Are Phosphatidylcholine and Lysophosphatidylcholine Body Levels Potentially Reliable Biomarkers in Obesity? A Review of Human Studies
Abstract
Phosphatidylcholines (PCs) are the major components of biological membranes in animals and are a class of phospholipids that incorporate choline as a headgroup. Lysophosphatidylcholines (LPCs) are a class of lipid biomolecules derived from the cleavage of PCs, and are the main components of oxidized low-density lipoproteins (oxLDLs) that are involved in the pathogenesis of atherosclerosis. Since obesity is associated with a state of chronic low-grade inflammation, one can anticipate that the lipidomic profile changes in this context and both PCs and LPCs are gaining attention as hypothetically reliable biomarkers of obesity. Thus, a literature search is performed on PubMed, Latin American and Caribbean Health Science Literature (LILACS), and Excerpta Medica DataBASE (Embase) to obtain the findings of population studies to clarify this hypothesis. The search strategy resulted in a total of 2403 reports and 21 studies were included according to the eligibility criteria. Controversial data on the associations of PCs and LPCs with body mass index (BMI) and body fat parameters have been identified. There is an inverse relationship between BMI and most species of PCs, and a majority of studies exhibited negative associations between BMI and LPCs. Other findings regarding the differences between PCs and LPCs in obesity are presented, and the associated uncertainties are discussed in detail.
1 Introduction
Obesity is a public health problem of multifactorial etiology that directly contributes to incident noncommunicable diseases (NCD), including diabetes mellitus, arterial hypertension, cardiovascular diseases, musculoskeletal disorders, and some types of cancer.[1] In 2016, approximately 39% of adults aged ≥18 years and over (39% of men and 40% of women) were overweight, and 13% of the world's adult population (11% of men and 15% of women) were obese, with at least 2.8 million people dying each year because of being overweight or obese.[1, 2]
Body mass index (BMI) is commonly used to classify overweight and obesity in adults. However, the accuracy of BMI as a diagnostic index is uncertain, as it does not consider the elements of body composition and metabolic changes that equate to cardiometabolic risk parameters, such as insulin resistance, endothelial dysfunction, and dyslipidemia.[3-5] In addition, some authors support the idea that obesity is not just about an increase in body fat mass but is associated with the dysfunction of adipose tissue and lipid homeostasis.[6]
Lipids have several very important functions, including metabolic and signaling, and make part of the cell membranes as major components. The great majority of lipids is composed from the fatty acids (FAs), and depending on the FAs carbon chain length and the degree of FAs desaturation, they can positively or negatively influence the production of inflammatory markers in overweight individuals, which is associated with the development of NCD.[7]
Different metabolomics approaches have been employed in nutrition research on food components, body fluids, and biological tissues analysis. Lipidomics has become an important branch of medical and clinical sciences. This approach has been used in the study of obesity to understand the regulatory and diagnostic value of the changes in the lipidome associated with obesity and cardiometabolic risk factors.[8-11]
Phosphatidylcholines (PCs) are the major components of biological membranes found in animals and plants. PCs contain two FAs esterified to a glycerol backbone, as well as a phosphodiester linkage connecting the third hydroxyl group to choline (Figure 1). PCs molecules contain a range of fatty acyl chains that vary in the length and position of the double bonds.[12] Most cells can synthesize PCs through the Kennedy pathway, which involves the transfer of phosphocholine onto the diacylglycerol (DG) moiety. The synthesized PCs predominantly contain saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA). Polyunsaturated fatty acids (PUFA) are incorporated into PCs via the Lands cycle through the hydrolysis of a single fatty acyl chain and the esterification of a free PUFA, resulting in lysophosphatidylcholines (LPCs).[13]
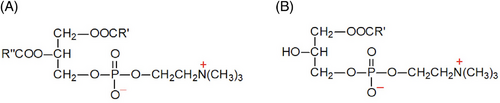
LPCs are a class of lipid biomolecules derived from the cleavage of PCs via the action of phospholipase A2. LPCs are the main components of oxidized low-density lipoproteins (oxLDLs) and are involved in the pathogenesis of atherosclerosis. Higher concentrations of LPCs disrupt mitochondrial integrity and enhance cytochrome C release from hepatocytes and the vascular system. In addition, LPCs induce prolonged endothelial activation and atherogenesis, modulate inflammatory chemokine expression in endothelial cells, impair arterial relaxation, increase oxidative stress, and inhibit endothelial cell migration and proliferation.[12, 13]
In this context, PCs and their metabolites appear to be useful biomarkers for identifying early cardiometabolic risk in overweight/obese individuals. Lipidomic analyses have shown that the concentrations of LPCs and PCs are positively associated with visceral and cardiac fat deposits.[14, 15] Furthermore, some PCs (C38:3, C38:4, and C40:5) were positively associated with waist circumference (WC).[16] Although there is a clear link between LPCs and obesity-associated comorbidities, findings in the literature are inconsistent.
During adipocyte expansion, fat cells undergo drastic remodeling of the lipidome composition to maintain plasma membrane integrity and functionality. In metabolically healthy obesity, lipid remodeling involves the accumulation of PUFA-containing phospholipids (PLs), in contrast to diminished MUFA- and SFA-containing PLs. However, the maintenance of membrane integrity occurs at the expense of increased concentrations of ethanolamine plasmalogens containing arachidonic acid, thus favoring a pro-inflammatory environment. Interestingly, the levels of PLs-containing PUFA are much lower in morbidly obese patients than in healthy obese subjects, indicating that the adipocytes were unable to compensate for the dramatic morphological changes in extreme obesity.[17] This mechanism suggests that lipidome remodeling in adipocytes may be necessary for cellular hypertrophy and tissue expansion.[18]
Nonetheless, these data provide evidence that nutritional status can influence circulating LPC levels, and should be considered when performing lipidomic profiling.[19] In this sense, lipidomics and metabolomics allow researchers to understand disease mechanisms and contribute to personalized dietary interventions, which, in turn, advance our knowledge of the relationships between diet and health and disease.[20]
This review aimed to discuss PCs and LPCs as reliable markers of obesity and their effects on the lipidome, examining associated uncertainties caused by factors such as species characteristics, body composition, sex, diet, analytical methods, and statistical analysis.
2 Experimental Section
The primary sources of potentially relevant literature were from the electronic database PubMed (Medical Literature Analysis and Retrieval System - MEDLINE), Latin American and Caribbean Health Sciences Literature (LILACS), and Excerpta Medica DataBASE (Embase) between January 2011 and October 2021. The following research question was formulated: Are body levels of PCs and LPCs potentially reliable biomarkers of obesity? The search was conducted using a combination of Medical Subject Heading Terms (Mesh Terms) and keywords. Three groups of keywords were combined by the Boolean operator “OR”: (“obesity” OR “BMI” OR “body mass index”), (“body fat” OR “adiposity”), and (“central obesity” OR “waist circumference” OR “waist-to-hip ratio”). These groups were combined using the Boolean operator “AND” with phosphatidylcholine descriptors: “phosphatidylcholines,” “lysophosphatidylcholines,” “LPC,” “lipoprotein-associated phospholipase A2,” “lysophosphatidylcholine acyltransferase” and “lysophosphatidylcholine acyltransferase,” and “lysophospholipase A1.”
The search was performed using English, Portuguese, or Spanish to select original articles whose participants were adults and/or older people. The search was conducted by two blinded researchers (PENRB and MNM). First, relevance based on the title and abstract was determined. Review studies, animal studies, and those involving children, adolescents, pregnant women, lactating women, or patients undergoing bariatric surgery were excluded. Duplicated studies and those that did not meet the eligibility criteria of the review were excluded based on the full reading of the text. The search resulted in 2403 studies, and 21 studies were included in this review according to the eligibility criteria, as shown in Figure 2.
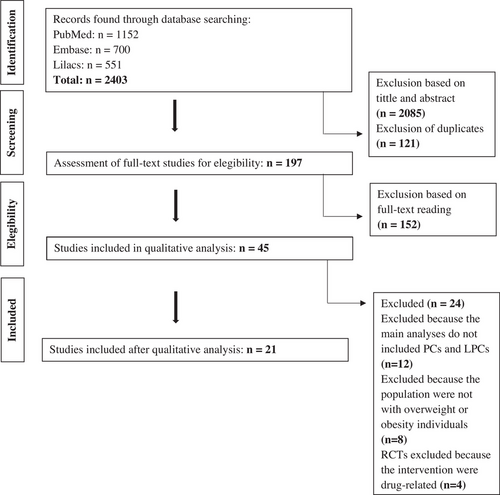
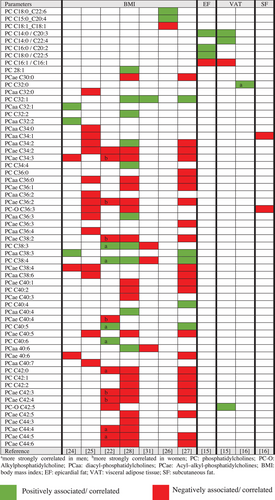
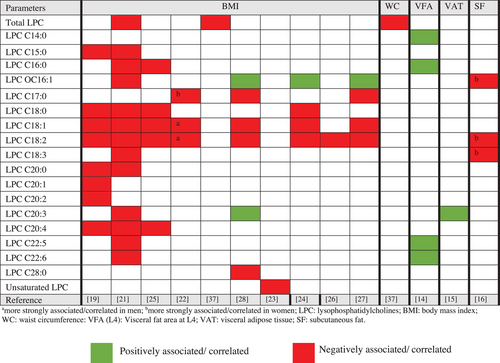
Data extracted from cross-sectional (Supplementary Material, Table S1) and intervention studies (Table 1) included authors, year of publication, study design, population, objective, biological matrix, analytical technique, body fat parameters, intervention, or main findings. The main results of the cross-sectional studies on the associations between body fat parameters and the PC and LPC species of overweight and obese individuals are summarized in Figures 3 and 4. This study used the terms negatively or positively associated/correlated, considering the statistical analyses described in these studies, namely, Spearman's correlation coefficient,[22, 23] Pearson's correlation coefficient,[14, 21, 30] or regression analysis.[15, 16, 19, 23-28, 31, 37]
| Authors | Study design/country | Population | Intervention/biological matrix | Main findings |
|---|---|---|---|---|
| Del Bas et al. (2016)[32] |
Randomized controlled trial United States |
34 Normal-weight and 38 obese subjects aged 18–65 years. |
Supplementation of corn oil (control) or n–3 PUFA–rich fish oil (3 g day−1) for 90 days Plasma Reverse-phase liquid chromatography and mass spectrometry |
Before intervention: ↑ LPC C20:2 in the obese group 90 days after 3 g (corn or fish oil): ↓ LPC C18:2 and LPC C20:3 in the n-3 PUFA-rich fish oil group 6 h after high-fat meal: n–3 PUFA treatment affected the response of LPC only in normal-weight subjects |
| Bagheri et al. (2018)[33] |
Randomized double-blind clinical trial Iran |
Men and women with obesity (BMI ≥30 kg m−2) aged between 18 and 50 years, classified into either MHO (n = 82) or MUHO (n = 78) |
Daily oral dose of one tablet containing either placebo or vitamin D (equivalent dose of 4000 IU) for 4 months Plasma LC-MS/MS |
↓ LPC C16:0/LPC C18:0/LPC C18:1/PC C32:0/PC C34:1/PC C38:3/PC C34:8 in the MUHO phenotype |
| Hernández-Alonso et al. (2018)[34] | Randomized, parallel, controlled, clinical trial Spain | 102 Subjects between 30 and 60 years of age, with BMIranging from 27 to 35 kg m−2 |
500-kcal energy-restricted LGI, HGI, or LF diet for 6 months Plasma 1H NMR, LC-MS, and GC-MS |
↓ Total LPC in the groups following a LGI or HGI diet ↑ LPC C18:2 in the LGI diet group ↑ LPC C20:3 in the LF diet group ↓ Total PC in all the intervention diets ↓ PC C32:1 and PC C34:2 (PCae) after the LGI diet |
| Shabrina et al. (2019)[35] |
Randomized controlled trial Taiwan |
21 Men and women aged ≥30 years, BMI ≥24 and ≤35 kg m−2, and score ≥3 in the MetS criteria based on the modified NCEP-ATP III |
Caloric restriction ( n = 12) or caloric restriction with fish oil (n = 9) for 12 weeks Plasma UPLC–MS/MS |
↓ PC C25:10 after intervention in both groups ↑ PC C34:1/PC C34:2/PC C36:2/PC C36:3/PC C38:4/PC C38:5/PC C40:6 after intervention in both groups |
| Papandreou et al. (2021)[36] |
Randomized multicenter trial Spain |
162 Women and men aged between 20 and 65 years with an initial BMI of 27–35 kg m−2 following the Modifast® formula diet to achieve at least 8% of weight reduction over 8-week LCD period |
12-Week supplementation of: (1) an active satiety-enhancing product or (2) a similar control product without satiety-enhancing properties Plasma 1H NMR LC-HRMS and GC-HRMS |
PC C32:1 was associated with ↓ in TAG PC C33:1 and PC C36:1 were associated with ↓ in TAG, TChol, and LDL-C. PC C35:1/PC C36:4/PC C38:3/PC C38:4/PC C40:6 was associated with ↓ in TChol and LDL-c PC C38:3/PC C36:4/PC C36:1/PC C33:1 were associated with ↑ in the levels of TChol over the maintenance period |
- GC-HRMS, gas chromatography coupled to high resolution mass spectrometry; HGI, high glycemic index; 1H NMR, proton nucelar magnetic resonance; LCD, low caloric diet; LC-HRMS, liquid chromatography coupled to high resolution mass spectrometry; LDL-C, low density lipoprotein cholesterol; LF, low fat; LGI, low-glycemic index; LC-MS, liquid chromatopraphy coupled to mass spectrometry; LC-MS/MS, liquid chromatography coupled to a triple quadrupole mass spectrometry; LPC, lysophosphatidylcholine; MHO, metabolically healthy obese; MUHO, metabolically unhealthy obese; NCEP-ATP III, National Cholesterol Education Program Adult Treatment Panel III; PC, phosphatidylcholine; PUFA, polyunsaturated fatty acid; TAG, triacylglycerols; TChol, total cholesterol, UPLC-MS/MS, ultra-high-performance liquid chromatography-tandem mass spectrometry
3 Results
Literature covering lipidomics in obesity shows that most of the studies have been published over the last five years. The publications were from 13 different countries that together add up to a population of 6907 adults or older. Most of the studies were conducted in European countries (10 studies), and seven studies were conducted in Asian countries. We found that 16 studies had a cross-sectional observational design, and 44% were case–control studies, in addition to five intervention studies. Overall, plasma and serum were used as the biological matrixes. However, two studies, in addition to plasma, evaluated tissue metabolites from muscles, adipose, and liver samples.[19, 29]
BMI is the preferred method for estimating body status,[19, 21-28] Dual-energy X-ray absorptiometry was used to assess body fat, abdominal fat distribution,[16] epicardial adipose tissues (EATs), intraperitoneal fat, subcutaneous fat tissue, visceral adipose tissue (VAT),[15] and computed tomography was used to evaluate abdominal fat distribution at L2/L3 and L4/L5,[14, 29] and visceral and subcutaneous fat area at L1 and L4[30] (Figures 3 and 4).
We found conflicting results in participants with overweight and obese individuals. Some metabolites of PCs were positively correlated with overweight individuals. There was a significant inverse relationship between BMI and most PC species, mainly acyl-alkylphosphatidylcholine (PCae) C30:0[28] C34:2,[22, 25, 28] PCae C34:3,[22, 24, 27, 28, 31] C36:2,[22, 25, 27, 28] C38:4,[22, 27, 28] C40:6,[24, 27, 28, 31] C42:3, C42:4, C44:4, C44:5, and C:44:6.[22, 28] Nonetheless, we observed positive associations between BMI and PC C18:0, C22:6, PC C15:0, C20:4,[26] unsaturated PCs, such as PC C28:1,[28] PC C32:1,[27, 28, 31] PC38:3[22, 28, 31] and PC38:4,[22, 28, 27] and most species of PC diacyl-phosphatidylcholine (PCaa) C32:1,[24] C32:2,[24] C36:3,[28] C38:3,[24] C40:4,[28] and C40:6.[28] Low levels of PCs C32:0, C34:2, C35:4, C36:2, and C38:5 have been found in individuals with obesity and insulin resistant individuals.[29] The results that attracted our attention were positive (PC C38:3, PC C38:4, PC C40:5, and PC C40:6[22]) or negative (PC C42:0, alkylphosphatidylcholine [PC-O] C42:5, PCae C44:4, PCae C44:5, and PCae C44:6[22]) associations between BMI and some PC species more intense in men. We also observed a positive association between PC32:0 and VAT in men[16] (Figure 3).
In relation to LPCs, most studies showed negative associations between BMI and LPC species, mainly LPC 15:0,[12, 21] LPC 16:0,[25] LPC 17:0,[23, 28, 29] LPC 18:0,[12, 25, 26] LPC 18:1, and LPC 18:2.[12, 21, 22, 24, 25, 27, 28] In addition, Baek and Kim[14] reported that an increase in visceral fat area (VFA) in the position of the fourth lumbar vertebra (L4) was associated with higher concentrations of plasma LPCs (C14:0, C16:0, C22:5, and C22:6). A positive association was only found between the metabolites lysoalkyphosphatidylcholine (LPC-O) C16:1[24, 27, 28] and LPC 20:3[28] with BMI (Figure 4). There were also differences in the metabolite profiles between males and females, with a negative association between subcutaneous fat and LPC-O C16:1, LPC C18:2, and LPC C18:3 in women,[16] and between BMI and LPC C18:1 and LPC C18:2 in men.[27]
The review also included a study that evaluated the differences in lipid metabolite concentrations between metabolically healthy obese (MHO) and metabolically unhealthy obese (MUO) individuals. The authors found that MHO individuals had a positive association with PCaa C32:1 and C38:3, but were negatively associated with LPCs (C18:1, 18:2, and 18:0), whereas the MUO group showed increases in LPC C16:1 and PCaa (C32:2, C34:2), but a decrease in LPCs (C18:1, C18:2C) and PCae C34:3.[31] Barber and Risis[19] found lower total LPCs levels in the plasma of MHO and obese individuals with type 2 diabetes mellitus (T2DM). LPCs (C15:0, C18:0, C18:1, C18:2, C20:0, C20:1, C20:2, and C20:4) were negatively associated with BMI. In addition, Tonks et al.[29] evaluated lipidomic signatures in the muscle and plasma of individuals with a BMI > 25 kg m−2 stratified into insulin-sensitive and insulin-resistant. Lower total plasma LPC levels were also observed in individuals with obesity and insulin resistance, mainly for LPCs C22:0 and C24:2. In skeletal muscle, lipid differences specific to insulin resistance in obesity included higher concentrations of PC(O-34:3). When comparing individuals with insulin resistance and sensitivity, PC(P32:1) and PC(P-34:1) were higher in resistant individuals, and LPC(22:6) was lower in this group.
The interventions included studies that tested the effect of corn oil or n–3 PUFA–rich fish oil[32] vitamin D,[33] low or high glycemic index (HGI) diet or low-fat diet[34] caloric restriction or n–3 PUFA–rich fish oil with caloric restriction,[35] and another study that evaluated the effect of an active satiety-enhancing product[36] (Table 1). We observed that there is no uniformity of species of lipid metabolites studied and therefore different results, depending on the intervention, for example, an increase in LPC C20:2[32] LPC C18:2 and LPC C20:3,[34] a decrease in total PC[34] or an increase in other PC species.[35] Changes in PC species were associated with lipid profile variables, suggesting that PCs could be potential biomarkers for a successful reduction in cardiovascular risk factors in diet-induced weight loss interventions and subsequent weight loss maintenance.[36]
4 Discussion
This review summarizes the main findings of observational and interventional studies on the lipidomic profiles of overweight and obese individuals, with a focus on PCs and LPCs. Body composition, sex, and diet are related to the synthesis of PCs and LPCs species. BMI is the most common anthropometric indicator of body mass used in the studies. Depending on the number of carbons, double bonds, and lipid polarity of the PCs and LPCs, different associations were found. Most species of PCs and LPCs showed lower concentrations when the BMI was increased. However, a number of authors have observed a positive association between obesity and certain species of unsaturated PCs (PCs C28:1, C32:1, C32:2, C34:4, C38:3 C38:4, C40:4, C40:5), PCaa (32:1, 32:2, 34:2, 36:3, 40:4, 40:6), LPCs O C16:1, and LPCs C20:3. Interesting findings were found regarding the different associations of certain species of PCs and LPCs between women and men with obesity. For example, PC 32:0 was positively associated with VAT in men, and the same was observed for BMI and PCs C38:3, C38:4, C40:5, and C40:6. In addition, a negative association between BMI and LPC C18:1 and LPC C18:2 was found in men, while women presented a negative association between subcutaneous fat and LPC-O C16:1, LPC C18:2, and LPC C18:3, and between BMI and certain PCae (PCae C36:2, C38:2, C40:4, C42:3, and C42:4). Moreover, the variability of analytical methods and statistical models should be emphasized as factors linked to the heterogeneity of these results.
Conflicting results were found regarding the behavior of PC species in the context of overweight and obesity. It is essential to point out that there are two types of structurally different PCs: PCaa has two FAs bound by ester bonds, whereas PCae has one of the FAs bound by an ester and one by an ether bond. PCaa consists of glycerol linked to PC and two FA residues. The removal of one FA produced LPCs. The corresponding acyl-alkyl-lysophosphatidylcholines (LPCae) consisted of an ether linkage to one alkyl chain and one PUFA.[38]
PCaa is essential for the hepatic secretion of very low density lipoprein (VLDL) particles and high density lipoprotein (HDL),[12] whereas PCae may act as a serum antioxidant to prevent lipoprotein oxidation.[39] PCae and LPCae levels were inversely correlated with plasma TAG levels. The excessive production of TAG in obesity can cause upregulation of glycerophospholipid synthesis, alterations in the saturation profile, and the phosphatidylcholine/phosphatidylethanolamine (PC/PE) ratio in the hepatic endoplasmic reticulum. These disorders affect metabolic consequences related to obesity, such as hyperglycemia and insulin resistance, especially in individuals with MUO.[40] In obesity, there is an increase in TAG-rich lipoproteins, such as VLDL, which probably explains the positive association between BMI and the main species of PCaa identified in this review. Another aspect that attracted our attention was the inverse association between BMI and most PCae species, indicating a higher risk of lipid peroxidation in obesity. These results suggest the importance of examining these PC subspecies as biomarkers of cardiovascular risk in the context of obesity.[41]
Additionally, FAs chains vary in length and position of double bonds,[42] and polarity,[24] making up a diverse spectrum of PC with different biological functions. The levels of PCs and LPCs reflect FAs turnover and the availability or depletion of saturated and unsaturated FAs, as well as changes in their chain length. It was previously reported that under pathological conditions, such as diabetes and obesity, the composition of the PCs and PC profiles in the liver and plasma changed in terms of chain length and saturation status.[19]
In particular, Bachlechner et al.[22] found that PCaa (⩽C40) were positively correlated with overall fatness, and longer-chained PCaa (C42) and all PCae and lysoPC were negatively correlated. In contrast, PCaa was more strongly related in men, whereas PCae was more strongly related in women. Similar associations have been found in T2DM.[43, 44] These authors suggested that lipids with a shorter chain length and SFA residues may trigger the development of T2DM, whereas those containing longer chains and unsaturated FA may offer protection. This was reinforced in a study that conducted lipidomic analysis of blood plasma in humans and demonstrated that lipids, mainly TAGs, with lower carbon number (<52) and double bond content (<2) were associated with an increased risk of diabetes, whereas lipids with a higher carbon number (>56) and double bond content (>2) were associated with decreased risk.[45]
An interesting example of the different behaviors of PCs between men and women was found by Szymańska and Bouwman[16] for PC C32:0, who were able to predict VAT only in men. The authors elucidated that the higher mean muscle mass in men may obscure the relationship between phenotypic parameters and body fat distribution. Nevertheless, other authors have already established a relationship between PC C32:0, obesity,[46] and hepatic steatosis, since PCs can be converted into TAGs in the liver.[47] In addition, relatively high concentrations of this metabolite were found in the adipose tissue compared to other tissues, which could explain its relationship with the parameters of body fat distribution.[48] For this reason, PC C32:0 appears to be a new potential indicator of VAT size and abdominal obesity in men.
LPCs are signaling molecules that play important and diverse roles in organisms, for example, the regulation of cell proliferation and inflammation. As obesity is associated with a state of chronic low-grade inflammation, it could be predicted that the concentrations of this molecule would be high in overweight and obese patients.[19] It is important to reinforce that Tonks et al.[29] suggested that overweight/obese lipidomic signature was only apparent in plasma and certify the usefulness of this matrix in studies of lipidomics in obesity. The authors confirmed that plasma LPCs were lower in insulin resistance, irrespective of obesity, whereas muscle insulin resistance was predominantly associated with higher C18:0 sphingolipids.
In this review, we also observed that most plasma LPC species were negatively associated with obesity parameters, particularly BMI. The authors commented that the exact mechanism that seems to explain the reduction in plasma LPCs in obesity is unknown, but a possible explanation is an increase in the degradation and/or clearance of circulating LPCs by metabolically active tissues such as the liver, adipose tissue, and muscle. In terms of obesity, glycosylphosphatidylinositol-anchored proteins (GPI-AP) are released into circulation. As a protective mechanism to balance the deleterious effect of circulating GPI-AP, glycosylphosphatidylinositol-specific phospholipase D is upregulated to degrade this protein.[49] Considering that LPCs and PCs are associated with GPI-AP in micelle-like complexes, a similar increase in lipolytic degradation could explain the reduction of this species in obesity.[27]
In relation to some species of LPCs, it was observed that in seven studies, inverse associations were identified between LPCs 18:1 and 18:2 and obesity. The same was observed for LPCs 18:0 in three manuscripts. Wallace et al.[25] found that individuals with decreased levels of LPCs C18:0, C18:1, and C18:2, who also had increased C-reactive protein (CRP) or leptin levels, had increased Homeostatic Model Assessment for Insulin Resistence (HOMA-IR) scores, suggesting a link between these lower lipid species and obesity, inflammation, and insulin resistance. Furthermore, LPC C18:2 can predict individuals with lower liver fat levels.[42, 44] LPC C18:1 has also been associated with a lower risk of insulin resistance via different glucose metabolism pathways. It was also reported that LPC C16:0 concentrations are different in insulin-sensitive individuals, insulin-resistant individuals, and those with hepatic steatosis.[25]
The authors emphasized that LPCs, especially LPC C18:1, may play an important role in glucose homeostasis by stimulating adipocyte glucose uptake and potentiating glucose-stimulated insulin secretion.[50] However, it remains to be determined whether the low LPC levels in obese and insulin-resistant individuals are due to greater clearance or decreased production.[25] The importance of different species of LPCs as biomarkers of metabolic changes can be explained by the fact that unsaturated LPCs hydrolyze to PUFAs, which have anti-inflammatory properties and positive health effects. Interestingly, this assumption cannot be extended to saturated LPCs.[23]
Abnormalities in LPC concentrations in obesity also involve obesity-related abnormalities in the activity of lecithin: cholesterol acyltransferase, the enzyme that generates almost all circulating LPCs, is a key enzyme in the metabolism of HDL.[51] These alterations increase the TAG content of HDL, which accelerates the hydrolysis of HDL particles by hepatic and lipoprotein lipases, promoting the formation of smaller HDL particles[52] which are less effective in the reverse cholesterol transport pathway.[53] These findings clarify that there is a low HDL phenotype commonly found in obese individuals, which may be related to changes in LPCs metabolism.
Another relevant finding is that women, in general, had lower concentrations of LPCs than men, in addition to a negative correlation between LPC 18:2-3 and subcutaneous adipose tissue, a relationship not found for men.[16] Considering the metabolic changes in LPC metabolism between body composition and sex, the relationship between LPCs, BMI, inflammation, and insulin resistance needs to be better studied to prove if this scenario is applicable to both sexes. Furthermore, obesity has strong genetic determinants that may be related to the different phenotypes of body composition, inflammation, and changes in lipid metabolism.[54]
Given these positive or negative associations between individual LPC and PC species and BMI, Pikó et al.[26] proposed the lipid species ratio as an interesting statistical approach to identify the most significant clusters of lipid molecules in obesity. The lipid species ratio had the highest sensitivity and specificity for obesity and a stronger association with BMI than individual lipid species alone. Some studies have used this data analysis technique to monitor obesity-related abnormalities in the human plasma lipidome.
In this review, another noteworthy fact was the importance of evaluating individuals with obesity using body composition assessment methods such as dual-energy X-ray absorptiometry, magnetic resonance imaging, or body scanning. Studies generally use anthropometric measurements, such as BMI, which are only approximate estimations of true body composition. However, direct methods for measurements of body composition are more accurate and precise for quantifying compartments of body composition. The relationship between PCs and LPCs and their metabolites appears to depend on body fat distribution as well, rather than BMI or total body fat percentage; however, only a few studies in this review have evaluated the effect of body fat distribution on the plasma circulation of PCs and LPCs.[14, 15, 30] Baek and Kim[14] and Kim and Yoo[30] assessed body fat distribution using computed tomography at the level of the fourth lumbar (L4) vertebrae. Both studies showed that VFA is strongly associated with a substantial increase in plasma lipid metabolites (higher LPC and lysophosphatidylethanolamine [LPE]) and atherogenic traits (higher HOMA-IR, high sensitivity CRP, oxidized LDL, lower HDL-c levels, and lower LDL particle size) in overweight subjects with a high VFA. Baek and Kim[14] found in this group of subjects LPC containing docosahexaenoic acid (DHA) and docosapentaenoic acid. LPC containing DHA in sn–1 position is known to exhibit antiinflammatory actions at noncytotoxic concentrations compared with other types of LPCs.[55] Thus, elevated DHA, as an inhibitor of prostaglandin synthesis, could partly explain the counter-regulatory mechanisms in subjects with high VFA. It was also observed that the plasma signatures of EAT and VAT reflected a depletion in relatively short-chain (30–36 carbon atoms) species containing PUFA, which more likely results from altered hepatic metabolism.[15]
In addition to adiposity, diet may have a greater contribution to modulating PCs and LPCs profiles. One study assessed the response of plasma LPCs to obesity, n–3 PUFA consumption, and a high-fat meal, showing that a high-fat meal altered the concentration of plasma LPCs in an oil treatment-dependent manner in normal-weight but not obese subjects, suggesting that obesity impairs the sensitivity of LPCs metabolism to n–3 PUFAs.[32] Another intervention with a caloric-restricted diet and n–3 PUFA–rich fish oil supplementation showed that PCs were responsive to dietary modification, mainly PCs identified as plasmalogens.[35] These metabolites have been inversely associated with obesity, liver fat, and insulin levels, and an increase in plasmalogens is associated with an increase in the dietary intake of n–3 PUFA.[56]
A caloric restriction andLGI diet in obese individuals suggests that the LGI diet could modulate certain circulating lipid levels marked by decreased values of PC C32:1 and increased LPC 18:2. Considering that PC C32:1 is positively correlated with T2DM and LPC18:2 has the opposite effect, this finding supports the beneficial role of LGI against T2DM by modulation of PC and LPC species.[34]
Additionally, the decreased concentration of several PC observed during intervention with a LCD was associated with a decrease in TChol, LDL-c, and TAG, indicating that the modulation of PC concentrations by diet could reduce cardiovascular risk.[36] Isolated nutrients, such as vitamin D supplementation, may also contribute to altering PC and LPC concentrations only in MUO subjects, suggesting that the effect of supplementation depends on the person's phenotype.[33]
We acknowledge some important limitations of this study. Most of these studies were related to the challenge of reviewing metabolomics studies. For example, misclassification of reported metabolites may occur because of the lack of uniformity in the description of the species. We emphasize that the associations listed in this review do not necessarily indicate causality. Furthermore, as most studies measured PC and LPC species in plasma/serum, the results likely reflect more acute variations due to recent food intake and circadian oscillations. The precision and accuracy of measurements are not harmonized between studies, making it difficult to assess the consistency of information. Another point that may explain the variation and some controversial results of lipidomes in patients with obesity is the analytical and statistical methods used.
Metabolite concentrations depend on biological variability and the precision of measurements, which might influence the metabolic panel. In addition, to ensure valid measurements, metabolites below the detection limit and those with high analytical variance must be excluded as they can compromise the reliability of the study.[22] Sampling, sample treatment and handling, extraction, and derivation of lipids prior to lipidomic evaluation are of the utmost importance for research that maps the lipid status of a given sample and identifies lipids qualitatively and quantitatively using different bioanalytical approaches. Most mass spectrometry (MS) methods have advantages in terms of detection limits in comparison to nuclear magnetic resonance (NMR), but need standardization in sample preparation, often are hyphenated with chromatographic separations, liquid or gas, and excellent standards for quantitative analysis are needed. On the other hand, NMR-based methods exhibit advantages in reproducibility and require very little or almost no sample modification prior to analysis but screen relatively high lipid levels; thus, detection limits are an issue.[57] The most recommended approach for elucidating PCs and LPCs in biological samples is a combination of NMR and MS to improve the identification of key lipids, and synergy could bring many advantages to lipidomics. We observed that only three studies explored in this review applied a combination of MS and NMR[16] or MS, NMR, and gas chromatography coupled with MS (GC-MS).[34, 36] In relation to statistical analysis, a diversity of different statistical methods is available to analyze data in the field of metabolomic. The main statistical methods applied were correlation and regression analysis, multiple tests with false discovery rate, principal component analysis, sparse partial least squares, linear discriminant analysis, and least absolute shrinkage and selection operator. The definition of statistical methods was based on the research question. Thus, the statistical analysis of complex data will be critical for extracting meaningful results from such metabolomics studies and may explain, in part, the diversity of results observed in the studies of this review.[58]
In conclusion, this review provides information about the complexity of PCs and LPCs body levels in the context of obesity, depending on a wide spectrum of factors, such as characteristics of the species, body composition, sex, diet, analytical methods, and statistical analysis. These findings suggest that PC and LPC levels may be potential biomarkers for overweight/obesity. However, further studies should be conducted with more homogeneous populations to elucidate the differences in PC and LPC species between sexes and ages, as well as further interventional studies, including diets with different lipid contents and FA profiles. In addition, large cohort studies should be conducted, including variables of genetics, diet, particularities of methods for body composition assessment, and traditional disease markers, such as WC, fasting glucose, and insulin. In addition, accurate analysis of the human lipidome is required to highlight the strong potential of lipidomics to better explain the mechanisms of obesity-related NCDs and identify sensitive predictive and prognostic biomarkers and targets for strategies for the prevention and treatment of obesity.
Acknowledgements
This study was funded in part by the Coordination of Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal do Nível Superior (CAPES) (Grant number 001) and the São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP) (Grant number #2018/24069-3).
Conflict of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization, K.C.M.S.E. and F.B.; Methodology, K.C.M.S.E., P.E.N.R.B., and M.N.M.; Validation, B.Z.R., F.B., K.C.M.S.E.; Investigation, P.E.N.R.B., M.N.M., and K.C.M.S.E.; Writing – Original Draft Preparation, P.E.N.R.B., B.Z.R., and K.C.M.S.E.; Writing – Review & Editing, K.C.M.S.E., B.Z.R., L.F.C.P., F.B., and L.T.; Visualization, F.B., L.T., L.F.C.P., B.Z.R., and K.C.M.S.E. All authors have read and agreed to the published version of the manuscript.
Biographies

Paula Emília Nunes Ribeiro Bellot is a nutritionist in a maternity hospital of the public health care service, Rio Grande do Norte. She obtained Master's degree at the Post-graduate Nutrition Programa of Federal University of Rio Grande do Norte, and specialization in Child Health Care at the Federal University of Rio Grande do Norte, She had an academic experience at the University of Toronto, Canada.

Melissa Nunes Moia nutritionist, specialized in Functional Clinical Nutrition from VP, São Paulo, Brazil. She is currently a Master's student of the Post-graduate Nutrition Program at the Universidade Federal do Rio Grande do Norte, Natal, RN, Brazil, in the field of nutrition diagnosis and nutrition intervention, in the context of micronutrients and chronic diseases. Clinical care in private practice.

Bruna Zavarize Reis nutritionist, has done master in Human Nutrition and PhD in Food Sciences at the Faculty of Pharmaceutical Sciences, University of São Paulo, Brazil. She is currently an Associate Professor in the Department of Nutrition at the Federal University of Rio Grande do Norte (UFRN) and a Professor of the Post-graduate Nutrition Program of UFRN. Works mainly in the following subjects: Micronutrients (Zinc, Selenium), Nutritional Genomics, microRNA, Metabolic inflammation, Obesity, and Metabolic Syndrome.

Lucia Fatima Campos Pedrosa is Senior Full Professor at the Federal University of Rio Grande do Norte, Natal, RN, Brazil. She obtained a doctorate at the University of São Paulo (USP) and earned her post-doctor degree from Vanderbilt University, Tennessee, USA. She has worked at Post-graduate Programs in Nutrition and Health Sciences and is the coordinator of Micronutrients, Nutritional Genomics, and Health Research Group (CNPq). Her primary research area is micronutrient status evaluation in children, older people, and patients with chronic diseases. She is a member of the International Society for Zinc Biology (ISZB).

Ljubica Tasic is a chemist and Professor at the Department of Organic Chemistry at the University of Campinas, Brazil. Our group research interests in the metabolic area are focused on human diseases, mostly ones with difficult diagnostics and unknown molecular basis, and magnetic resonance-based investigations. We seek information about the composition of mixtures or structures of different biomolecules to understand biological systems and how they change because of the disease and/or some drug use through effects on metabolites.

Fernando Barbosa Jr is Professor of toxicology at the University of São Paulo, Brazil. His primary research interest focuses on Human Biomonitoring Studies and Multi-omics signatures of the Exposome in Public Health.

Karine Cavalcanti Maurício Sena-Evangelista is a nutritionist and Professor of Clinical Nutrition area at the Department of Nutrition and Post-graduate Nutrition Program at the Federal University of Rio Grande do Norte, Brazil. She deepened her knowledge on nutrition and chronic diseases during her PhD at the University of São Paulo, Brazil. Her current research interests are focused on studies about micronutrients and nutrimetabolomic in the context of chronic diseases, mostly diabetes, obesity, metabolic syndrome, and cardiovascular disease (heart failure). She aims to identify nutritional biomarkers related to prevention and treatment of chronic diseases.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




