Childhood adiposity, adult adiposity, and bone health
ABSTRACT
Importance
Childhood and adolescence are critical periods for lifelong bone mineral accrual, but few studies have determined the impact of childhood adiposity on adult bone density.
Objective
To determine the long-term impact of childhood adiposity on adult areal bone mineral density (aBMD) and the effect of adult adiposity on this relationship.
Methods
We conducted a longitudinal study of 1156 adults (56.3% men), for whom skinfold thickness (SFT) had been measured during childhood (6–18 years) and fat mass percentage (FMP) and aBMD were measured during adulthood (29–43 years). Adult aBMD in the lumbar spine (LS), femoral neck (FN), arms, and legs was measured using dual-energy X-ray absorptiometry. The direct effect of childhood SFT and its indirect effect through adult FMP on adult aBMD were estimated using general linear regression and a causal steps approach.
Results
Significant positive associations between childhood SFT and adult aBMD were found in the LS in men (β = 0.089, P = 0.044) and in all the skeletal sites in women. With respect to the adult fat–bone relationship, high adult FMP was associated with low aBMD in most of the sites in men, but with high FN aBMD in women (β = 0.144, P = 0.002). Moreover, suppressive effects of adult FMP on the associations between childhood SFT and adult aBMD in the LS (−34.8%) and legs (−67.1%) of men, and a positive effect on the FN aBMD in women (17.0%) were identified.
Interpretation
Childhood adiposity appears to have a positive long-term effect on adult aBMD, which may be reduced by adiposity in adult men but reinforced by adiposity in adult women.
1 INTRODUCTION
Osteoporosis affects millions of people worldwide and has enormous public health consequences because of the morbidity and mortality associated with the resulting fractures.1, 2 Bone density is an established indicator of subsequent osteoporotic fracture risk3, 4 that tracks strongly from childhood to adulthood.5, 6 Therefore, the elimination of risk factors that affect childhood bone development should contribute significantly to the prevention of osteoporosis in later life.
Body mass is closely associated with bone health, a relationship that is thought to be mediated via mechanical loading during growth.7 However, this relationship was identified principally in cross-sectional studies, using indices such as body mass index (BMI).8 However, because lean mass and fat mass have distinct effects on bone metabolism,9, 10 it is essential to study the relationship between fat per se and bone using an accurate measure of adiposity and adjustment for lean mass. Furthermore, because fat mass tracks from childhood to adulthood, childhood adiposity might influence adult bone health through the degree of adiposity in adulthood. However, to date, any direct or indirect effects of childhood adiposity on adult bone density have not been well characterized.
In the present study, we aimed to investigate the long-term relationship between childhood adiposity and adult BMD and quantify the role of adult adiposity on this association using data from the Beijing Blood Pressure Study (BBS).
2 METHODS
2.1 Ethical approval
This study was approved by the Institutional Review Board and Ethics Committee of Capital Institute of Pediatrics, Beijing, China (2010068). Written informed consents were obtained from all participants or from a parent/guardian in those under 18 years of age at both childhood and adulthood surveys.
2.2 Study population
The BBS was a population-based prospective cohort study of hypertension and its risk factors from childhood that commenced in 1987. This has been described in detail previously.11 Briefly, 2442 children and adolescents aged 6–18 years were recruited between April 1987 and October 1988 using a random cluster sampling method from 12 schools located in urban areas of Beijing. Then, between March 2010 and June 2011, all the participants were invited by mail and telephone to undergo a health examination, and 1261 were successfully followed up. We excluded 105 participants with missing data for bone density in adulthood or body fatness in childhood; therefore, data from 1156 participants were analyzed in the present study (Figure S1). The mean duration of the follow-up period was 22.9 ± 0.6 years. No significant difference in the baseline characteristics of the included and excluded participants was found, except that the excluded participants were younger at baseline than those that were included (Table S1).
2.3 General examination
At each study visit, body mass and height were measured twice, with the participants wearing light clothing and no shoes, using an automatic measuring instrument (BSM330, Biospace Co., Ltd., Seoul, Korea), and the mean value was used to calculate BMI as body mass in kilograms divided by the square of height in meters. Childhood height and BMI values were converted to z -scores according to the WH O Child Growth Standards.12 Participants were classified as underweight (z-score < −2), normal (−2 ≤ z-score ≤ 1), overweight (1 < z-score ≤ 2), and obese (z-score > 2), based on the BMI z-score in childhood, and using the established BMI cut-off values (18.5, 25, and 30 kg/m2) in adulthood. Subscapular skinfold thickness (SFT) was measured twice below the inferior angle of the left scapula using Japanese Eiken-type skinfold caliper (Shanghai Medical Instrument Development Co., Ltd., Shanghai, China) in accordance with standard protocols, during their childhood visit.
A standardized questionnaire was used to collect data regarding lifestyle factors in adulthood (smoking, alcohol consumption, and physical activity). Participants were defined as smokers or drinkers if they smoked or consumed alcohol, respectively, on >3 days per week during the preceding 12 months. Physical activity was assessed using questions regarding the intensity, frequency, and duration of physical activity per week, and further quantified as metabolic equivalents (MET) according to the 2011 Compendium.13
2.4 Body composition and density measurements
Adult body composition and areal bone mineral density (aBMD) we r e assessed using dual-energy X-ray absorptiometry (DXA) (Hologic Explorer, Hologic Inc., Bedford, MA, USA). Scans of the whole body, lumbar spine (LS) from L1 to L4, and femoral neck (FN) were conducted by a trained technician in accordance with a standardized protocol. All DXA scans were automatically analyzed using Apex version 4.0 software (https://www.apexwin.com/), which provided values for whole-body fat mass, lean mass, and site-specific bone mineral content (BMC) and bone area (BA). Whole-body lean mass (excluding bone mineral content) was converted to lean mass index (LMI) by dividing it by the square of height (m2). Fat mass percentage (FMP) was calculated as total body fat mass (kg)/total body mass (kg) × 100%. Leg and arm aBMD were each calculated as (BMCleft limb + BMCright limb)/(BAleft limb + BAright limb).
2.5 Statistical analyses
Data are presented as mean ± standard deviation (SD), median (interquartile range), or numbers (percentage). Characteristics of male and female participants were compared using χ2 tests for categorical variables and t-tests for continuous variables except for physical activity, which was tested by the Mann-Whitney U test due to skewness. We used multivariable linear regression to evaluate the relationships between childhood adiposity, adult adiposity, and adult bone mass. Prior to these regression analyses, childhood BMI and SFT and adulthood BMI and FMP were adjusted for the corresponding age, and adult BMC and aBMD were adjusted for adult age and height by regression residual analyses for each sex and then standardized by Z-transformation (mean = 0, SD = 1). Adult age, height, LMI, physical activity, and smoking and drinking status were included as covariates in the regression models. The variance inflation factor in all the models was less than two, indicating that multicollinearity was highly unlikely.
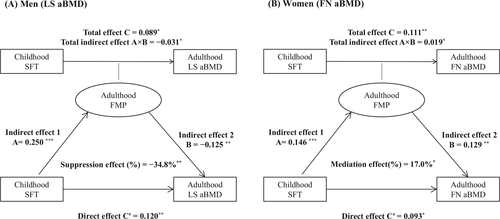
To quantify the indirect effect of adult FMP on the relationship between childhood SFT and adult BMD, the causal steps approach was adopted, as previously described.14 Multivariable-adjusted linear regression models were used to calculate standardized regression coefficients, taking into account adult age, height, LMI, physical activity, and smoking and drinking status. In the pathway diagram for the mediation models (Figure S2), the predictor variable (X) was childhood SFT; the mediator or suppressor variable (M) was adult FMP; and the outcome variables (Y) were adult LS aBMD, FN aBMD, arm aBMD, or leg aBMD in the models. For each model, four steps were used to identify mediation or suppression effects: (1) showing that childhood SFT determines adult aBMD (Model Y = C × X) (C = total effect); (2) showing that childhood SFT affects adult FMP (Model M = A × X) (A = indirect effect 1); (3) showing that childhood SFT determines adult aBMD, with additional adjustment for adult FMP (Model Y = B × M + C′ × X) (B = indirect effect 2, C′ = direct effect); and (4) determining the mediation or suppression effect and calculating its magnitude. If C > C′, a mediation effect was implied; otherwise, a suppression effect was implied. The proportion of the total indirect effect (A × B) in the total effect (C) was used to estimate the mediation or suppression effect (%) = (A × B/C) × 100%, and its significance was tested using the Sobel test. All analyses were performed using R software (V.3.4.0; www.r-project.org), and a two-tailed P of < 0.05 was considered to represent statistical significance.
3 RESULTS
The study cohort consisted of 651 (56.3%) male and 505 (43.7%) female participants with a mean age of 11.7 ± 3.7 years at baseline and 34.6 ± 3.7 years at follow-up (Table 1). The prevalence of overweight/obesity, according to BMI, was 10.5% in childhood and 44.8% in adulthood. The BMIs of most of the participants tracked from childhood to adulthood. Only six (7.5%) men who had been overweight or obese by BMI during childhood had BMIs in the normal range in adulthood. Furthermore, 313 (54.8%) boys and 98 (21.1%) girls who were underweight or normal-weight in childhood had become overweight or obese adults.
| Variables |
Total (n = 1156) |
Male (n = 651) |
Female (n = 505) |
|---|---|---|---|
| Childhood | |||
| Age (year) | 11.7 ± 3.7 | 11.9 ± 3.7 | 11.4 ± 3.6 |
| Height z-score | −0.20 ± 0.95 | −0.18 ± 1.00 | −0.23 ± 0.87 |
| BMI z-score | −0.53 ± 1.18 | −0.44 ± 1.23 | −0.66 ± 1.11 |
| BMI category | |||
| Underweight | 99 (8.5) | 55 (8.5) | 44 (8.7) |
| Normal | 936 (81.0) | 516 (79.3) | 420 (83.2) |
| Overweight | 84 (7.3) | 53 (8.1) | 31 (6.1) |
| Obesity | 37 (3.2) | 27 (4.1) | 10 (2.0) |
| SFT (mm) | 9.3 ± 5.0 | 8.6 ± 4.2 | 10.2 ± 5.9 |
| Adulthood | |||
| Age (year) | 34.6 ± 3.7 | 34.8 ± 3.7 | 34.3 ± 3.6 |
| Height (cm) | 167.9 ± 8.5 | 173.1 ± 6.3 | 161.2 ± 5.6 |
| Weight (kg) | 70.1 ± 15.0 | 78.3 ± 13.1 | 59.7 ± 10.1 |
| BMI (kg/m2) | 24.7 ± 4.2 | 26.1 ± 3.9 | 23.0 ± 3.9 |
| BMI category | |||
| Underweight | 49 (4.2) | 11 (1.7) | 38 (7.5) |
| Normal | 589 (51.0) | 253 (38.9) | 336 (66.6) |
| Overweight | 394 (34.1) | 294 (45.1) | 100 (19.8) |
| Obesity | 124 (10.7) | 93 (14.3) | 31 (6.1) |
| FMP (%) | 31.8 ± 5.9 | 28.8 ± 4.8 | 35.7 ± 4.8 |
| LMI (kg/m2) | 15.7 ± 2.6 | 17.3 ± 2.0 | 13.6 ± 1.7 |
| LS aBMD (g/cm2) | 0.991 ± 0.122 | 0.977 ± 0.125 | 1.008 ± 0.117 |
| FN aBMD (g/cm2) | 0.798 ± 0.117 | 0.825 ± 0.121 | 0.763 ± 0.102 |
| Arms aBMD (g/cm2) | 0.726 ± 0.075 | 0.777 ± 0.054 | 0.660 ± 0.038 |
| Legs aBMD (g/cm2) | 1.097 ± 0.123 | 1.162 ± 0.112 | 1.014 ± 0.078 |
| Smokers† | 373 (37.3) | 338 (58.5) | 35 (8.3) |
| Drinkers‡ | 488 (50.6) | 354 (62.5) | 134 (33.6) |
| Physical activity (MET × hour/week)§ | 12.0 (6.0–24.0) | 15.5 (8.0–28.0) | 8.0 (4.0–16.0) |
- Data are presented as mean ± standard deviation, n (%), or median (interquartile range). Characteristics of participants between sexes were compared using χ2 tests for categorical variables and t-tests for continuous variables except for physical activity, which was tested by the Mann-Whitney U test due to skewness. †Data on smoking status were only available for 578 men and 422 women. ‡Data on drinking status were only available for 566 men and 399 women. §Data on physical activity were only available for 534 men and 390 women. BMI, body mass index; SFT, skinfold thickness; FMP, fat mass percentage; LMI, lean mass index; LS, lumbar spine; FN, femoral neck; aBMD, areal bone mineral density; MET, metabolic equivalents.
Tables 2 and S2 present the results of linear regression analyses for independent associations of childhood SFT and adult FMP with adult aBMD in the LS, FN, arms, and legs, adjusted for adult covariates (age, height, LMI, physical activity, and smoking and drinking status). When adult FMP was not adjusted for (Model 1), significant positive associations between childhood SFT and adult aBMD were found in the LS in men (β = 0.089, P = 0.044), and in all the skeletal sites in women. When adult FMP was also included in the model (Model 2), all these positive associations remained statistically significant. Moreover, the coefficient for leg aBMD in men changed from non-significant in Model 1 (β = 0.050, P = 0.191) to positive in Model 2 (β = 0.083, P = 0.033) (Table S2). For the adult fat–bone relationship, adult FMP was negatively associated with aBMD at most sites in men, except in the FN (β = −0.057, P = 0.136). In contrast, there was a positive relationship between adult FMP and LS aBMD in women (β = 0.144, P = 0.002).
| Variables | LS aBMD | FN aBMD | ||
|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |
| Male | ||||
| Childhood SFT | ||||
| Model 1 (No adjustments) | 0.089 (0.002, 0.175) | 0.044 | 0.020 (−0.060, 0.100) | 0.625 |
| Model 2 (Adjustment for adulthood FMP) | 0.120 (0.032, 0.208) | 0.008 | 0.036 (−0.046, 0.118) | 0.390 |
| Adulthood FMP | −0.099 (−0.180, −0.018) | 0.017 | −0.057 (−0.131, 0.018) | 0.136 |
| Female | ||||
| Childhood SFT | ||||
| Model 1 (No adjustments) | 0.174 (0.087, 0.261) | <0.001 | 0.111 (0.028, 0.194) | 0.009 |
| Model 2 (Adjustment for adulthood FMP) | 0.174 (0.086, 0.262) | <0.001 | 0.093 (0.010, 0.176) | 0.029 |
| Adulthood FMP | 0.027 (−0.068, 0.122) | 0.579 | 0.144 (0.055, 0.234) | 0.002 |
- aBMD were adjusted for adult age and height, childhood SFT and adulthood FMP were adjusted for corresponding age by regression residual analyses in each sex and then standardized with Z-transformation (mean = 0, SD = 1). Covariates in all models included adult age, height, lean mass index, physical activity and status of smoking and drinking. SFT, skinfold thickness; FMP, fat mass percentage; LS, lumbar spine; FN, femoral neck; aBMD, areal bone mineral density; CI, confidence interval.
The standardized regression coefficients (A, B, C, and C′) and effects of adult FMP on the childhood SFT–adult aBMD associations, with adjustment for covariates, are shown in Figure 1 and Table S3. In men, the direct effects of childhood SFT on adult aBMD at LS and legs were larger than the total effects (LS aBMD: C′ = 0.120 vs. C = 0.089; leg aBMD: C′ = 0.083 vs. C = 0.050). Thus, the childhood SFT–adult aBMD associations were suppressed by adult FMP in men (−34.8% for LS aBMD and −67.1% for leg aBMD). In women, the childhood SFT–adult aBMD relationship for FN aBMD was positively affected by adult FMP (17.0%), but no significant effects at the other skeletal sites were found.
4 DISCUSSION
Several previous studies have investigated the short-term impact of body fat on bone health during childhood and adolescence.15-19 However, few studies have examined the relations hips between childhood adiposity and adult bone density. In the present study, which involved measurements made a mean of 23 years apart, independent significant positive associations were identified between childhood adiposity and adult bone density in both sexes, suggesting a beneficial effect of adiposity on bone development. However, it should be noted that SFT only reflects the quantity of subcutaneous adipose tissue, and therefore the possibly harmful impact of visceral adiposity on bone growth could not be analyzed in the present study.19-21 Therefore, future prospective studies are needed to determine the depot-specific effects of childhood adiposity on bone health in later life.
Consistent with the results of a previous meta-analysis,22 sexual dimorphism was identified in the relationships between fat mass and bone density at various ages in the present study. In men, the association between adiposity and bone density changed from being positive during childhood to negative during adulthood, whereas in contrast, consistent positive relationships were identified in women. Therefore, it may be that sustained excess adiposity from childhood to adulthood is beneficial for the bone health of women but not men. This difference may be, at least in part, because of the influence of sex hormones. Estrogen and testosterone have different effects on bone metabolism,23 and on fat distribution as well.24 Because sexual dimorphism with respect to fat distribution becomes more marked with age,25 it is plausible that the relationships between fat and bone would differ between the sexes in adulthood.
The present cohort, which was recruited through schools, provides a unique opportunity to determine the long-term impact of childhood adiposity on adult aBMD at different skeletal sites. However, the present study had several limitations. First, information regarding childhood
SFT was limited to a measurement made close to the scapula, which only reflects the degree of peripheral adiposity. Second, data regarding physical activity and nutrient intake during childhood were not available, and therefore their influences on adult bone health could not be evaluated. Finally, the prevalence of childhood obesity, determined using BMI only in the study sample, was relatively low (3.2%); therefore, the present findings should be generalized with caution to populations of children with a higher prevalence of excess adiposity, which is now more common.
In summary, childhood adiposity has a positive effect on adult bone mass. This positive impact is suppressed by excess adiposity in adult men but is reinforced in women. These findings suggest that age- and sex-related strategies are needed to improve bone mass accrual and reduce subsequent age-related bone loss.
5 Funding source
National Natural Science Foundation of China (81973110, 71904132)
ACKNOWLEDGMENTS
The authors are grateful to all study members and the whole research team of the Beijing Blood Pressure Cohort Study for their contribution and continuing support.
CONFLICT OF INTEREST
None.




