Effectiveness and tolerability of liraglutide for the management of weight regain following sleeve gastrectomy
Abstract
Background
There is currently very little research evidence on the benefits and safety of liraglutide in the management of weight regain or inadequate weight loss following metabolic and bariatric surgery. This study aimed to determine the clinical effectiveness and tolerability of liraglutide as an adjunct therapy for managing weight regain and inadequate weight loss following sleeve gastrectomy (SG).
Methods
This was a retrospective analysis of medical records conducted at a private clinic in Kuwait.
Results
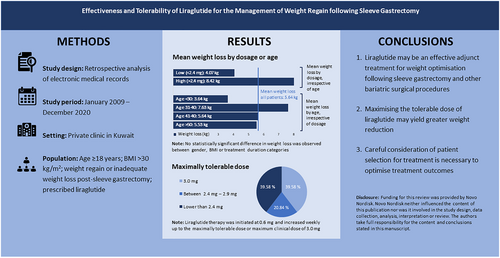
Data of 57 post-SG patients were included in the analysis. The mean (±SD) pre-treatment weight was 96.12 (29.26) kg. Following a median liraglutide treatment duration of approximately 3 months, the mean post-treatment weight was 90.19 (26.82) kg. This represents a statistically significant mean weight loss of 5.94 (6.31) kg (p < 0.001), corresponding to a loss of 6.20% of pre-treatment weight. Patients aged 31–40 years achieved a greater post-treatment weight loss of 7.63 (7.41) kg, a loss of 7.80%, relative to age groups after treatment (p = 0.047). Patients who tolerated ≥2.4 mg of liraglutide recorded a higher mean weight loss of 8.42 (7.63) kg, a loss of 8.10% (p = 0.010).
Conclusion
The use of liraglutide may be an effective adjunct treatment for weight optimization following SG. Maximizing the tolerable dose may yield greater weight reduction.
Graphical Abstract
This study adds to the current body of evidence supporting the clinical effectiveness of liraglutide for weight reduction in patients with obesity, particularly in those regaining weight following SG. Our finding of statistically significant differences in weight loss across age groups underscores the importance of considering patient selection for treatment.
1 INTRODUCTION
Metabolic and bariatric surgery (MBS) is considered one of the most effective treatment modalities for achieving weight loss and managing obesity associated medical problems in patients with obesity.1, 2 Sleeve gastrectomy (SG) is the most common MBS procedure performed in Kuwait, reflecting global trends.3 Evidence shows that, on average, patients achieve 30%–45% weight loss with MBS.4 However, evidence from literature has also demonstrated that a substantial proportion (up to 20%) of patients do not achieve long-term desirable weight reduction goals or regain weight even after successful MBS procedures.5 In addition to the likelihood of inadequate weight loss or weight regain, MBS has also been associated with the risk of surgical complications and the need for additional surgical procedures.6 Hence, pharmacotherapy is increasingly being explored as an adjunct treatment option for managing inadequate weight loss or regain after MBS.6, 7
Liraglutide is a glucagon-like peptide-1 (GLP-1) analog approved by the Food and Drug Administration of the United States for the treatment of obesity in 2014.8 As a standalone therapy this treatment approach has demonstrated success in sustaining weight loss among patients, compared with other weight loss treatment programmes such as diet therapy.7, 9 It is also used for the treatment of obesity in several other settings, including for weight recurrence after MBS, pre-operative weight loss in clinically severe obesity, concurrent use postoperatively with MBS and for further weight loss when weight plateau occurs within the first year after MBS.7, 8 The use of liraglutide for managing weight regain following SG has been adopted in some countries and was introduced recently in Kuwait. However, there is currently limited evidence on this treatment approach's clinical utility, effectiveness, and safety. Moreover, there is a dearth of evidence from real-world studies investigating the use of liraglutide for managing excessive weight regain or insufficient weight loss in post-SG patients. Therefore, this retrospective study of electronic medical records of patients treated with liraglutide aimed to determine the effectiveness and tolerability of liraglutide as an adjunct weight gain management therapy following SG.
2 METHODS
2.1 Study design
A retrospective analysis of electronic medical records was performed on patients who underwent SG surgery at a private clinic in Kuwait between January 2009 and December 2020, and were managed using liraglutide from January 2019 onwards for weight loss after SG surgery. Health history data, laboratory investigations data, and all treatment data, including surgical procedures, were extracted for each patient using a data extraction tool designed for this study.
2.2 Study setting and population
The study involved patients managed at a private clinic in Kuwait. In addition to regular medical and surgical services, the clinic manages patients with obesity and obesity complications. Patients are managed according to international standards and clinical best practices for treating obesity in adults. Services are funded through patients' private medical schemes or out-of-pocket payment. On average, the clinic sees 300–600 post-MBS patients per year. Liraglutide was initiated in consenting patients who had weight regain or inadequate weight loss following SG, as recommended using international guidelines. Liraglutide therapy followed a dosing schedule starting at 0.6 mg and adjusted weekly until reaching the maximum clinical dose of 3.0 mg. In line with international standards, treatment was kept at a lower dose or discontinued as necessary when there were tolerability concerns. Following liraglutide initiation, patients attended follow-up appointments. Patients were encouraged to do at least 10,000 steps per day and were encouraged to have a high protein diet with a maximum of 1500 Kcal/day. All patients were assigned to receive a follow-up session with a clinical nutritionist. During each follow-up appointment, patients' treatment adherence and tolerability/safety, dietary plan, weight/anthropometric progress and general clinical status were monitored by clinicians.
2.3 Participant enrollment and informed consent process
The study population comprised consenting adults ≥18 years of age, who had undergone SG, had a BMI greater than 30 kg/m2, and were prescribed liraglutide to manage weight regain post-SG surgery. Specifically, participants who met the study eligibility criteria were those prescribed liraglutide due to: (1) a ≥10% weight regain from the lowest post-SG weight and (2) patients experiencing inadequate weight loss (<20% weight loss from preoperative weight).
Patients who were on any other weight loss regimen and those with a history of medullary thyroid cancer were excluded. Participants who discontinued the liraglutide therapy before 4 weeks or initiated normal feeding before 4 weeks were also excluded from the study. All patients undergoing SG surgery consented to the use of their medical records for research purposes. Additionally, all patients were informed that their treatment was not dependent on participation or lack of consent for the use of their medical records.
2.4 Data collection process and outcome measurements
The study involved a retrospective analysis of routinely collected patients' medical records. During the initial visit to the clinic, patients with obesity or obesity complications completed a comprehensive intake questionnaire on demographics, medical and weight management history. Baseline anthropometric measurements were performed on all patients on treatment, including those on liraglutide. During each follow-up appointment, clinicians monitored patients' treatment adherence and tolerability/safety, dietary plan, weight/anthropometric progress, and general clinical status.
2.5 Data analysis
Continuous variables were summarized as means and standard deviations (SDs) or medians and interquartile ranges (IQRs) as appropriate. Categorical variables were summarized as relative frequencies and percentages. Intergroup differences in weight changes were compared using Paired samples t-test for normally distributed data and the Wilcoxon signed-rank test for non-normally distributed data. Where appropriate, the proportion was compared using the χ2 test or Fischer's exact test. A one-way ANOVA or Kruskal-Wallis test was used to compare weight changes across variable categories. p values < 0.05 were considered statistically significant. Analysis was conducted using STATA version 16, and R Version 3.8.9 software.10, 11
2.6 Ethical considerations
The study was conducted in accordance with the principles expressed in the Declaration of Helsinki.12 The study protocol and tools conformed to research ethics standards and were approved by the relevant Research Ethics Committee in Kuwait (Reference Number: 1776/2021). All patients whose medical records were used gave informed consent for the use of their electronic medical data for research purposes. The research dataset was anonymized and stored securely in a password-protected device accessible only to the research team. Each participant was assigned a unique study identity number.
3 RESULTS
3.1 Baseline sociodemographic and clinical characteristics of patients
Data for 57 patients, who had SG between January 2009 and December 2020 and received liraglutide treatment from January 2019 onwards for weight gain following surgery, were available and retrieved from the clinic's medical record database. The pre-SG weight of the patients was 121.5 (34.5) kg, which went down to 77.8 (22.5) kg post-SG in 1–2 years, a weight loss of 36.0%. During liraglutide treatment, the median (IQR) age of the patients was 36.71 (9.86) with the majority (54.39%) of them being in the 30–49 age group and 42 (73.68%) being female. About a quarter (26.32%) of the patients reported one or more underlying chronic conditions; 2 (3.51%) had Type 2 diabetes, 3 (5.26%) had hypertension, 2 (3.51%) had liver diseases, and the remaining 8 (14.04%) had other chronic conditions (including polycystic ovary syndrome, hypothyroidism, multiple sclerosis, hypercholesterolemia, glucose-6-phosphate dehydrogenase deficiency, asthma, hepatic hydatid disease, pulmonary embolism, deep venous thrombosis, thalassemia minor and obstructive sleep apnea). The mean maximum tolerated liraglutide dose was 2.21 (0.83) mg, while the median (IQR) treatment duration was 85 (103) days (Table 1).
| Variable (n = 57) | N (%) |
|---|---|
| Median age (years ± IQR) (n = 57) | 36.71 (9.86) |
| 20–29 | 13 (22.81) |
| 30–39 | 28 (49.12) |
| 40–49 | 09 (15.79) |
| >50 | 07 (12.28) |
| Gender (%) | |
| Male | 15 (26.32) |
| Female | 42 (73.68) |
| Chronic conditions | |
| Type 2 diabetes | 02 (03.51) |
| Hypertension | 03 (05.26) |
| Liver disease | 02 (03.51) |
| Other chronic conditions | 08 (14.04) |
| No chronic conditions | 42 (73.68) |
| Maximum dose tolerated (mg) (n = 48) | |
| 3.0 | 19 (39.58) |
| 2.4–2.9 | 10 (20.84) |
| <2.4 | 19 (39.58) |
| Mean liraglutide maximally tolerated dose (mg ± standard deviation) | 2.21 (0.83) |
| Median treatment duration in days (IQR) | 85 (103) |
| Weight | |
| Pre-SG weight (kg ± SD) | 121.5 (34.5) |
| Post-SG weight (kg ± SD) | 77.8 (22.5) |
- Abbreviations: IQR, interquartile range; SD, standard deviation; SG, sleeve gastrectomy.
3.2 Weight changes across patient characteristics following liraglutide treatment
Mean (±SD) sample weight pre-treatment was 96.12 (29.26) kg, a weight regain of 23.6% post SG. Table 2 shows the distribution of pre-treatment weight, post-treatment weight and weight changes across patient variables. Following a median liraglutide treatment duration of approximately 3 months, the mean post-treatment weight was 90.19 (26.82) kg across all patients included in the analysis. This represents a statistically significant mean weight loss of 5.94 (6.31) kg (p < 0.001), corresponding to a loss of 6.20% of pre-treatment weight. There was a statistically significant difference in weight reduction across age groups, with patients aged 31–40 years achieving the highest mean weight loss of 7.80% after treatment (p = 0.047). Similarly, patients who tolerated ≥2.4 mg of liraglutide recorded a higher mean weight loss of 8.10% (p = 0.010) than those who received lower doses (Table 2).
| Variable | Mean weight (kgs) before treatment mean (SD) | Mean weight (kgs) after treatment mean (SD) | Mean (SD) post-treatmentweight change | Mean (SD) percentage post-treatment weight change | p-value |
|---|---|---|---|---|---|
| All patients | 96.12 | 90.19 | −5.94 | −6.20 (6.0) | <0.001* |
| Age | |||||
| <30 | 93.45 (31.24) | 89.81 (30.75) | −3.64 (4.59) | −3.50 (3.77) | 0.047* |
| 31–40 | 94.87 (26.86) | 87.25 (24.63) | −7.63 (7.41) | −7.80 (5.47) | |
| 41–50 | 97.92 (29.06) | 92.28 (23.47) | −5.64 (7.50) | −4.80 (6.39) | |
| >50 | 105.45 (31.08) | 99.92 (30.99) | −5.53 (1.84) | −5.50 (2.16) | |
| Sex | |||||
| Male | 128.84 (30.49) | 119.86 (27.38) | −8.98 (9.63) | −6.50 (6.10) | 0.558 |
| Female | 84.44 (17.89) | 79.59 (17.00) | −4.85 (4.26) | −5.60 (4.70) | |
| Treatment duration | |||||
| ≤3 months | 103.56 (30.91) | 91.46 (23.41) | −12.09 (11.06) | −10.70 (7.00) | 0.256 |
| >3 months | - | - | - | - | |
| BMI categories | |||||
| <35 | 82.38 (10.37) | 78.71 (11.18) | −3.68 (3.56) | −4.50 (4.50) | 0.678 |
| 35–40 | 97.45 (23.81) | 90.02 (20.02) | −7.43 (8.09) | −6.90 (8.50) | |
| >40 | 137.63 (39.19) | 132.93 (40.30) | −4.70 (2.81) | −3.80 (2.40) | |
| Max tolerated dose | |||||
| High(>2.4 mg) | 96.46 (29.50) | 88.05 (24.84) | −8.42 (7.63) | −8.10 (5.50) | 0.010* |
| Low (<2.4 mg) | 97.78 (30.80) | 93.71 (30.69) | −4.07 (2.45) | −4.40 (2.70) | |
| Patients achieving a weight loss of ≥5% of pre-treatment weight | 31/57 (54.39%) | ||||
- *p-value <0.05.
Among males, the mean pre-treatment weight was 128.60 (30.49) kg, whereas the pre-treatment weight for females was 84.44 (17.89) kg. The mean post-treatment weights were 119.86 (27.38) and 79.59 (17.00) kg for males and females, respectively. However, there was no statistically significant difference in weight loss between the two genders. Likewise, no statistically significant difference in weight loss was observed across BMI and treatment duration categories (see Table 2). The percentage post-treatment weight changes are presented in Table 2 and illustrated in Figure 1 for all patients and by variables.
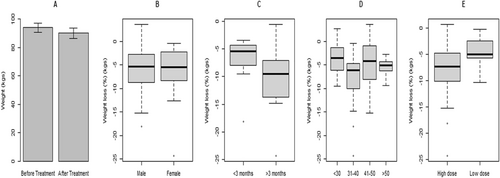
Box and whisker plots of mean post-treatment percentage weight changes after 3 months. (A) Pre-treatment and post-treatment weight of patients. (B) Weight loss percentage of male and female patients post-liraglutide treatment. (C) Weight loss percentage of patients based on treatment duration. (D) Weight loss percentage across age group. (E) Weight loss percentage based on liraglutide dosage.
3.3 Patients achieving a weight loss of ≥5% of pre-treatment weight
A slight majority (54.39%) of the patients achieved a weight loss of ≥5% of their pre-treatment weight (see Table 2).
3.4 Tolerability of liraglutide
The mean maximally tolerated liraglutide dose was 2.21 mg. Only about two-fifths (39.58%) of patients received up to the optimally recommended daily dose of 3.0 mg per day, while a fifth (20.84%) received doses ranging from 2.4 to 2.9 mg and another two-fifths (39.58%) received doses lower than 2.4 mg. The medical records used for this study did not have sufficient data on adverse events and reasons for lowering doses or discontinuing liraglutide (Table 1).
4 DISCUSSION
Findings from this study add to the current body of evidence supporting the clinical effectiveness of liraglutide for weight reduction in patients with obesity, particularly in those regaining weight following SG. Although the study's retrospective nature and sample size limit the interpretation of its findings, the results are consistent with those of previous studies investigating the clinical effectiveness of liraglutide for weight loss across different patient populations. Particularly, the mean weight loss of 6% of pre-treatment weight following a median treatment duration of 3 months in the current study is consistent with those found in both previous trials and real-world studies.13-15
Previous evidence suggested that MBS may be superior to pharmacologic treatment in terms of weight loss in patients with obesity; however, recent evidence has shown that a considerable proportion of MBS patients have unsatisfactory postoperative weight response in the long term.16 Moreover, treatment options for patients with insufficient weight loss or excessive weight regain following MBS are often limited to revisional surgeries, which may have higher rates of complications than the initial surgery itself.17, 18 However, there is growing evidence that, compared with revisional surgery, weight regain after MBS can be effectively reversed with pharmacotherapy using liraglutide, with long-term weight reduction benefits, lower risks of complications and additional benefits of improvement in hypertension and dyslipidemia.19
Results from randomized and observational studies indicate that the use of GLP-1 analogs in addition to surgery can provide significant benefits in weight loss and improvements of comorbidities in patients with suboptimal postoperative outcomes.15, 20, 21 In a retrospective analysis of 207 patients treated for post-MBS weight recurrence, Gadza et al. compared weight loss outcomes between an intensive lifestyle modification (ILM) group, a non-GLP-1 receptor agonist-based weight loss pharmacotherapy (WLP) group, and a GLP-1 receptor agonist-based WLP group. GLP-1 receptor agonist–based WLP therapies were found to be more effective for treating post-MBS weight recurrence than non–GLP-1 receptor agonist–based WLP or ILM irrespective of the type of surgery.22 Thus, findings from the current study support the existing evidence base, suggesting that liraglutide may be an effective treatment for managing excessive weight regain or insufficient weight loss in post-MBS patients.6, 7, 23, 24
A slight majority (54.39%) of the patients achieved a weight loss of ≥5% of their pre-treatment weight. This is similar to the findings from a previous study of post-MBS patients.25 Current guidelines for the use of liraglutide as a weight management pharmaceutical stipulate that treatment with liraglutide should be discontinued after 12 weeks on the 3.0 mg/day dose if a patient has not lost at least 5% of their initial body weight.26 However, whether these guidelines apply to the off-label prescription of liraglutide and other weight management pharmaceuticals in patients who underwent SG remains uncertain.
In this study, the mean weight loss was higher in the patients who tolerated up to 2.4 mg of liraglutide compared with those who tolerated lower doses. This is in keeping with the dose-response findings observed in earlier studies.6, 23, 24, 27 This dose-response relationship could reflect the tolerability of liraglutide and other GLP-1 analogs.28 However, suboptimal doses may also be influenced by other factors such as financial considerations due to the cost of treatment and injection site concerns.15 The tolerability of liraglutide was assessed with respect to the maximally tolerated dose. While previous studies have shown that liraglutide is generally well tolerated,29 the current study found that only a third (33.33%) of patients received up to the optimally recommended dose of 3.0 mg per day, while about half (49.51%) received doses lower than 2.4 mg. The medical records used for this study lacked sufficient data on adverse events and reasons for lowering doses or discontinuing liraglutide. A few patients for whom adverse event data were available reported side effects such as nausea, constipation, and headaches. Previous studies found that liraglutide treatment was discontinued in about 5% of patients due to adverse events, most commonly gastrointestinal complaints such as nausea, vomiting, and diarrhea.6
While this study shows the real-world effectiveness of liraglutide for weight management, the finding of statistically significant differences in weight loss across age groups underscores the importance of considering patient selection for treatment. This warrants a careful assessment of post-SG patients by a multidisciplinary obesity clinic team to identify the best candidates for adjunct pharmacological treatment with liraglutide. The findings also underscore the need for future research efforts to investigate and determine patient populations for whom weight loss and clinical benefits are likely to be maximized.
There was no statistically significant difference between weight loss and patients' variables such as gender, BMI categories and presence of obesity associated medical problems. This is in contrast to the findings of previous studies demonstrating a range of sociodemographic, clinical and other patient-level factors as important predictors of clinical efficacy and effectiveness.6, 23, 24, 27 The absence of a significant finding in this regard may be due to the sample size and duration of follow-up limitations of the current study. Thus, further studies with larger sample sizes and longer follow-up periods are needed to ascertain the relationships between these potential predictors of treatment outcomes, and short- and long-term clinical benefits and safety of liraglutide in patients regaining weight or not achieving adequate weight loss following SG.
The current study has several strengths and limitations that warrant mentioning. A notable strength of this study includes being one of the few studies to examine the use of liraglutide in a population of patients who had undergone SG globally and the first such study in Kuwait. In addition, the routine clinical setting of the study helps to account for real-world factors such as lower therapeutic adherence levels than those typically seen in experimental settings of randomized controlled trials. As such, the current study provides important evidence that may reflect the general patient population and the realities of routine clinical practice. Notwithstanding these strengths, this study has some limitations worth noting. First, it is a retrospective study with a modest sample size, short duration of follow-up, limited data on baseline patient characteristics and lack of a control group. These methodological limitations reflect the trend in most real-world studies of this nature, particularly those investigating weight outcomes of GLP-1 analogs in post-MBS patients. As alluded to earlier, although the results are consistent with those of previous studies, these limitations have implications for the interpretation and applicability of study findings. Finally, owing to the sample size limitations and the lack of data on some important co-variates, it was not feasible to fit multivariable regression models to further explore the association between weight changes and patient-level characteristics such as patients' diet, exercise, co-medications and socio-economic status, which might influence their treatment outcomes. Therefore, it is important for future studies to address the aforementioned limitations, including the assessment of the benefits of liraglutide in terms of obesity-related metabolic and cardiovascular comorbidities as observed in SG patients treated with liraglutide in previous studies.13-15
5 CONCLUSION
Findings from the current study are in keeping with the existing evidence base suggesting that liraglutide may be an effective treatment for managing excessive weight regain or insufficient weight loss in patients who underwent SG. Maximizing the tolerable dose is likely to achieve greater weight reduction. Findings also underscore the importance of carefully assessing patients to identify the best candidates for adjunct pharmacological treatment. Further studies with larger sample sizes and longer follow-up periods are needed to strengthen the evidence on the benefits and safety of this treatment approach.
AUTHOR CONTRIBUTIONS
Conceptualization: Mohammad Jamal. Protocol development: Chukwudi A. Nnaji, Paul Otiku and Mohammad Jamal. Data curation: Mohammad Jamal, Paul Otiku and Chukwudi A. Nnaji. Data analysis and interpretation: Chukwudi A. Nnaji, Paul Otiku and Mohammad Jamal. The preparation of the first draft of the manuscript: Mohammad Jamal, Chukwudi A. Nnaji and Paul Otiku. The provision of critical insights and refinement of the manuscript: Mohammad Jamal, Paul Otiku, Wafa Qasem, Fatima Hamshari, Carol Dsouza and Nagi Alqallaf. Guarantor: Mohammad Jamal. All authors have read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
Medical writing, statistical analysis and editorial assistance were provided by Last Mile agency and financially funded by Novo Nordisk. The authors take full responsibility for the content and conclusions stated in this manuscript. Novo Nordisk neither influenced the content of this publication nor was it involved in the study design, data collection, analysis, interpretation, or review.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare.