Optimization modeling for pandemic vaccine supply chain management: A review and future research opportunities
Shibshankar Dey
Department of Industrial Engineering and Management Sciences, Northwestern University, Evanston, Illinois
Center for Engineering and Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois
Search for more papers by this authorAli Kaan Kurbanzade
Department of Industrial Engineering and Management Sciences, Northwestern University, Evanston, Illinois
Center for Engineering and Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois
Search for more papers by this authorEsma S. Gel
Department of Supply Chain Management and Analytics, University of Nebraska-Lincoln, Lincoln, Nebraska
Search for more papers by this authorJoseph Mihaljevic
School of Informatics, Computing and Cyber Systems Northern Arizona University, Flagstaff, Arizona
Search for more papers by this authorCorresponding Author
Sanjay Mehrotra
Department of Industrial Engineering and Management Sciences, Northwestern University, Evanston, Illinois
Center for Engineering and Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois
Correspondence
Sanjay Mehrotra, Department of Industrial Engineering and Management Sciences, Northwestern University, Evanston, IL, USA.
Email: [email protected]
Search for more papers by this authorShibshankar Dey
Department of Industrial Engineering and Management Sciences, Northwestern University, Evanston, Illinois
Center for Engineering and Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois
Search for more papers by this authorAli Kaan Kurbanzade
Department of Industrial Engineering and Management Sciences, Northwestern University, Evanston, Illinois
Center for Engineering and Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois
Search for more papers by this authorEsma S. Gel
Department of Supply Chain Management and Analytics, University of Nebraska-Lincoln, Lincoln, Nebraska
Search for more papers by this authorJoseph Mihaljevic
School of Informatics, Computing and Cyber Systems Northern Arizona University, Flagstaff, Arizona
Search for more papers by this authorCorresponding Author
Sanjay Mehrotra
Department of Industrial Engineering and Management Sciences, Northwestern University, Evanston, Illinois
Center for Engineering and Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois
Correspondence
Sanjay Mehrotra, Department of Industrial Engineering and Management Sciences, Northwestern University, Evanston, IL, USA.
Email: [email protected]
Search for more papers by this authorAbstract
During various stages of the COVID-19 pandemic, countries implemented diverse vaccine management approaches, influenced by variations in infrastructure and socio-economic conditions. This article provides a comprehensive overview of optimization models developed by the research community throughout the COVID-19 era, aimed at enhancing vaccine distribution and establishing a standardized framework for future pandemic preparedness. These models address critical issues such as site selection, inventory management, allocation strategies, distribution logistics, and route optimization encountered during the COVID-19 crisis. A unified framework is employed to describe the models, emphasizing their integration with epidemiological models to facilitate a holistic understanding. This article also summarizes evolving nature of literature, relevant research gaps, and authors' perspectives for model selection. Finally, future research scopes are detailed both in the context of modeling and solutions approaches.
1 INTRODUCTION
The COVID-19 pandemic emerged as the largest global health crisis in recent history, with an staggering incidence of over 750 million reported cases and death toll approaching seven million individuals worldwide (World Health Organization, 2023). Beyond the profound health implications, the pandemic has presented an array of multifaceted challenges encompassing social, technological, economic, environmental, political, legal, and ethical domains (Klemeš et al., 2020; Saberi Isfeedvajani et al., 2020; Sampath et al., 2021; Shah et al., 2021; Taskinsoy, 2020). In response, numerous researchers have delved into crucial issues such as resource allocation for essential care resources like ventilators and hospital beds/capacity (Bozkir et al., 2023; Mehrotra et al., 2020; Yang, Sürer, et al., 2021), as well as investigating the impact of community mitigation strategies like social distancing and mask-wearing (Gel et al., 2020; Keskinocak et al., 2020) during the initial stages of the pandemic. However, with the rapid development of highly effective vaccines, the research efforts have progressively shifted towards studying operational dilemmas related to vaccine allocation and distribution.
The COVID-19 pandemic underscored the significance of timely and widespread vaccine deployment, particularly when highly effective vaccines are shown to prevent infections and hospitalizations to achieve herd immunity against novel pathogens (MacIntyre et al., 2022). However, the operational challenges associated with rapidly developed vaccines, such as those created and approved for COVID-19, necessitate careful consideration to ensure efficient, effective, and equitable deployment (Huang et al., 2012). Primarily, vaccine availability is typically limited, especially in the early stages of production, requiring prioritized allocation of scarce doses among various subgroups and geographical regions. Additionally, the diverse range of vaccines developed by different manufacturers offer varying levels and types of immunity against different pathogen strains. These vaccines also present different storage requirements (shelf life, doses per vial, cold storage, etc.) and dosing protocols (single or multiple shots), thereby complicating transportation, allocation, and distribution logistics. From a global perspective, addressing these challenges requires tailored solutions in different regions due to factors such as varying vaccine availability, healthcare infrastructure, socioeconomic pressures, education levels, and risk perceptions among citizens. Moreover, different countries may prioritize subpopulations and employ conflicting economic measures, leading to the adoption of diverse vaccine deployment and delivery strategies (Saadi et al., 2021).
In response to the various operational challenges encountered during the COVID-19 pandemic, the Operations Research/Management Science (OR/MS) community has generated a significant body of research comprising modeling and optimization studies, introducing novel dimensions and capabilities to address operational problems in this domain. Some of these optimization models incorporate dynamic considerations of disease transmission and infection stages, enabling accurate estimation of population-level vaccination requirements. Considering this diverse range of work on optimization models for pandemic vaccine management, this literature review offers a comprehensive overview of these papers using a systematic framework for the first time in COVID-19 literature. The primary objectives of this review are to (i) advocate for the adoption of such models in decision-making processes by public health officials in future pandemics and (ii) identify research gaps that hinder ongoing pandemic preparedness efforts undertaken by our nation and various global agencies. Furthermore, we categorize solution methodologies used in literature, encompassing heuristics, global and local optimization techniques, followed by research questions for operations research/management scientists applicable beyond pandemic vaccine deployment. Real life interpretation and importance of such models are also demonstrated. Thus, this review work provides a comprehensive view of pandemic vaccine supply chain models, spanning from their conceptualization to execution and fostering connections among interdisciplinary communities.
1.1 Search methodology and organization
Our search methodology involved identifying relevant articles published or made available online between the years 2020 and 2023, employing various combinations of keywords listed in the first, second, and third columns of Table 1. Through this approach, we identified a total of 109 articles that met the inclusion criteria. Additionally, to ensure comprehensive coverage of pertinent topics, we incorporated a selection of highly cited articles published between 2011 and 2019, as they offered valuable insights into issues of interest such as equitable vaccine allocation, vial size, and the vaccine supply chain. Figure A1 in the Appendix outlines the paper selection and classification process as a flow chart with Figure A2 showing the countrywise classification of real-life cased studies used by the surveyed optimization-articles. Abbreviation used throughout Appendix are spelled out in (Table A1).
| Keyword set 1 | Keyword set 2 | Keyword set 3 |
|---|---|---|
| Coronavirus vaccine | Allocation | Mathematical modelling |
| COVID-19 vaccine | Distribution | Mathematical programming |
| Influenza vaccine | Equity | Network |
| Pandemic vaccine | Facility location problem | Optimization |
| Vaccination | Inventory | Optimization model |
| Supply chain management | Simulation |
The articles covered in this review can be categorized into three main groups (i) Optimization articles, which encompass resource allocation, location-allocation, inventory management, supply chain management, and routing problems, employing at least one mathematical optimization model. Some of these articles incorporate simulation and compartmental epidemiological dynamics models, such as Susceptible-Infected-Recovered (SIR) models, integrated with the optimization models. (ii) Simulation-based articles, which focus on prioritization, management of multiple vaccine, and inventory control, utilizing simulation modeling to compare various scenarios or interventions of interest. These articles do not include an optimization model but rely on simulation-based approaches. (iii) Other articles, involving descriptive analysis, conceptualization, vaccine hesitancy, and waste management. These articles do not involve optimization or simulation models but offer valuable insights to guide future research in this field. In total, our review covers 77 optimization model-based articles, 17 simulation-based articles, and 15 articles employing alternative approaches. Notably, 76 out of the 94 articles involving optimization and simulation modeling specifically pertain to COVID-19 vaccine studies, highlighting the significant recent interest in pandemic vaccine management prompted by the COVID-19 pandemic.
The subsequent sections of this paper are organized as follows: Section 2 presents the vaccine supply chain framework along with the nomenclature adopted throughout the paper. In Sections 3, and 4, we propose a comprehensive optimization modeling framework for COVID-19 vaccine allocation and distribution management. These sections respectively address supply chain and location problems using mixed-integer linear programming techniques. Section 5 reviews the optimization technique found in vaccine logistics literature in presence of uncertainties. Additionally, Section 6 provides an overview of how equity and sustainability are incorporated into the models. In Section 7, we outline a generalized epidemiological modeling framework utilizing an extended version of the compartmental SIR model called DELPHI-V and present a framework for integrating optimization and epidemiological models. In Section 8, we provide a classification of methodological considerations and highlight the important insights from case studies. In Section 9, we offer an overall discussion of the literature as well as our own perspectives for model selection. In Section 10, we provide a critique and suggestions for future research directions. Appendix B covers routing problems. Finally, Appendix C covers simulation-based and other relevant articles in alignment with the optimization-based articles.
2 VACCINE SUPPLY CHAIN FRAMEWORK AND MODEL NOTATIONS
- Location: Decisions regarding locating facilities, such as mass vaccination centers.
- Inventory: Decisions on managing various aspects of vaccine inventory.
- Allocation: Joint decisions on vaccine allocation and assignment of demand nodes to locations.
- Distribution: Decisions regarding distribution of vaccines to demand nodes.
- Routing: Decisions regarding the management of vehicles/mobile vaccination clinics visiting depots, vaccination centers (VCs) and so forth.
Table 2 classifies the articles based on the specific type of decision being modeled, such as location, inventory, allocation, distribution, or routing. It also indicates whether a compartmental Susceptible–Infected–Recovered (SIR) type epidemiological model was utilized to capture the dynamic nature of the infectious disease being studied. It is worth noting that papers incorporating a SIR-type model often consider demand uncertainty within the modeling framework.
| Article | Type of decision | |||||
|---|---|---|---|---|---|---|
| Location | Inventory | Allocation | Distribution | Routing | SIR | |
| Supply chain models (See Section 3) | ||||||
| Resource allocation | ||||||
| Abbasi et al. (2020) | ||||||
| Anahideh et al. (2022) | ||||||
| Balcik et al. (2022) | ||||||
| Bandi et al. (2021) | ||||||
| Barth et al. (2023) | ||||||
| Bennouna et al. (2022) | ||||||
| Bertsimas et al. (2020) | ||||||
| Enayati and Özaltin (2020) | ||||||
| Hu et al. (2023) | ||||||
| Huang et al. (2017) | ||||||
| Jadidi et al. (2021) | ||||||
| Jarumaneeroj et al. (2022) | ||||||
| Li and Aprahamian (2023) | ||||||
| Minoza et al. (2021) | ||||||
| Munguía-López and Ponce-Ortega (2021) | ||||||
| Orgut et al. (2023) | ||||||
| Rao and Brandeau (2021) | ||||||
| Roy et al. (2021) | ||||||
| Shukla et al. (2022) | ||||||
| Thul and Powell (2023) | ||||||
| Yang et al. (2022) | ||||||
| Yarmand et al. (2014) | ||||||
| Inventory management | ||||||
| Azadi, Eksioglu, and Geismar (2020) | ||||||
| Azadi, Gangammanavar, and Eksioglu (2020) | ||||||
| Bonney and Jaber (2011) | ||||||
| Fadaki et al. (2022) | ||||||
| Georgiadis and Georgiadis (2021) | ||||||
| Hovav and Tsadikovich (2015) | ||||||
| Işik and Yildiz (2023) | ||||||
| Jahani et al. (2022) | ||||||
| Karakaya and Balcik (2023) | ||||||
| Mak et al. (2022) | ||||||
| Mohammadi et al. (2022) | ||||||
| Shah et al. (2022) | ||||||
| Sinha et al. (2021) | ||||||
| Supply chain management | ||||||
| Abbasi et al. (2023) | ||||||
| Basciftci et al. (2023) | ||||||
| Chowdhury et al. (2022) | ||||||
| Gilani and Sahebi (2022) | ||||||
| Goodarzian, Ghasemi, et al. (2022) | ||||||
| Goodarzian, Navaei, et al. (2022) | ||||||
| Habibi et al. (2023) | ||||||
| Kohneh et al. (2023) | ||||||
| Lai et al. (2021) | ||||||
| Li, Pan, et al. (2021) | ||||||
| Lim et al. (2022) | ||||||
| Manupati et al. (2021) | ||||||
| Rahman et al. (2023) | ||||||
| Rastegar et al. (2021) | ||||||
| Sazvar et al. (2021) | ||||||
| Shiri and Ahmadizar (2022) | ||||||
| Tang et al. (2022) | ||||||
| Tavana et al. (2021) | ||||||
| Wang et al. (2023) | ||||||
| Xu et al. (2021) | ||||||
| Yang, et al. (2021) | ||||||
| Location and routing models (See Section 4 and Appendix B) | ||||||
| Location-allocation | ||||||
| Bertsimas et al. (2022) | ||||||
| Bravo et al. (2022) | ||||||
| Cabezas et al. (2021) | ||||||
| Cao et al. (2023) | ||||||
| Dastgoshade et al. (2022) | ||||||
| Emu et al. (2021) | ||||||
| Enayati, Li, et al. (2023) | ||||||
| Enayati, Campbell, and Li (2023) | ||||||
| Kumar et al. (2022) | ||||||
| Leithäuser et al. (2021) | ||||||
| Lim et al. (2016) | ||||||
| Luo and Mehrotra (2023) | ||||||
| Lusiantoro et al. (2022) | ||||||
| Polo et al. (2015) | ||||||
| Soria-Arguello et al. (2021) | ||||||
| Srivastava et al. (2021) | ||||||
| Zhang et al. (2022) | ||||||
| Routing | ||||||
| Shamsi Gamchi et al. (2021) | ||||||
| Yang and Rajgopal (2021) | ||||||
| Yucesoy et al. (2022) | ||||||
Optimization articles are further grouped into five categories based on the decisions studied: (i) resource allocation, (ii) location-allocation, (iii) inventory management, (iv) supply chain management, and (v) routing. Further details on each paper in each category (model type, uncertainty type, commodity, period, echelons, objective, case study, and solution methodology) are compiled in Tables A2–A5 and B1 in the Appendix. Table 3 provides an overall summary for all of the optimization articles.
| Decision levels | Model type | Commodity type | Period | Case study | Solution method | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | Total | Location | Inventory | Allocation | Distribution | Routing | SIR | Deterministic | Uncertain | Single | Multiple | Multiple dose | Single | Multiple | Real Life | Synthetic | Commercial solver |
| Supply chain models | |||||||||||||||||
| Resource allocation | 23 | 0 (0%) | 0 (0%) | 23 (100%) | 10 (43%) | 0 (0%) | 13 (57%) | 9 (39%) | 14 (61%) | 17 (74%) | 6 (26%) | 2 (9%) | 10 (43%) | 13 (57%) | 18 (78%) | 5 (22%) | 4 (17%) |
| Inventory management | 13 | 0 (0%) | 13 (100%) | 9 (69%) | 7 (54%) | 0 (0%) | 1 (8%) | 4 (31%) | 9 (69%) | 8 (62%) | 5 (38%) | 4 (31%) | 2 (15%) | 11 (85%) | 10 (77%) | 3 (23%) | 1 (8%) |
| Supply chain management | 20 | 20 (100%) | 18 (90%) | 20 (100%) | 16 (80%) | 2 (10%) | 0 (0%) | 9 (45%) | 11 (55%) | 13 (65%) | 8 (40%) | 0 (0%) | 7 (35%) | 14 (70%) | 19 (95%) | 1 (5%) | 4 (20%) |
| Location and routing models | |||||||||||||||||
| Location-allocation | 18 | 18 (100%) | 0 (0%) | 16 (89%) | 5 (28%) | 0 (0%) | 1 (6%) | 12 (67%) | 6 (33%) | 15 (83%) | 2 (11%) | 0 (0%) | 14 (78%) | 3 (17%) | 16 (89%) | 2 (11%) | 7 (39%) |
| Routing | 3 | 2 (67%) | 0 (0%) | 0 (0%) | 3 (100%) | 3 (100%) | 1 (33%) | 1 (33%) | 2 (67%) | 3 (100%) | 0 (0%) | 1 (33%) | 2 (67%) | 1 (33%) | 3 (100%) | 0 (0%) | 2 (67%) |
| Total | 77 | 40 (52%) | 31 (40%) | 68 (88%) | 41 (53%) | 5 (6%) | 16 (21%) | 35 (45%) | 42 (55%) | 56 (73%) | 21 (27%) | 7 (9%) | 35 (45%) | 42 (55%) | 66 (86%) | 11 (14%) | 18 (23%) |
Tables 4–6 provide the index sets, parameters, and decision variables employed in our overarching optimization model. We strongly suggest the reader to refer to these tables when reading the technical expressions appearing in this review, since they are not necessarily described in the main text. Note that, in the above notation convention, the subscripts refer to the indexes. Letters , , , , and so forth are used as superscripts in many variables or parameters to highlight their association with the manufacturer (M), distribution center (DC), and vaccination center (VC), respectively. The manufacturer set can also be defined as where is its cardinality. All sets are defined using calligraphic letters. As necessary, we also interchangeably use to represent an index set with the entry ‘’ representing the last entry of that set starting from 1. Decision variable types are indicated with letters B (binary), C (continuous) and I (integer). I/C refers to decision variables that should have been integer but were treated as continuous to reduce complexity.
| Notation | Description |
|---|---|
| Set of manufacturers (M)/suppliers/vendors, indexed by | |
| Set of distribution centers (DC), indexed by | |
| Set of vaccination centers (VC), indexed by | |
| Set of vaccination centers (VC) that can be served by distribution center (DC) , indexed by | |
| Set of risk-based population groups, indexed by | |
| Set of different types of vaccines, indexed by | |
| Set of vaccine types that can only come from manufacturer (M) , indexed by | |
| Set of discrete time periods, indexed by | |
| Set of vehicles, indexed by | |
| Set of regions, indexed by or | |
| Set of population sites (PS), indexed by | |
| Set of coverage levels to serve population sites (PS)/villages , indexed by | |
| Set of outreach centers/teams, indexed by | |
| Set of scenarios representing uncertainty, indexed by | |
| Ambiguity set in distributionally robust optimization (DRO) |
| Notation | Description | Equations |
|---|---|---|
| Capacity | ||
| Production capacity of manufacturer at time period for type of vaccine | 1 | |
| Capacity of distribution center (DC) | 8 | |
| Capacity of vaccination center (VC) (Overall capacity/availability over all VCs) | 34-63 | |
| Fleet size availability | 15 | |
| Safety stock level of distribution center (DC) | 8 | |
| Capacity of outreach center (OC) | 37 | |
| Capacity of vehicle | B1 | |
| Cold refrigeration capacity of distribution center (DC) | 10 | |
| Very cold refrigeration capacity of distribution center (DC) | 11 | |
| Ultra cold refrigeration capacity of distribution center (DC) | 11-12 | |
| Cost | ||
| Shortage cost for vaccine at vaccination center (VC) | In Text | |
| Environmental impact cost for establishing a facility | 48 | |
| Amount of carbon emmission by transshipping a unit of vaccine | 48 | |
| Cost of locating vaccination center (VC) at | 35-36 | |
| Total budget | 35-36 | |
| Demand | ||
| Demand of vaccine type among population group at location in time period | 28-45 | |
| Demand for vaccine type at vaccination center (VC) at time period | 16-17 | |
| Demand of region for risk group | B1,46-47 | |
| Demand (average demand rate) of distribution center (DC) | 38 | |
| Minimum demand satisfaction ratio | 44-45 | |
| Demand of population site (PS) | 34 | |
| Coverage coefficient at population site (PS) | 35-36 | |
| Coverage fraction attainable when the nearest serving center is between and | 36 | |
| Population size of region (of population site (PS) ) | 63 | |
| Inventory | ||
| Lead time to deliver order to distribution center (DC) | 38 | |
| Ordering cost to order from manufacturer (M) by distribution center (DC) | 38 | |
| Holding cost to hold inventory at facility | 38 | |
| Logistics | ||
| 1, if vaccine coming from manufacturer (type ) needs very cold or ultra-cold refrigeration | 10-13 | |
| 1, if vaccine coming from manufacturer (type ) needs ultra-cold refrigeration | 11-13 | |
| 1, if vaccination center (VC) is feasible to be assigned to distribution center (DC) | 18 | |
| 1, if population site (PS) is feasible to be assigned to vaccination center (VC) | 34 | |
| Distance from node to node (general index notation and ) | 34-48 | |
| Travel time from region to region | B1 | |
| Service time of vehicle (mobile vaccination clinic) at region | B1 | |
| Maximum allowable distance | 35-37 | |
| Distance at coverage level | 37 | |
| Estimated equitable quantity that should be allocated to region for priority group | 47 | |
| Others | ||
| Weight such that | 46 | |
| Big- value | ||
| Context specific penalty term (not necessarily a large value) or importance factor | 41-46 | |
| Vaccine | ||
| Vaccine vial shelf life | 20-24 | |
| Vaccine safe-use-life after opening the vial | 26-29 | |
| Number of vaccines contained in a vial size | 25 | |
| Number of vaccines contained in a vial size , for group | 26-27 | |
| Workforce | ||
| Number of people a health worker can serve within time | 19 | |
| Existing workforce in vaccination center (VC) in time | 19 | |
| Uncertainty modeling | ||
| Significance level such that | 38-43 | |
| score of standard normal distribution at confidence level | 38-43 | |
| Mean (standard deviation) estimate of an uncertain parameter | 38-43 | |
| Ambiguity in empirical estimate of a parameter vaccination center (VC) in time | 42 | |
| Factor to scale an ambiguously estimated parameter such that | 42 | |
| Probability that a scenario would happen | 41 | |
| Notation | Description | Type | Equations |
|---|---|---|---|
| Allocation | |||
| 1, if vaccination center (VC) is assigned to distribution center (DC) | B | 18-45 | |
| Assignment variable (or percentage, Continuous). Assigning population site (PS) to vaccination center (VC) | B/C | 34 | |
| 1, if population site (PS) is covered by a facility for vaccinating its population | B | 35 | |
| 1, if population site (PS) is covered by a facility at its distance based level | B | 36-37 | |
| 1, if supply happens to vaccination center (VC) by distribution center (DC) at time period | B | In Text | |
| Number of people of group vaccinated by vaccine type at vaccination center (VC) during time period | I/C | 16-44 | |
| Number of people vaccinated by vaccine type , at vaccination center (VC) during time | I/C | 17 | |
| Number of people of group vaccinated by vaccine type at vaccination center (VC) during time period from a vial opened at time period | I/C | 26-29 | |
| Number of vaccine type available to fulfill demand or exactly meeting demand of priority group in vaccination center (VC) at time period | I/C | 30-33 | |
| Amount of shortages of vaccine type in vaccination center (VC) at time period by group | I/C | 29-48 | |
| Amount of shortages of vaccine type in vaccination center (VC) at time period | I/C | 17 | |
| Number of type vaccine vials allocated to vaccination center (VC) at time and opened in time | I/C | 20-23 | |
| Number of type vaccine vials opened at vaccination center (VC) in time | I/C | 22-23 | |
| Number of type vaccine vial of size opened at vaccination center (VC) in time | I/C | 25-27 | |
| 1, if vaccination center (VC) served by an outreach center/team | B | 37 | |
| Number of type vaccine vials available in vaccination center (VC) at initial time period and opened in time | I/C | 23-24 | |
| Positive and negative deviation from a quantity particular to a region for group | C | 46-47 | |
| Positive difference between minimum percentage of demand to be met and allocation in region for group | C | 46 | |
| Fraction of total population in region (of risk group ) | C | 63 | |
| Inventory | |||
| Inventory available at distribution center (DC) at the end of time period | I/C | 3-9 | |
| Inventory available at vaccination center (VC) at the end of time period | I/C | 16-33 | |
| Number of type vaccine vials available at initial time period at vaccination center (VC) | I/C | 24 | |
| Order quantity of distribution center (DC) | I/C | 38 | |
| Location | |||
| 1, if distribution center (DC) at location with usual cold storage facility is launched | B | 10-48 | |
| 1, if distribution center (DC) at location with very cold storage facility is launched | B | 13-48 | |
| 1, if ultra-cold storage facility is added to an existing distribution center (DC) | B | 11-48 | |
| 1, if distribution center (DC) having only usual cold storage facility is launched | B | 5-48 | |
| 1, if vaccination center (VC) is established | B | 34-48 | |
| Routing | |||
| 1, if region and are served in order in a vehicle routing problem | B | B1 | |
| 1, if region is served by vehicle | B | B1 | |
| Service start time of region | C | B1 | |
| 1, if region is visited by a vehicle | B | In Text | |
| Transshipment | |||
| Number of vaccine-type arriving from manufacturer to distribution center (DC) at the beginning of time period | I/C | 1-48 | |
| Number of vaccine-type arriving from manufacturer to distribution center (DC) at the beginning of time period , ordered at | I/C | 3-6 | |
| Amount of vaccines shipped from manufacturer to distribution center (DC) , ordered at time , received at time | I/C | 10-14 | |
| Number of vaccine type transferred from distribution center (DC) to vaccination center (VC) at time (using vehicle ) | I/C | 3-48 | |
| Number of vaccine type transshipped from distribution center (DC) to another distribution center (DC) at time period | I/C | 3-48 | |
| Number of vaccine type arriving to distribution center (DC) from another distribution center (DC) via transshipment at time period | I/C | 3-7 | |
| Number of vaccine type transshipped from vaccination center (VC) to another vaccination center (VC) at time period | I/C | 30-48 | |
| Number of vaccine type arriving to vaccination center (VC) from another vaccination center (VC) via transshipment at time period | I/C | 30-31 | |
| 1, if selection of manufacturer at time for vaccine production occurs | B | 1 | |
| 1, if vaccine type ordered from manufacturer at time to be delivered to distribution center (DC) at the beginning of time | B | 2 | |
| Vaccine wastage | |||
| Wasted amount of type vaccine vial at distribution center (DC) at time | I/C | 3-9 | |
| Wasted amount of type vaccine vial at vaccination center (VC) at time | I/C | 16-31 | |
| 32-33 | |||
| Amount of type vaccine vials available in vaccination center (VC) at initial time period and wasted in time due to time expiration | I/C | 24 | |
| Wasted dosage of vaccine type in vaccination center (VC) at time period from opened vial | I/C | 25-28 | |
| Workforce | |||
| Required number of health care worker in vaccination center (VC) at time period | I | 19 | |
| Additional healthcare workers required in vaccination center (VC) at time period | I | 19 | |
| Uncertainty modeling | |||
| Probability that a scenario would happen | C | 42 | |
In the next five sections of mathematical models for pandemic vaccine supply chain, the categorization can be seen into two broad classes: (i) traditional supply chain model, with specific considerations for handling vaccines (Sections 3–6), and (ii) models in epidemiological dynamics while building upon traditional vaccine supply chain models (Section 7). This is important for endemic/pandemic management. If the focus of a work is on location (e.g., coverage model) or routing decision without much consideration for other details in the entire supply chain, we position them in a separate section (Section 4). In vaccine supply chain model, it is important to take uncertainty into account in practice. This uncertainty can be modeled in various ways, and hence we delve into these diverse approaches in Section 5, offering some details and illustrating their broad applicability. Finally, to highlight the importance—equity and multi-criteria decision-making in pandemic vaccine distribution, especially in the early stage, we separately discuss them in Section 6.
Note that we first present core mathematical models pertinent to the scope of each section/subsection. If some of the models are unique to the vaccine supply chain, we also present them separately. Models dealing with cold storage refrigeration requirements, vaccine vial shelf life, relationship between vaccine vials, dosages and open vial wastage, priority group, equity, epidemiological dynamics, and herd immunity are some of those considerations. Given the scope of this review some of the advanced models are only discussed to ensure greater completeness.
3 SUPPLY CHAIN MODELS
In this section, we present a comprehensive perspective on the diverse factors considered in the existing literature concerning the COVID-19 vaccine supply chain across multiple echelons. We achieve this by examining various objective functions and constraints that have been utilized for different components of the supply chain. Specifically, we emphasize key constraints derived from mathematical models documented in relevant literature, while also highlighting the potential challenges associated with modeling the intricate network of vaccine allocation and distribution for manufacturers (Section 3.1), distribution centers (Section 3.2), and vaccination centers (Section 3.3).
3.1 Manufacturers
The capacity constraint can take various forms depending on the specific problem context. To achieve a more aggregated representation of the constraints in terms of vaccine type, time, and facility, the decision variables, parameters, summations, and set indexes in (1) can be redefined. This allows for a more generalized formulation of the capacity constraint, accommodating different variations based on the problem.
While the majority of research on COVID-19 focuses on shipments from manufacturers to distribution centers (DCs), it is worth noting that manufacturers can also directly transship vaccines to vaccination centers (VCs). Additionally, in the context of COVID-19, all manufacturers typically produce a single type of vaccine. For example, Johnson & Johnson plants exclusively manufacture the Janssen Vaccine, while Pfizer plants exclusively produce the BioNTech Vaccine. However, in cases where a manufacturing plant produces multiple types of vaccines, the set can be incorporated into (1) and (2) to account for this variation. Furthermore, some articles consider the flow of raw materials from suppliers to manufacturers (Chowdhury et al., 2022; Gilani & Sahebi, 2022; Sazvar et al., 2021), the manufacturing process itself (Shah et al., 2022), and the location decisions of manufacturers with different technology levels (Sazvar et al., 2021). These aspects contribute to a more comprehensive understanding of the COVID-19 vaccine supply chain.
3.2 Distribution center
In this section, we present the considerations related to distribution centers (DCs) in terms of flow conservation (Section 3.2.1), cold chain requirements (Section 3.2.2), and fleet size (Section 3.2.3). These considerations are based on optimization models proposed in recent COVID-19 literature addressing resource allocation (Abbasi et al., 2020; Anahideh et al., 2022; Balcik et al., 2022; Bennouna et al., 2022; Bertsimas et al., 2020; Enayati & Özaltin, 2020; Huang et al., 2017; Jadidi et al., 2021; Jarumaneeroj et al., 2022; Minoza et al., 2021; Munguía-López & Ponce-Ortega, 2021; Rao & Brandeau, 2021; Roy et al., 2021; Shukla et al., 2022; Thul & Powell, 2023; Yang et al., 2022; Yarmand et al., 2014), inventory management (Azadi, Eksioglu, & Geismar, 2020; Azadi, Gangammanavar, & Eksioglu, 2020; Bonney & Jaber, 2011; Fadaki et al., 2022; Georgiadis & Georgiadis, 2021; Hovav & Tsadikovich, 2015; Karakaya & Balcik, 2023; Mak et al., 2022; Mohammadi et al., 2022; Shah et al., 2022; Sinha et al., 2021), and supply chain management (Basciftci et al., 2023; Chowdhury et al., 2022; Gilani & Sahebi, 2022; Goodarzian, Ghasemi, et al., 2022; Goodarzian, Navaei, et al., 2022; Habibi et al., 2023; Kohneh et al., 2023; Lim et al., 2022; Manupati et al., 2021; Rahman et al., 2023; Rastegar et al., 2021; Sazvar et al., 2021; Shiri & Ahmadizar, 2022; Tavana et al., 2021; Wang et al., 2023; Xu et al., 2021; Yang, Bidkhori, & Rajgopal, 2021). These models contribute to a comprehensive understanding of the distribution center (DC) aspects within the COVID-19 vaccine supply chain.
3.2.1 Flow conservation
Distribution centers (DCs) play a critical role in the vaccine supply chain, facilitating both inflows and outflows of vaccines. The inflows to distribution centers (DCs) include procurement from manufacturers, their own inventory carried over from previous time periods, and shipments received from other distribution centers (DCs). Conversely, the outflows involve transshipments to vaccination centers (VCs), maintaining inventory for future time periods, shipments to other distribution centers (DCs), and addressing vaccine waste resulting from expiration, damage (e.g., physical or temperature-related), or mishandling. If distribution centers (DCs) can also function as vaccination centers (VCs), an additional vaccine outflow can be the administration of vaccines to individuals.
To maintain flow conservation in distribution centers (DCs) (3) to (8) are utilized. Equations (3) and (4) represent the inventory flow conservation constraints for distribution centers (DCs). Equation (5) ensures that if there is an outflow from a distribution center (DC), it must correspond to an existing outflow location. Similarly, (6) and (7) ensure that if there is an inflow to a distribution center (DC), it must originate from an existing inflow location. It should be noted that not all distribution centers (DCs) may serve all vaccination centers (VCs). In such cases, the flow balance constraints can be modified by incorporating the index set , which represents the set of vaccination centers (VCs) exclusively served by distribution center (DC) .
In Fadaki et al. (2022), flow units of different decision variables or parameters differ. For example, if inflow is in terms of vaccine lots and outflow is in terms of vaccine vials, corresponding variables must be multiplied by the appropriate conversion coefficient. Moreover, Hinojosa et al. (2000) consider fractional flow (i.e., using continuous decision variables instead of discrete decision variables).
3.2.2 Cold storage refrigeration requirement
3.2.3 Fleet and drones
Vaccine vials can be transported between different echelons using various refrigerated vehicles, each with its own capacity for different levels of refrigeration (Chowdhury et al., 2022; Dastgoshade et al., 2022; Goodarzian, Ghasemi, et al., 2022; Habibi et al., 2023; Hovav & Tsadikovich, 2015; Lim et al., 2022). However, it is worth noting that the majority of existing studies in the literature do not consider modeling of vehicles and exclude them from their scope. Among the works that do incorporate vehicles, refrigerated trucks are commonly utilized as the primary transportation mode. Similarly, Lim et al. (2022) explore the use of motorbikes.
Notable studies by Wang et al. (2023), Enayati, Li, et al. (2023), and Enayati, Campbell, and Li (2023) explore the use of drones for vaccine distribution. While Wang et al. (2023) optimizes drone capacity with vaccination scheduling in facility location problems, models in the latter two works involve location decision of drone base, drone relay station, and distribution centers (DCs) layering each possible origin-destination path for drone delivery with controlled drone stops, drone range, and cold-time.
3.3 Vaccination center
In this section, we discuss the vaccination center (VC) considerations in terms of flow conservation (Section 3.3.1), DC-VC assignment (Section 3.3.2), healthcare personnel capacity/scheduling (Section 3.3.3), vaccine shelf life (Section 3.3.4), vaccine vials (Section 3.3.5), and priority groups (Section 3.3.6), based on the optimization models presented in the recent COVID-19 literature.
3.3.1 Inventory and flow balancing
Vaccination centers (VCs), similar to distribution centers (DCs), experience both inflows and outflows of vaccines. The inflows of vaccines at vaccination centers (VCs) can originate from several sources, including procurement from manufacturers, their own inventory carried over from previous time periods, and shipment from other vaccination centers (VCs) (lateral transshipment) or distribution centers (DCs). Conversely, the outflows from vaccination centers (VCs) involve shipment to other vaccination centers (VCs), maintaining inventory for future periods, returning leftover vaccines to distribution centers (DCs), managing vaccine waste (such as open vial wastage or improper administration), and administering vaccines to individuals. In the context of this article, we assume that a vaccination center (VC) can only have inventory, vaccination, and waste as outflows. Additionally, the center can only receive inflows from its own inventory from the previous periods and distribution centers (DCs). It is important to note that vaccine demand can be either deterministic or stochastic. Furthermore, when managing lateral shipment to minimize open vial wastage, the flow should be considered at both the dosage and vial levels. In this formulation, the demand is expressed in terms of vaccine vials. If the demand were stated in terms of vaccine doses, appropriate conversion coefficients would need to be included for accurate calculations.
3.3.2 Assigning a vaccination center to a distribution center
Once the distribution center (DC) assignment is specified, the next step is to allocate vaccines from the distribution center (DC) to various destinations, such as regional depots (Balcik et al., 2022; Huang et al., 2017; Munguía-López & Ponce-Ortega, 2021), vaccination centers (VCs) (Abbasi et al., 2020; Anahideh et al., 2022; Bertsimas et al., 2020; Roy et al., 2021; Shukla et al., 2022; Thul & Powell, 2023; Yang et al., 2022; Yarmand et al., 2014), or specific sub-population groups (Bennouna et al., 2022; Enayati & Özaltin, 2020; Jadidi et al., 2021; Jarumaneeroj et al., 2022; Minoza et al., 2021; Rao & Brandeau, 2021). Different allocation rules and prioritization strategies are used in these models to determine the amount of vaccine to be allocated, with the objective of minimizing the spread of infection while considering the limited availability of vaccines. In addition to efficiency concerns, equity among the served subpopulations is often a key objective or constraint in these models. For more detailed information on resource allocation articles, refer to Table A2 in the Appendix.
3.3.3 Healthcare personnel capacity and vaccination scheduling
Wang et al. (2023); Zhang et al. (2022) focus on vaccination scheduling. Zhang et al. (2022) propose a mass vaccination appointment scheduling model that has the potential to be extended to healthcare personnel scheduling, including overtime requirements. Their linear ordering formulation considers location, population size, and time window inputs to determine optimal vaccination center (VC) location, appointment acceptance, assignment of appointments to selected vaccination centers (VCs), and the scheduling of the vaccination timetable while accounting for possible tardiness. The formulation is further enhanced using dominance rules and valid inequalities, and is solved using a logic-based Benders approach. Bandi et al. (2021) emphasize the importance of efficient appointment slot management, second-dose reserves, and flexible vaccine preferences for optimizing vaccination rates during a two-dose roll-out.
3.3.4 Vaccine vial shelf life
3.3.5 Relationship between vaccine vials, dosages and open vial wastage
Finally, Mak et al. (2022) assess the inventory dynamics of the rollout process for three strategies: (i) holding back second doses, (ii) releasing second doses, and (iii) stretching the lead time between doses.
3.3.6 Priority group focus
4 LOCATION AND ROUTING MODELS
Decisions on distribution center (DC) (Dastgoshade et al., 2022; Emu et al., 2021; Soria-Arguello et al., 2021; Srivastava et al., 2021) and vaccination center (VC) locations (Bertsimas et al., 2022; Bravo et al., 2022; Cabezas et al., 2021; Kumar et al., 2022; Leithäuser et al., 2021; Lim et al., 2016; Luo & Mehrotra, 2023; Lusiantoro et al., 2022; Polo et al., 2015; Zhang et al., 2022) are important for vaccine supply-demand matching in the context of COVID-19. Models are based on demand, distance, coverage, priority, required service levels, and fairness. A recent article considers locating waste disposal center (Cao et al., 2023). In this section, we present studies that focus on a variant of the assignment type (Section 4.1) or coverage type (Section 4.2) facility location models. In the formulations used in this section, vaccination center (VC) is the facility and population site (PS) is the demand source. Similar formulations can be used for the case where distribution center (DC) is the facility and vaccination center (VC) is the demand source. We present vehicle routing in Appendix B.
4.1 Facility location models
Bravo et al. (2022) consider a formulation similar to the one given in (34), where the second objective function is not considered and the decision variable is a binary assignment variable, which captures percent coverage in the current formulation. In addition to the classical facility location factors, Polo et al. (2015) consider healthcare workforce planning and spatial accessibility using GIS data (Radke & Mu, 2000). Cabezas et al. (2021) use stochastic optimization to incorporate patient preference of vaccination centers (VCs) in terms of distance. Bertsimas et al. (2022) is the only paper that considers an epidemiological model within a location model. Dastgoshade et al. (2022) develop an allocation optimization framework using Rawls' theory, Sadr's theory, and utilitarianism fairness theories. Unlike other studies, Soria-Arguello et al. (2021) locate intermediate distribution centers (DCs) based on production plants and existing distribution centers (DCs). Kumar et al. (2022) compute number of optimal days a vaccination center (VC) should operate. Luo and Mehrotra (2023) integrate linear utility function with COVID-19 service center location decision that depends on travel distance, waiting time and location based service features. Zhang et al. (2022) merge appointment scheduling with vaccination center (VC) location problem. The location decisions are dependent on the accepted appointments and vaccination sequencing in each site. Cao et al. (2023) find the best locations for COVID-19 waste disposal centers by considering stochastic infection risk, while carrying the waste and the population around candidate disposal centers.
4.2 Coverage models
5 UNCERTAINTY MODELING IN COVID-19 VACCINE SUPPLY CHAIN MODELS
In COVID-19 vaccine supply chain literature, demand uncertainty is the predominant form of uncertainty (Azadi, Eksioglu, & Geismar, 2020; Azadi, Gangammanavar, & Eksioglu, 2020; Basciftci et al., 2023; Bennouna et al., 2022; Bertsimas et al., 2020; Bertsimas et al., 2022; Chowdhury et al., 2022; Dastgoshade et al., 2022; Enayati & Özaltin, 2020; Goodarzian, Ghasemi, et al., 2022; Habibi et al., 2023; Jadidi et al., 2021; Jarumaneeroj et al., 2022; Kohneh et al., 2023; Lai et al., 2021; Mak et al., 2022; Manupati et al., 2021; Mehrotra et al., 2020; Minoza et al., 2021; Rao & Brandeau, 2021; Sazvar et al., 2021; Shamsi Gamchi et al., 2021; Shiri & Ahmadizar, 2022; Sinha et al., 2021; Thul & Powell, 2023; Yang et al., 2022; Yang & Rajgopal, 2021). However, research has also considered uncertainty in supply (Chowdhury et al., 2022; Dastgoshade et al., 2022; Jahani et al., 2022; Karakaya & Balcik, 2023; Mohammadi et al., 2022; Wang et al., 2023), lead time (Habibi et al., 2023; Jahani et al., 2022; Karakaya & Balcik, 2023; Manupati et al., 2021; Yang & Rajgopal, 2021), infection (Basciftci et al., 2023; Cao et al., 2023; Roy et al., 2021), vaccination location choice (Cabezas et al., 2021; Luo & Mehrotra, 2023), vaccine accessibility (Gilani & Sahebi, 2022; Yarmand et al., 2014) and vaccine wastage (Rahman et al., 2023; Shah et al., 2022). While demand from a distribution center (DC) to manufacturer (Gilani & Sahebi, 2022; Goodarzian, Ghasemi, et al., 2022; Manupati et al., 2021; Shah et al., 2022) and manufacturing lead time (Manupati et al., 2021) impact the DC-manufacturer level uncertainty, vaccination center (VC) level demand uncertainty and their variation across regions is influenced by numerous factors including gender, education, employment, exposure to misinformation, and hesitancy (Seboka et al., 2021). These uncertainties affect both DC-VC and VC-PS related echelons.
In terms of mathematical modeling of such uncertainties, stochastic inventory model (Chick et al., 2008; Mak et al., 2022; Manupati et al., 2021; Shah et al., 2022; Sinha et al., 2021; Yarmand et al., 2014), two-stage stochastic programming (Azadi, Gangammanavar, & Eksioglu, 2020; Basciftci et al., 2023; Karakaya & Balcik, 2023; Lai et al., 2021; Mohammadi et al., 2022; Shiri & Ahmadizar, 2022), robust and distributionally robust optimization approaches (Basciftci et al., 2023; Gilani & Sahebi, 2022; Luo & Mehrotra, 2023; Mohammadi et al., 2022; Wang et al., 2023), chance-constraint modeling (Azadi, Eksioglu, & Geismar, 2020; Goodarzian, Ghasemi, et al., 2022; Rahman et al., 2023) are noteworthy. Forecasting, predictive analytics, machine learning methods, simulation tools as well as their ensembles (Bennouna et al., 2022) are also used to obtain estimates of the uncertain parameters and aid optimization under various types of uncertainties. Other practices of modeling uncertainty found in COVID-19 vaccine literature include queuing (Jahani et al., 2022), fuzzy programming (Kohneh et al., 2023), game theory (Sinha et al., 2021), decision trees (Manupati et al., 2021), epidemiological modeling (Bertsimas et al., 2020; Enayati & Özaltin, 2020; Jadidi et al., 2021; Jarumaneeroj et al., 2022; Minoza et al., 2021; Rao & Brandeau, 2021; Thul & Powell, 2023; Yang et al., 2022) and so forth. The latter is separately explored in Section 7. In this section, we present stochastic inventory models (Section 5.1), two stage stochastic programming approaches (Section 5.2), robust/distributional robust approaches (Section 5.3), and chance constrained modeling (Section 5.4).
5.1 Stochastic inventory models
In response to supply chain uncertainties, vaccine literature, besides leveraging the inventory balancing equations discussed earlier, also uses to continuous or periodic inventory review policies. Mak et al. (2022) focus on the scenario of very limited supply during the early period of COVID-19, examine varied two-dose vaccine rollout policies by analyzing the related inventory dynamics.
Periodic review policy-based inventory decisions that review stocks periodically are considered using a newsvendor modeling framework. Yarmand et al. (2014) propose a closed-form solution to Phase-1 allocation of a two-phase vaccine allocation problem using the newsvendor approach. This work uses the ratio of underage cost to summation of underage and overage cost and finds a closed-form solution for vaccine allocation. The newsvendor structure is also utilized to model contracts for supply chain coordination (Chick et al., 2008).
Sinha et al. (2021) use inventory balancing equations similar to those described in the earlier sections to model uncertainties. For instance, using forecasts, Sinha et al. (2021) replace the term by in (16) for critical nodes of a supply chain network. Due to budget and infrastructure inefficiencies (especially in developing countries), vaccination centers (VCs) may not get sufficient number of vaccines from distribution centers (DCs) on a regular basis. A model in such a context adds binary variables to decide at which time period a distribution center (DC) should supply vaccine to a vaccination center (VC). For a similar reason, lead time can be uncertain as well. Sinha et al. (2021) analyze major disruption scenarios and store additional vaccine supplies in strategic higher-echelon nodes to prevent transshipment lead time uncertainty.
5.2 Two-stage stochastic programming approach
Mehrotra et al. (2020) were the earliest to use a TSSP framework in the context of ventilator allocation. The TSSP model proposed by Lai et al. (2021) minimizes setup costs by considering the opening of vaccination centers (VCs), vaccination center (VC) assignment to population sites, and required healthcare personnel as first-stage variables. Each discrete scenario of the second stage model incorporates multi-period planning and considers vaccine transportation, inventory, shortages, and satisfied demand at each location within the planning horizon as decision variables. The number of personnel required at each vaccination center (VC) in each time period is also considered, ensuring that the minimum average realized demand threshold is met based on their service rate. Additionally, a coupling constraint prevents the number of healthcare personnel needed to meet demand from exceeding the total workers assigned in the strategic stage at any given time period. Similarly, demand of a population site is satisfied by a vaccination center (VC) if only if the first stage establishes a one-to-one assignment between them.
In the work of Basciftci et al. (2023), the TSSP model minimizes setup and operational costs of different distribution centers (DCs), while considering inventory balancing and lead time. However, it does not account for the connection between facility requirements and healthcare workers. Karakaya and Balcik (2023), on the other hand, propose a TSSP model that focuses on supply level uncertainty and aims to minimize the expected weighted sum of deviations from the vaccination timeline for each priority group made in the first stage. In each scenario, their second stage model calculates earliness and lateness deviation, taking into account factors such as scenario-specific vaccination starting and completion time, dosage scheme, inventory availability, and other factors with necessary coupling of the first stage decisions. Mohammadi et al. (2022) present a TSSP model that incorporates uncertainty in both supply and demand, including parameters related to pandemic progression, vaccination effectiveness, social contacts, and uncertain death rates. Their objective is to minimize cost and the number of deaths, with the added consideration of vaccination center (VC) capacity determined through a queuing system.
5.3 Robustness and distributionally robust approaches
Motivated by Bertsimas et al. (2016), Gilani and Sahebi (2022) propose a dynamic robust optimization approach based on uncertainty sets and cutting hyperplanes, to reduce conservatism compared to its static counterpart while maintaining robustness. Wang et al. (2023) develop a two-stage robust optimization model for facility location and scheduling under supply uncertainty, with the goal of distributing vaccines to remote areas using drones.
5.4 Chance-constrained modeling
6 EQUITY, SUSTAINABILITY, AND MULTI-OBJECTIVE MODELING
6.1 Equity considerations
In public health decision-making, equity plays a significant role. A significant number of papers has studied this concern in the context of COVID-19 (Balcik et al., 2022; Basciftci et al., 2023; Bennouna et al., 2022; Bertsimas et al., 2020; Dastgoshade et al., 2022; Enayati & Özaltin, 2020; Fadaki et al., 2022; Lim et al., 2016; Mohammadi et al., 2022; Munguía-López & Ponce-Ortega, 2021; Rastegar et al., 2021; Shiri & Ahmadizar, 2022; Tavana et al., 2021; Wang et al., 2023). Equity can be integrated into decision models by incorporating it into the objective function and/or the constraints. Within the COVID-19 literature, two main dimensions of equity are examined: priority group-based (Basciftci et al., 2023; Enayati & Özaltin, 2020; Fadaki et al., 2022; Mohammadi et al., 2022; Shiri & Ahmadizar, 2022), geography-based (Bertsimas et al., 2020; Lim et al., 2016; Munguía-López & Ponce-Ortega, 2021; Wang et al., 2023) or both (Balcik et al., 2022; Bennouna et al., 2022; Dastgoshade et al., 2022; Rastegar et al., 2021; Tavana et al., 2021). Priority group equity involves distributing vaccines equitably among diverse sub-populations, taking into account factors like risk, age, occupation, and other demographic characteristics. This approach ensures that individuals with higher vulnerability or essential roles have equitable access to vaccines. Geography-based equity aims to ensure that vaccines are distributed equitably across different geographical locations. Decision makers consider factors such as population density, healthcare infrastructure, and disease prevalence to achieve an equitable allocation of vaccines.
The approaches discussed in Munguía-López and Ponce-Ortega (2021) include Rawlsian justice and the Social Welfare II approaches. Rawlsian justice principle maximizes the minimum allocated vaccine over all regions. Social Welfare II ensures vaccine allocation per region by maximizing the sum of their squared deviations from the allocation quantity possible in each region without equity consideration to achieve the overall maximum allocation. Bertsimas et al. (2022) consider equity in deciding the location of vaccination centers (VCs) at a strategic level. The number of sites opened in each state is determined based on the total target facility setup weighted by the state's population share, with efficiency-fairness trade-offs controlled through model parameters. Enayati and Özaltin (2020) incorporate equity using the well-known Gini coefficient (or index) expressed as , where is a decision variable representing the fraction of population covered from population class .
6.2 Sustainability considerations
Bonney and Jaber (2011) use carbon emissions, waste disposal cost and social cost, in addition to ordering cost per replenishment cycle, to derive the optimal order quantity of an environmentally-friendly EOQ model. Moreover, Shah et al. (2021) consider carbon tax and amount of carbon emissions during manufacturing, holding, preparation, purchase and inspection processes to build an inventory model that minimizes total cost of an inventory system per unit time.
6.2.1 Waste management
There are two optimization papers that consider waste management in the context of COVID-19 vaccine supply chain. Abbasi et al. (2023) emphasize the importance of building a green SCM that not only addresses the environmental impact of biomedical and plastic waste but also considers the hygiene costs associated with waste management. In parallel, Işik and Yildiz (2023) delves into provincial-level waste management in Turkey, with a specific focus on medical waste generated during the vaccine distribution process.
6.3 Multi-objective considerations
Equity, sustainability, and economic objectives often conflict. Several papers consider conflicting multiple objective functions in resource allocation (Anahideh et al., 2022; Minoza et al., 2021), location-allocation (Bertsimas et al., 2022; Cao et al., 2023; Dastgoshade et al., 2022; Leithäuser et al., 2021; Lusiantoro et al., 2022; Polo et al., 2015), inventory management (Fadaki et al., 2022; Jahani et al., 2022; Mohammadi et al., 2022), supply chain management (Chowdhury et al., 2022; Gilani & Sahebi, 2022; Goodarzian, Ghasemi, et al., 2022; Goodarzian, Navaei, et al., 2022; Kohneh et al., 2023; Li, Pan, et al., 2021; Mohammadi et al., 2022; Sazvar et al., 2021; Tang et al., 2022; Wang et al., 2023) and routing (Shamsi Gamchi et al., 2021). For example, expressions in (48) are conflicting, since, in order to prevent vaccine shortage, more vaccine should be shipped and more facilities should be located. In the bi-objective TSSP and robust optimization model of Mohammadi et al. (2022), death and cost minimization are also conflicting to each other. To handle the conflicting nature of the models, different multi-objective approaches have been employed. Regardless of the number of objectives in these multi-objective models, most literature eventually works with a single objective after using other objective(s) as constraints.
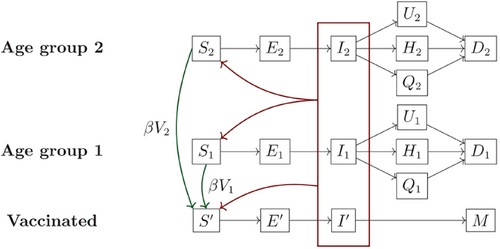
7 MODELS CONSIDERING EPIDEMIOLOGICAL DYNAMICS
| Notation | Description |
|---|---|
| Vaccine effectiveness percentage | |
| Nominal infection rate | |
| Governmental and societal response function modulating infection rate over different time | |
| Infection progression rate | |
| Detection rate | |
| Death rate | |
| Transition rate to undetected compartment for risk group | |
| Hospitalization rate for risk-based population risk group | |
| Quarantine rate for risk-based population risk group | |
| Number of susceptible people in region , risk group (vaccinated class, see Figure 2) and time | |
| Number of eligible people in region , risk group and time | |
| Number of vaccinated people in region , risk group and time | |
| Number of exposed people in region , risk group (vaccinated class) and time | |
| Number of infectious people in region , risk group (vaccinated class) and time | |
| Number of undetected people in region , risk group and time | |
| Number of dead people in region , risk group and time | |
| Number of immunized people in region , risk group and time | |
| Number of quarantined people in region , risk group and time | |
| Number of hospitalized people in region , risk group and time |
7.1 Embedding SIR dynamics into optimization modeling
Bertsimas et al. (2022) also decide where to locate mass vaccination centers (VCs) and how to assign each state to a vaccination center (VC) by embedding an epidemiological model into a facility location optimization model. The model aims to minimize total mortality and hospitalizations. Finally, their analytical approach determines the quantity of vaccines allocated to each vaccination center (VC) and the eligibility of individuals within a particular risk category in a state for receiving the vaccination. Several SIR related literature can be considered in the context of the general structure sketched above. The optimization model in Jarumaneeroj et al. (2022), for instance, is similar but considers an infectious case minimization objective. Barth et al. (2023) incorporated epidemiological dynamics with time-varying contact rate. The corresponding model is thus able to reflect multiple waves during pandemic. Note that other than an SIR type structure, a vaccine allocation model can reflect infection spread dynamics by incorporating subject-specific risk and contact information accounting social network, such as the model presented by Li and Aprahamian (2023) to underscore the benefits of customized vaccine distribution strategies.
7.2 Graph Laplacian-based dynamics representation
Epidemiological dynamics are also represented by graph Laplacian-based dynamic models. Enayati and Özaltin (2020) compute the minimum number of required vaccines to prevent the virus spread, while ensuring sufficient vaccine coverage. They model the infection growth in terms of generation instead of time (Hill & Longini Jr, 2003). Using the current population in each compartment, their model gives the number of new infections due to contact with exposed or infectious individuals.
7.3 Model parameterization and herd immunity
Embedding a discretized SIR model results in a large optimization model that can be computationally expensive to solve. Consequently, several papers incorporate SIR dynamics indirectly into their model. For example, Rao and Brandeau (2021) use a SIR model-generated patient population over time in each compartment as parameters in their optimization model. Their models examine different objectives separately including minimization of infections, deaths, life years lost, and quality adjusted life years. The model requires vaccination of a minimum fraction of population in each region. Minoza et al. (2021) also use the output from a SIR model as parameters in an optimization model that focuses on risk-class based prioritization for front-line workers and the elderly. As an alternative approach to incorporate an SIR model into optimization framework, Shamsi Gamchi et al. (2021) leverage optimal control theory to determine the proportion of susceptible population targeted for vaccination in a region.
Instead of using a herd immunity function, MacIntyre et al. (2022); Rahman et al. (2023) use vaccine coverage coefficient, , as a measure for herd immunity. When vaccine effectiveness is small and nominal infection rate is large, vaccine coverage coefficient becomes larger than 1, implying that we need to vaccinate more people to achieve the desired herd-immunity level. Vaccine effectiveness is also addressed in Mohammadi et al. (2022) while minimizing expected number of deaths resulting from unvaccinated, single-dose and double-dose vaccinated groups.
7.4 Vaccine roll-out policy evaluation with SIR dynamics
Hu et al. (2023) propose a vaccine allocation model motivated by the trade-offs between partially vaccinating a segment of population versus fully vaccinating half of that segment by splitting each full vaccine dose into two under supply scarcity. Their work considers an SIR model with two vaccinated compartments, containing individuals with fractional and full dosage. Mak et al. (2022) evaluated vaccine roll-out policies such as hold-back, release, and single-dose-first strategies using SEIR dynamics, focusing on symptomatic cases, hospitalizations, and deaths.
8 METHODOLOGICAL CONSIDERATIONS AND CASE STUDIES
8.1 Methodological considerations
Initial studies for COVID-19 vaccine supply chain management literature predominantly used commercial solvers to solve the analytical models developed. As the pandemic evolved, researchers integrated more realistic features into existing models or introduced new representations of previously studied features while adapting existing solution approaches. The following sections outline decomposition-based global optimization approaches (Section 8.1.1), decomposition-based heuristic approaches (Section 8.1.2), other heuristic and meta-heuristic approaches (Section 8.1.3), non-linear optimization approaches (Section 8.1.4) and multi-objective optimization algorithms (Section 8.1.5).
8.1.1 Decomposition and global optimization approaches
These methods guarantee solution quality using a defined value for the optimality gap. Azadi, Gangammanavar, and Eksioglu (2020) use the L-shaped method to solve their two-stage problem. Motivated by their problem structure, Zhang et al. (2022) utilize logic-based Benders to decompose location-assignment and appointment scheduling decisions. Wang et al. (2023) use a column-and-cut generation (C&CG) algorithm to solve their two-stage robust optimization problem, working with both primal and dual forms of C&CG. The primal approach requires exploring vertices of a specially structured uncertainty set, while a vertex-traversing algorithm helps obtain the worst-case scenario among all vertices. The dual approach dualizes the innermost problem, and the resultant bilinear problem is reformulated as a mixed-binary linear program by exploiting a vertex-traversal algorithm. This approach avoids the traditional Big-M approach. Enayati and Özaltin (2020) use the multi-parametric-disaggregation technique, a global optimization approach, to solve their proposed bi-linear problem. This method introduces two new sets of variables (continuous and binary) for each bilinear term, in addition to a variable representing each bi-linear term. These representative variables are then expressed as a summation of a continuous set of variables with varying parameters as coefficients.
8.1.2 Decomposition-based heuristic approaches
These methods, while not assuring solution quality like global optimization, can still efficiently achieve good solutions depending on the problem and decomposition type. Lai et al. (2021) utilize a Benders decomposition-based heuristic to solve a two-stage stochastic model. Their method solves the LP relaxation of a mixed-integer linear subproblem to generate an optimality cut. Once a targeted optimality gap is reached, the fractional solution of the second stage is rounded up for integer components providing the heuristic solution. Georgiadis and Georgiadis (2021) treat hub/DC-VC level allocation as a subproblem in a vaccine supply chain network from manufacturer to VC. Their method first allocates VCs to nearby hubs and then condenses the model by clustering VCs within existing political boundaries, subsequently addressing the smaller hub-VC assignment problems with aggregated parameters. By treating binary solutions from this assignment as incumbent, original large-scale MILP subproblems become LPs, consequently reducing computational burden.
The heuristic decomposition method by Yang, Sürer, et al. (2021) first breaks down the main network using a hierarchical clustering technique, ensuring the resulting sub-network can handle a MILP problem. After solving the sub-problem corresponding to a sub-network, the method sequentially aggregates other sub-networks, keeping certain optimal hub-opening decisions fixed, while allowing adjustments to others. The nodes that are reconsidered as decision variables in the merged sub-networks depend on their positions. The aggregation-based algorithm by Karakaya and Balcik (2023), on the other hand, performs aggregation on the priority groups. The algorithm for priority group based vaccination timeline decision simultaneously deals with two groups: the highest priority group and the group of all others post-aggregation. The timeline established for the first group serves as a parameter in the subsequent iteration, which focuses on the second priority class and the rest in an aggregated form. To prevent potential delays in vaccinating the aggregated group due to its high demand (due to aggregation), the minimum coverage threshold is also suitably adjusted, guaranteeing an optimal timeline for each class at the end. Karakaya and Balcik (2023) also offer comprehensive guidance on modifying the initial vaccination calendar in response to unforeseen events, such as severe vaccine side effects during the planned period.
8.1.3 Other heuristics and meta-heuristics
Heuristics are problem-specific strategies or rules of thumb that guide the search for scalable solutions but offer no performance guarantee and sometimes result in very poor solution quality. In comparison to heuristic approaches, meta-heuristics often yield better solutions, but they are generally computationally more intensive for exploring a large search space.
A commonly-practiced algorithm by local and state governments during the COVID-19 pandemic was the pro-rata heuristic, which divides the available vaccines equally based on the population size. The vaccine allocation heuristic by Orgut et al. (2023) distributes vaccines to population sites following capacity-to-demand ratio in ascending order. If a site's demand is unmet, the heuristic checks neighboring sites in the order of proximity, adjusting available capacity with each allocation. After fulfilling a region's entire demand, the process repeats for the next region, using the updated capacity-to-demand sequence. The greedy approach outlined in Li and Aprahamian (2023) ranks vaccination candidates based on a value derived from multiplying the associated risk measure, the likelihood of post-infection fatality, and the reduction in fatality rate after vaccination. Vaccine needs of a selected candidate are then fully met unless constrained by vaccine availability or budget. Although such an approach is optimal when restricted to a single period design, due to its myopic nature, it can be always far from an optimal allocation in a multi-period framework.
Enayati, Campbell, and Li (2023), Enayati, Li, et al. (2023) in their layered feasible path network formulation leverages depth-first-search algorithm to construct all viable paths between each origin-destination pair. Subsequent heuristic reduces layered network size through parameters that control the number of transshipments, and drone launch-landings in a vaccine delivery path. Thus, the computational cost to solve the resultant model is reduced. In Zhang et al. (2022), a meta-heuristic approach is proposed for a combined location-assignment and scheduling problem, which mitigates NP-hardness of the scheduling subproblem by using a heuristic sequencing policy. This policy allows for the elimination of binary variables that determine task sequences. The heuristic prioritizes tasks based on the smallest values of the earliest start and latest completion times. Consequently creating an approximation of the original MILP is created, which, in addition to other feasibility constraints, includes constraints for the start times of each vaccination task utilizing the predetermined sequences.
8.1.4 Nonlinear optimization approaches
A two-stage stochastic formulation of the facility location problem with location preference order by Cabezas et al. (2021) uses the accelerated dual ascent (ADA) algorithm. ADA in the first step solves the Lagrangian dual problem by a subgradient algorithm while the semi-Lagrangian-dual problem in the second step is solved by a dual ascent algorithm using the solution from the first step, followed by a heuristic for accelerating the entire procedure. Iterative coordinate descent algorithm, which takes a gradient descent step with respect to only one variable at a time, is used in Bertsimas et al. (2020, 2022) to solve the bilinear model, resulting from epidemiological dynamics considerations. Li and Aprahamian (2023) also employed the coordinate descent method, alternating between assignment variable and the product term associated to risk over multiple time periods, exploiting their multiplicative relationship. Among other methods, Barth et al. (2023) used sequential quadratic programming (SQP) to solve a discretized version of continuous time model that, alongside bilinearity also involves non-linearity resulting from time-dependent contact-rate considerations. We note that these non-linear optimization approaches for non-convex problems typically converges to a local solution, but the quality of the solution can be enhanced by exploring different initial solutions.
8.1.5 Algorithms for multi-objective optimization
Among the types of algorithms used in multi-objective vaccine supply chain literature, especially including the sustainability criteria, weighted-sum approach, and epsilon-constraint approach are the two simplest ones used (e.g., Tang et al. (2022)). Nature-inspired and evolutionary-type algorithms, also known as meta-heuristics, appear next in the list of most common algorithm types. Under this category, gray-wolf optimization and its modifications (e.g., Goodarzian, Navaei, et al. (2022)), particle swarm optimization (PSO) (e.g., Chowdhury et al. (2022)), teaching-learning-based optimization (TLBO) and its hybrid with PSO and genetic algorithm (GA) (e.g., Goodarzian, Ghasemi, et al. (2022)), non-dominated sorting genetic algorithm II (NSGA-II) (e.g., Tang et al. (2022)), evolutionary strategies (ES) (e.g., Lim et al. (2022)), can be found in the literature. Other methods include, but not limited to, variable neighborhood search and LP-metric method (e.g., Goodarzian, Navaei, et al. (2022)). We note that constructing a Pareto frontier is an integral part of these works. We also note that evolutionary type algorithms are better regarded for building a Pareto frontier with a diverse set of non-dominated solutions compared to other multi-objective optimization approaches used.
8.2 Insights from numerical studies
In this section, we outline key highlights from representative numerical or case studies in the literature. While some studies use synthetic data to emphasize their main points, others integrate real data (e.g., Hajibabai et al. (2022)), supplemented with rough approximations. These studies either provide an artificial view of national vaccine supply chains, like those in the U.S. or India, or focus on specific regional details. In deterministic modeling, some papers validate models using a range of artificially generated parameter data and assessing their interpretational consistency, whereas under uncertainty, model outcomes are assessed under various uncertainty scenarios. Another aspect of model validation, observed in a few studies, involves stating modeling assumptions and evaluating their reasonableness in reflecting real-world conditions without oversimplification or misrepresentation. Regardless, sensitivity analysis is often performed with respect to central parameters such as budget in facility number, cost budget, vaccine availability, supplier/DC/VCs capacity, healthcare personnel service rate, minimum coverage threshold, minimum service ratio, cost-coefficient, parameters defining uncertainty/ambiguity set and so forth.
Case studies also highlight the importance of using an optimal allocation policy compared to the pro-rata policy by using metrics such as region or risk class-wise number of active cases, deaths, (e.g., Bertsimas et al. (2020)), infected days (infection rate multiplied by length of time horizon in days) (e.g., Barth et al. (2023), percentage of unused vaccines, percentage of unmet demand (e.g., Orgut et al. (2023)) and so forth. For example, Bertsimas et al. (2020) showed that optimized vaccine allocation following their model can reduce 10%–25% of the fatalities over the 90-day planning horizon. Balcik et al. (2022) compared several coverage-based key performance indicators (KPIs) across allocation policies systematically, factoring in priority groups and/or socio-economic differences. Barth et al. (2023) additionally assessed the efficiency of different heuristic vaccine allocation strategies, remarking on the consistency of the pro-rata policy compared to others. For more on such policies, see section 4.1 of Barth et al. (2023).
8.2.1 Insights from parameter variation effect
Mohammadi et al. (2022) demonstrated how decisions such as the number of vaccine centers, vaccine orders, transfers, trans-shipments, and first-dose and second-dose vaccinations per risk class vary across different uncertainty scenarios. Their study revealed that as vaccine campaign parameters (like warehouse capacity, dose time-lag, and manufacturer trust rate) enhance, deaths decrease and first-dose vaccinations increase. Longer vaccine delivery lead times, however, have the opposite effect. Mortality also increases with increased contact and death rates but reduces with vaccine effectiveness. The authors suggest prioritizing high-risk individuals for vaccination when supplies are limited but high-contact individuals otherwise. Vaccination strategy and dosing intervals depend on the situation: in the face of a lethal and highly contagious virus variant, it is important to fully vaccinate the high-risk group rather than just giving one dose to many. Boosting vaccination capacity can combat such variants, and resource sharing offers flexible capacity planning.
Sensitivity analyses in the literature explicitly show that equity can be improved via a change in facility capacity (e.g., Balcik et al. (2022), Orgut et al. (2023)). In addition to evaluating fairness and efficiency through case studies, many studies assess other performance metrics as indicated earlier in this section. When optimization models introduce or emphasize specific features, assessing their outcomes numerically is standard to highlight their importance. For instance, Balcik et al. (2022) evaluated the fairness measure they use against other known equity measures. Barth et al. (2023) analyzed how the ratio of available vaccine percentage to population size affects infected days, Gini-coefficients of vaccinations as well as Gini-coefficients of infected days.
9 DISCUSSION
This section provides a comprehensive discussion of the above discussed models and their usefulness in a future pandemic. The discussion includes unique features of a vaccine supply chain during a pandemic (Section 9.1), trends in the literature (Section 9.2), and authors' perspectives for model selection (Section 9.3).
9.1 Unique challenges in pandemic vaccine supply chain
The vaccine supply chain presents unique challenges that distinguish it from other supply chains. First, it requires rigorous cold chain management, especially for vaccines necessitating ultra-cold storage. Secondly, it necessitates the development of adaptable distribution strategies that take into account limited manufacturing sites and unpredictable epidemiological patterns. Third, complex inventory management is needed due to diverse factors such as vaccine dose policies, vial sizes, vial shelf life, open vial shelf life, wastage, and the need for specialized healthcare staff. Additionally, the supply chain should maintain a delicate balance between equity and efficiency while addressing vaccine hesitancy and priority groups. Extending vaccine access to remote areas poses another hurdle, wherein drone delivery does not fully resolve the issue of healthcare personnel availability. Moreover, the integration of demand into allocation models is critical, considering vaccine preferences and epidemiological dynamics in quantifying demand.
9.2 Temporal trends in the literature
In consideration of the above mentioned distinctions, several temporal trends in the literature can be noted. In the initial phases of the pandemic, models considered are similar to those of conventional supply chains, albeit with some added emphasis on cold chain management. Additionally, real-life case studies were rare, most probably due to limitations in the availability of data. However, as the pandemic progressed, the number of real-life case studies increased, providing a rich repository of insights and experiences. In this stage, researchers also produced more realistic models that consider different sources of uncertainty and epidemiological dynamics, enhancing the relevance of these tools in public health decision-making.
A later wave of studies has aimed to advance equity and efficiency in vaccine deployment beyond that enabled by prior work. Recent work also features models that address vaccine efficacy in preventing transmission and infections, as well as vaccine hesitancy to take the vaccine among different segments of the population. Hence, we see a strong interest in modeling characteristics pertaining to human behavior and social dynamics as well as clinical performance of the developed vaccines against mutations of the vaccine.
Despite these advances, however, there are still important issues to study. For example, the more general question of how to prioritize the inoculation of various segments of the population (e.g., fractional dosing, prioritizing individuals of high-risk occupations/social interaction) while at the same time considering realistic models of vaccine characteristics (e.g., type, dosing, storage requirements) population mobility, social determinants of health, and access to healthcare are lacking.
Regardless of the specific circumstances, it is clear that vaccine allocation models that incorporate epidemiological dynamics and realistic mobility behavior can greatly enhance the effectiveness of public health decision-making. However, even without the explicit consideration of these aspects of the problem, optimization models that facilitate resource sharing among different geographical areas and possess the flexibility to adapt to changing environments can still prove to be highly valuable.
9.3 Perspectives for model selection
Models that primarily address location selection and opening decisions, as these are strategic choices that are not easily altered once made, should incorporate different practical factors. In parallel, there should be solution approaches that provide solutions of high quality. On the other hand, operational-level decision-making models, which prioritize enhancing coverage in hard-to-reach regions or fortifying vulnerable supply chains to mitigate the aftermath of a major impact, warrant attention for enhancing global vaccination efforts. Furthermore, it is advisable to develop models that considers epidemiological dynamics, either via compartment or network structure, to account for long-term effects. These models should focus on the time-varying nature of a pandemic and should be capable of optimizing resource allocations, while also considering factors such as vaccine availability, quarantine, lockdown measures, and other preventive strategies. Although such models may pose computational challenges, they are deemed to be ideal, even when epidemiological dynamics is represented in approximate or simulated forms.
Effective utilization of Operations Research often demands a high level of expertise, and without it, there exists a risk of improperly applying models and techniques. Hence, models should be carefully developed, providing clear and simple guidelines for public health officers to facilitate implementation and execution. Additionally, caution is required as recommendations from models based on unreliable or insufficient data can deviate significantly from reality, particularly in scenarios with rapidly changing dynamics such as the COVID-19 pandemic. It is also worth noting that vaccine supply chains can be influenced by cultural, social, or behavioral factors, which may prove challenging to quantify within a model's framework. Moreover, if the chosen solution approach to address the modeled problem lacks quality or fails to align with the specific purpose, the effort and expenses invested in the model may outweigh the benefits it provides.
10 CONCLUDING REMARKS ON FUTURE RESEARCH DIRECTIONS
A pandemic vaccine supply chain can be viewed in three phases: Phase-I represents the initial period following vaccine development, characterized by a pronounced gap between vaccine supply and demand for inoculation. During Phase-II, the supply of vaccines increases, meeting a substantial portion of the demand but still falling short of full coverage. Phase-III defines the state in which the vaccine supply matches or even surpasses the demand. Note that the pandemic vaccine deployment literature, including the planning of distribution center (DC) and vaccination center (VC) locations, is predominantly centered on problems pertaining to Phase-II. Below, we include some concluding remarks on fruitful research directions in modeling (Sections 10.1 and 10.2) and solution methods (Section 10.3).
10.1 Models with explicit consideration of epidemiological dynamics
Traditionally, an estimated size of the susceptible population is regarded as the demand in vaccine allocation models. However, several factors, such as the administration of multiple vaccine doses, variations in vaccine efficacy, and fundamental epidemiological dynamics strongly influence the size of the population susceptible to infection over time. Hence, it is imperative that such decision making models incorporate effective epidemiological dynamics models to estimate the demand for vaccines over time in different geographical regions that may be going through different phases of the pandemic.
Although several papers incorporate compartmentalized epidemiological models (e.g., SIR-type models) into vaccine allocation models, present studies exhibit several limitations. As discussed in Section 7, directly integrating SIR dynamics into optimization models or employing separate models with diverse SIR parameters results in computational complexity or challenges in achieving interpretability. This leads to a critical question: is it possible to develop a computationally efficient SIR-surrogate that preserves essential epidemiological dynamics?
Moreover, the ever-evolving nature of virus challenges the accuracy of models incorporating epidemiological dynamics, even using advanced machine-learning models. Addressing this uncertainty underscores the need for using multiple prediction models. However, current studies lack a unified approach for integrating these models into optimization algorithms. Additionally, concerning the dynamic environment of a pandemic, it is worth exploring whether prediction and optimization should be performed sequentially or concurrently in a decision-making framework. Furthermore, in a sequential decision-making framework, the potential utilization of an integrated approach that combines offline and online learning for a fast-yet-more informed decision-making warrants new studies. The question of how contextual optimization model outcomes, modeled either with real or simulated data, respond to contextual changes should also be examined. The OR/MS community needs to address these critical issues to advance the field beyond superficial adjustments to carefully-studied vaccine supply chain models with potential for real-world impact.
10.2 Additional modeling considerations
Several additional gaps exist in the modeling of vaccine supply chain models. The uncertainty-driven dynamism of these problems poses the question of whether distribution networks aided by novel comprehensive topological metrics and graph representation learning can yield decisions that are more adaptable. Research examining the interplay between quarantine/isolation policies and vaccine allocation is notably scarce, as is the development of models that account for time-varying contact and mortality rates. Recognizing that vaccination does not uniformly reduce the risks of infection and mortality across various subpopulations in different risk categories, equity metrics based on demand or coverage may prove inadequate, prompting the exploration of alternative methods. Exploring vaccine rollout strategies that consider time-varying contact rates and the occurrence of multiple infection spikes, and utility-driven location models that factor in diverse determinants such as distance, regional income, and education, present promising avenues for further research. Modeling regulated by risk-neutral or risk-aversion type utility can further serve as a viable alternative to conventional vaccine hesitancy models.
Current discrete scenario-based approaches to two-stage stochastic programming fall short of addressing multiple spikes of infections during a pandemic. Multi-stage stochastic programming, while computationally intensive, could more accurately capture epidemiological dynamics over time. The challenge lies in devising effective surrogates for this approach and rigorously assessing their performance.
Finally, further developments on model validation approaches are required for ascertaining the adaptability of a vaccine supply chain network to rapid shifts in infection dynamics. Defining criteria for allocating limited vaccine supplies in a prioritized order during the initial vaccination phase (Phase-I) necessitates systematic analysis, streamlining long-term risks and benefits, while considering access to healthcare, social determinants of health, human behavior, mobility and adherence to non-pharmaceutical interventions such as social distancing and vaccine hesitancy.
10.3 Solution methodologies
The majority of reviewed work directly adopts solution methods from similar literature, which, regrettably, hampers the advancement of comprehensive models equipped with practical features. To address this gap, the OR/MS community needs to innovate effective solution strategies that leverage the inherent problem structure, decomposition techniques (e.g., graph-based methodologies), and valid inequalities synchronized with model condensation techniques, and harness combinatorial heuristic algorithms to solve realistic problems with large network structures. Such methods will be particularly crucial to efficiently solve multi-objective models with conflicting objectives as well as bilinear/biconvex models resulting from robust optimization formulations that are needed to represent the plethora of sources of uncertainties in this problem domain.
ACKNOWLEDGMENTS
This research is supported by the NIH Grant R01AI168144. We would like to thank Jiaqi Lei for helping proofread this article. We also sincerely thank the Associate Editor and the two anonymous reviewers for their insightful comments and recommendations, which allowed us to improve the manuscript significantly.
APPENDIX A: DETAILED FEATURES OF OPTIMIZATION AND SIMULATION ARTICLES IN PANDEMIC VACCINE MANAGEMENT
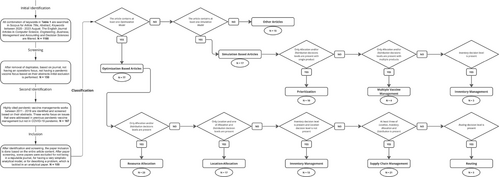
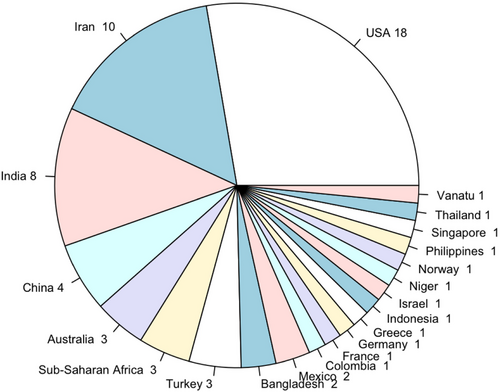
| Model | |||
|---|---|---|---|
| CCP: Chance Constraint Programming | CoP: Copositive Programming | CSP: Constraint Satisfaction Programming | DT: Decision Trees |
| FP: Fuzzy Programming | GT: Game Theory | I: Inventory Modeling | LP: Linear Programming |
| LRP: Location Routing Problem | MC: Markov Chains | MILP: Mixed Integer Linear Programming | MINLP: Mixed Integer Non-linear Programming |
| NLP: Non-linear Programming | OC: Optimal Control | Q: Queuing | RL: Reinforcement Learning |
| RO: Robust Optimization | S: Simulation | SCP: Set Covering Problem | SN: Social Networks |
| SP: Stochastic Programming | U: Utility Function | VRP: Vehicle Routing Problem | |
| Uncertainty | |||
| D: Demand | DR: Deterioration Rate | I: Infection | VLC: Vaccination Location Choice |
| LT: Lead Time | S: Supply | VA: Vaccine Accessibility | VW: Vaccine Wastage |
| Commodity | |||
| Quantity | M: Multiple | S: Single | |
| Type | ChV: Childhood Vaccine | CP: COVID-19 Pills | CT: COVID-19 Test |
| CV: COVID-19 Vaccine | CW: COVID-19 Waste | GHS: General Health Service | |
| GV: General Vaccine | IV: Influenza Vaccine | ||
| Characteristics | MD: Multiple Doses | P: Perishable | SD: Single Dose |
| Period | |||
| M: Multiple | S: Single | ||
| Echelon | |||
| DC: Distribution Center | GHSC: General Health Service Center | M: Manufacturer | MVC: Mobile Vaccination Clinic |
| OC: Outreach Center | PS: Population Sub-group | RT: Refrigerated Truck | VC: Vaccination Center |
| WDC: Waste Disposal Center | |||
| Objective function | |||
| Quantity | [Max]: Maximize | [Min]: Minimize | []: Different Separate Objective Functions |
| Operator | M: Multiple Objectives | S: Single Objective | |
| Vehicle | |||
| Type | MVC: Mobile Vaccination Clinic | RT: Refrigerated Truck | |
| Characteristics | He: Heterogeneous | Ho: Homogeneous |
| Article | Model | Prioritization | Uncertainty | Commodity | Period | Echelon: From | Echelon: To | Objective | Case study | Solution method |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbasi et al. (2020) | MILP | S, CV, SD | S | DC | PS | S, [Min] Vaccine oversupply, transshipment time | Victoria, Australia | Risk-based heuristic algorithm | ||
| Anahideh et al. (2022) | MILP | S, GV, SD | S | DC | VC | M, [Min] Geographical diversity, [Min] social fairness gap | Baltimore, Chicago, New York, USA | LP Relaxation, Penalty Method, Binary Search | ||
| Balcik et al. (2022) | MILP | M, CV, SD | S | DC | DC | S, [Min] Deviation from the fair coverage levels | Turkey | Commercial Solver | ||
| Bandi et al. (2021) | CoP | D | M, CV, MD, P | M | VC | PS | M, [Max] Vaccination rates, [Min] appointment waiting times | Singapore | Copositive Program Reformulation | |
| Barth et al. (2023) | OC | D | S, CV, SD | M | VC | PS | S, [Min] Total number of infected people | Synthetic | NLP reformulation by discretizing the time dimension | |
| Bennouna et al. (2022) | SP | D | M, CV, MD | M | DC | PS | S, [Min] Expected number of deaths | Massachusetts, USA | ML-based aggregation method | |
| Bertsimas et al. (2020) | NLP | D | S, CV, SD | M | DC | PS | S, [Min] Number of deaths | New York, USA | Iterative Coordinate Descent | |
| Enayati and Özaltin (2020) | NLP | D | S, IV, SD | M | DC | PS | S, [Min] Number of vaccine doses distributed | Synthetic (Asian Flu Pandemic) | Discretization with Multi-parametric Disaggregation Algorithm | |
| Hu et al. (2023) | OC | D | S, CV, SD | M | VC | PS | S, [Min] Total number of infected people | World | Optimal Control with Binary Search | |
| Huang et al. (2017) | MILP | M, IF, SD | S | DC | DC | S, [Max] Equal access | Texas, USA | Commercial Solver | ||
| Jadidi et al. (2021) | NLP, S | D | S, CV, SD | S | DC | PS | S, [Max] Total immunity among populations | Synthetic (Another Paper) | Graph Theory Algorithm | |
| Jarumaneeroj et al. (2022) | NLP | D | M, CV, SD | M | DC | PS | S, [Min] Stress on healthcare system | Thailand | Iterative Algorithm | |
| Li and Aprahamian (2023) | NLP, SN, S | S, CV, SD | M | VC | PS | S, [Min] Number of total fatalities | Texas | Greedy solution scheme, solution algorithm using coordinate descent | ||
| Minoza et al. (2021) | LP,S | D | S, CV, SD | S | DC | PS | M, [Max] Vaccine to be allocated for each location, [Max] priority factor | Quezon City, Philippines | Simulating Scenarios | |
| Munguía-López and Ponce-Ortega (2021) | LP, NLP | S, CV, SD | S | DC | DC | [3], [Max] Total vaccines allocated, [Max] smallest allocated vaccines, [Max] social welfare | Mexico | Commercial Solver | ||
| Orgut et al. (2023) | MILP | S, CV, SD | S | DC | VC | S, [Min] Maximum travel duration of patients | Alabama | Pro Rata Vaccine Allocation Heuristic | ||
| Rao and Brandeau (2021) | MILP | D | S, CV, SD | S | DC | PS | [4], [Min] New infections, [Min] deaths, [Min] life years lost, [Min] quality-adjusted life years | New York, USA | Exact Analytical Conditions | |
| Roy et al. (2021) | LP | I | S, CV, SD | M | DC | PS | S, [Min] Transportation cost | New York, USA | Exact Solution Algorithm | |
| Shukla et al. (2022) | MILP | M, CV, SD | M | DC | VC, MVC | S, [Max] Vaccine distribution efficiency | USA | Python NetworkX, Minimum Cost Flow Problem | ||
| Thul and Powell (2023) | SP, RL | D | S, CV, SD, CT | M | DC | PS | S, [Max] Expected sum of cumulative rewards | USA | Partially-Observable Markov Decision Process | |
| Yang et al. (2022) | SP | D | S, CV, SD | S | DC | PS | S, [Min] Total number of confirmed cases | Synthetic | Commercial Solver | |
| Yarmand et al. (2014) | SP | VA | S, GV, SD | M | DC | PS | S, [Min] Phase-I vaccination cost, expected Phase-II vaccination cost | North Carolina | Newsvendor Closed Form Solution, Heuristic Algorithm |
| Article | Model | Uncertainty | Commodity | Period | Facility echelon | Demand echelon | Objective | Case study | Solution method |
|---|---|---|---|---|---|---|---|---|---|
| Bertsimas et al. (2022) | NLP | D | S, CV, SD | M | VC | PS | M, [Min] Deaths, [Min] exposed, [Min] distance | New York, USA | Iterative Coordinate Descent |
| Bravo et al. (2022) | MILP | S, CV, SD | S | VC | PS | S, [Max] Aggregate vaccinations | California, USA | Commercial Solver | |
| Cabezas et al. (2021) | SP | VLC | M, CV, SD | S | VC | PS | S, [Min] Demand coverage and facility cost | Synthetic | Lagrangian Relaxation Heuristic |
| Cao et al. (2023) | RO | I | S, CW | M | WDC | VC | M, [Min] Transportation and waste disposal cost, [Min] infectious risk | Maharashtra, India | KKT, Branch and Bound, Polyhedral Uncertainty Set |
| Dastgoshade et al. (2022) | SP | D, S | S, CV, SD | M | VC | PS | M, [Min] Network cost, [Max] social equity | Iran | Sample Average Approximation, Lexicographic Goal Programming |
| Emu et al. (2021) | CSP | S, CV, SD | S | DC | PS | [4], [Max] Overall vaccine distribution, [Max] vaccine distribution among the higher priority groups, [Max] vaccine distribution among people located closer to DCs, [Max] vaccine distribution among the higher priority groups and people located closer to DCs | Chennai, India | K-medoids Algorithm | |
| Enayati, Li, et al. (2023) | MILP | S, CV, SD, P | S | DC | DC | S, [Min] Total operating costs | Synthetic | Commercial Solver | |
| Enayati, Campbell, and Li (2023) | MILP | S, CV, SD | S | DC | VC | S, [Min] Total operating costs | Vanatu Islands | Commercial Solver | |
| Kumar et al. (2022) | MINLP, I | S, CV, SD | S | VC | PS | S, [Min] Operational (transportation, location, salary) costs and travel distance | Chandigarh, India | Two-stage MILP | |
| Leithäuser et al. (2021) | MILP | S, CV, SD | S | VC | PS | M, [Min] Number of vaccination centers, [Min] number of physicans, [Min] sum of patient travel distance | Germany | Different Assignment Heuristics (Greedy, Same State) | |
| Lim et al. (2016) | MILP | S, ChV, SD | S | OC | PS | S, [Max] Demand coverage | Bihar, India | Commercial Solver | |
| Luo and Mehrotra (2023) | RO, U | VLC | S, CT | S | VC | PS | S, [Max] Total utility of the service received by the customers | San Diego, USA | Mixed 0-1 second-order cone (MISOCP) formulation and cutting-plane algorithm |
| Lusiantoro et al. (2022) | MILP | S, CV, SD | S | VC | PS | M, [Max] Coverage, [Min] weighted distance | Yogyakarta, Indonesia | Commercial Solver | |
| Polo et al. (2015) | MILP | S, GHS | S | GHSC | PS | M, [Max] Coverage, [Min] total distance | Bogota, Colombia | Commercial Solver | |
| Soria-Arguello et al. (2021) | MILP | M, CV, SD | S | M, DC | DC | S, [Min] Total operational cost | Mexico | Commercial Solver | |
| Srivastava et al. (2021) | MILP | S, CV, SD | S | DC | VC | S, [Max] Coverage of demand points, within a maximum time | Madhya Prades, India | Greedy Adding Algorithm-Based Optimization | |
| Zhang et al. (2022) | MILP | S, CV, SD | S | VC | PS | S, [Min] Operational cost (location, traveling distance, appointment rejection, tardiness) | Yiwu, China | Logic Based Benders Decomposition and Metaheuristic |
| Article | Model | Uncertainty | Commodity | Period | Echelons | Objective | Case study | Solution method |
|---|---|---|---|---|---|---|---|---|
| Azadi, Eksioglu, and Geismar (2020) | CCP | D | M, ChV, SD | M | DC, VC | S, [Max] Number of fully immunized children, vaccine availability | Niger | Sample Average Approximation |
| Azadi, Gangammanavar, and Eksioglu (2020) | SP | D | M, ChV, SD | M | DC, VC | S, [Min] Cost and open vial waste | Bangladesh | Stochastic Benders Decomposition, L-Shaped Method |
| Bonney and Jaber (2011) | I | S, GHS | S | DC | S, [Min] Environmental cost | Closed Form | ||
| Fadaki et al. (2022) | MINLP | S, CV, SD | M | DC, VC | M, [Min] Weighted risk of unvaccinated population, transshipment mechanism and return mechanism | Victoria, Australia | Augmented Multi-period Algorithm | |
| Georgiadis and Georgiadis (2021) | MILP | M, CV, SD, P | M | M, DC, VC | S, [Min] Distribution, inventory and wastage cost | Greece | Two-Step Decomposition Algorithm | |
| Hovav and Tsadikovich (2015) | LP | S, IV, SD | M | M, DC, VC | S, [Min] Operational and shortage cost | Israel | Commercial Solver | |
| Işik and Yildiz (2023) | RO | D, DR, S | S, CV, MD, P | M | DC, VC, WDC | M, [Max] Total vaccinations, [Min] holding, transhipment, waste management cost | Izmir, Turkey | -constraint Method |
| Jahani et al. (2022) | NLP, Q | S, LT | M, CV, SD | M | M, VC | M, [Min] Expected vaccine waiting time, [Min] holding and ordering cost | Melbourne, Australia | Goal Attainment Algorithm, Non-dominated Sorting Genetic Algorithm |
| Karakaya and Balcik (2023) | SP | S, LT | M, CV, MD | M | DC, PS | S, [Min] Deviations from the announced COVID-19 calendar | Norway | Aggregation-based algorithm |
| Mak et al. (2022) | I | D | S, CV, MD | M | DC, VC | Three roll-out scenarios are compared: (i) holding back second doses, (ii) releasing second doses, and (iii) stretching the lead time between doses | Synthetic | Proof Based |
| Mohammadi et al. (2022) | RO, Q | S | S, CV, MD | S | DC, VC, PS | M, [Min] Total expected number of deaths among the population, [Min] total distribution cost | France | Scenario-based robust-stochastic optimization approach |
| Shah et al. (2022) | I | VW | S, CV, SD, P | M | M, DC | S, [Min] Ordering, manufacturing, holding, inspection and carbon emission cost | Synthetic | Exact Solution Algorithm |
| Sinha et al. (2021) | I, GT | D | S, CV, SD, P | M | DC, VC | [3], [Min] Total cost, under no disruption scenario, [Min] total cost, under disruption, [Min] excess inventory cost | Gorakhpur, India (Japanese Encephalitis Vaccine) | Heuristic Algorithm |
| Article | Model | Uncertainty | Commodity | Period | Echelons | Objective | Case study | Solution method |
|---|---|---|---|---|---|---|---|---|
| Abbasi et al. (2023) | MILP | S, CV, SD | S | M, DC, VC,PS,WDC | M, [Min] Economic cost, [Min] environmental cost | Iran | Multi-objective gray wolf optimizer algorithm | |
| Basciftci et al. (2023) | SP, RO | D, I | S, CV, SD | M | DC, PS | S, [Min] Total cost of locating DCs, installing their capacities, expected cost of shipping, holding inventory, back-orders | Michigan, USA | Moment-based ambiguity set approach |
| Chowdhury et al. (2022) | MILP | D, S | S, CV, SD, P | M | M, DC, VC | M, [Min] Operational cost, [Min] environmental cost, [Max] job opportunities | Bangladesh | Two heuristic algorithms: multi-objective social engineering optimizer and multi-objective feasibility enhanced particle swarm optimization |
| Gilani and Sahebi (2022) | RO | VA | M, CV, SD | M | M, DC, VC | M, [Min] Economical cost, [Min] environmental cost, [Min] social cost | Iran | Cutting Planes Approach |
| Goodarzian, Ghasemi, et al. (2022) | MILP, CCP, S | D | M, CP | M | M, DC, VC | M, [Min] Operational cost [Min] environmental cost, [Max] social responsibility | USA | Multi-Objective Teaching–learning-based Optimization Heuristic, Particle Swarm Optimization Heuristic, Genetic Algorithm Heuristic |
| Goodarzian, Navaei, et al. (2022) | MILP | M, CV, SD | M | DC, VC | M, [Min] Operational, shortage, surplus cost, [Min] unmet demand, [Min] delivery time, [Min] environmental impact | Iran | Gray Wolf Optimization Heuristic, Variable Neighborhood Search Heuristic | |
| Habibi et al. (2023) | SP, MC | D, LT | S, CV, SD | S | DC, VC | S, [Min] Operational cost | Iran | Two heuristics: population-based simulated annealing and improved grey wolf optimizer |
| Kohneh et al. (2023) | FP | D | M, CV, SD, P | M | DC, VC, PS | M, [Min] System cost, [Max] vaccination service level | Mashhad, Iran | Model Linearization, Defuzzification |
| Lai et al. (2021) | SP | D | M, CV, SD | M | VC, PS | S, [Min] Operational cost | Synthetic | Benders decomposition-based heuristic algorithm |
| Li, Pan, et al. (2021) | MINLP | S, CV, SD | S | VC, PS | M, [Min] Travel distance, cost, [Max] number of fully opened vaccination stations | Shenzhen, China | Two-stage MILP Reformulation, -Constraint Approach | |
| Lim et al. (2022) | MILP | S, CV, SD | S | DC, VC | S, [Min] Operational, logistical cost | Sub-Saharan Africa | Commercial Solver, Hybrid Heuristics for Large Scale | |
| Manupati et al. (2021) | MILP, DT | D, LT | S, CV, SD | M | M, DC, VC | S, [Min] Overall cost | India | Commercial Solver |
| Rahman et al. (2023) | MILP, CCP | VW | M, CV, SD | M | DC, VC, PS | S, [Min] Operational cost | Canberra, Australia | Heuristic Genetic Algorithm |
| Rastegar et al. (2021) | MILP | S, IV, SD | M | DC | S, [Max] Minimum vaccine distributed-to-demand ratio | Iran | Commercial Solver, Linearization | |
| Sazvar et al. (2021) | RO, FP | D | S, IV, SD | M | M, DC, PS | M, [Min] Economic cost, [Min] environmental cost, [Max] social responsibility, [Max] resilience | Iran | Multi-choice goal programming with a utility function approach |
| Shiri and Ahmadizar (2022) | SP | D | M, CV, SD | M | DC, VC | S, [Min] Transportation, operation, shortage cost | Iran | Scenario Reduction Algorithm |
| Tang et al. (2022) | MILP | S, CV, SD | S | VC, PS | M, [Min] Operational, inventory cost, [Min] total travel distance | Beijing City, China | Tailored Genetic Algorithm, DP Based Heuristic | |
| Tavana et al. (2021) | MILP | M, CV, SD | M | M, DC | S, [Max] Minimum delivery-to-demand ratio | India | Commercial Solver | |
| Wang et al. (2023) | RO | S | S, CV, MD | M | DC, VC | M, [Max] vaccine benefits, [Min] cost | Linshui, China | Column-and-Constraint Generation, Vertex Traversal and Dual Methods |
| Xu et al. (2021) | MILP | S, CV, SD | S | DC, OC | [3], [Max] Total coverage using three different coverage measures | New Jersey, USA | Commercial Solver | |
| Yang, Bidkhori, and Rajgopal (2021) | MILP | S, ChV, SD | S | DC, VC | S, [Min] Annual hub facility, transportation costs | Sub-Saharan Africa | Disaggregation and Merging Algorithm |
APPENDIX B: VEHICLE ROUTING CONSIDERATIONS FOR VACCINE DISTRIBUTION
One major feature in both Yang and Rajgopal (2021) and Yucesoy et al. (2022) is that the model selects population sites (PSs) to visit under demand information at hand, with the binary decision variable . Subsequently, the routing constraints are expressed as and . Service time in each population site (PS) is treated as a decision variable and the total time budget constraint is formulated. Moreover, some articles consider routing within the entire supply chain design (Chowdhury et al., 2022; Habibi et al., 2023). Further information on routing articles can be found in Table B1.
| Article | Model | Uncertainty | Commodity | Period | Vehicle | Echelons | Objective | Case study | Solution method |
|---|---|---|---|---|---|---|---|---|---|
| Shamsi Gamchi et al. (2021) | VRP | D | S, GV, SD | S | RT, He | DC, PS | M, [Min] Social cost, [Min] routing cost | Tehran, Iran | Dynamic Programming |
| Yang and Rajgopal (2021) | VRP, SCP | D, LT | S, ChV, SD | M | MVC, Ho | DC, PS | S, [Min] Vehicle and outreach trip cost | Sub-Saharan Africa | MILP Commercial Solver, Multi-period Stochastic Modeling Approach |
| Yucesoy et al. (2022) | LRP | S, CV, SD | S | MVC, Ho | DC, PS | S, [Max] Vaccination service levels | Van, Turkey | Commercial Solver |
APPENDIX C: ADDITIONAL LITERATURE
We now review papers that do not use mathematical optimization models but use simulation approaches (Section C.1) to support decisions (Section C.2) in vaccine management using descriptive statistics, observation, or other relevant methods in order to identify and conceptualize crucial steps. These papers highlight some additional, less studied aspects of vaccine management. Typically, these studies involve testing different scenarios using a simulation model and offering managerial insights for public health authorities, by observing the trends in the identified Key Performance Indicators (KPIs).
C.1 Simulation-based articles
Table C1 contains information about the decision levels, type of the simulation model, commodity, duration of the simulation, priority groups, and case studies used in simulation-based articles.
Prioritization: A large number of the simulation papers analyzed questions on the prioritization of different population subgroups, due to vaccine scarcity at the early stages. The most common KPIs studied under a number of alternative prioritization schemes (e.g., by age groups, by profession, or by infection risk/immunity) are the number of infections, hospitalizations, and mortality. Chen et al. (2020) and Rosenstrom et al. (2022) also consider fairness by using the Gini index and race-based sub-population grouping, respectively. Papers that employ system dynamics models (i.e., SIR-type) addressed prioritization issues through the use of additional compartments that reflect differences between different population subgroups, but typically agent-based simulation models are deployed when analyzing the impact of interactions that may result in infections (Chen et al., 2021; Oruc et al., 2021).
The simulation based studies resulted in valuable insights shaping the COVID-19 vaccine deployment, such as prioritization of the elderly to minimize mortality, prioritization of younger and working individuals to minimize spread, benefits of dynamic prioritization (first vaccinate elderly, then switch to young), importance of equity objectives in reducing health disparities and so forth. In addition, simulation approaches provided timelines for the duration of the pandemic as well as required number of vaccine doses to reach herd immunity (Chaturvedi & Chakravarty, 2021).
Managing multiple vaccines: COVID-19 vaccines have different, possibly time-dependent, efficacy, resource, storage and dosing requirements, and became available at different times during the pandemic. While most papers have considered a single type of vaccine, a few focused on issues related to managing multiple vaccines. For example, Kim et al. (2021) provide the insight that a vaccine with fewer resource requirements and becoming available later can reduce the virus spread as much as a vaccine that becomes available earlier but requires more resources. In subsequent work, Kim et al. (2022) showed that if a vaccine with lower initial efficacy can be distributed fast, then it would be as effective as a vaccine with higher initial efficacy with a slower distribution rate, assuming that vaccine efficacy drops by time. In a similar vein, Shim (2021) show that to minimize deaths under scarce supply of vaccines, COVID-19 vaccines with lower efficacy should be given to younger people and COVID-19 vaccines with higher efficacy should be given to older people, highlighting an important principle in prioritization with multiple vaccines. Finally in Romero-Brufau et al. (2021) a simulation model was constructed to compare standard COVID-19 vaccination with a delayed second dose strategy, prioritizing the first dose, for vaccines with varying degrees of effectiveness. The results indicate that implementing a delayed second dose vaccination plan, specifically for individuals under the age of 65, could potentially lower the overall number of cumulative deaths under specific circumstances.
Management of vaccine inventory: Vaccine vial size, recommended dosing, and population targeted for deployment are parameters that affect vaccine inventory management. For example, there are significant operational differences between storing and administering vaccines with 10-dose vials versus those with 2-dose vials. Assi et al. (2011, 2012) build discrete-event simulation models where every vaccine with its storage location, refrigerator, freezer, and transport device is modeled as an entity to study the impact of vial size, vaccination period duration, and target vaccination population on supply chain bottlenecks.
C.2 Additional considerations
Conceptual articles: Dai and Song (2021) outline the COVID-19 supply chain research agenda under supply, demand, and supply-demand matching and highlight important areas such as cold chain management, demand eligibility, and vaccine hesitancy. Forman et al. (2021) outline the obstacles to successful and coordinated international vaccine initiatives to prevent COVID-19, as well as proposed strategies to overcome them. Schmidt et al. (2021) use descriptive statistics to assess the equity of COVID-19 vaccine allocation within the US, and conclude that policymakers at the federal, state, and municipal levels should widely adopt the use of disadvantage indices and associated place-based indicators to ensure that fairness plays a prominent role in allocation strategies. Alam et al. (2021) use the decision-making trial and evaluation laboratory framework to examine the main challenges of the COVID-19 vaccine supply chain and come up with practical policy guidelines for the decision-makers. The article identifies the main bottlenecks of the COVID-19 vaccine supply chain as (i) limited vaccine producers, (ii) improper collaboration with local organizations, (iii) lack of vaccine monitoring (iv) difficulties in regulating vaccine temperature, and (v) high cost of vaccinations. Li, Norman, et al. (2021) describe the main methods used by a sizable hospital pharmacy department to overcome the challenges of preparing a large number of COVID-19 vaccine doses in a short period of time. Cano-Marin et al. (2023) utilize social media data and natural language processing to demonstrate that vaccine supply chain challenges, especially in developing countries, significantly affect COVID-19 healthcare responses, emphasizing the critical need for improved coordination in vaccine supply chains to ensure equitable access.
Vaccine hesitancy: As mentioned in Dai and Song (2021), a major obstacle to vaccination adoption is vaccine hesitancy, which the World Health Organization named as of the top threats to world health. Vaccine hesitancy is quantified using surveys. Bogart et al. (2021) performed a questionnaire among HIV-infected African Americans about COVID-19 vaccine hesitancy and found that more than half of the participants supported at least one COVID-19 treatment reluctance or vaccination stance. Silva et al. (2021) deployed a comparative questionnaire at a college campus to quantify COVID-19 and influenza vaccine hesitancy among college students. The results show that if the vaccinations are shown to be both safe and effective, college students are generally willing to get the vaccine. Sallam (2021) reviewed the vaccine hesitancy surveys and came up with a vaccine hesitancypercentage for each country. Troiano and Nardi (2021) performed a narrative review to find the underlying factors of vaccine hesitancy (i.e., age, gender, race, belief, education, work status) in the context of COVID-19. Finally, Hegde et al. (2023) proposed a framework with two distinct operating modes to examine service rates, which signify the capacity of the system to administer vaccinations under the first mode and the throughput affected by vaccine hesitancy under the second mode.
Waste management: Mass COVID-19 vaccination practices increase medical waste dramatically. The typical waste is vaccine vials, needles, syringes, and plastic vaccine administration equipment. Hasija et al. (2022) conceptualize and classify the types of vaccine waste and provide guidelines for vaccine waste management and treatment. Similarly, Rayhan et al. (2022) assess the COVID-19 vaccine-related waste management practices in Bangladesh.
| Decision level | Model characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Article | Inventory | Allocation | Distribution | Type | SIR | Commodity | Period | Priority groups | Case study |
| Prioritization | |||||||||
| Bubar et al. (2021) | SEIR simulation | Single COVID-19 vaccine | One year | Age based | USA | ||||
| Chaturvedi and Chakravarty (2021) | SEIR simulation | Single COVID-19 vaccine | 9 months | India, Brazil, USA | |||||
| Chen et al. (2020) | SAPHIRE simulation | Single COVID-19 vaccine | 3 months | Age based | New York, USA | ||||
| Chen et al. (2021) | Agent based simulation | Single COVID-19 vaccine | 4 months | Social contact based | Virginia, USA | ||||
| Foy et al. (2021) | SEIR simulation | Single COVID-19 vaccine | Five years | Age based | India | ||||
| Fujimoto et al. (2021) | SEIR simulation | Single COVID-19 vaccine | 550 days | Antibody testing based | Synthetic | ||||
| MacIntyre et al. (2022) | SEIR simulation | Single COVID-19 vaccine | 700 days | Age, health worker based | New South Wales, Australia | ||||
| Oruc et al. (2021) | Agent based simulation | Single COVID-19 vaccine | 16 months | Age, risk based | Georgia, USA | ||||
| Rosenstrom et al. (2022) | Agent based simulation | Single COVID-19 vaccine | 17 months | Income, race based | North Carolina, USA | ||||
| Walker et al. (2022) | SEPIR simulation | Single COVID-19 vaccine | 731 days | Age, health worker based | USA | ||||
| Multiple vaccine management | |||||||||
| Kim et al. (2021) | SIRD simulation | Two different COVID-19 vaccines | One year | Synthetic | |||||
| Kim et al. (2022) | SIRD simulation | COVID-19 vaccines with different efficacy | One year | Synthetic | |||||
| Romero-Brufau et al. (2021) | Agent based simulation | COVID-19 vaccines with different efficacy | 180 days | Age, health worker based | USA | ||||
| Shim (2021) | SEIR simulation | COVID-19 vaccines with different efficacy | One year | Age based | South Korea | ||||
| Management of vaccine inventory | |||||||||
| Assi et al. (2011) | Discrete event simulation | Multiple vial sized measles vaccines | One year | Niger | |||||
| Assi et al. (2012) | Discrete event simulation | Ten different influenza vaccines | 1–6 months | Age based | Trang Province, Thailand | ||||
| Govindan et al. (2020) | Fuzzy inference system | General demand management | N/A | Age, disease, risk based | Synthetic | ||||
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- Abbasi, B., Fadaki, M., Kokshagina, O., Saeed, N., & Chhetri, P. (2020). Modeling vaccine allocations in the COVID-19 Pandemic: A case study in Australia. Available at SSRN 3744520. https://doi.org/10.2139/ssrn.3744520
10.2139/ssrn.3744520 Google Scholar
- Abbasi, S., Zahmatkesh, S., Bokhari, A., & Hajiaghaei-Keshteli, M. (2023). Designing a vaccine supply chain network considering environmental aspects. Journal of Cleaner Production, 417, 137935. https://doi.org/10.1016/j.jclepro.2023.137935
- Alam, S. T., Ahmed, S., Ali, S. M., Sarker, S., & Kabir, G. (2021). Challenges to COVID-19 vaccine supply chain: Implications for sustainable development goals. International Journal of Production Economics, 239, 108193. https://doi.org/10.1016/j.ijpe.2021.108193
- Anahideh, H., Kang, L., & Nezami, N. (2022). Fair and diverse allocation of scarce resources. Socio-Economic Planning Sciences, 80, 101–193. https://doi.org/10.1016/j.seps.2021.101193
- Assi, T.-M., Brown, S. T., Djibo, A., Norman, B. A., Rajgopal, J., Welling, J. S., Chen, S.-I., Bailey, R. R., Kone, S., Kenea, H., Connor, D. L., Wateska, A. R., Jana, A., Wisniewski, S. R., van Panhuis, W. G., Burke, D. S., & Lee, B. Y. (2011). Impact of changing the Measles vaccine vial size on Niger's vaccine supply chain: A computational model. BMC Public Health, 11(1), 1–9. https://doi.org/10.1186/1471-2458-11-425
- Assi, T.-M., Rookkapan, K., Rajgopal, J., Sornsrivichai, V., Brown, S. T., Welling, J. S., Norman, B. A., Connor, D. L., Chen, S.-I., Slayton, R. B., Laosiritaworn, Y., Wateska, A. R., Wisniewski, S. R., & Lee, B. Y. (2012). How Influenza Vaccination policy may affect vaccine logistics. Vaccine, 30(30), 4517–4523. https://doi.org/10.1016/j.vaccine.2012.04.041
- Azadi, Z., Eksioglu, S. D., & Geismar, H. N. (2020). Optimization of distribution network configuration for pediatric vaccines using chance constraint programming. arXiv preprint, arXiv:2006.05488, https://doi.org/10.48550/arXiv.2006.05488.
10.48550/arXiv.2006.05488 Google Scholar
- Azadi, Z., Gangammanavar, H., & Eksioglu, S. (2020). Developing childhood vaccine administration and inventory replenishment policies that minimize open vial wastage. Annals of Operations Research, 292(1), 215–247. doi:10.1007/s10479–019–03164–8
- Balcik, B., Yucesoy, E., Akca, B., Karakaya, S., Gevsek, A. A., Baharmand, H., & Sgarbossa, F. (2022). A mathematical model for equitable in-country COVID-19 vaccine allocation. International Journal of Production Research, 60, 1–25. https://doi.org/10.1080/00207543.2022.2110014
- Bandi, C., Gao, S. Y., Moorthy, R., Tan, K. B., & Teo, C.-P. (2021). Optimal scheduling and policy design of two-dose vaccination rollout. https://doi.org/10.2139/ssrn.3909792
10.2139/ssrn.3909792 Google Scholar
- Barth, J., Li, S., Aprahamian, H., & Gupta, D. (2023). Spatiotemporal vaccine allocation policies for epidemics with behavioral feedback dynamics. Naval Research Logistics (NRL), 71, 109–139. https://doi.org/10.1002/nav.22142
10.1002/nav.22142 Google Scholar
- Basciftci, B., Yu, X., & Shen, S. (2023). Resource distribution under spatiotemporal uncertainty of disease spread: Stochastic versus robust approaches. Computers & Operations Research, 149, 106028. https://doi.org/10.1016/j.cor.2022.106028
- Bennouna, A., Joseph, J., Nze-Ndong, D., Perakis, G., Singhvi, D., Lami, O. S., Spantidakis, Y., Thayaparan, L., & Tsiourvas, A. (2022). COVID-19 prediction, prevalence, and the operations of vaccine allocation. Manufacturing & Service Operations Management, 25, 1013–1032. https://doi.org/10.1287/msom.2022.1160
10.1287/msom.2022.1160 Google Scholar
- Bertsimas, D., Allison, K., & Pulleyblank, W. R. (2016). The analytics edge. Dynamic Ideas LLC Charlestown.
- Bertsimas, D., Digalakis, V., Jr., Jacquillat, A., Li, M. L., & Previero, A. (2022). Where to locate COVID-19 mass vaccination facilities? Naval Research Logistics (NRL), 69(2), 179–200. https://doi.org/10.1002/nav.22007
- Bertsimas, D., Ivanhoe, J., Jacquillat, A., Li, M., Previero, A., Lami, O. S., & Bouardi, H. T. (2020). Optimizing vaccine allocation to combat the COVID-19 Pandemic. medRxiv. Preprint. https://doi.org/10.1101/2020.11.17.20233213
10.1101/2020.11.17.20233213 Google Scholar
- Bogart, L. M., Ojikutu, B. O., Tyagi, K., Klein, D. J., Mutchler, M. G., Dong, L., Lawrence, S. J., Thomas, D. R., & Kellman, S. (2021). COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among black Americans living with HIV. Journal of Acquired Immune Deficiency Syndromes (1999), 86(2), 200. https://doi.org/10.1097/QAI.0000000000002570
- Bonney, M., & Jaber, M. Y. (2011). Environmentally responsible inventory models: Non-classical models for a non-classical era. International Journal of Production Economics, 133(1), 43–53. https://doi.org/10.1016/j.ijpe.2009.10.033
- Bozkir, C. D., Ozmemis, C., Kurbanzade, A. K., Balcik, B., Gunes, E. D., & Tuglular, S. (2023). Capacity planning for effective cohorting of hemodialysis patients during the coronavirus pandemic: A case study. European Journal of Operational Research, 304(1), 276–291. https://doi.org/10.1016/j.ejor.2021.10.039
- Bravo, F., Hu, J., & Long, E. (2022). Optimal COVID-19 Vaccination facility location. Available at SSRN 4008669. https://doi.org/10.2139/ssrn.4008669
10.2139/ssrn.4008669 Google Scholar
- Bubar, K. M., Reinholt, K., Kissler, S. M., Lipsitch, M., Cobey, S., Grad, Y. H., & Larremore, D. B. (2021). Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science, 371(6532), 916–921. https://doi.org/10.1126/science.abe6959
- Cabezas, X., García, S., Martin-Barreiro, C., Delgado, E., & Leiva, V. (2021). A two-stage location problem with order solved using a Lagrangian algorithm and stochastic programming for a potential use in COVID-19 Vaccination based on sensor-related data. Sensors, 21(16), 5352. https://doi.org/10.3390/s21165352
- Cano-Marin, E., Ribeiro-Soriano, D., Mardani, A., & Gonzalez-Tejero, C. B. (2023). Exploring the challenges of the COVID-19 vaccine supply chain using social media analytics: A global perspective. Sustainable Technology and Entrepreneurship, 2(3), 100047. https://doi.org/10.1016/j.stae.2023.100047
10.1016/j.stae.2023.100047 Google Scholar
- Cao, C., Liu, J., Liu, Y., Wang, H., & Liu, M. (2023). Digital twin-driven robust bi-level optimisation model for COVID-19 medical waste location-transport under circular economy. Computers & Industrial Engineering, 186, 109107. https://doi.org/10.1016/j.cie.2023.109107
- Chaturvedi, D., & Chakravarty, U. (2021). Predictive analysis of COVID-19 eradication with vaccination in India, Brazil, and USA. Infection, Genetics and Evolution, 92, 104834. https://doi.org/10.1016/j.meegid.2021.104834
- Chen, J., Hoops, S., Marathe, A., Mortveit, H., Lewis, B., Venkatramanan, S., Haddadan, A., Bhattacharya, P., Adiga, A., & Vullikanti, A. (2021). Prioritizing allocation of COVID-19 vaccines based on social contacts increases vaccination effectiveness. MedRxiv. Preprint. https://doi.org/10.1101/2021.02.04.21251012
10.1101/2021.02.04.21251012 Google Scholar
- Chen, X., Li, M., Simchi-Levi, D., & Zhao, T. (2020). Allocation of COVID-19 vaccines under limited supply. MedRxiv. Preprint. https://doi.org/10.1101/2020.08.23.20179820
10.1101/2020.08.23.20179820 Google Scholar
- Chick, S. E., Mamani, H., & Simchi-Levi, D. (2008). Supply chain coordination and Influenza Vaccination. Operations Research, 56(6), 1493–1506. https://doi.org/10.1287/opre.1080.0527
- Chowdhury, N. R., Ahmed, M., Mahmud, P., Paul, S. K., & Liza, S. A. (2022). Modeling a sustainable vaccine supply chain for a healthcare system. Journal of Cleaner Production, 370, 133423. https://doi.org/10.1016/j.jclepro.2022.133423
- Dai, T., & Song, J.-S. (2021). Transforming COVID-19 vaccines into vaccination. Health Care Management Science, 24(3), 455–459. https://doi.org/10.2139/ssrn.3797192
- Dastgoshade, S., Shafiee, M., Klibi, W., & Shishebori, D. (2022). Social equity-based distribution networks design for the COVID-19 vaccine. International Journal of Production Economics, 250, 108684. https://doi.org/10.1016/j.ijpe.2022.108684
- Emu, M., Chandrasekaran, D., Mago, V., & Choudhury, S. (2021). Validating optimal COVID-19 vaccine distribution models. In International Conference on Computational Science (pp. 352–366). Springer. https://doi.org/10.48550/arXiv.2102.04251
- Enayati, S., Campbell, J. F., & Li, H. (2023). vaccine distribution with drones for less developed countries: A case study in Vanuatu. vaccine: X, 14, 100312. https://doi.org/10.1016/j.jvacx.2023.100312
- Enayati, S., Li, H., Campbell, J. F., & Pan, D. (2023). Multimodal vaccine distribution network design with drones. Transportation Science, 57, 839–1114. https://doi.org/10.1287/trsc.2023.1205
- Enayati, S., & Özaltin, O. Y. (2020). Optimal Influenza vaccine distribution with equity. European Journal of Operational Research, 283(2), 714–725. https://doi.org/10.1016/j.ejor.2019.11.025
- Fadaki, M., Abareshi, A., Far, S. M., & Lee, P. T.-W. (2022). Multi-period vaccine allocation model in a pandemic: A case study of COVID-19 in Australia. Transportation Research Part E: Logistics and Transportation Review, 161, 102689. https://doi.org/10.1016/j.tre.2022.102689
- Forman, R., Shah, S., Jeurissen, P., Jit, M., & Mossialos, E. (2021). COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy, 125(5), 553–567. https://doi.org/10.1016/j.healthpol.2021.03.013
- Foy, B. H., Wahl, B., Mehta, K., Shet, A., Menon, G. I., & Britto, C. (2021). Comparing COVID-19 vaccine allocation strategies in India: A mathematical modelling study. International Journal of Infectious Diseases, 103, 431–438. https://doi.org/10.1016/j.ijid.2020.12.075
- Fujimoto, A. B., Keskinocak, P., & Yildirim, I. (2021). Significance of SARS-CoV-2 specific antibody testing during COVID-19 vaccine allocation. Vaccine, 39(35), 5055–5063. https://doi.org/10.1016/j.vaccine.2021.06.067
- Gel, E. S., Jehn, M., Lant, T., Muldoon, A. R., Nelson, T., & Ross, H. M. (2020). COVID-19 healthcare demand projections: Arizona. PLoS One, 15(12), e0242588. https://doi.org/10.1371/journal.pone.0242588
- Georgiadis, G. P., & Georgiadis, M. C. (2021). Optimal planning of the COVID-19 vaccine supply chain. Vaccine, 39(37), 5302–5312. https://doi.org/10.1016/j.vaccine.2021.07.068
- Gilani, H., & Sahebi, H. (2022). A data-driven robust optimization model by cutting hyperplanes on vaccine access uncertainty in COVID-19 vaccine supply chain. Omega, 110, 102637. https://doi.org/10.1016/j.omega.2022.102637
- Goodarzian, F., Ghasemi, P., Gunasekaren, A., Taleizadeh, A. A., & Abraham, A. (2022). A sustainable-resilience healthcare network for handling COVID-19 Pandemic. Annals of Operations Research, 312(2), 761–825. https://doi.org/10.1007/s10479-021-04238-2
- Goodarzian, F., Navaei, A., Ehsani, B., Ghasemi, P., & Muñuzuri, J. (2022). Designing an integrated responsive-green-cold vaccine supply chain network using Internet-of-Things: Artificial intelligence-based solutions. Annals of Operations Research, 312, 1–45. https://doi.org/10.1007/s10479-022-04713-4
- Govindan, K., Mina, H., & Alavi, B. (2020). A decision support system for demand management in healthcare supply chains considering the epidemic outbreaks: A case study of coronavirus disease 2019 (COVID-19). Transportation Research Part E: Logistics and Transportation Review, 138, 101967. https://doi.org/10.1016/j.tre.2020.101967
- Gralla, E., Goentzel, J., & Fine, C. (2014). Assessing trade-offs among multiple objectives for humanitarian aid delivery using expert preferences. Production and Operations Management, 23(6), 978–989. https://doi.org/10.1111/poms.12110
- Habibi, F., Abbasi, A., & Chakrabortty, R. K. (2023). Designing an efficient vaccine supply chain network using a two-phase optimization approach: A case study of COVID-19 vaccine. International Journal of Systems Science: Operations & Logistics, 10(1), 2121623. https://doi.org/10.1080/23302674.2022.2121623
10.1080/23302674.2022.2121623 Google Scholar
- Hajibabai, L., Hajbabaie, A., Swann, J., & Vergano, D. (2022). Using COVID-19 data on vaccine shipments and wastage to inform modeling and decision-making. Transportation Science, 56, 1111–1408. https://doi.org/10.1287/trsc.2022.1134
- Hasija, V., Patial, S., Raizada, P., Thakur, S., Singh, P., & Hussain, C. M. (2022). The environmental impact of mass coronavirus vaccinations: A point of view on huge COVID-19 vaccine waste across the globe during ongoing vaccine campaigns. Science of the Total Environment, 813, 151881. https://doi.org/10.1016/j.scitotenv.2021.151881
- Hegde, S. J., Mahmassani, H., & Smilowitz, K. (2023). A two-regime analysis of the COVID-19 vaccine distribution process. Journal of Humanitarian Logistics and Supply Chain Management, 13, 111–124. https://doi.org/10.1108/JHLSCM-10-2021-0106
- Hill, A. N., & Longini, I. M., Jr. (2003). The critical vaccination fraction for heterogeneous epidemic models. Mathematical Biosciences, 181(1), 85–106. https://doi.org/10.1016/S0025-5564(02)00129-3
- Hinojosa, Y., Puerto, J., & Fernández, F. R. (2000). A multiperiod two-echelon multicommodity capacitated plant location problem. European Journal of Operational Research, 123(2), 271–291. https://doi.org/10.1016/S0377-2217(99)00256-8
- Hovav, S., & Tsadikovich, D. (2015). A network flow model for inventory management and distribution of Influenza vaccines through a healthcare supply chain. Operations Research for Health Care, 5, 49–62. https://doi.org/10.1016/j.orhc.2015.05.003
10.1016/j.orhc.2015.05.003 Google Scholar
- Hu, M., Zhang, C., & Zhou, Y. (2023). First or second doses first? vaccine allocation under limited supply. SSRN. Preprint. https://doi.org/10.2139/ssrn.4411818
10.2139/ssrn.4411818 Google Scholar
- Huang, H.-C., Singh, B., Morton, D. P., Johnson, G. P., Clements, B., & Meyers, L. A. (2017). Equalizing access to pandemic Influenza vaccines through optimal allocation to public health distribution points. PLoS One, 12(8), e0182720. https://doi.org/10.1371/journal.pone.0182720
- Huang, M., Smilowitz, K., & Balcik, B. (2012). Models for relief routing: Equity, efficiency and efficacy. Transportation Research Part E: Logistics and Transportation Review, 48(1), 2–18. https://doi.org/10.1016/j.tre.2011.05.004
- Işik, E. E., & Yildiz, S. T. (2023). Optimizing the COVID-19 cold chain vaccine distribution network with medical waste management: A robust optimization approach. Expert Systems with Applications, 229, 120510. https://doi.org/10.1016/j.eswa.2023.120510
- Jadidi, M., Jamshidiha, S., Masroori, I., Moslemi, P., Mohammadi, A., & Pourahmadi, V. (2021). A two-step vaccination technique to limit COVID-19 spread using mobile data. Sustainable Cities and Society, 70, 102886. https://doi.org/10.1016/j.scs.2021.102886
- Jahani, H., Chaleshtori, A. E., Khaksar, S. M. S., Aghaie, A., & Sheu, J.-B. (2022). COVID-19 vaccine distribution planning using a congested queuing system—A real case from Australia. Transportation Research Part E: Logistics and Transportation Review, 163, 102749. https://doi.org/10.1016/j.tre.2022.102749
- Jarumaneeroj, P., Dusadeerungsikul, P. O., Chotivanich, T., Nopsopon, T., & Pongpirul, K. (2022). An epidemiology-based model for the operational allocation of COVID-19 vaccines: A case study of Thailand. Computers & Industrial Engineering, 167, 108031. https://doi.org/10.1016/j.cie.2022.108031
- Karakaya, S., & Balcik, B. (2023). Developing a national pandemic vaccination calendar under supply uncertainty. Available at SSRN 4380147. https://doi.org/10.2139/ssrn.4380147
10.2139/ssrn.4380147 Google Scholar
- Keskinocak, P., Oruc, B. E., Baxter, A., Asplund, J., & Serban, N. (2020). The impact of social distancing on COVID-19 spread: State of Georgia case study. PLoS One, 15(10), e0239798. https://doi.org/10.1371/journal.pone.0239798
- Kim, D., Keskinocak, P., Pekgün, P., & Yildirim, I. (2022). The balancing role of distribution speed against varying efficacy levels of COVID-19 vaccines under variants. Scientific Reports, 12(1), 1–8. https://doi.org/10.1038/s41598-022-11060-8
- Kim, D., Pekgün, P., Yildirim, İ., & Keskinocak, P. (2021). Resource allocation for different types of vaccines against COVID-19: Tradeoffs and synergies between efficacy and reach. Vaccine, 39(47), 6876–6882. https://doi.org/10.1016/j.vaccine.2021.10.025
- Klemeš, J. J., Van Fan, Y., Tan, R. R., & Jiang, P. (2020). Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renewable and Sustainable Energy Reviews, 127, 109883. https://doi.org/10.1016/j.rser.2020.109883
- Kohneh, J. N., Amirdadi, M., & Teimoury, E. (2023). An optimization framework for COVID-19 vaccine allocation and inventory management: A case study. Applied Soft Computing, 132, 109801. https://doi.org/10.1016/j.asoc.2022.109801
- Kumar, A., Kumar, G., Ramane, T. V., & Singh, G. (2022). Optimal COVID-19 vaccine stations location and allocation strategies. Benchmarking: An International Journal, 30, 3328–3356. https://doi.org/10.1108/BIJ-02-2022-0089
10.1108/BIJ?02?2022?0089 Google Scholar
- Lai, X., Lu, X., Yu, X., & Zhu, N. (2021). Multi-period integrated planning for vaccination station location and medical professional assignment under uncertainty. Computers & Industrial Engineering, 161, 107673. https://doi.org/10.1016/j.cie.2021.107673
- Leithäuser, N., Schneider, J., Johann, S., Krumke, S. O., Schmidt, E., Streicher, M., & Scholz, S. (2021). Quantifying COVID-19 vaccine location strategies for Germany. BMC Health Services Research, 21(1), 1–18. https://doi.org/10.1186/s12913-021-06587-x
- Li, Q., Norman, S., Guthrie, P., Gachoya, J., Sebakijje, J., Leftridge, Z., Willis, C. G., Kishore, A., Breakenridge, M., & Spence, C. (2021). Strategies used to meet the challenges of mass COVID-19 Vaccination by the pharmacy department in a large academic medical center. American Journal of Health-System Pharmacy, 78(18), 1724–1731. https://doi.org/10.1093/ajhp/zxab184
- Li, S., & Aprahamian, H. (2023). Quantifying the benefits of customized vaccination strategies: A network-based optimization approach. Naval Research Logistics (NRL), 71, 64–86. https://doi.org/10.1002/nav.22134
10.1002/nav.22134 Google Scholar
- Li, X., Pan, Y., Jiang, S., Huang, Q., Chen, Z., Zhang, M., & Zhang, Z. (2021). Locate vaccination stations considering travel distance, operational cost, and work schedule. Omega, 101, 102236. https://doi.org/10.1016/j.omega.2020.102236
10.1016/j.omega.2020.102236 Google Scholar
- Lim, J., Claypool, E., Norman, B. A., & Rajgopal, J. (2016). Coverage models to determine outreach vaccination center locations in low and middle income countries. Operations Research for Health Care, 9, 40–48. https://doi.org/10.1016/j.orhc.2016.02.003
- Lim, J., Norman, B. A., & Rajgopal, J. (2022). Redesign of vaccine distribution networks. International Transactions in Operational Research, 29(1), 200–225. https://doi.org/10.1111/itor.12758
- Luo, F., & Mehrotra, S. (2023). Service center location with decision dependent utilities with an application to early stage testing and vaccination in epidemic planning. Naval Research Logistics, 71, 3–26. https://doi.org/10.1002/nav.22112
10.1002/nav.22112 Google Scholar
- Lusiantoro, L., Mara, S., & Rifai, A. (2022). A locational analysis model of the COVID-19 vaccine distribution. Operations and Supply Chain Management: An International Journal, 15(2), 240–250. https://doi.org/10.31387/oscm0490344
10.31387/oscm0490344 Google Scholar
- MacIntyre, C. R., Costantino, V., & Trent, M. (2022). Modelling of COVID-19 vaccination strategies and herd immunity, in scenarios of limited and full vaccine supply in NSW, Australia. Vaccine, 40(17), 2506–2513. https://doi.org/10.1016/j.vaccine.2021.04.042
- Mak, H.-Y., Dai, T., & Tang, C. S. (2022). Managing two-dose COVID-19 vaccine rollouts with limited supply: Operations strategies for distributing time-sensitive resources. Production and Operations Management, 31, 4424–4442. https://doi.org/10.1111/poms.13862
- Manupati, V. K., Schoenherr, T., Subramanian, N., Ramkumar, M., Soni, B., & Panigrahi, S. (2021). A multi-echelon dynamic cold chain for managing vaccine distribution. Transportation Research Part E: Logistics and Transportation Review, 156, 102542. https://doi.org/10.1016/j.tre.2021.102542
- Mehrotra, S., Rahimian, H., Barah, M., Luo, F., & Schantz, K. (2020). A model of supply-chain decisions for resource sharing with an application to ventilator allocation to combat COVID-19. Naval Research Logistics (NRL), 67(5), 303–320. https://doi.org/10.1002/nav.21905
- Minoza, J. M. A., Bongolan, V. P., & Rayo, J. F. (2021). COVID-19 agent-based model with multi-objective optimization for vaccine distribution. arXiv preprint, arXiv:2101.11400 https://doi.org/10.48550/arXiv.2101.11400
10.48550/arXiv.2101.11400 Google Scholar
- Mohammadi, M., Dehghan, M., Pirayesh, A., & Dolgui, A. (2022). Bi-objective optimization of a stochastic resilient vaccine distribution network in the context of the COVID-19 pandemic. Omega, 113, 102725. https://doi.org/10.1016/j.omega.2022.102725
- Munguía-López, A. d. C., & Ponce-Ortega, J. M. (2021). Fair allocation of potential COVID-19 vaccines using an optimization-based strategy. Process Integration and Optimization for Sustainability, 5(1), 3–12. https://doi.org/10.1007/s41660-020-00141-8
10.1007/s41660-020-00141-8 Google Scholar
- Orgut, I. S., Freeman, N., Lewis, D., & Parton, J. (2023). Equitable and effective vaccine access considering vaccine hesitancy and capacity constraints. Omega, 120, 102898. https://doi.org/10.1016/j.omega.2023.102898
- Oruc, B. E., Fujimoto, A., Breiman, R., & Keskinocak, P. (2021). Impact of prioritizing elderly and high-risk individuals during COVID-19 vaccine allocation. https://doi.org/10.21203/rs.3.rs-944201/v1
10.21203/rs.3.rs?944201/v1 Google Scholar
- Polo, G., Acosta, C. M., Ferreira, F., & Dias, R. A. (2015). Location-allocation and accessibility models for improving the spatial planning of public health services. PLoS One, 10(3), e0119190. https://doi.org/10.1371/journal.pone.0119190
- Radke, J., & Mu, L. (2000). Spatial decompositions, modeling and mapping service regions to predict access to social programs. Geographic Information Sciences, 6(2), 105–112. https://doi.org/10.1080/10824000009480538
10.1080/10824000009480538 Google Scholar
- Rahimian, H., & Mehrotra, S. (2019). Distributionally robust optimization: A review. arXiv preprint, arXiv:1908.05659 https://doi.org/10.48550/arXiv.1908.05659
10.48550/arXiv.1908.05659 Google Scholar
- Rahman, H. F., Chakrabortty, R. K., Paul, S. K., & Elsawah, S. (2023). Optimising vaccines supply chains to mitigate the COVID-19 pandemic. International Journal of Systems Science: Operations & Logistics, 10(1), 2122757. https://doi.org/10.1080/23302674.2022.2122757
10.1080/23302674.2022.2122757 Google Scholar
- Rao, I. J., & Brandeau, M. L. (2021). Optimal allocation of limited vaccine to control an infectious disease: Simple analytical conditions. Mathematical Biosciences, 337, 108621. https://doi.org/10.1016/j.mbs.2021.108621
- Rastegar, M., Tavana, M., Meraj, A., & Mina, H. (2021). An inventory-location optimization model for equitable Influenza vaccine distribution in developing countries during the COVID-19 Pandemic. Vaccine, 39(3), 495–504. https://doi.org/10.1016/j.vaccine.2020.12.022
- Rayhan, M. R. I., Liza, J. M., & Rahman, M. M. (2022). Assessment of COVID-19 vaccination-related medical waste management practices in Bangladesh. PLoS One, 17(8), e0273053. https://doi.org/10.1371/journal.pone.0273053
- Romero-Brufau, S., Chopra, A., Ryu, A. J., Gel, E., Raskar, R., Kremers, W., Anderson, K. S., Subramanian, J., Krishnamurthy, B., & Singh, A. (2021). Public health impact of delaying second dose of (BNT)162b2 or m(RNA)-1273 COVID-19 vaccine: Simulation agent based modeling study. BMJ, 373, 1–9. https://doi.org/10.1136/bmj.n1087
- Rosenstrom, E. T., Mele, J., Ivy, J. S., Mayorga, M. E., Patel, M. D., Lich, K. H., Johnson, K., Delamater, P., Keskinocak, P., & Boyce, R. (2022). Can vaccine prioritization reduce disparities in COVID-19 burden for historically marginalized populations? PNAS Nexus, 1(1), pgab004. https://doi.org/10.1093/pnasnexus/pgab004
- Roy, S., Dutta, R., & Ghosh, P. (2021). Optimal time-varying vaccine allocation amid pandemics with uncertain immunity ratios. IEEE Access, 9, 15110–15121. https://doi.org/10.1109/ACCESS.2021.3053268
- Saadi, N., Chi, Y., Ghosh, S., Eggo, R. M., McCarthy, C. V., Quaife, M., Dawa, J., Jit, M., & Vassall, A. (2021). Models of COVID-19 vaccine prioritisation: A systematic literature search and narrative review. BMC Medicine, 19(1), 1–11. https://doi.org/10.1186/s12916-021-02190-3
- Saberi Isfeedvajani, M., Fares, F., & Moqaddam, Z. I. S. (2020). Ethical issues in COVID-19 pandemic. Hospital Practices and Research, 5(4), 126–133. https://doi.org/10.34172/hpr.2020.25
10.34172/hpr.2020.25 Google Scholar
- Sallam, M. (2021). COVID-19 vaccine hesitancy Worldwide: A concise systematic review of vaccine acceptance rates. Vaccine, 9(2), 160. https://doi.org/10.3390/vaccines9020160
- Sampath, S., Khedr, A., Qamar, S., Tekin, A., Singh, R., Green, R., & Kashyap, R. (2021). Pandemics throughout the history. Cureus, 13(9), 1–9. https://doi.org/10.7759/cureus.18136
- Sazvar, Z., Tafakkori, K., Oladzad, N., & Nayeri, S. (2021). A capacity planning approach for sustainable-resilient supply chain network design under uncertainty: A case study of vaccine supply chain. Computers & Industrial Engineering, 159, 107406. https://doi.org/10.1016/j.cie.2021.107406
- Schmidt, H., Weintraub, R., Williams, M. A., Miller, K., Buttenheim, A., Sadecki, E., Wu, H., Doiphode, A., Nagpal, N., & Gostin, L. O. (2021). Equitable allocation of COVID-19 vaccines in the United States. Nature Medicine, 27(7), 1298–1307. https://doi.org/10.1038/s41591-021-01379-6
- Seboka, B. T., Yehualashet, D. E., Belay, M. M., Kabthymer, R. H., Ali, H., Hailegebreal, S., Demeke, A. D., Amede, E. S., & Tesfa, G. A. (2021). Factors influencing COVID-19 vaccination demand and intent in resource-limited settings: Based on health belief model. Risk Management and Healthcare Policy, 14, 2743–2756. https://doi.org/10.2147/RMHP.S315043
- Shah, N. H., Patel, E., & Rabari, K. (2022). Investigation of carbon emissions due to COVID-19 vaccine inventory. International Journal of System Assurance Engineering and Management, 13(1), 409–420. https://doi.org/10.1007/s13198-021-01285-7
- Shah, S. M. A., Mohammad, D., Qureshi, M. F. H., Abbas, M. Z., & Aleem, S. (2021). Prevalence, psychological responses and associated correlates of depression, anxiety and stress in a global population, during the coronavirus disease (COVID-19) pandemic. Community Mental Health Journal, 57(1), 101–110. https://doi.org/10.1007/s10597-020-00728-y
- Shamsi Gamchi, N., Torabi, S. A., & Jolai, F. (2021). A novel vehicle routing problem for vaccine distribution using SIR epidemic model. OR Spectrum, 43(1), 155–188. https://doi.org/10.1007/s00291-020-00609-6
- Shim, E. (2021). Optimal allocation of the limited COVID-19 vaccine supply in South Korea. Journal of Clinical Medicine, 10(4), 591. https://doi.org/10.3390/jcm10040591
- Shiri, M., & Ahmadizar, F. (2022). An equitable and accessible vaccine supply chain network in the epidemic outbreak of COVID-19 under uncertainty. Journal of Ambient Intelligence and Humanized Computing, 14, 1–25. https://doi.org/10.1007/s12652-022-03865-2
- Shukla, S., Fressin, F., Un, M., Coetzer, H., & Chaguturu, S. K. (2022). Optimizing vaccine distribution via mobile clinics: A case study on COVID-19 vaccine distribution to long-term care facilities. Vaccine, 40(5), 734–741. https://doi.org/10.1016/j.vaccine.2021.12.049
- Silva, J., Bratberg, J., & Lemay, V. (2021). COVID-19 and Influenza vaccine hesitancy among college students. Journal of the American Pharmacists Association, 61(6), 709–714. https://doi.org/10.1016/j.japh.2021.05.009
- Sinha, P., Kumar, S., & Chandra, C. (2021). Strategies for ensuring required service level for COVID-19 herd immunity in Indian vaccine supply chain. European Journal of Operational Research, 304, 339–352. https://doi.org/10.1016/j.ejor.2021.03.030
10.1016/j.ejor.2021.03.030 Google Scholar
- Soria-Arguello, I., Torres-Escobar, R., Pérez-Vicente, H. A., & Perea-Rivera, T. G. (2021). A proposal mathematical model for the vaccine COVID-19 distribution network: A case study in Mexico. Mathematical Problems in Engineering, 2021, 1–11. https://doi.org/10.1155/2021/5484101
10.1155/2021/5484101 Google Scholar
- Srivastava, V., Ratna, M., Ray, A., Shukla, S., Shrivastava, V., Kothari, N., Gupta, A., Kukreja, M., & Jandu, H. S. (2021). Strengthening the immunization supply chain: A time-to-supply based approach to cold chain network optimization & extension in Madhya Pradesh. Vaccine, 39(45), 6660–6670. https://doi.org/10.1016/j.vaccine.2021.09.062
- Tang, L., Li, Y., Bai, D., Liu, T., & Coelho, L. C. (2022). Bi-objective optimization for a multi-period COVID-19 vaccination planning problem. Omega, 110, 102617. https://doi.org/10.1016/j.omega.2022.102617
- Taskinsoy, J. (2020). COVID-19: Is the great outbreak a sign of what the future has stowed for the human race? Available at SSRN 3597434. https://doi.org/10.2139/ssrn.3597434
10.2139/ssrn.3597434 Google Scholar
- Tavana, M., Govindan, K., Nasr, A. K., Heidary, M. S., & Mina, H. (2021). A mathematical programming approach for equitable COVID-19 vaccine distribution in developing countries. Annals of Operations Research, 301, 1–34. https://doi.org/10.1007/s10479-021-04130-z
10.1007/s10479?021?04130?z Google Scholar
- Thul, L., & Powell, W. (2023). Stochastic optimization for vaccine and testing kit allocation for the COVID-19 Pandemic. European Journal of Operational Research, 304(1), 325–338. https://doi.org/10.1016/j.ejor.2021.11.007
- Troiano, G., & Nardi, A. (2021). vaccine hesitancy in the era of COVID-19. Public Health, 194, 245–251. https://doi.org/10.1016/j.puhe.2021.02.025
- Walker, J., Paul, P., Dooling, K., Oliver, S., Prasad, P., Steele, M., Gastañaduy, P. A., Johansson, M. A., Biggerstaff, M., & Slayton, R. B. (2022). modeling strategies for the allocation of SARS-CoV-2 vaccines in the united states. Vaccine, 40(14), 2134–2139. https://doi.org/10.1016/j.vaccine.2022.02.015
- Wang, X., Jiang, R., & Qi, M. (2023). a robust optimization problem for drone-based equitable pandemic vaccine distribution with uncertain supply. Omega, 119, 102872. https://doi.org/10.1016/j.omega.2023.102872
- Westerink-Duijzer, E. (2017). Mathematical optimization in vaccine allocation.
- world health organization. (2023). WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/
- Xu, X., Rodgers, M. D., & Guo, W. G. (2021). A hub-and-spoke design for ultra-cold COVID-19 vaccine distribution. Vaccine, 39(41), 6127–6136. https://doi.org/10.1016/j.vaccine.2021.08.069
- Yang, H., Sürer, Ö., Duque, D., Morton, D. P., Singh, B., Fox, S. J., Pasco, R., Pierce, K., Rathouz, P., & Valencia, V. (2021). Design of COVID-19 staged alert systems to ensure healthcare capacity with minimal closures. Nature Communications, 12(1), 3767. doi:10.1038/s41467–021–23989–x
- Yang, T., Deng, W., Liu, Y., & Deng, J. (2022). Comparison of health-oriented cross-regional allocation strategies for the COVID-19 vaccine: A mathematical modelling study. Annals of Medicine, 54(1), 941–952. https://doi.org/10.1080/07853890.2022.2060522
- Yang, Y., Bidkhori, H., & Rajgopal, J. (2021). Optimizing vaccine distribution networks in low and middle-income countries. Omega, 99, 102197. https://doi.org/10.1016/j.omega.2020.102197
10.1016/j.omega.2020.102197 Google Scholar
- Yang, Y., & Rajgopal, J. (2021). Outreach strategies for vaccine distribution: A multi-period stochastic modeling approach. In Operations Research Forum (Vol. 2, pp. 1–26). Springer. https://doi.org/10.1007/s43069-021-00064-1
10.1007/s43069-021-00064-1 Google Scholar
- Yarmand, H., Ivy, J. S., Denton, B., & Lloyd, A. L. (2014). Optimal two-phase vaccine allocation to geographically different regions under uncertainty. European Journal of Operational Research, 233(1), 208–219. https://doi.org/10.1016/j.ejor.2013.08.027
- Yucesoy, E., Coban, E., & Balcik, B. (2022). Mobile vaccination clinic routing problem. In 12th Annual International Conference on Industrial Engineering and Operations Management (Vol. 12, pp. 1207–1216). IEOM Society International. https://doi.org/10.46254/AN12.20220212
- Zhang, C., Li, Y., Cao, J., & Wen, X. (2022). On the mass COVID-19 vaccination scheduling problem. Computers & Operations Research, 141, 105704. https://doi.org/10.1016/j.cor.2022.105704




