Oxygen tension modulates extracellular vesicles and its miRNA contents in bovine embryo culture medium
Abstract
The biotechnology for in vitro embryo production is becoming increasingly popular, being applied to humans and domestic animals. Embryo development can be achieved with either 20% or 5% oxygen tension. The extracellular vesicles (EVs) are secreted by different cell types and carry bioactive materials. Our objective was to determine the secretion pattern and micro RNA (miRNA) contents of EVs released in the bovine embryo culture environment—embryo and cumulus cell monolayer—on Days 3 and 7 of in vitro culture under two different oxygen tensions: High (20%) and low (5%). The EVs were isolated from the medium and analyzed to determine size, concentration, and miRNA levels. EVs concentration in low oxygen tension increased on Day 3 and decreased on Day 7. Additionally, altered EV miRNAs derived from the embryo-cumulus culture medium were predicted to regulate survival and proliferation-related pathways on Days 3 and 7. Moreover, miR-210 levels decreased in EVs isolated from the culture medium under high oxygen tension suggesting that this miRNA can be used as a marker for normoxia since it is associated with low oxygen tension. In summary, this study provides knowledge of the oxygen tension effects on EVs release and content, and potentially, on cell-to-cell communication during in vitro bovine embryo production.
1 INTRODUCTION
The culture medium obtained after in vitro embryo production contains a repertoire of messages secreted by the cells enclosed in the same environment. Culturing embryos in group improves their development rates compared to single embryo cultures (Neill, 2008). The molecules responsible for this are the embryotrophins (Thatcher, Bazer, Sharp, & Roberts, 1986), which are involved in various physiological and biochemical responses that sustain early pregnancy promoting the activation of growth and survival factors (Neill, Li, & Jin, 2012; Paria & Dey, 1990). Embryotrophins are produced and secreted by preimplantation embryos from active secretion, passive outflow, binding to carrier molecules, and transported within extracellular vesicles (EVs; Wydooghe et al., 2015). Additionally, in coculture systems bovine embryo culture contains remaining cumulus cells that create a microenvironment, allowing the crosstalk between embryos and the cumulus monolayer. Thus, changing culture conditions can interfere with the embryo-cumulus cell crosstalk and reflect on the culture medium.
EVs (exosomes and microvesicles) are secreted by different types of cells in the body fluids or cell culture medium, and are considered biomarkers based on the contents such as proteins, microRNAs (miRNAs), and messenger RNAs (mRNAs), originated from the secreting cell (Kosaka, Iguchi, & Ochiya, 2010; Simpson, Lim, & Moritz, 2009). These nanoparticles have a phospholipid bilayer structure similar to the producer cell and are capable of transporting molecules between nearby or distant cells through the extracellular environment; however, the secretion mechanism is different and can reflect their contents (Cocucci, Racchetti, Podini, & Meldolesi, 2007). Usually, microvesicles are larger (100–1,000 nm) than exosomes (EXOs; 30–120 nm). Microvesicles are formed by the cell membrane shedding while EXOs are produced by the endosome (Heijnen, Schiel, Fijnheer, Geuze, & Sixma, 1999; Muralidharan-chari, Clancy, Sedgwick, & Souza-schorey, 2010). Importantly, the mechanism for forming cell-secreted vesicles can dictate the contents of each EV population (Cocucci, Racchetti, & Meldolesi, 2009).
EXOs contain mRNAs, proteins, microRNAs, growth factors and cytokines, which are routed to specific target cells, corresponding to the cell-specific ligands on the EXO surface (Barkalina, Jones, Wood, & Coward, 2015). The protein content of endometrial-derived EXOs is associated with the enhanced adhesive capacity of trophoblast during implantation (Greening, Nguyen, Elgass, Simpson, & Lois, 2016). Additionally, microRNAs from endometrial EXOs modulate different biological functions, such as adhesive junctions at the implantation sites, as well as activating multiple cytokines important for the implantation process (Aplin, 2006; Ng et al., 2013). Also, trophoblast-derived EXOs were correlated with proinflammatory monocyte recruitment, thus increasing the endometrium receptivity (Greening et al., 2016). This evidence suggests that EV activity is involved in the dynamic changes during early pregnancy.
MicroRNAs are small noncoding RNAs fragments (18–25 nucleotides), which are transcribed by RNA Pol III and processed by Drosha; after that, miRNAs are transferred to the cytoplasm and further processed into mature sequences, which are loaded into the RISC complex (Ha & Kim, 2014). The RISC complex loaded with the mature miRNA can bind to the 3′-untranslated region (3′-UTR) of target mRNAs (Ameres & Martinez, 2007). Based on the base pairing miRNA–mRNA complementarity, partial or complete, mRNA molecule is blocked or degraded, respectively (Guo, Ingolia, Weissman, & Bartel, 2010; Iwakawa & Tomari, 2013). In mammals, the partial complementarity sequence between miRNA and mRNA is the most frequent regulation mechanism, resulting in deadenylation, which impairs mRNA circulation (5′CAP and poly (A) tail synergy) thereby inhibiting translation (Wakiyama, Takimoto, Ohara, & Yokoyama, 2007). Besides that, Petersen, Bordeleau, Pelletier and Sharp (2006) have shown that incomplete pairing regulation can induce ribosome drop-off. Conversely, the perfect complementarity determines mRNA cleavage and occurs mainly in plants (Llave, Xie, Kasschau, & Carrington, 2002). The miRNAs can be found outside cells, free or enclosed in cell-secreted vesicles. Thus, the miRNAs contents of EVs secreted in the body fluids or cell culture medium can serve as noninvasive biomarkers.
EVs were described as mediators of cell communication among neighboring embryos (Saadeldin, Kim, Choi, & Lee, 2014) in swine in vitro embryo production. We have previously reported the miRNA expression changes due to oxidative stress during bovine in vitro embryo culture (Bomfim et al., 2017). The high atmospheric oxygen tension (20%) during early embryo development promotes oxidative stress induced by reactive oxygen species (ROS). Recent studies have demonstrated the influence of oxidative stress on the secretion of EXOs release (King, Michael, & Gleadle, 2012; Salomon et al., 2013). However, the effects of atmospheric oxygen tension during in vitro embryo production and its relationship with EV secretion and contents have not been established yet. The aim of the present study was to identify the consequences of different atmospheric oxygen tensions on EVs release and miRNA contents in the embryonic culture medium in the presence of cumulus cell monolayer. Therefore, we hypothesized that modifying the oxygen tension changes the EVs pattern and the contents in the culture medium during in vitro embryo culture in the coculture system. To address this question, the EVs miRNA contents were isolated from the culture medium on Days 3 and 7 under two different conditions, high (20% O2) and low (5% O2) atmospheric oxygen tensions. Our results demonstrated that EVs concentration is modulated by the oxygen tension since the results were significantly different on Days 3 and 7 studied.
2 RESULTS
2.1 In vitro embryo production
Embryos produced under high (20%) and low (5%) oxygen tensions were used in another manuscript from our research group (Bomfim et al., 2017). As described in this previous work, the cleavage (D3) and blastocyst (D7) rates were not statistically different. The percentages of cleavage were 82.9 ± 6.3 and 83.2 ± 4.2, and blastocyst rates were 27.2 ± 11.0 and 24.0 ± 9.4 for high (20%) and low (5%) oxygen tensions, respectively (Bomfim et al., 2017).
2.2 EVs characterization
The EVs were characterized by transmission electronic microscopy (TEM) and Western blot analysis. TEM allowed observing the presence of EVs in all experimental groups (Figure 1a) whereas Western blot analysis indicated the presence of CD63, an EVs marker protein, and the absence of GPR78, an endoplasmic reticulum protein (Figure 1b). Therefore, it can be concluded that the isolation protocol was adequate.
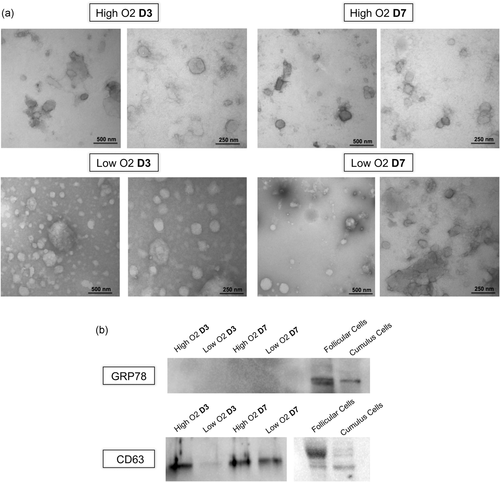
Characterization of extracellular vesicles (EVs). Transmission electron microscopy of embryo culture medium in high or low atmospheric oxygen (O2) tension on Day 3 (D3) and Day 7 (D7) of culture. Left pictures (50 K magnification) and right pictures (100 K magnification; a). Western blot analysis analyses of CD63, an extracellular vesicle marker protein, and GRP78 as a negative control, in embryo culture medium EVs, follicular and cumulus cells (b)
2.3 Effects of oxygen tension on EVs size and concentration
EVs were isolated from embryo-cumulus culture media in high and low oxygen tensions. The NanoSight NS300 was used to investigate the effects of oxygen tension on EVs, the captured videos were used to determine both EVs size and concentration. No detectable size difference was observed between the EVs derived from culture medium on Days 3 and 7 (Figure 2a). Additionally, the nanoparticle analysis performed to calculate EVs concentration demonstrated significant differences between high and low oxygen tensions on Days 3 and 7. On Day 3, a total of 178,000,000.00 ± 28.3 × 106 particles per ml on average was obtained for high oxygen tension compared to 283,933,333.33 ± 72.1 × 106/ml for low tension. On Day 7, the EVs concentration was 393,133,333.33 ± 14.1 × 108 in high oxygen tension compared to 252,466,666.67 ± 11.7 × 107 in low tension (Figure 2b).
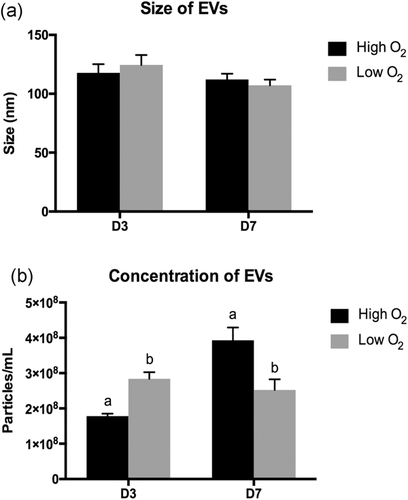
Extracellular vesicles size and concentration. Graphic representations of (a) EVs size. (b) EVs concentration. Size and concentration were calculated automatically by nanoparticle track analysis. EVs were isolated from culture medium on Days 3 and 7 of in vitro embryo culture. Error bar represents the standard error of the mean. Different letters indicate p ≤ .05. EVs: extracellular vesicles
2.4 EV miRNAs contents on Day 3
In this experiment, 281 miRNAs were identified in EVs from the embryo-cumulus culture media on Day 3. A total of 10 miRNAs were exclusively detected in the high oxygen tension samples, and 19 miRNAs exclusively detected in the low oxygen tension, while 252 miRNAs were common to both groups. Among the 252 miRNAs, a total of 4 miRNAs were upregulated (bta-miR-129-5p, bta-miR-433, bta-miR-429, and bta-miR-656) and 11 downregulated (bta-miR-15a, bta-miR-16b, bta-miR-125a, bta-miR-125b, bta-miR-195, bta-miR-215, bta-miR-346, bta-miR-374b, bta-miR-425-5p, bta-miR-491, and bta-miR-664b) in high oxygen tension compared to low tension on Day 3 of embryo-cumulus culture media (Figure 3).
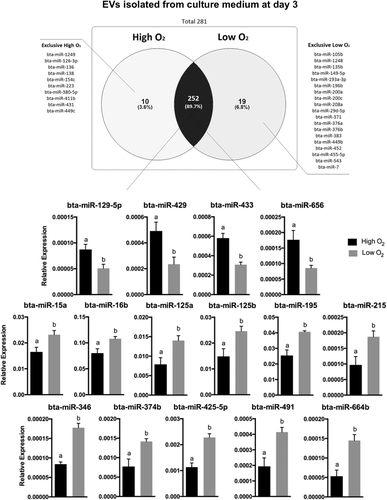
Extracellular vesicle miRNA contents detected in embryo-cumulus culture medium from bovine embryo production in high and low oxygen tensions on Day 3. Venn diagram indicating the distribution of the 281 miRNAs detected in EVs from Day 3 culture media of high or low oxygen tension conditions. A total of four miRNAs were upregulated while 11 downregulated in high oxygen tension compared to low tension. Data are presented as mean of 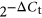 ± SEM. Different letters indicate p ≤ .05. EV: extracellular vesicle; miRNA: micro RNA; SEM: standard error of the mean
± SEM. Different letters indicate p ≤ .05. EV: extracellular vesicle; miRNA: micro RNA; SEM: standard error of the mean
Based on the altered miRNAs, we performed bioinformatics analysis using Tarbase v7.0 tool from the DIANA DNA LAB software (http://diana.imis.athena-innovation.gr). A total of two out of four upregulated miRNAs (bta-miR-129-5p and bta-miR-429) were predicted to be involved in the regulation of 36 cellular pathways (Table S2). A total of 9 out of 11 miRNAs (bta-miR-15a, bta-miR-16b, bta-miR-125a, bta-miR-125b, bta-miR-195, bta-miR-346, bta-miR-374b, and bta-miR-491) were predicted to be involved in the regulation of 54 cellular pathways (Table S2). For the purpose of the present manuscript, we considered the first 20 enriched pathways ranked by −log10 of the p value data based on enrichment score (Figure 4).
Enrichment analysis of predicted pathways regulated by extracellular vesicle miRNAs detected differently in embryo-cumulus culture medium in high and low oxygen tensions on Day 3. (a) Pathways identified based on two EVs miRNAs upregulated under high oxygen tension on Day 3. (b) Pathways identified based on nine EVs miRNAs downregulated under high oxygen tension on Day 3. The decrease in the number of analyzed miRNAs is due to differences in miRNA homology with humans. The left Y-axis values represent the number of genes enriched by the miRNAs for the respective pathways, while the right Y-axis represents the enrichment score for each pathway. The enrichment score value is the −log10 of the p value, which was extracted from the DIANA TOOLS software algorithm based on the proportion of target simulations for the miRNAs. EV: extracellular vesicle; miRNA: micro RNA [Color figure can be viewed at wileyonlinelibrary.com]
2.5 EV miRNA contents on Day 7
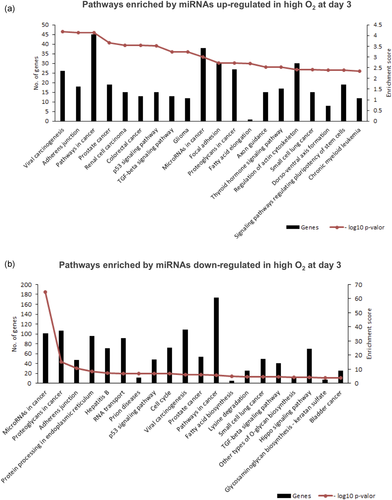
A total of 378 miRNAs were identified in EVs from the embryo-cumulus culture media, of which 25 were exclusive to high tension, and 14 exclusive to low tension (Figure 5). A total of 286 miRNAs were common to both groups, while 18 miRNAs were significantly different between groups: 16 upregulated (bta-let-7c, bta-miR-23a, bta-miR-34a, bta-miR-96, bta-miR-98, bta-miR-182, bta-miR-204, bta-miR-211, bta-miR-221, bta-miR-224, bta-miR-301b, bta-miR-302d, bta-miR-449a, bta-miR-450a, bta-miR-454, and bta-miR-1224) and 2 downregulated (bta-miR-132 and bta-miR-210) in high oxygen tension compared to low tension on Day 7 of embryo-cumulus culture media (Figure 5).
Extracellular vesicle miRNA contents detected in embryo-cumulus culture medium from bovine embryo production in high and low oxygen tensions on Day 7. Venn diagram indicating the distribution of the 325 miRNAs between high or low oxygen tension conditions. A total of 16 miRNAs were upregulated while two downregulated in high oxygen tension compared to low tension. Data are presented as mean of 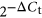 t ± SEM. Different letters indicate p ≤ .05. EV: extracellular vesicle; miRNA: micro RNA; SEM: standard error of the mean
t ± SEM. Different letters indicate p ≤ .05. EV: extracellular vesicle; miRNA: micro RNA; SEM: standard error of the mean
Based on the altered miRNAs, we performed bioinformatics analysis using the Tarbase v7.0 tool from the DIANA DNA LAB software (http://diana.imis.athena-innovation.gr). A total of the 12 miRNAs out of the 16 upregulated miRNAs (bta-miR-23a, bta-miR-34a, bta-miR-96, bta-miR-182, bta-miR-204, bta-miR-211, bta-miR-221, bta-miR-224, bta-miR-450a, bta-miR-449a, bta-miR-454, and bta-miR-1224) were predicted to regulate 71 signaling pathways (Table S3). While only bta-miR-132, from the two downregulated miRNAs, was predicted to regulate eight signaling pathways (Table S3). For the purpose of the present manuscript, we considered the first 20 enriched pathways ranked by −log10 of the p value data based on enrichment score (Figure 6).
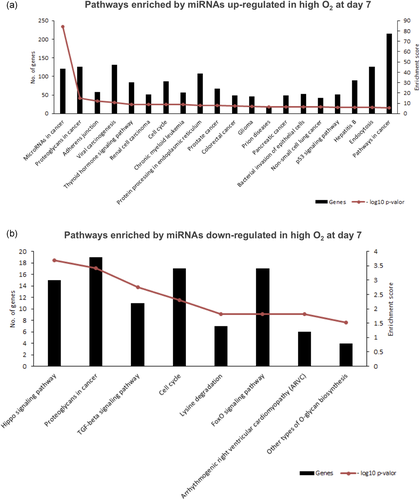
Enrichment analysis of predicted pathways regulated by extracellular vesicles miRNAs detected differently in embryo-cumulus culture media in high or low oxygen tensions on Day 7. (a) Pathways predicted based on 12 EVs miRNAs upregulated in high oxygen embryo culture media on Day 7. (b) Pathways predicted by EVs miR-132 downregulated in high oxygen embryo culture media on Day 7. The decrease in the number of analyzed miRNAs is due to differences in miRNA homology with humans. The left Y-axis values represent the number of genes enriched by the miRNAs for the respective pathways, while the right Y-axis represents the enrichment value of the score for each pathway. The enrichment score value is the −log10 of the p value, which was extracted from the DIANA TOOLS software algorithm based on the proportion of target simulations for the miRNAs. EV: extracellular vesicle; miRNA: micro RNA; SEM: standard error of the mean [Color figure can be viewed at wileyonlinelibrary.com]
3 DISCUSSION
In the present study, we evaluated the changes in EVs size and concentration, as well as miRNA levels, in the culture medium of the in vitro coculture of the bovine embryo and cumulus cell monolayer, under different oxygen tensions on Days 3 and 7. The results indicated that the concentration of EVs and miRNAs contents was affected by the oxygen tension. The bioinformatics analysis of the EVs miRNAs showed that the high oxygen tension changed the miRNAs involved in the regulation of signaling pathways, such as TGF-beta, forkhead transcription factors family subclass O (FOXO), p53, cancer-related and fatty acid elongation. Additionally, the miR-210 (a low oxygen tension stimulated miRNA) increased in EVs collected on Day 7 from the low oxygen tension medium compared to high oxygen tension. Thus, our results indicate that oxygen tension can modulate EVs concentrations and miRNA contents.
Initially, we investigated the EVs profiles in embryo-cumulus culture medium under high and low oxygen conditions on Days 3 and 7 since studies correlating EVs with oxygen tension of the environment are still scarce. Nanoparticle analysis demonstrated that EVs had a similar size on Day 3 in both oxygen tensions evaluated. On Day 3, EVs sizes were 1,178 nm and 1,246 nm on average for the high and low oxygen tensions, respectively. Importantly, EVs in this size range can cross the embryo zona pellucida (ZP), since the ZP has pores of about 200 nm in diameter, ranging between ~223 nm in the zygote and ~203 nm in the eight-cell stage (Vanroose et al., 2000).
Similarly, no differences were observed in EVs size on Day 7. On Day 7, the EVs measured 112.2 nm and 107.1 nm on average in high and low oxygen tensions, respectively. Likewise, EVs of this size on Day 7 can virtually fit in the ZP pores, which measure ~155 nm in the morula stage (Vanroose et al., 2000). EVs sizes were not significantly different for the studied high and low oxygen tensions on Day 7 compared to those isolated from the embryo-cumulus culture medium on Day 3. Unlike our results, Saadeldin et al. (2014) reported that EXOs size increased from Day 3 to Day 7 under low oxygen tension; such heterogeneity can be explained by different starting material volumes, collection methods, isolation protocols, size measurement methods, culture conditions, presence of cumulus cells or even may reflect the differences between species since their study investigated the EXOs size changes in swine in vitro produced (IVP) embryos (Saadeldin et al., 2014). Together, these results suggest that EVs present in early embryo-cell culture medium could move through the ZP pores and possibly modulate the crosstalk between embryos and surrounding cells.
In addition to size, the EVs concentration was also determined in the cell culture medium isolated on Days 3 and 7. Our analysis demonstrated that EVs concentration in embryo-cumulus culture medium on Day 3 is lower in high oxygen tension compared to low oxygen tension. Similarly, tumor cells have increased EVs secretion in low oxygen tension, which is involved with cancer metastatic capacity and tumor progression (King et al., 2012; Salomon et al., 2013). Apparently, these differences in EVs concentrations may be related to cell proliferation, thus an important process for embryo development (Desrochers, Bordeleau, Reinhart-King, Cerione, & Antonyak, 2016). Interestingly, EVs concentrations reversed on Day 7. On Day 7, the EVs concentration increased in high oxygen tension compared to the low oxygen tension. These data suggest that increased EVs release from Day 3 to Day 7 in high oxygen tension is associated with a microenvironment alteration during the in vitro embryo development. Unlike the observed results, trophoblast cells during in vitro culture, under other conditions, released more EVs at 1% oxygen tension compared to 8% on Day 7 (Truong et al., 2017). Thus, the observed results showed that EVs secretion in the embryo-cumulus culture medium is being modulated by oxygen tension. These effects can be observed directly by the influence of oxygen tension on the embryo, on monolayer cells and even indirectly, influencing the cells and, then, altering embryo secretion.
To investigate the effects of the oxygen tension during bovine embryo IVP, we isolated EVs from the embryo-cumulus culture medium and performed miRNA profile analysis. Initially, we identified a total of 15 miRNAs expressed differently on Day 3; 4 miRNAs upregulated (bta-miR-129-5p, bta-miR-433, bta-miR-429, and bta-miR-656), and 11 downregulated (bta-miR-15a, bta-miR-16b, bta-miR-125a, bta-miR-125b, bta-miR-195, bta-miR-215, bta-miR-346, bta-miR-374b, bta-miR-425-5p, bta-miR-491, and bta-miR-664b) in high compared to low oxygen tension. The miRNA target analysis revealed that three out of four upregulated miRNAs in high tension (miR129-5p, miR-433, and miR-656) were predicted to regulate pathways involved in cell proliferation, invasion, and differentiation in cancer cells (Dong, Ihira, Xiong, Watari, & Hanley, 2016; Li, Mao, Wang, Miao, & Li, 2017; Zhang et al., 2016; Zhang, Zhang, Zhang, Zhang, & Jiang, 2016). The miR-429 was studied in mouse embryos and associated with differentiation of epithelial-mesenchymal cells during implantation (Z. Li, Gou, Jia, & Zhao, 2015).
Out of the 11 miRNAs downregulated in EVs from high oxygen tension on Day 3, miR-15a, miR-125a, miR-195, miR-125b, and miR-346 were previously investigated in embryonic or placental cells. The miRNA-346 has been described as a regulator of trophoblast invasion and migration in humans, while miR-195 is associated with an important paracrine effect on angiogenesis (Almeida et al., 2015; Su, Tsai, Tsai, Chen, & Kuo, 2016). The miR-125 family members, miR-125a and miR-125b play a role in cellular differentiation in mouse embryonic stem cells (K. Kim et al., 2016). It is known that serotonin protects the placenta against cellular apoptosis (Hadden et al., 2017), whereas the miRNA-15a, which was lower in EVs from high oxygen tension, has been reported as a regulator of serotonin transporter levels in human placenta (Moya, Wendland, Salemme, Fried, & Murphy, 2013). Similar to our results, another study demonstrated the increase of miR-15a in low oxygen condition in chicken eggs (Hao, Hu, Wu, & Li, 2014). Thus, the presence of altered miRNAs in high oxygen tension on Day 3 of embryo development suggests disturbed regulation of important pathways associated with the success of embryonic development compared to miRNA profile in EVs isolated from media in low oxygen tension, which is close to physiological conditions.
The miRNA analysis on Day 7 revealed 18 miRNAs differently expressed in EVs contents. A total of 16 miRNAs were upregulated (bta-let-7c, bta-miR-23a, bta-miR-34a, bta-miR-96, bta-miR-98, bta-miR-182, bta-miR-204, bta-miR-211, bta-miR-221, bta-miR-224, bta-miR-301b, bta-miR-302d, bta-miR-449a, bta-miR-450a, bta-miR-454 and bta-miR-1224) while two miRNAs were downregulated (bta-miR-132, and bta-miR-210) in high compared to low oxygen tension. The miRNA-96 regulates glutathione (GSH), which when inhibited causes an increase of GSH levels and increases the cell viability in neuronal mouse cells (Kinoshita et al., 2014). GSH is a nonenzymatic cellular antioxidant, which removes ROS excess by reducing peroxides and hydroperoxides in inactive products (Matos et al., 1996). The increase of miRNA-96 in EVs from high oxygen tension on Day 7 could indicate a disturb in the antioxidant function of GSH to neutralize ROS. The ROS increase induces an increase in miR-182, which is related to the maintenance of DNA integrity in human fallopian tube cells exposed to in vitro oxidative stress (Liu et al., 2015). Besides that, FOX1 mRNA 3′-UTR is a direct target of miR-182 (K. M. Kim et al., 2012). FOX1 plays an important role in oxidative stress response and inhibition of cellular apoptosis (Lam, Francis, & Petkovic, 2006; Stahl, Dijkers, Kops, & Medema, 2002). Based on these data, the upregulation of miR-96 and miR-182 in EVs from high oxygen tension on Day 7 indicates a possible disturb on cellular homeostasis. Moreover, the overexpression of miR-96 or miR-182 was associated with repression of cellular differentiation of human embryonic stem cells (Du, Ma, Phillips, & Zhang, 2013).
In addition, miRNAs let-7c and miR-23a also increased in EVs of the high oxygen IVP embryo culture on Day 7. The let-7c miRNA was described as an essential regulator involved in the progress of embryo development (Lin et al., 2007). Similarly, miR-23a regulates cellular maintenance and differentiation in mouse embryonic stem cells (Gioia et al., 2014). Among the already mentioned miRNAs, miR-96, let-7c, and miR-23a were identified in the human placenta of in vivo and successful pregnancies (Li et al., 2015). Thus, environment disturbance of miRNA levels can influence the communication between the early embryo and surrounding cells, suggesting an important role for EVs contained miRNAs. Prior studies described blastocyst rates generally lower under high oxygen tension compared to low oxygen tension (Amin et al., 2014; Dumoulin et al., 1999; Yoon et al., 2014). Importantly, other studies demonstrated a decrease in pregnancy rates when embryos were cultured in high oxygen tension (Q. Li et al., 2015). Morula compaction and embryo implantation depend on tight junction proteins, which play a role establishing embryonic polarity and promoting adherent junction and focal adhesion (Bazer et al., 2010; Duranthon, Watson, & Lonergan, 2007). Tight junction proteins can be regulated by miRNAs (Cichon, Sabharwal, Christian, & Schmidt, 2014). We found increased levels of miR-98 in EVs isolated from the culture medium of embryo IVP in high compared to low oxygen tension. The miRNA-98 regulates negatively tight junction mRNAs reducing protein levels (Zhuang, Peng, Mastej, & Chen, 2016). These results suggest that high oxygen tension has a detrimental effect on delivered microRNAs mediated by EVs and might be involved in delayed embryo development during the implantation phase.
Lastly, the miR-210 levels were downregulated in EVs isolated from embryo-cumulus culture medium under high oxygen tension compared to low tension on Day 7. The increase in miR-210 under low oxygen tension promotes antiapoptotic functions and, consequently, is correlated with poor prognosis in tumor cells (King et al., 2012). The miR-210 increase has been considered a signature of hypoxia (Kulshreshtha et al., 2007). In the context of the cultural environment of in vitro embryo production, miR-210 can be indicative of normoxia state, since the oviduct and maternal uterus present physiological oxygen levels close to 5–7% (Fischer & Bavister, 1990; Harvey, 2007). Curiously, the miR-210 did not differ in EVs contents from culture medium on Day 3 suggesting that miR-210 levels in EVs may reflect a later response of the cultured cell to the in vitro environment at low oxygen tension. Altogether, we suggest that miR-210 has potential as a noninvasive biomarker of normoxia state in bovine blastocyst produced in vitro and with further studies could be explored as a tool to improve bovine IVP.
Nevertheless, this is the first report on EVs miRNAs contents from embryo-cumulus culture medium regulating cellular functions such as proliferation, mitosis, and apoptosis. Another enriched pathway was FOXO signaling, which was predicted to be regulated by the miRNAs isolated EVs from culture medium under high oxygen tension on Days 3 and 7. FOXO signaling pathway plays an important role in oxidative stress response triggering the transcription of antioxidant enzymes (Klotz et al., 2015). Enrichment analysis of EVs miRNAs under high oxygen tension, suggests that besides the cell response against oxidative stress, they can secrete vesicles signaling this response to other cells. The crosstalk mediated by EVs could improve embryo development for all embryos within the same culture drop. A similar effect was observed in embryos cultured in groups compared to single embryos (Wydooghe et al., 2015). Signaling pathways associated with survival increases the potential of embryo development, thus suggesting an involvement of EXOs mediated transport of embryotrophins (Wydooghe et al., 2015). FOXO signaling pathway may be considered a marker of bovine embryo oxidative stress response, thus the modulation of this pathway might improve the in vitro production under high oxygen tension.
Finally, it is known that embryos release EVs during the early embryonic development (Saadeldin et al., 2014) but there is still controversy regarding the function of embryo secreted vesicles and its consequences on further development (Mellisho et al., 2017). The release of EVs by the embryos and the content of these vesicles, especially miRNAs, undergo several changes, which may be caused by the culture conditions directly on the embryo or influenced by the cells present in the microenvironment, in alignment with previous reports that oxidative stress can alter the composition of granulosa cells from the EVs (Saeed-zidane et al., 2017).
4 CONCLUSION
In the present study, we demonstrated that exposure of bovine embryos with cell monolayers to different oxygen tensions changed the EVs concentration and the EVs miRNA contents. Based on EVs size, it is possible to conclude that these vesicles may transpose the ZP pores and potentially modulate embryo-embryo or embryo-cell communication. Overall, the microRNAs contents in the EVs are associated with modulation of cell survival and proliferation activity, with a potential role in embryo progression during development or even in cells present in the environment. EVs concentration was lower on Day 3 and higher on Day 7 under high oxygen tension compared to low oxygen tension, suggesting that the culture under different oxygen tensions can induce changes in the released EVs. The increase in EVs concentration, combined with the upregulation of miRNAs targeting the FOXO signaling pathway in high oxygen tension on Day 7, suggests that cultured cells are responding to oxidative stress and propagating this response in EVs miRNA contents. We also demonstrated that miR-210 can serve as a noninvasive biomarker to normoxia state during in vitro embryo development in cattle. Overall, the study contributed to the understanding of the cellular activity after exposure to different oxygen tensions and the effects on EVs size, concentration and miRNA contents.
5 MATERIALS AND METHODS
The EVs were isolated from the culture medium of bovine embryos produced under high (20%) or low (5%) oxygen tension on Days 3 and 7 and analyzed to determine EVs size and concentration, as well as miRNA contents. The experimental protocol (number 5763200215) was approved by the Ethics Committee on Animal Use of the University of São Paulo (CEUA-FZEA). Reagents and culture medium used were purchased from Sigma–Aldrich Chemical Company (St. Louis, MO) unless otherwise stated.
5.1 In vitro embryo production and culture medium sampling
In the present study, the medium used for the embryo culture was analyzed regarding the content of EVs while the produced embryos were used in a different study (Bomfim et al., 2017). Bovine ovaries were collected at a slaughterhouse near São Paulo, Pirassununga, Brazil, and the cumulus-oocyte complexes (COCs) were obtained through ovarian follicles aspiration (3–6 mm) using 18-gauge needles. Grade I and II COCs presenting compact cumulus and homogeneous cytoplasm were selected and matured in vitro in TCM199 culture medium, to which we added 26 mM sodium bicarbonate, 22 μg/ml sodium pyruvate, 50 μg/ml gentamicin, 0.5 μg/ml follicle stimulating hormone (Folltropin®; Bioniche Animal Health, Belleville, ON, Canada), 50 μg/ml human chorionic gonadotropin (hCG; Vetecor® 5000 U.I. Intervet, São Paulo, SP, Brazil) plus 10% custom made EV-depleted fetal bovine serum (FBS), previously described in other experiments (da Silveira et al., 2017). The COCs were matured at 38.5°C and 5% CO2 for 22 hr.
In vitro fertilization was performed with frozen-thawed semen (CRV Lagoa, Sertãozinho, SP, Brazil) processed by percoll gradient (45% and 90%). Matured oocytes and sperm were incubated in IVF-TALP (Tyrode-lactate stock) supplemented with 50 µg/ml gentamicin, 22 µg/ml sodium pyruvate, 40 µl/ml PHE (2 mM D-penicillamine, 1 mM hypotaurine, and 245 µM epinephrine), 5.5 UI/ml heparin, and 6 mg/ml bovine serum albumin (BSA), as previously described by Bomfim et al. (2017), at 38.5°C and 5% CO2 for 20 hr.
Presumptive zygotes were gently denuded preserving a cumulus cell monolayer to constitute the pseudo-coculture for improving embryo development (Wydooghe et al., 2015). Culture drops of 100 μl contained ≅ 25 embryos in SOFaaci (Bomfim et al., 2017) culture medium supplemented with 5 mg/ml BSA, 22 μg/ml sodium pyruvate, 50 μg/ml gentamicin, and 2.5% EV-depleted FBS, covered with mineral oil. Embryos were divided into two groups under the atmospheric conditions, high (20%) and low (5%) oxygen tension, at 38.5°C and 5% CO2.
On Day 3, after assessing the cleavage rate, a 50 μl culture medium aliquot was collected for further analysis while 50 μl of new culture medium was added to each drop in both high and low oxygen tension conditions. From Day 3 to Day 7, the embryos remained in the same drop in the presence of cell monolayers. On Day 7, the blastocyst rates were evaluated and the culture medium was collected.
It is important to highlight that the medium samples used to analyze EVs and miRNAs were obtained from the in vitro coculture of the embryo and cumulus cells, referred to as embryo-cumulus culture medium.
5.2 Isolation of EVs
The EVs were isolated from three biological replicates, obtained from culture medium on Days 3 and 7 under high and low oxygen tension. Each biological replicate was composed of a pool of culture media of three replicates of in vitro embryo production with a 300 μl starting volume. Embryo-cumulus culture medium samples were centrifuged to remove live cells at 300g for 10 min, cell debris at 2,000g for 10 min, and large microvesicles at 16,500g for 30 min. To isolate the EVs, 300 μl embryo-cumulus culture medium was mixed with 300 μl Exoquick-TC (1:1, #EXOQ20A1, System Biosciences, CA), incubated overnight at 4°C, followed by centrifugation at 1,500g for 30 min. The EVs pellets were diluted in 50 μl of PBS, of which 10 μl was used for nanoparticle tracking analysis and 40 μl for total RNA extraction. The EVs pellets were diluted in 2 ml PBS and, to remove the remaining Exoquick, centrifuged 2 times at 1,00,000g for 70 min each, and further used.
5.3 Western blot analysis
EVs were diluted in 20 μl Ripa buffer and used for gel analysis. The used control samples consisted of ~15 μg of protein from follicular and cumulus cells, which were collected during the in vitro embryo production runs. The aspirated follicular fluid was centrifuged at 2,500g for 5 min to isolate the follicular cells while the cumulus cells were collected from the remaining immature COCs that were not used in the IVM experiments. Both cell samples were lysed with Ripa buffer for 30 min, alternating between vortex and ice, and centrifuged at 13,000g for 10 min to discard the sediment and the supernatant was used in the Western blot analysis.
Samples were separated on 12% sodium dodecyl sulfate-polyacrylamide gels. Proteins were then transferred to a polyvinylidene difluoride membrane using Bio-Rad products and system (Trans-Blot® Turbo-Bio-Rad). These membranes were blocked with 5% BSA (Sigma–Aldrich) in TBST (100 mM NaCl, 0.1% Tween 20, 50 mM Tris, pH 7.4) for 1 hr and incubated overnight at 4°C with the primary antibody in TBST containing 1% BSA. Herein, we identified the presence of CD63 (sc-15363; Santa Cruz Biotechnology), an EV marker. Additionally, we verified the absence of GRP78 (sc-376768; Santa Cruz Biotechnology), a reticulum endoplasmic protein, which serves as a negative control for cell contamination. Blots were then washed with TBST three times, incubated with a secondary antibody diluted in TBST containing 1% BSA at room temperature for 1 hr, and washed with TBST three more times. Finally, the blots were treated with ClarityTM Western ECL substrate (Bio-Rad) to visualize the proteins.
5.4 Transmission electron microscopy
After ultracentrifugation, the EVs pellet was diluted in fixative solution (0.1 M cacodylate, 2.5% glutaraldehyde, 4% paraformaldehyde, pH 7.2–7.4) at room temperature for 2 hr. Then, 2 ml of milli-Q ultrapure water was added to perform the third centrifugation (119,700g, 4°C, 70 min) to remove the fixative solution. The obtained pellet was diluted in 20 μl of ultrapure milli-Q water and kept refrigerated until the analysis. The contents were placed in a pioloform-coated copper grid for 5 min and the excess was removed with moist filter paper. The grid was then inserted into a drop of 2% aqueous uranyl acetate for 3 min. The excess was removed with wet filter paper, followed by the transmission electron microscope analysis (FEI 200 kV, Tecnai 20, emitter LAB6).
5.5 Nanoparticle tracking analysis
EVs concentration and size were determined using NanoSight NS300 (NanoSight, Salisbury, UK) with 10 μl of EVs isolated from the embryo-cumulus culture medium diluted 1:50 in PBS total of 500 μl. Instrument calibration was completed with 50, 100, and 150 nm beads to verify particle size and concentration. EVs analyses were considered valid if at least 500 valid tracks were observed per frame. Video files (n = 5) were captured using a camera level of 14 for 30 s. The screen gain was standardized for both groups, Days 3 and 7, and no screen gain variation was observed between groups. The Stokes–Einstein equation was used to determine the size distribution and number of particles (concentration) within each sample. We compared three samples from high and low oxygen tension on Days 3 and 7.
5.6 Total RNA isolation
Total RNA including small RNA molecules were isolated from the EVs using QIAzol Lysis reagent and immediately purified by miRNeasy® Micro Kit (50; Qiagen, Venlo, Limburg, Holanda), according to the manufacturer's instructions. RNA pellets were dissolved in 14 μl RNase-free water. The RNA concentrations were measured using a NanoDrop 2000 (Applied Biosystems, Forster City, CA).
5.7 miRNA reverse transcription and real-time polymerase chain reaction analysis
Relative levels of 378 miRNA sequences were examined by reverse transcription polymerase chain reaction (RT-PCR) for three biological replicates of EVs preparation isolated from the embryo-cumulus culture medium on Days 3 and 7. Reverse-transcribed mRNA was generated using 100 ng of total RNA and kit miScriptII RT (Qiagen) according to the manufacturer's instructions. We opted to use HiFlex buffer, which allows reverse transcription of precursor and mature microRNAs.
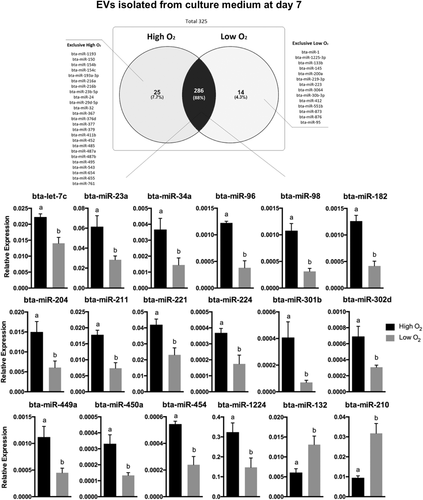
Real-time RT-PCR was performed with the resulting cDNA using SYBR Green PCR Master Mix (#1020722), sequence-specific forward primers (Table S1) and universal reserve primer (Qiagen). The PCR cycle conditions were as follows: step at 95° for 15 min, then 45 cycles of 94°C for 15 s, 55°C for 30 s, 70°C for 30 s by QuantStudio6 Flex system (Applied Biosystems). Melt curve analyses confirmed the amplification of specific cDNA products. Samples with three or more melting curves and raw Ct values higher than 35 were discarded. To identify differences in miRNA levels in EVs isolated from embryo-cumulus culture medium under high or low oxygen tension, raw Ct values were normalized to the geometrical mean of two internal controls (Hm/Ms/Rt U1 snRNA and bta-miR-99b). The relative expression values for each miRNA were calculated using the ∆Ct method (Schmittgen & Livak, 2008). Finally, data used for illustration was transformed using 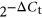 formula.
formula.
5.8 Bioinformatics analysis
Bioinformatics analyses were based on bovine miRNAs sequences, only with bovine miRNAs which presented 100% homology to human miRNAs sequences, according to mirBase (http://www.mirbase.org). Enriched pathways were identified using DIANA DNA LAB software (http://diana.imis.athena-innovation.gr) in Tarbase v7.0 tool to human miRNAs (Vlachos et al., 2015).
5.9 Statistical analysis
Statistical analyses were performed independently. The parameters EVs size, concentration, and EVs miRNA contents were compared regarding the high and low oxygen tensions on Days 3 and 7 of in vitro embryo culture. The statistical differences were assessed using Student's t test, according to the criteria of normality and homoscedasticity using the SAS software. p < .05 was considered significant.
ACKNOWLEDGMENTS
The authors are thankful to the College of Animal Science and Food Engineering (FZEA) of the University of Sao Paulo (USP) for providing the infrastructure support; the CRV Lagoa for donating the semen; Slaughterhouse Olhos D'água for donating the ovaries; and the funding agencies: São Paulo Research Foundation (FAPESP), the National Council of Technological and Scientific Development (CNPq), and the Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES). This study was supported by the São Paulo Research Foundation-FAPESP (grant numbers 2014/21042-6, 2015/21829-9, 2014/22887-0, 2014/21034-3, 2012/50533-2, and 2013/08135-2) and by the National Council of Technological and Scientific Development – CNPq (grant number 306349/2017-5). This study was financed in part by the Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES) - Finance Code 001.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.