expression)] signature achieved an improved area under the receiver operating characteristic curve (AUC, 0.825; 95% CI: 0.735–0.915), compared with the AUC generated from individual host RNA. A combination of the DRS and the C-reactive protein (CRP) level achieved an AUC of 0.896 (95% CI: 0.825–0.966). Optimal cutoffs for the DRS and CRP level were –3.18 and 19.80 mg/L, respectively.
Interpretation
The DRS was significantly more accurate than the CRP level in distinguishing between bacterial and viral infections; the combination of these two parameters exhibited greater sensitivity and specificity. This study provides information that could be useful for the clinical application of FAM89A and IFI44L in terms of distinguishing between viral and bacterial infections.
INTRODUCTION
Currently, there are no effective rapid tests for distinguishing between bacterial and viral infections. Thus, most infections are misdiagnosed, leading to increased misuse of antibiotics. Biomarkers such as complete white blood cell (WBC) counts, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin are used in routine practice to distinguish between bacterial and viral infections. However, because these markers are not sufficiently sensitive and specific, they cannot effectively diagnose bacterial infections.1, 2 Other methods used to diagnose bacterial infections include cultures, rapid antigen detection tests, and polymerase chain reaction (PCR) assays, as well as microbial pathogen analyses. Microbial cultures, which are considered the “gold standard” method for confirming pathogenic microorganisms, are laborious and time-consuming; however, treatment timing is crucial for the successful eradication of infectious diseases. Moreover, cultures may yield false-negative results if a patient has already received antibiotics,3 while rapid antigen detection tests may yield false results (particularly if the organism is not present in blood or other easily accessible sites). Thus, researchers have developed more sensitive molecular diagnostic methods to improve the detection of viral infections.4
Multiple studies of host responses to infections have been performed to develop better diagnostic tools. For instance, Herberg et al5 used a 38-transcript signature to create a two-transcript RNA (FAM89A and IFI44L) transferred disease risk score (DRS) signature that exhibited high sensitivity and specificity for differentiating between well-defined viral and bacterial infections in children. This signature was further validated in infants aged ≤ 60 days,6 although it has not been confirmed in other studies. A major challenge associated with using transcriptomic signatures for diagnosis is the translation of multi-transcript signatures into clinical tests that can be applied in hospital laboratories or at the bedside. Therefore, the DRS signature, which distinguishes between viral and bacterial infections by using only two transcripts, can potentially be translated into a clinically applicable test using current technologies (e.g., PCR). Quantitative real-time polymerase chain reaction (qRT-PCR) is the preferred method for assaying small sets of known genes.7 This method is also considered the “gold standard” for RNA quantification; thus, it is ideal for validation studies.
This study aimed to validate the two-transcript signature in a larger cohort of children with febrile illness caused by various etiologic agents in China. It included distinct clinical scenarios involving qRT-PCR assessment, which can be implemented in low- and middle-income country populations. Findings from this study are expected to clarify the ability of the two-transcript signature to distinguish between bacterial and viral infections in children with febrile illness.
METHODS
Ethical approval
This study was reviewed and approved by the Ethics Committee of Beijing Children’s Hospital, Capital Medical University (2018-k-100) and the Medical Ethical Committee of Shenzhen Children’s Hospital (201900802). Written informed consent was obtained from a parent or legal guardian for each subject before inclusion into the study.
Study population and sample collection
This prospective study recruited febrile patients admitted to the Infectious Disease Departments of Beijing Children’s Hospital and Shenzhen Children’s Hospital from July 1, 2018 to June 30, 2019. The inclusion criteria were fever (axillary temperature ≥38°C) and perceived illness of sufficient severity to warrant a blood routine test in children <14 years of age. Patients with comorbidities that might affect gene expression (e.g., bone marrow transplant, immunodeficiency, or immunosuppressive treatment) were excluded, in accordance with the approach described by Herberg et al.5
Case definition
Currently, no single reference standard test exists for identifying the cause of infection in patients with febrile illness.8 Therefore, we followed England’s National Health Service’s standard for assessing diagnostic tests, which involved compiling an expert panel reference standard. The procedures were performed as previously reported.9, 10 Briefly, a panel of three experienced pediatricians performed reference standard diagnoses of all patients (i.e., bacterial or viral infection) using available clinical and laboratory data, including a 28-day follow-up assessment. Moreover, microbiologically confirmed viral or bacterial diagnosis was defined as the detection of at least one causative organism combined with a unanimous panel diagnosis.
RNA preparation
Blood samples from children were collected into EDTA-containing tubes on the first day of admission, irrespective of prior antibiotic use. Thereafter, peripheral white blood cells (PBWCs) were isolated from blood using the RNAprep Pure Hi-Blood Kit (Tiangen, Beijing, China), in accordance with the manufacturer’s instructions. The PBWCs were stored at −20°C for 24 h, then stored at −80°C until RNA extraction. Subsequently, PBWCs were added into 1.5-mL microcentrifuge tubes (RNase, Axygen) for RNA extraction, in accordance with the manufacturer’s instructions. RNA quality was determined using an electropherogram (showing 28S, 18S, and 5S bands); RNA Integrity Number (RIN; generally, a RIN score >7 indicates good quality RNA) was determined using an Agilent 2100 Bioanalyzer (Agilent). Finally, the total RNA concentration was determined using a NanoDrop ND-100 spectrometry instrument (NanoDrop Inc.).
The NCBI BLAST tool, Clustal software, and Primer Express software were used to design primer and probe sequences for FAM89A (NM_198552), IFI44L (XM_006710304), and ACTB (NM_001101). Primers were synthesized by Sangon Bioengineering Technology, Ltd (Shanghai, China). Primer and probe sequences are shown in Table 1.
| Name | Sequence | Retouch |
|---|---|---|
| FAM89A-F | CCTTGCTCTGCCAACTGTACAG | |
| FAM89A-R | CTGGCATGCCCCCTTGT | |
| FAM89A-P | CTCTACGAGTCGATTCAG | 5’ FAM 3’ MGB |
| IFI44L-F | CTTTTGTTCGTTTTGCCTTCTGT | |
| IFI44L-R | CCCACCGCTTCTCAGGTTT | |
| IFI44L-P | CAGTCATATCTCAAGTTCAAA | 5’ VIC 3’ MGB |
| actin-F | CGAGAAGATGACCCAGAT | |
| actin-R | GATAGCACAGCCTGGATA | |
| actin-P | CTTCAACACCCCAGCCAT | 5’ CY5 3’ MGB |
qRT-PCR
Absolute quantification of cDNA species (synthesis protocol described below) was performed using a combination of extremely accurate double-stranded DNA quantification and a plasmid reference curve, as previously reported.11 Additionally, the three gene fragments were ligated into the pBlunt vector, to ensure consistency of the positive standard then synthesized by Sangon Biotech Company (Shanghai, China). Plasmid size was 3461 bp. To generate the standard curve, 10-fold serial dilutions of purified plasmid standard DNA were prepared.
First-strand cDNA was synthesized using the SuperScript™ III First-Strand Synthesis System kit (Thermo Fisher, USA), in accordance with the manufacturer’s instructions. Briefly, genomic DNA was removed using DNase I at 42°C. Thereafter, 5 μL of total RNA from blood samples were added into the reverse transcription mix and incubated at 42°C for 15 min. The enzyme was inactivated at 95°C for 3 min; samples were then placed on ice and directly used for qRT-PCR. The synthesized plasmid cDNA was used as a template for qRT-PCR. The primers shown in Table 1 were used for qRT-PCR. Moreover, qRT-PCR was performed using the Roche LightCycler 480II (Roche Diagnostics, Basel, Switzerland). Βeta-actin served as the control gene and relative expression was calculated using the 2−∆∆Ct method.
Data management and statistical analysis
Patient data were stored in a computer database using EpiData software; Statistical data processing and figure generation were conducted using SPSS Statistics, version 25.0 (SPSS Inc. Chicago, IL, USA)and GraphPad Prism, version 7.0 (GraphPad Software Inc.).
The DRS for each sample was calculated as described by Kaforou et al.6 Individual DRS values were first obtained using normalized values (∆Ct). Thereafter, the scale of ∆Ct values was increased 10-fold to avoid negative values when logarithmic transformation was performed. The final DRS formula was log2(FAM89A expression) – log2(IFI44L expression).
Normally distributed data were presented as (mean ± standard deviation), whereas data with skewed distributions were presented as median (interquartile range). Statistical analysis was performed using the t-test, χ2 analysis, and the nonparametric rank-sum test. Receiver operator characteristic (ROC) curves were generated using GraphPad Prism, version 7.0; areas under the ROC curve (AUC) were compared among experiments using the Z test, while joint predictors were analyzed using a logistic regression model. P <0.05 was considered statistically significant.
RESULTS
Characteristics of the study population
In total, 133 children with febrile illness were screened for inclusion between 1 July 2018 and 30 June 2019 from Shenzhen Children’s Hospital and Beijing Children’s Hospital; the final follow-up visits were performed in July 2019. Of these 133 children, 35 were excluded because of failure to meet the inclusion criteria, absence of a blood sample, or withdrawal of consent. Consequently, the final analysis included 98 patients (Figure 1). Baseline characteristics were compared between children with bacterial infections and children with viral infections; these data are summarized in Table 2. The children’s ages ranged from 0.8 to 192 months, the age was younger in bacterial group as compared with that in viral group (P <0.001). Sex ratio did not significantly differ between the two groups (P = 0.386). Moreover, patients presented with a wide range of clinical syndromes (e.g., respiratory tract infection, urinary tract infection and gastrointestinal infection), as well as various intervals from the onset of symptoms. Various pathogens were also present in each group.
| Characteristics | Viral infection (n = 59) | Bacterial infection (n = 39) | Z | P |
|---|---|---|---|---|
| Age (month) | 48 (18–84) | 5 (2–23) | 4.935 | <0.001 |
| Male | 34 (57.6) | 19 (48.7) | 0.750 | 0.386 |
| Yellow race | 59 (100.0) | 39 (100.0) | NA | NA |
| Time from symptom onset to blood sampling (day) | 4 (3–6) | 4 (2–10) | 2.560 | 0.800 |
| CRP (mg/L) | 4.0 (4.0–12.7) | 41.0 (8.9–94.9) | 5.244 | <0.001 |
| Pathogen (cases) | RV(3), RSV(1), HSV(1), IFA(12), IFB(16), EV(9), EBV(10), ADV(1) | SP(3), SA(2), GAS(1), LM(1), PA(1), E.coli(6) | NA | NA |
| The use of antibiotics | 29 (49.2) | 39 (100.0) | NA | NA |
| Infection sites | ||||
| CNS infection | 20 | 25 | NA | NA |
| URTI | 17 | 1 | NA | NA |
| LRTI | 13 | 6 | NA | NA |
| GI | 3 | 3 | NA | NA |
| UTI | 0 | 4 | NA | NA |
| BI | 0 | 15 | NA | NA |
| Others | 11 | 0 | NA | NA |
- Data are shown as n (%) or median (interquartile range). Some patients were co-infected in more than one sites. CRP, C-reactive protein; RV, rotavirus; HSV, herpes simplex virus; IFA, influenza virus type A; IFB, influenza virus type B; EV, enterovirus; EBV, Epstein-Barr virus; ADV, adenovirus; SP, Streptococcus pneumonia; SA, Staphylococcus aureus; GAS, group A streptococcus; LM, Listeria monocytogenes; E. coli, Escherichia coli; PA, Pseudomonas aeruginosa; CNS, central nervous system; GI, gastrointestinal infection gastroenteritis; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection, UTI, urinary tract infection; BI, blood infection; NA, not applicable.
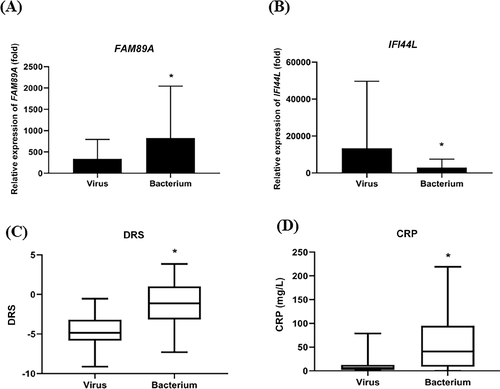
Expression levels of FAM89A and IFI44L significantly differed between patients with viral infections and patients with bacterial infections
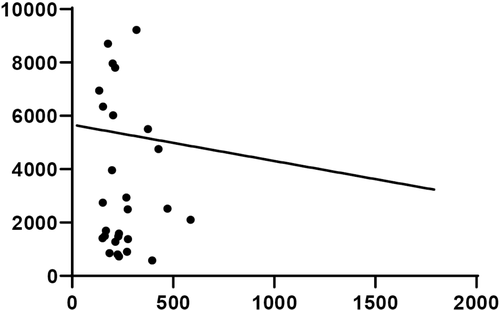
The FAM89A expression level was significantly higher in the bacterial infection group than in the viral infection group (Figure 2A). In contrast, the IFI44L expression level was significantly higher in the viral infection group than in the bacterial infection group (Figure 2B). Additionally, the DRS (based on a combination of FAM89A and IFI44L) was significantly higher in the bacterial infection group than in the viral infection group (Figure 2C). Furthermore, CRP level was significantly higher in children with bacterial infections than in children with viral infections (Figure 2D), consistent with previous findings. Figure 3 shows that there was no correlation between IFI44L and FAM89A expression levels in patients with viral infections and patients with bacterial infections (r = 0.0785, P = 0.467).
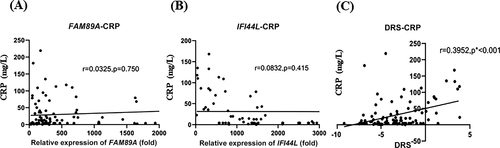
Correlations between CRP level and the expression levels of two host transcripts
Spearman’s correlation test was performed to evaluate the correlations between the CRP level and the expression levels of the two host transcripts. Figure 4 shows a significant positive correlation between the DRS and the CRP level (r = 0.3952) (Figure 4C). However, the CRP level was not significantly correlated with the FAM89A expression level (r = 0.0325; P = 0.750) or the IFI44L expression level (r = 0.0832; P = 0.415), as shown in Figure 4A and B.
Diagnostic potentials of the two host RNA biomarkers
ROC curve analysis was performed to evaluate the diagnostic potentials of the two host RNAs in distinguishing between bacterial and viral infections (Figure 5). The AUCs of FAM89A and IFI44L were 0.694 (95% CI: 0.583–0.804) and 0.751 (95% CI: 0.651–0.851), respectively. The DRS signature displayed an improved ROC curve (AUC, 0.825; 95% CI: 0.735–0.915), compared with the curves generated from individual host RNAs. The combination of the DRS and the CRP level exhibited an AUC value of 0.896 (95% CI: 0.825–0.966), whereas the combination of the IFI44L expression level and the CRP level exhibited an AUC value of 0.809 (95% CI: 0.715–0.904). Optimal cutoffs for the DRS and CRP level were –3.18 and 19.80 mg/L, respectively.
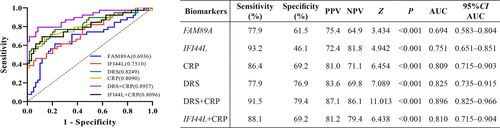
DISCUSSION
Currently, there are no reliable rapid test s for distinguishing between bacterial and viral infections. This has led to infection misdiagnosis and subsequent antibiotic misuse. Analysis of baseline data in this study revealed that the prevalence of antibiotic misuse in the viral infection group was 49.2% (Table 2). Therefore, it is important to develop interventions to increase early differentiation between viral and bacterial infections in children with febrile illness, thereby reducing unnecessary treatment.
A novel biomarker that integrates measurements of bloodborne host genes (FAM89A and IFI44L) was developed to differentiate between bacterial and viral infections. The results demonstrated that the two transcripts (FAM89A and IFI44L) can distinguish between viral and bacterial infections in children with febrile illness. These findings are consistent with previous reports.5, 6 Therefore, FAM89A and IFI44L can be used to detect infections throughout the entire infectious cycle. Moreover, the DRS was more accurate than the CRP level in terms of distinguishing between viral and bacterial infections; the DRS was significantly correlated with the CRP level. Therefore, the DRS provides a more effective alternative to CRP level.
The two transcripts, IFI44L and FAM89A, exhibit reciprocal expression level sinviral and bacterial infections. IFI44L is reportedly upregulated during antiviral responses mediated by type I interferons,12 whereas FAM89A is reportedly elevated in children with septic shock.13 In ROC curve analysis, the ordinate indicates sensitivity, while the abscissa represents 1− specificity.14, 15 Here, ROC curve and logistic regression analyses were used to evaluate the diagnostic accuracy of the DRS in children with febrile symptoms. The DRS was significantly more accurate than the CRP level; furthermore, a combination of the DRS and the CRP level exhibited greater sensitivity and specificity, consistent with results from previous studies.5, 6 This is presumably because the CRP level is elevated in many inflammatory conditions (e.g., autoimmune diseases, trauma, and neurological disorders).16-19 In this study, the individual expression levels of IFI44L and FAM89A were not correlated with CRP level. However, a combination of the two (i.e., the DRS) was associated with CRP level. Notably, CRP assessment can be used to distinguish between viral and bacterial responses; the DRS is associated with CRP level and exhibits significantly greater accuracy than the CRP level in terms of ROC curve analysis. Therefore, the DRS comprises a more reliable marker for distinguishing between bacterial and viral infections.
Our results provide insights regarding the clinical applications of FAM89A and IFI44L in terms of distinguishing between viral and bacterial infections on the first day of hospitalization. However, additional studies are needed to validate these findings and to inform decisions regarding antimicrobial treatment. This study used samples from hospitals in both northern and southern China, as well as samples from patients with extended fever durations. Notably, the DRS exhibited satisfactory diagnostic accuracy, implying that it can be used in both acute and non-acute infectious phases. Despite the small sample size, the qRT-PCR analysis method was highly sensitive and specific. These findings indicate that the DRS has high clinical value in the diagnosis of viral and bacterial infections.
This study had some limitations. The main limitation was that the small sample size may have decreased the statistical power of the results, and more data should be collected to verify the role of these two host RNA in distinguishing mixed infections, inflammation disease or severity of illness. For a previous study of patients with moderate to severe diarrhea showed that the two-transcript host RNA signature was positively correlated with greater disease severity, greater inflammation, and increased mortality in patients with bacterial or viral infections.20 Other host RNA signatures have been shown to distinguish between bacterial infections and other conditions (e.g., childhood inflammatory diseases, systemic lupus erythematosus, juvenile idiopathic arthritis, and Henoch-Schönlein purpura). These signatures could also distinguish between bacterial and viral infections in adult patients.21-24 We found that the DRS was strongly associated with the CRP level; thus, it may also provide useful insights regarding the development of inflammatory diseases and may be useful in the prediction of clinical outcomes among patients with tumors or autoimmune diseases, further studies are needed. Considering the lack of available gold-standard tests and the small sample size, the approach used in this study (consistent with a previously published method10) involved diagnostic test assessment in combination with an expert panel reference standard. What’s more, there is a need for further exploration of the mechanisms underlying the roles of IFI44L and FAM89A in infectious diseases. Thus, more studies with larger sample sizes are needed to further evaluate the two-transcript host RNA signature.
In conclusion, our results demonstrated that the DRS (combined assessment of FAM89A and IFI44L expression levels) is more accurate than the CRP level in terms of distinguishing between bacterial and viral infections. Moreover, a combination of the DRS and the CRP level had greater sensitivity and specificity. Therefore, the DRS can potentially be applied in clinical practice to distinguish between viral and bacterial infections. Nonetheless, additional studies are required to validate these findings and to inform clinical decisions regarding antimicrobial treatment.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Ke Cao, Gang Xu and Qiru Su from the Shenzhen Children’s Hospital for facilitating sample collection and statistical analyses. We would also like to thank Dr. Yuejie Zheng and Yonghong Yang who were in charge of the Sanming Project of Medicine in Shenzhen (SZSM201512030).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




