Differential effect of gastric bypass versus sleeve gastrectomy on insulinotropic action of endogenous incretins
This article relates to:
-
Insulinotropic effect of endogenously secreted incretins after gastric bypass and sleeve gastrectomy
- Volume 31Issue 11Obesity
- pages: 2723-2724
- First Published online: October 19, 2023
Corresponding Author
Marzieh Salehi
Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, San Antonio, Texas, USA
South Texas Veteran Health Care System, Audie Murphy Hospital, San Antonio, Texas, USA
Correspondence
Marzieh Salehi, Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, 7703 Floyd Curl Dr., mail code 7886, San Antonio, TX 78229, USA.
Email: [email protected]
Search for more papers by this authorRichard Peterson
Department of Surgery, University of Texas Health at San Antonio, San Antonio, Texas, USA
Search for more papers by this authorDevjit Tripathy
Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, San Antonio, Texas, USA
Search for more papers by this authorSamantha Pezzica
Cardiometabolic Risk Unit, CNR Institute of Clinical Physiology, Pisa, Italy
Search for more papers by this authorRalph DeFronzo
Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, San Antonio, Texas, USA
Search for more papers by this authorAmalia Gastaldelli
Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, San Antonio, Texas, USA
Cardiometabolic Risk Unit, CNR Institute of Clinical Physiology, Pisa, Italy
Search for more papers by this authorCorresponding Author
Marzieh Salehi
Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, San Antonio, Texas, USA
South Texas Veteran Health Care System, Audie Murphy Hospital, San Antonio, Texas, USA
Correspondence
Marzieh Salehi, Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, 7703 Floyd Curl Dr., mail code 7886, San Antonio, TX 78229, USA.
Email: [email protected]
Search for more papers by this authorRichard Peterson
Department of Surgery, University of Texas Health at San Antonio, San Antonio, Texas, USA
Search for more papers by this authorDevjit Tripathy
Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, San Antonio, Texas, USA
Search for more papers by this authorSamantha Pezzica
Cardiometabolic Risk Unit, CNR Institute of Clinical Physiology, Pisa, Italy
Search for more papers by this authorRalph DeFronzo
Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, San Antonio, Texas, USA
Search for more papers by this authorAmalia Gastaldelli
Division of Diabetes, Department of Medicine, University of Texas Health at San Antonio, San Antonio, Texas, USA
Cardiometabolic Risk Unit, CNR Institute of Clinical Physiology, Pisa, Italy
Search for more papers by this authorAbstract
Objective
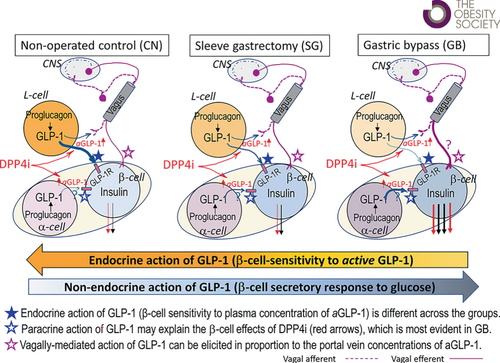
Prandial hyperinsulinemia after Roux-en-Y gastric bypass surgery (GB), and to lesser degree after sleeve gastrectomy (SG), has been attributed to rapid glucose flux from the gut and increased insulinotropic gut hormones. However, β-cell sensitivity to exogenous incretin is reduced after GB. This study examines the effect of GB versus SG on prandial glycemia and β-cell response to increasing concentrations of endogenous incretins.
Methods
Glucose kinetics, insulin secretion rate (ISR), and incretin responses to 50-g oral glucose ingestion were compared between ten nondiabetic participants with GB versus nine matched individuals with SG and seven nonoperated normal glucose tolerant control individuals (CN) with and without administration of 200 mg of sitagliptin.
Results
Fasting glucose and hormonal levels were similar among three groups. Increasing plasma concentrations of endogenous incretins by two- to three-fold diminished prandial glycemia and increased β-cell secretion in all three groups (p < 0.05), but insulin secretion per insulin sensitivity (i.e., disposition index) was increased only in GB (p < 0.05 for interaction). However, plot of the slope of ISR (from premeal to peak values) versus plasma glucagon-like peptide-1 concentration was smaller after GB compared with SG and CN.
Conclusions
After GB, increasing incretin activity augments prandial β-cell response whereas the β-cell sensitivity to increasing plasma concentrations of endogenous incretin is diminished.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
Supporting Information
| Filename | Description |
|---|---|
| oby23872-sup-0001-FigureS1.tifTIFF image, 876.1 KB | Figure S1. Plasma concentrations of acetaminophen after oral glucose ingestion during OGTT with (dashed line) and without (solid line) administration of sitagliptin in subjects who underwent gastric bypass (top panel) or sleeve gastrectomy (middle panel) and non-operated controls (bottom panel). |
| oby23872-sup-0002-FigureS2.tifTIFF image, 2.9 MB | Figure S2. The slope of post OGTT ISR plotted against increasing plasma concentrations of active GLP-1 (aGLP-1) (left), GIP (middle), and glucose (right) during control (solid line) and sitagliptin (dashed line) conditions in subjects who underwent gastric bypass (black line) or sleeve gastrectomy (red line) and non-operated controls (blue line). |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Bradley D, Magkos F, Eagon JC, et al. Matched weight loss induced by sleeve gastrectomy or gastric bypass similarly improves metabolic function in obese subjects. Obesity (Silver Spring). 2014; 22: 2026-2031.
- 2Yoshino M, Kayser BD, Yoshino J, et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N Engl J Med. 2020; 383: 721-732.
- 3Ferrannini E, Camastra S, Gastaldelli A, et al. Beta-cell function in obesity: effects of weight loss. Diabetes. 2004; 53(Suppl 3): S26-S33.
- 4Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012; 303: E122-E131.
- 5Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008; 93: 2479-2485.
- 6Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009; 250: 234-241.
- 7Iaconelli A, Gastaldelli A, Chiellini C, et al. Effect of oral sebacic acid on postprandial glycemia, insulinemia, and glucose rate of appearance in type 2 diabetes. Diabetes Care. 2010; 33: 2327-2332.
- 8Camastra S, Muscelli E, Gastaldelli A, et al. Long-term effects of bariatric surgery on meal disposal and beta-cell function in diabetic and nondiabetic patients. Diabetes. 2013; 62: 3709-3717.
- 9Jacobsen SH, Bojsen-Moller KN, Dirksen C, et al. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia. 2013; 56: 2250-2254.
- 10Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014; 146: 669-680 e2.
- 11Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption, and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014; 22: 2003-2009.
- 12Braghetto I, Davanzo C, Korn O, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009; 19: 1515-1521.
- 13Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007; 30: 1709-1716.
- 14Nannipieri M, Baldi S, Mari A, et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013; 98: 4391-4399.
- 15Salehi M, Gastaldelli A, D'Alessio DA. Evidence from a single individual that increased plasma GLP-1 and GLP-1-stimulated insulin secretion after gastric bypass are independent of foregut exclusion. Diabetologia. 2014; 57: 1495-1499.
- 16Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007; 92: 4678-4685.
- 17Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018; 103: 2815-2826.
- 18Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011; 60: 2308-2314.
- 19Hindso M, Hedback N, Svane MS, et al. The importance of endogenously secreted GLP-1 and GIP for postprandial glucose tolerance and beta-cell function after Roux-en-Y gastric bypass and sleeve gastrectomy surgery. Diabetes. 2023; 72: 336-347.
- 20Shah M, Law JH, Micheletto F, et al. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. 2014; 63: 483-493.
- 21Salehi M, Gastaldelli A, D'Alessio DA. Beta-cell sensitivity to insulinotropic gut hormones is reduced after gastric bypass surgery. Gut. 2019; 68: 1838-1845.
- 22Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006; 91: 4612-4619.
- 23Salehi M, Gastaldelli A, DeFronzo R. Prandial hepatic glucose production during hypoglycemia is altered after gastric bypass surgery and sleeve gastrectomy. Metabolism. 2022; 131:155199.
- 24Gastaldelli A. Measuring and estimating insulin resistance in clinical and research settings. Obesity (Silver Spring). 2022; 30: 1549-1563.
- 25Salehi M, DeFronzo R, Gastaldelli A. Altered insulin clearance after gastric bypass and sleeve gastrectomy in the fasting and prandial conditions. Int J Mol Sci. 2022; 23: 7667.
- 26Salehi M, Gastaldelli A, D'Alessio DA. Beta-cell sensitivity to glucose is impaired after gastric bypass surgery. Diabetes Obes Metab. 2018; 20: 872-878.
- 27Kjems LL, Holst JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003; 52: 380-386.
- 28Mingrone G, Panunzi S, De Gaetano A, et al. Insulin sensitivity depends on the route of glucose administration. Diabetologia. 2020; 63: 1382-1395.
- 29von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014; 12: 1495-1499.
- 30Aoki K, Masuda K, Miyazaki T, Togashi Y, Terauchi Y. Effects of miglitol, sitagliptin or their combination on plasma glucose, insulin and incretin levels in non-diabetic men. Endocr J. 2010; 57: 667-672.
- 31Ohlsson L, Alsalim W, Carr RD, et al. Glucose-lowering effect of the DPP-4 inhibitor sitagliptin after glucose and non-glucose macronutrient ingestion in non-diabetic subjects. Diabetes Obes Metab. 2013; 15: 531-537.
- 32Wu T, Ma J, Bound MJ, et al. Effects of sitagliptin on glycemia, incretin hormones, and antropyloroduodenal motility in response to intraduodenal glucose infusion in healthy lean and obese humans and patients with type 2 diabetes treated with or without metformin. Diabetes. 2014; 63: 2776-2787.
- 33Alsalim W, Goransson O, Carr RD, et al. Effect of single-dose DPP-4 inhibitor sitagliptin on beta-cell function and incretin hormone secretion after meal ingestion in healthy volunteers and drug-naive, well-controlled type 2 diabetes subjects. Diabetes Obes Metab. 2018; 20: 1080-1085.
- 34Rhee NA, Ostoft SH, Holst JJ, Deacon CF, Vilsboll T, Knop FK. The impact of dipeptidyl peptidase 4 inhibition on incretin effect, glucose tolerance, and gastrointestinal-mediated glucose disposal in healthy subjects. Eur J Endocrinol. 2014; 171: 353-362.
- 35Ahren B, Schweizer A, Dejager S, et al. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009; 94: 1236-1243.
- 36Andersen DK, Elahi D, Brown JC, Tobin JD, Andres R. Oral glucose augmentation of insulin secretion. Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest. 1978; 62: 152-161.
- 37Qualmann C, Nauck MA, Holst JJ, Orskov C, Creutzfeldt W. Insulinotropic actions of intravenous glucagon-like peptide-1 (GLP-1) [7-36 amide] in the fasting state in healthy subjects. Acta Diabetol. 1995; 32: 13-16.
- 38Pilichiewicz AN, Chaikomin R, Brennan IM, et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2007; 293: E743-E753.
- 39Salehi M, Gastaldelli A, D'Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014; 99: 2008-2017.
- 40Svane MS, Bojsen-Moller KN, Martinussen C, et al. Postprandial nutrient handling and gastrointestinal hormone secretion after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology. 2019; 156: 1627-1641 e1621.
- 41Svane MS, Bojsen-Moller KN, Nielsen S, et al. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab. 2016; 310: E505-E514.
- 42Omar BA, Liehua L, Yamada Y, Seino Y, Marchetti P, Ahren B. Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia. 2014; 57: 1876-1883.
- 43Kim KS, Hutch CR, Wood L, Magrisso IJ, Seeley RJ, Sandoval DA. Glycemic effect of pancreatic preproglucagon in mouse sleeve gastrectomy. JCI Insight. 2019; 4:e129452.
- 44Vahl TP, Tauchi M, Durler TS, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007; 148: 4965-4973.
- 45Moore MC, Connolly CC, Cherrington AD. Autoregulation of hepatic glucose production. Eur J Endocrinol. 1998; 138: 240-248.