A Review on Dietary Flavonoids as Modulators of the Tumor Microenvironment
Abstract
The tumor microenvironment (TME) is the local environment where malignant cells strive and survive, composed of cancer cells and their surroundings, regulating essential tumor survival, and promotion functions. Dietary flavonoids are abundantly present in common vegetables and fruits and exhibit good anti-cancer activities, which significantly inhibit tumorigenesis by targeting TME constituents and their interaction with cancer cells. This review aims to synthesize information concerning the modulation of TME by dietary flavonoids, as well as to provide insights into the molecular basis of its potential anti-tumor activities, with an emphasis on its ability to control intracellular signaling cascades that regulate the TME processes, involving cell proliferation, invasion and migration, continuous angiogenesis, and immune inflammation. This study will provide a theoretical basis for the development of the leading compound targeting TME for anti-cancer therapies from these dietary flavonoids.
1 Introduction
TME is an extremely complicated system surrounding cancer cells. TME mainly consists of tumor cells, vasculature, extracellular matrix (ECM), fibroblasts, immune cells, and signaling molecules.[1] Acquiring and maintaining the hallmarks of cancer, which include sustaining proliferative signaling, activating invasion, and metastasis, inducing angiogenesis, tumor-promoting inflammation, and evading immune destruction, benefit from TME in various degrees.[2] TME is increasingly thought to contribute to the development of tumors by activating many oncogenic signal transduction factors via mediating multiple oncogenic signaling pathways.[3] Accordingly, the studies on the important role and regulatory molecular mechanism of the TME provide the basis for novel therapeutic intervention strategies by targeting TME elements or its signaling pathways in human cancer.[4] Given the increased understanding of the TME in tumor growth, considerable efforts have been devoted to identify the drugs targeting components of the TME, improving clinical efficacy in the treatment of advanced tumor patients.
Natural compounds (NPs) have supplied a rich source for drug development. There is plenty of evidence that many NPs and their derivatives exhibit immense therapeutic efficacy towards various types of diseases, favorable bioavailability, excellent safety, and good tolerability in humans.[5] Among the diverse range of NPs, flavonoids have received considerable attention due to their anti-inflammatory, anti-oxidative, anti-mutagenic, and anti-carcinogenic properties coupled with their capacity to modulate key cellular enzyme functions. Flavonoids, one of the common plant secondary metabolites in the human diet, are found in common ingredients including vegetables, fruits, chocolate, wine, flowers, tea, and other plant sources, hence commonly referred to as dietary flavonoids.[6] The main dietary flavonoids and their sources are shown in Table 1. Flavonoids are the building blocks of polyphenol compounds that can be found in various foods. They commonly have a generic structure consisting of two aromatic rings linked by carbons that are usually in an oxygenated hetero-cycle ring. Differences in the generic structure of the heterocycle C ring classify them as flavones, flavonols, flavanones, flavanols, isoflavonoids, and anthocyanidins.[7] In recent years, the roles of flavonoids as protective dietary constituents have become an increasingly essential part of human nutrition research. Dietary flavonoids displayed anti-tumor activity in multiple tumors (Table S1, Supporting Information). Accumulating evidence indicates that dietary flavonoids play an essential role in tumor suppression through different mechanisms, including those relevant to inhibition of cellular proliferation, induction of apoptosis, impairment of cell migration and invasion, suppression of tumor angiogenesis, regulation of immune inflammation, and so on.[8] During the past decade, as our understanding of the essential role of TME in tumor development and progression grows, increasing studies have also begun to reveal the working mechanisms of dietary flavonoids on the regulation of TME. The present review aims to focus primarily on the ant-cancer activity of dietary flavonoids as well as their underlying molecular mechanisms via regulating TME in recent years. A discussion on the potential of flavonoids in the reversal of drug resistance and combined medication is also presented, which provides new insights into the application of dietary flavonoids in the treatment or prevention of tumors.
| Structure | Compounds | Major dietary source | Reference |
|---|---|---|---|
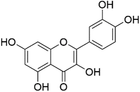 |
Quercetin | Tea, Capers, angelica, apples, red onions, red grapes, citrus, Tomatoes, broccoli | [61] |
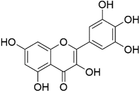 |
Myricetin | Fruits, vegetables, tea, and wine | [13] |
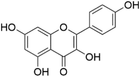 |
Kaempferol | Tea, broccoli, delphinium, witch hazel, grapefruit, brussels sprouts, apples | [18] |
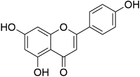 |
Apigenin | Chamomile, melissa, perilla, verbena, yarrow | [34] |
 |
Hesperetin | Oranges, grapefruit, Tangerines, etc. | [44] |
 |
Naringenin | Grapefruit, bergamot, lime, sour cherry, tomato, cocoa, Greek oregano, water mint | [68] |
 |
Epigallocatechin gallate | Green tea, cocoa based products, pome fruits | [93] |
 |
Puerarin | Pueraria lobata, Pueraria phaseoloides | [74] |
2 Inhibition of Tumor Proliferation
The interaction of TME with cancer cells plays a crucial role in regulating the proliferation and anti-apoptosis of cancer cells. Stimulation of tumor cell death is one of the critical goals of tumor therapy. Apoptosis is triggered in two distinct ways: the extrinsic death receptor pathway and the intrinsic mitochondrial pathway.[9, 10] The external apoptosis pathway transmits signals from the extracellular death ligands to the apoptosis mechanism of cells through appropriate death receptors. The tumor necrosis factor (TNF) family of proteins is the most clearly described death ligand. The intrinsic apoptosis pathway also referred to as the “stress” or “mitochondrial” apoptosis pathway, is tightly regulated by anti-apoptotic proteins (Bcl-2 and Bcl-xL) and pro-apoptosis proteins (Bax, Bid, and Bim), which interact on the mitochondrial outer membrane. Meanwhile, there are some critical signaling pathways including JNK/ERK, NF-κB, MAPK, PI3K/Akt/mTOR, and PTEN/PI3K, involved in apoptosis. Most flavonoids have been shown to delay or block the development of cancer cells in vitro and in vivo by triggering apoptosis via extrinsic and intrinsic signaling pathways.[11] And the core structure of the dietary-polyphenol flavone 2-phenyl-4 H-1-benzopyran-4-one is closely related to the proliferation, apoptosis, and cell cycle regulation of cancer cells.[12]
2.1 Myricetin
Myricetin (3,5,7-trihydroxy-2[3,4,5-trihydroxyphenyl]-4-chromenone, MYR) is a flavonol component abundantly found in dietary sources such as berries, vegetables, fruits, red wine, tea, and other foods (Table 1).[13] MYR is characterized by the pyrogallol Bring, and the more hydroxylated structure is known to be capable of its increased biological properties compared with other flavonols.[14] Accumulating evidence demonstrates that MYR exhibits excellent anti-proliferative and pro-apoptotic potential in the treatment of breast cancer. MYR induces apoptosis by regulating autophagy, at the molecular level, it upregulates apoptosis and autophagy proteins, including Bcl-2, p-ERK, light chain 3 (LC3), and Beclin 1, while decreasing, Cl-PARP, Bax, p-JNK, and p-p38.[15] Furthermore, Mondal et al.[16] reported that MYR also binds to the 30-end of the G-quadruplex telomeric structure in human breast cancer MCF-7 cells, suppressing human telomerase reverse transcriptase mRNA and telomerase in a time and dose-dependent manner, thereby inhibiting the proliferation of breast cancer cells. It has been shown that MYR induces apoptosis in hepatocellular carcinoma (HCC) cells by directly phosphorylating yes-associated protein (YAP) on serine residues, repressing the expression of the downstream target p21 activated kinase 1 (PAK1) of Ras signaling pathway, and accelerating the activation of the large tumor suppressor 1/2 (LATS1/2) kinase.[17, 18] In human placental choriocarcinoma cells, MYR significantly enhances oxygen species production, glutathione depletion, lipid peroxidation, loss of mitochondrial membrane potential (MMP), and modulation of endoplasmic reticulum stress, while promoting apoptosis by inhibiting MAPK and PI3K/Akt signaling pathways.[19] MYR inhibition of the PI3K/Akt/mTOR pathway has also been reported in colon cancer that MYR-induced cell apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signaling pathway.[20] A complete list of anti-proliferative molecules affected by MYR in different cancer cell lines is included in Table 2. Unfortunately, the result of the pharmacokinetic evaluation showed poor absorption after oral administration, which limited the application of MYR severely.[21] Currently, microemulsion formulations and pharmaceutical cocrystals were paid attention to overcome this issue.[22]
| Compounds | Efficacy | Cancer types | Mechanism | Reference |
|---|---|---|---|---|
| Myricetin | Proliferation | Hepatocellular cancer | ↑ P-YRP, LATS1/2 kinase | [18] |
| Breast cancer | ↓ hTERT expression | [16] | ||
| Placental choriocarcinoma |
↑ ROS production, lipid peroxidation, glutathione depletion; ↓ mitochondrial membrane potentials |
[19] | ||
| Breast cancer | ↑ p-JNK, ↓ Bcl-2, and ↑ LC 3-II/I | [15] | ||
| Migration and invasion | Breast cancer | ↓ MMP-2/9 and ST6GALNAC5 | [58] | |
| Cholangiocarcinoma |
↓ the activation of the STAT3 pathway; ↑ ICAM-1, MMP-9, iNOS, and COX-2 |
[49] | ||
| Prostate cancer | ↓ PIM1, CXCR4 | [60] | ||
| Angiogenesis | Ovarian cancer | ↑ p21, ↓ HIF-1α, VEGF | [85] | |
| Breast cancer | ↓ VEGFR2, p38 MAPK, and p-p38 MAPK | [86] | ||
| Hepatocellular carcinoma | ↑ E-cadherin; ↓ VE-adherin, VEGFR1/2 | [87] | ||
| Immunity inflammation | Bone marrow macrophage | ↓ TNF-α and NF-κB activation; ↑ IL-6 and IL-8 expression. | [102] | |
| Colon cancer | ↓ TNF-α, IL-1β, IL-6, NF-κB, p-NF-κB, COX-2, PCNA, and Cyclin D1 | [104] | ||
| RAW264.7 cells | ↓ GP91 phosphorylation, p47 phosphorylation, JAKs, and STAT1 | [106] | ||
| Kaempferol | Proliferation | Ovarian cancer | ↑ DR4, DR5, CHOP, JNK, ERK1/2, p38 | [26] |
| Gastric cancer | ↓ NF-κB and MAPK signaling pathways | [27] | ||
| Colon cancer | ↓ CDK2, CDK4, cyclin A/D/E | [28] | ||
| Ovarian cancer | ↑ p21, p-Chk2, p-Cdc25C, p-Cdc2 | [29] | ||
| Apigenin | Proliferation | Prostate cancer |
↓ Rb, p-Rb (Ser807/811), phospho-Rb (Ser780), p-ELK-1, c-FOS, and cyclin D1; ↑ p-ERK1/2, p-JNK1/2 |
[41] |
| Melanoma | ↓ p-ERK1/2, p-AKT, and p-mTOR | [43] | ||
| Breast cancer |
↑ cl-caspase-8, cl-caspase-3, and cl-PARP; ↓ p-JAK2 and p-STAT3 |
[40] | ||
| Cervical cancer |
↑ p16, INK4A; ↓ cyclin A/D/E and CDK2/6; ↓ the PTEN/PI3K/AKT pathway |
[42] | ||
| Angiogenesis | Esophageal cancer | ↓ VEGF and IL-6 expression | [91] | |
| Melanoma | ↓ IL-6, IL-10 and TNF-ɑ | [92] | ||
| Immunity inflammation | Colonic inflammation | ↓MPO, inflammatory cytokines, and COX-2 | [108] | |
| Pancreatic cancer | ↓ SK-3β/NF-κB signaling pathway | [10] | ||
| NSCLC | ↓ IFN-γ-induced PD-L1 expression | [112] | ||
| Hesperetin | Proliferation | Breast cancer | ↓ HER2, MMP-9, Rac1; arrested cell cycle | [46] |
| ↑ p53, NOTCH1, PPARG; ↓ β-catenin | [48] | |||
| Gastric cancer | ↓PI3K/Akt signaling pathway; ↑PTEN | [50] | ||
| Immunity inflammation | Hepatic cancer | ↑ Nrf2, PPARγ, and HO-1 | [106] | |
| Gastric cancer | ↑cl-caspase3, cl-PARP, and P53↓ Akt and p-Akt | [116] | ||
| Quercetin | Migration and invasion | Hepatocellular carcinoma | ↓ MAPK/ERK and PI3K/Akt signaling pathways | [63] |
| Colon cancer | ↓ p38, JNK, and ERK signaling pathways | [64] | ||
| Melanoma | ↓ STAT3, Mcl-1, MMP-2, MMP-9, and VEGF | [65] | ||
| Angiogenesis | Prostate cancer | ↓Akt, mTOR, and p70S6K, which are called VEGFR2 downstream signaling molecules are suppressed | [89] | |
| ↓ TSP-1 signaling pathway | [88] | |||
| Stomach cancer | ↓ β-catenin, Twist1 and ITGβ6 | [90] | ||
| Naringenin | Migration and invasion | Glioblastoma | ↓ MMP-2, MMP-9, and Naringenin decreased mesenchymal markers | [70] |
| Chondrosarcoma cancer | ↓VCAM-1; ↑ miR-126 expression | [72] | ||
| Gastric cancer | ↓ MMP2, MMP9 | [73] | ||
| Puerarin | Migration and invasion | Breast cancer | ↓ CCR7, CXCR4, MMP-2, MMP-9, ICAM, and VCAM; ↓ the NF-κB activation, ↓ p-p65 and p-IκBα | [77] |
| Lung cancer | ↓ the MEK/ERK 1/2 pathway | [78] | ||
| Hepatocellular carcinoma | ↓ the miR-21/PTEN/EMT signaling pathway | [9] | ||
| Epigallocatechin gallate | Angiogenesis | Hepatocellular carcinoma | ↓ HIF1α and VEGF | [95] |
| ↓ MAPK/ERK1/2, PI3K/AKT, HIF-1α, and VEGF | [96] | |||
| ↓ STAT3 and VEGF expression | [97] | |||
| Gastric cancer | ↓ VEGF | [98] |
2.2 Kaempferol
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a tetrahydroxy flavone substance mostly found in more than 400 species of fruits and vegetables such as tea, broccoli, hazelnut, propolis, grapefruit, etc. (Table 1).[23] The presence of 3’-OH moiety on the B ring of kaempferol and suggests that eating a customized range of green vegetables and fruits could offer enhanced protection against cancer.[24] It has been shown for the oral bioavailability of kaempferol that there was a positive therapeutic effect in the nano- or microgram per mL range.[25] There was a significant inhibitory effect of kaempferol in various signaling pathways associated with the initiation or progression of many cancer types. Zhao et al.[26] revealed that kaempferol drastically upregulates the expression of the apoptotic proteins, caspase-3, caspase-8, caspase-9, and Bax, whereas declines the expression of anti-apoptotic proteins, Bcl-xL, Bcl-2, survivin, XIAP, Cl-FLIP, ultimately inhibiting human ovarian cancer cells proliferation via JNK/ERK-CHOP pathway. In another study, kaempferol has been shown to induce autophagic cell death in gastric cancer via inhibiting the NF-κB and MAPK signaling pathways.[27] In addition to its pro-apoptotic potential, kaempferol also halts cell cycle progression in different cancer cell lines. It causes cell cycle arrest in the G2/M phase, with strong downregulation of cyclin-dependent kinase 2 (CDK2), cyclin-dependent kinase 4 (CDK4), cyclins D1, cyclin E, and cyclin A in human colon cancer HT29 cells.[28] A similar effect on cell cycle progression has also been observed in ovarian cancer and non-small cell lung cancer (NSCLC) cells.[29, 30] Lastly, kaempferol also inhibits the PI3K/Akt/mTOR signaling pathway and the expression of estrogen receptor alpha (ERα), thus modulating p53, Bax, Bcl-2, and Caspase-3/7 protein activity, which all contribute to its anti-tumor effects.[29, 31-33] Table 2 summarizes the effect of molecules affected by kaempferol treatment in different cancer cells.
2.3 Apigenin
Apigenin (4′,5,7-trihydroxyflavone) is a naturally occurring flavonoid present abundantly in oranges, garlic, parsley, propolis, celery, carrot, and artichokes (Table 1).[34] Despite apigenin displaying poor oral bioavailability, it was able to accumulate in tissues which seemed to provide a novel approach to the application of apigenin.[35] Sufficient studies have found that apigenin significantly inhibits tumor cell proliferation through different molecular or mechanism signal pathways.[36-39] Shukla et al. revealed that apigenin induces extrinsic caspase-dependent apoptosis by increasing Cl-caspase-3/8 and inducing the cleavage of PARP in HER2-overexpressing BT-474 breast cancer cells.[40] On the other hand, Liu et al. reported that apigenin inhibits the proliferation and induces apoptosis of human prostate cancer cells by decreasing Rb phosphorylation and suppressing cell cycle progression.[41] Zhao et al. also proved apigenin can induce G0/G1 phase arrest, reduce MMP, and upgrade intracellular reactive oxygen species (ROS) production via inhibiting the PTEN/PI3K/Akt pathway, resulting in cervical carcinoma HeLa cell apoptosis.[42] In addition, apigenin also has been reported to suppress the proliferation, metastasis, and invasion of melanoma cells A375 and C8161 via inactivating the p-ERK1/2, p-Akt, and p-mTOR signaling pathways.[43] Therefore, apigenin can prevent cancer cell proliferation by triggering apoptosis, which leads to cell cycle regulation, and can also reduce cancer cell motility, thereby preventing cancer cell migration and invasion. Overview of apigenin's anti-cancer mechanism against numerous types of cancer is summarized in Table 2.
2.4 Hesperetin
5,7,3′trihydroxyl-4′-methoxyl-flavanone, also known as hesperetin (HSP), is found in oranges, grapefruit, tangerines, etc. (Table 1) and possesses good anti-oxidant, anti-carcinogenic, and anti-inflammatory properties.[44] In a previous study, researchers found that HSP induces esophageal cancer Eca109 cellular death by decreasing GSH accumulation and increasing ROS levels and cytochrome C expression, confirming intrinsic mitochondrial cell apoptosis.[45] Additionally, HSP also exhibits similar pro-apoptotic effects in colon cancer, and breast cancer cells.[46, 47] Wang et al. indicated that HSP induces cell cycle arrest and cell apoptosis by elevating the mRNA levels of p53, Notch-1, and PPARG while reducing the mRNA level of β-catenin in human breast cancer MCF-7 cells.[48] Besides this, HSP is also able to activate endoplasmic reticulum stress pathways and alter cell cycle progression via upregulation levels of CHOP and GRP78, inhibiting HeLa cell proliferation.[49] Notably, HSP is underlined by inhibition of the PI3K/Akt signaling pathway, and induction of the mitochondrial pathway via upregulating PTEN expression, thereby significantly enhancing cisplatin's anti-tumor effect in gastric cancer HGC-27, SGC-7901, and MGC-803 cells.[50] In spite of wide range of therapeutic efficacy, HSP displayed higher absolute bioavailability than hesperidin. However, the way of its extensive metabolism ultimately led to lower bioavailability.[51]
3 Inhibition of Tumor Migration and Invasion
Tumor migration and invasion are complex multistep processes influenced by non-malignant components of the TME, involving phenotypic alterations and genetic changes in cancer cells, as well as responsible for 90% of tumor deaths in tumor therapy.[52, 53] In the last several decades, a tremendous number of NPs with multiple biological activities and industrial potentials have been demonstrated to interrupt the invasive processes of cancer cells in a specific tumor or environment, via targeting several TME components, such as ECM, fibroblasts, immune cells, and so on.[54, 55] Moreover, more and more dietary flavonoids have been revealed to directly regulate tumor invasion and metastasis, suppressing the tumorigenesis of various cancer types, including breast, prostate, liver, colon, lung cancers, etc.[55-57] Elucidating the underlying molecular mechanisms of these dietary flavonoids for suppressing the migration and invasion of tumors is of great significance for identifying novel therapeutic targets and improving survival in patients with tumors.
3.1 Myricetin
MYR exerts a significant inhibitory effect on tumor migration and invasion in different types of cancer cells. Tuponchai et al. reported that MYR suppresses migration and invasion of the MDA-Mb-231 breast cancer both in vitro and in vivo by reducing the protein expression levels of matrix metalloproteinase-2/9 (MMP-2/9) and ST6GALNAC5.[58] Moreover, Ye et al. demonstrated that MYR has been proved to suppress the signal transducer and activator of the transcription 3 (STAT3) pathway, downregulate the expression of metastasis-related genes, such as ICAM-1, MMP-9, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS), thus inhibiting cytokine-induced cholangiocarcinoma KKU-100 cells migration and invasion.[59] In addition, Moloney murine leukemia virus-1 (PIM1) and C-X-C chemokine receptor type 4 (CXCR4) are co-expressed, which can lead to poor prognosis and aggressive clinicopathologic traits in prostate cancer patients. Recent studies have demonstrated that MYR remarkably inhibits invasion and migration of prostate cancer cells via eliminating the protein levels of PIM1, disrupting PIM1/CXCR4 interaction.[60] Up to now, the anti-metastatic mechanisms of MYR are most confirmed by in vitro and animal studies, which is a significant limiting factor to its use in clinical settings. Therefore, it is an emergency to conduct well-designed clinical trials to determine the underlying mechanisms in the future.
3.2 Quercetin
Quercetin (3, 3′, 4′, 5, 7-pentahydroxyflvanone), a major flavonoid found in many commonly consumed fruits and vegetables, has unique biological properties, which is a pleiotropic molecule with anti-proliferation, anti-metastatic, anti-inflammatory, and anti-cancer activities (Table 1).[61] Importantly, quercetin has been approved to applicate to humans, indicating its safety.[62] It has been found that quercetin remarkably downregulates MMP-2 and MMP-9 protein levels by suppressing the PI3K/Akt/AMPK signaling pathway, resulting in inhibition of cell migration and invasion in HCCLM3 cells.[63] NguyetTran et al. reported that quercetin increases the protein expression of N-cadherin and vimentin via activating the p38/JNK/ERK signaling pathways, finally inducing the human colon cancer RKO cells migration and invasion.[64] For another, treatment of A375 melanoma cells with quercetin inhibits cell migration and invasion via decreasing STAT3 transcription activity and its targeted gene expression, including Mcl-1, MMP-2, MMP-9, and VEGF.[65] In addition, quercetin has been proved to suppress the epithelial-to-mesenchymal transition (EMT) process, also confirming its inhibitory effect on migration and invasion in prostate cancer cells.[66] A similar effect was observed in colon cancer SW480 cells treated with TGF-β1 (which induces EMT transition), quercetin treatment rescues the morphological changes, and EMT-like phenotypes lower via the suppression of Twist1 and E-cadherin expression.[67] Finally, the reduced EMT process in cultured cells and cancer xenograft tumor models, further confirmed the good anti-metastatic effect of quercetin.
3.3 Naringenin
Naringenin (2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is an abundant polyphenolic compound in citrus fruits, such as mandarin, grapefruit, and orange (Table 1).[68] Notably, the evaluation of its safety and pharmacokinetics was performed in human vivo, suggesting that 150–900 mg ingestion was the safe dose for healthy adults.[69] Numerous studies indicate that naringenin exhibits remarkable health-promoting activities, such as anti-metastatic, anti-oxidant, anti-inflammation, and anti-cancer properties. A recent study showed that naringenin can eliminate the migration and invasion of glioblastoma cancer through reduction of EMT markers, snail and slug protein expression, and inhibition of MMPs, ERK, and p38 activities.[70] Han et al. observed that naringenin, as a potential anti-cancer compound, is capable of upregulating E-cadherin protein expression, downregulating the protein levels of vimentin, SNAI1, SNAI2, and TWIST1, thus inhibiting migration and invasion in prostate cancer PC-3 cells.[71] Similarly, it has been demonstrated that naringenin inhibits cell migration and invasion via downregulation of VCAM-1 expression by increasing miR-126 expression in human chondrosarcoma JJ012 cells.[72] Additionally, treatment with apigenin in vitro significantly suppressed the migration and invasion of gastric cancer SGC-7901 cells by decreasing the expression of MMP-2 and MMP-9 via inhibition of Akt phosphorylation.[73] However, the molecular mechanism of naringin regulating tumor migration and invasion is still not fully elucidated, therefore, more studies are needed to explore additional molecular events for anti-metastatic effects.
3.4 Puerarin
Puerarin (8-β-d-grapes pyranose-4, 7-dihydroxy isoflavone), a natural flavonoid, exhibits various pharmacological properties including anti-vascular, anti-inflammatory, and anti-cancer, and could be extracted from Pueraria lobata and Pueraria phraseologies (Table 1).[74] A meta-analysis provided a piece of clear evidence of relative safe of puerarin in clinic.[75] Recently, growing evidence suggests a crucial involvement of puerarin in the processes of cancer cell migration, adhesion, and invasion, providing the different mechanisms of puerarin on the malignant behavior of tumor cells.[76] Kang et al. noted that puerarin significantly regulates lipopolysaccharide (LPS)-induced MCF-7 and MDA-MB-231 cells migration, invasion, and adhesion via remarkably lowering the expression of CXCR4, MMP-2, MMP-9, ICAM, and VCAM, inactivating NF-κB and ERK signaling pathways.[77] Moreover, puerarin can suppress the invasion and migration of lung cancer macrophages by inhibiting the activation of the MEK/ERK 1/2 pathway via inhibition of ERK nucleus translocation, resulting in antagonizing tumor growth and tumor volumes in the lung cancer xenograft model.[78] Puerarin has been proved to suppress the EMT process of HCC cells by blocking the miR-21/PTEN/EMT signaling pathway.[79] Overall, these findings demonstrated that puerarin might be a dietary supplement for cancer patients’ treatment.
4 Regulation of Tumor Angiogenesis
Angiogenesis is defined as a process of sprouting and remodeling of small new capillaries from the preexisting vessels and is characterized as an essential step for tumor growth as well as development from the premalignant to the invasive and malignant phenotype.[80] TME consists of numerous pro-angiogenic factors, including TGF-β, VEGF, VEGFR, PDGF, COX-2, HIF-1, and angiopoietins. These factors are secreted by tumor cells or macrophages, and can activate pro-angiogenic signaling pathways to promote tumor angiogenesis, growth, invasion, and metastasis.[81, 82] The main pro-angiogenic factor VEGF, not only promotes the formation of new vessels stimulated by hypoxia but also increases microvascular permeability and divides vascular endothelial cells.[83] Additionally, many cytokines such as TGF-β, IFNs, TNF-α, and ILs acting in paracrine and autocrine fashion secreted by tumor cells in TME, play a critical role in regulating tumor angiogenesis. It is worth noting that dietary flavonoids have shown great potential anti-cancer activities through inhibiting the angiogenesis regulated via affecting various pro-angiogenic and anti-angiogenic factors.[84]
4.1 Myricetin
MYR is a dietary flavonoid extracted from fruits and vegetables, exhibiting anti-angiogenic activity in various human cancers, as confirmed in vitro and in vivo.[85, 86] VEGF is a crucial protein-peptide family in the mediation of tumor vascular development and maintenance. A result of a prior study showed that MYR plays a prominent anti-angiogenic role by inhibiting the secretion of VEGF via blocking the Akt/p70S6K/HIF-1α pathway in human ovarian cancer cells.[85] Meanwhile, cell migration is an important factor in the procedure of angiogenesis and the formation of a tumor vascular network. It has been demonstrated that MYR suppresses the migration of human umbilical vein endothelial cells but not proliferation, suggesting that MYR may exhibit potential for the chemoprevention of breast cancer.[86] Another study showed that MYR can effectively inhibit vasculogenic mimicry and angiogenesis of HCC via reversing the expression of epithelial-endothelial transition markers by targeting PAR1, resulting in the suppression of HCC progression.[87] In summary, MYR exhibits a particular inhibitory effect on angiogenesis, which means that MYR has a high potential for being developed as a leading compound for targeting tumor angiogenesis.
4.2 Quercetin
In the last 10 years, quercetin has also been widely studied in different cancer cells and has shown good anti-angiogenetic potential. Quercetin can effectively inhibit angiogenesis by upregulating its expression on the endogenous thrombospondin-1, resulting in antagonizing human prostate cancer growth in vitro and in vivo.[88] The activation of Akt, mTOR, and p70S6K, called VEGFR2 downstream signaling molecules, is suppressed by quercetin in a dose-dependent manner, which inhibits the growth of prostate cancer.[89] A similar effect was observed in stomach cancer: irinotecan combined with quercetin treatment is proven to be capable of suppressing angiogenesis to treat stomach cancer by significantly downregulating the concentration of VEGF-A and VEGFR2. Quercetin is also able to be used with metformin to treat colon cancer through inhibiting VEGF/PI3K/Akt signaling pathway.[90] These studies suggest that the combination of quercetin and chemotherapy drugs on the market can effectively improve anti-angiogenesis ability, contributing to inhibiting the development of malignant tumors.
4.3 Apigenin
One prominent participant in acute inflammation is interleukin-6 (IL-6), which can promote the angiogenesis-osteogenesis coupling process. It is remarkable that apigenin, as a novel IL-6 transcription inhibitor, is becoming increasingly important for esophageal cancer therapy.[91] Qiu et al. noted that the expression of VEGF and IL-6 in esophageal cancer is decreased by apigenin in vivo and in vitro, suppressing tumor angiogenesis. Interestingly, the inhibitory effect on angiogenesis induced by apigenin may be inversely correlated with the treatment concentration. When tested on the human melanoma model in the chorioallantois membrane assay, it has been found that compared with the higher concentration, the lower concentration of apigenin treatment reduces the number of capillaries in the application area more effectively.[92]
4.4 Epigallocatechin Gallate
Epigallocatechin gallate (((2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl),4, 5-trihydroxybenzoate, EGCG), abundantly found in cocoa-based and green tea products, is the most studied molecule of the flavanol class (Table 1).[93] EGCG exhibited well tolerated confirmed by clinical trials.[94] It has been found that hypoxia is one of the characteristics of many tumors, which affects gene expression, metabolism, and ultimately tumor biology-related processes, especially angiogenesis. Notably, EGCG has demonstrated its capacity to inhibit angiogenesis under hypoxic conditions by reducing the protein levels of the HIF-1α and its downstream target gene VEGF of gastric cancer.[95] Its similar inhibitory effect on tumor angiogenesis via decreasing VEGF expression also has been further confirmed by Liao et al. in 2020.[96] Sartippour et al. further demonstrated that the inhibitory effect of EGCG on VEGF is achieved by suppressing the DNA-binding activity of activator protein-1 (AP-1) to the VEGF promoter.[97] More interestingly, a novel mechanism is revealed that EGCG also can inhibit IL-6-induced VEGF expression and angiogenesis of human gastric cancer by suppressing STAT3-DNA binding activity and STAT3 nucleus translocation.[98] The results mentioned above imply that EGCG may have a great potential to be developed as a VEGF inhibitor.
5 Regulation of Tumor Immunity and Inflammation
There is a strong relationship between immune-inflammation and cancer. Inflammatory cells and their mediators are an essential component of TME, contributing to virtually all aspects of tumor progression. Several immune pro-tumor effector mechanisms are up-regulated by chronic inflammation, leading to the hypothesis that inflammation promotes carcinogenesis and tumor growth by altering the balance between pro- and anti-tumor immunity.[99, 100] During the last few years, dietary flavonoids have been well documented for their great anti-inflammatory activity by suppressing the excessive production of inflammatory mediators TNF-α, IL-6, MAPK, NF-κB, and iNOS.[101] Here, we summarize the immunomodulatory, anti-inflammation effects, and their underlying molecular mechanisms of different dietary flavonoids in different cancer cells.
5.1 Myricetin
MYR-mediated SIRT1 activation can effectively ameliorate their inflammatory response in NSCLC A549 cells by inhibiting TNF-α, NF-κB activation, and upregulating the IL-6 and IL-8 expression.[102] A report had also shown that MYR inhibits the expression of PD-L1 and IDO1 induced by IFN-γ in human lung cancer cells and reduces the production of kynurenine, a catalytic product of IDO1, while restoring T cell activity and promoting T cell proliferation.[103] Another study showed that MYR significantly reduces the occurrence of azoxymethane/dextran sulfate sodium-induced colitis by interfering with the inflammatory factors TNF-α, IL-1β, IL-6, NF-κB, COX-2, PCNA, and Cyclin D1 and increasing CD8+T and CD4+T cells in the colonic tissues, ultimately improving the inflammatory TME and reducing the risk of colon cancer.[104] MAPK, an important transmitter of signals from the cell surface to the interior of the nucleus, is thought to be a key regulator of inflammation.[105] Further mechanism research demonstrated that MYR attenuates LPS-induced inflammation in RAW 264.7 macrophages by reducing the secretion of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β via mitigation of MAPK and NF-κB signaling pathways. And some of these findings have been confirmed in vivo.[106] As mentioned above, although MYR is capable of regulating the inflammatory response, more studies are needed to explore additional molecular events behind these effects.
5.2 Apigenin
Since the anti-cancer activity of apigenin was found, amounting studies have been conducted to explore the mechanism of action underlying immunomodulatory induced by apigenin in various types of cancers.[107] In colitis model group mice, apigenin can reduce bone marrow peroxidase, inflammatory cytokines, and COX-2 levels, showing good anti-inflammatory activity.[108] A similar effect has also been observed in human melanoma A375 cells. Moreover, it seems that the effect of apigenin on inflammatory response is concentration-dependent.[109] The study by Ghitu et al. revealed that high concentrations of apigenin treatment remarkably eliminate about 60% TNF-α secretion, confirming its great anti-inflammatory activity.[92] Another study provided the first direct evidence that apigenin treatment contributes to the suppression of pancreatic cancer growth by upregulating the gene expression of the pro-inflammatory cytokines, IL17s and LTA.[110] Furthermore, a whole transcriptomic analysis was also performed to evaluate the effects of apigenin on breast cancer cells. The result of microarrays indicated that apigenin can inhibit TNF-α-induced IκB-α protein phosphorylation and reduces IL-1, CCL2, IL-8, and IL-6 mRNA expression, collectively resulting in the enhancement of T cell-mediated tumor killing.[111] More recently, it has been reported that apigenin suppresses the progression of NSCLC with KRAS mutant by inhibiting IFN-γ-induced PD-L1 expression.[112] Additionally, as an inhibitor of multiple kinases, apigenin can induce the expression of inositol 5′ -phosphatase-1, reduce the production of tumor-derived factor, increase the proportion of killer macrophages, enhance anti-tumor immune responses, and reduce tumor load in mice with pancreatic cancer.[113] On the whole, apigenin is able to modulate the expression of inflammatory mediators and the immune systems to show promising anti-cancer activity.
5.3 Hesperetin
HSP has received considerable attention for its good anti-inflammation and anti-cancer activities. Furthermore, in oleic acid-induced HepG2 cells, HSP also has been proved to suppress hepatic oxidative stress and inflammation by blocking NF-κB activation and decreasing the expression of NF-κB-regulated TNF-α and IL-6 genes.[114] Mahmoud et al. performed an assessment analysis of the chemopreventive effect of HSP against DEN/CCl4-induced hepatocarcinogenesis. HSP was shown to inhibit oxidative stress, inflammation, and cell proliferation by inhibiting the expression of Nrf2, PPAR, and HO-1 via abolishing NF-κB and TGF-γ1/Smad3 signaling pathways, ultimately suppressing hepatocarcinogenesis in DEN/CCl4-induced rats.[115] Similar effects that inhibit cancer progression were observed in the benzo(a)pyrene-induced lung carcinoma mice model, in addition to inhibiting the NF-κB signaling pathway, HSP decreases the expression of PCNA and CYP1A1, while reducing the activities of anti-oxidant enzymes SOD, CAT, GPx, GST, GR by alleviating the status of lipid peroxidation.[116] However, due to the poor water solubility, HSP conjugated gold nanoparticles show better anti-inflammatory and anti-carcinogenic properties compared with the control pure HSP.[117] Hence, nanoparticle encapsulation strategies for targeted drug delivery are exciting new directions for treating cancer that deserved to pay more attention. Taken together, these findings are fully confirmed the chemo preventive potential of HSP in vivo and in vitro.
6 Conclusion and Future Perspectives
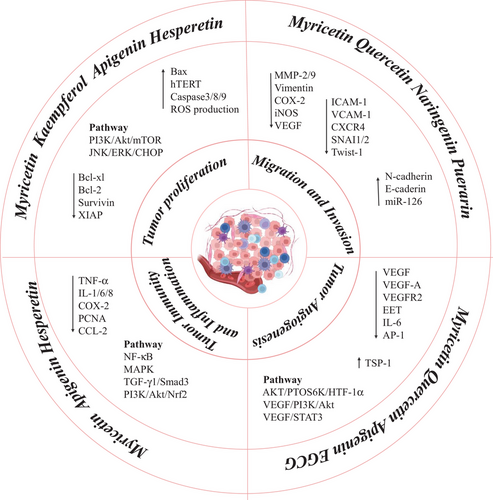
Malignancy development is highly dependent on TME, which influences the tumor initiation, progression, metastasis, and response to therapies of tumor cells by interactions between tumor cells and TME. Currently, targeting TME is increasingly being explored as a novel approach to improving cancer therapeutics. However, therapeutic strategies targeting TME are far from satisfactory because of relatively low response rates and adverse side effects. Meanwhile, the highly heterogeneous and dynamic nature of the TME imposes vast challenges to the development of effective drugs to target them. The tumor is a multifactorial disease, as it is influenced by TME in multi-pathway and multi-link. In comparison to synthetic compounds, NPs offer lower toxicity, fewer adverse reactions, more diverse structures, and more abundant active sites. Studies have shown that NPs can inhibit tumor cell growth, angiogenesis, immune inflammation, metastasis, etc., via regulating TME constituents as well as their interaction with cancer cells, thereby suppressing the occurrence and progression of tumors. In particular, there is a growing body of evidence showing that bioactive dietary flavonoids play a pleiotropic role in many aspects of the TME via regulating PI3K/Akt, NF-κB, JNK/STAT, p38/MAPK, and VEGF pathways, suggesting that dietary flavonoids may have potential multi-target anti-tumor effects (Figure 1). Accordingly, NPs have gradually been deemed a treasure trove of anti-tumor drug development. In this review, we summarized the regulatory effects and the underlying mechanisms of dietary flavonoids that target the TME, supplying new therapeutic possibilities to overcome tumor diseases induced by multiple factors.
To date, lots of flavonoids are now under clinical trials to treat certain cancers, such as apigenin (NCT03139227), quercetin (NCT03493997, NCT03476330, NCT02989129, NCT01912820, NCT01538316, NCT00003365), and EGCG (NCT02891538, NCT01317953), implying their potential of these compounds in cancer treatment and prevention. As a chemo preventive agent, EGCG or green tea has been extensively examined in clinical trials for various cancers, such as gastric, prostate, colon, pancreatic, liver, lung, and breast cancer. In a pilot clinical study assessing the efficacy of EGCG capsules (400 mg day−1 in three divided doses for 2–8 weeks), the capsules were found to enlarge the efficacy of radiotherapy in breast cancer patients.[118] In regard to flavonoids, many compounds are therapeutic adjuvants that ameliorate the side effects of chemotherapy, but relative clinical trials have lacked exploration of the ability of flavonoids to strengthen or overcome resistance to chemotherapy agents in cancer. Meanwhile, TME is essential for regulating drug resistance, progression, and metastasis of malignant tumors. Reconstructions of TME and acquisition of drug resistance often occur as a consequence of reciprocal interactions between cancer cells and non-malignant cells. Recent studies uncovered dietary flavonoids sensitize drug-resistant cancer cells to chemotherapy and coordinate the effects of drugs in non-resistant cancer cells, which provide possibilities for overcoming drug resistance in the future. Quercetin overcomes ABT-737 resistance by inhibiting Mcl-1 protein expression and potentiates the effect of adriamycin against multidrug-resistant leukemia, supplying a therapeutic option to reverse the multidrug resistance in the future.[119] In addition, dietary flavonoids have also shown prodigious synergistic effects by combining flavonoids and current anti-cancer drugs. Erdogan et al. reported that naringin-paclitaxel combination therapy increases the protein expression of tumor suppressor PTEN and decreases the protein level of NF-κB p50 in human acute promyelocytic leukemia cells, contributing to improving the overall efficacy of cancer treatment.[120] However, the detailed molecular mechanism of flavonoid dietary compounds regulating drug resistance through TME is not yet fully understood, which limits the clinical development and application. Furthermore, additional studies indicated dietary flavonoids modulate one facet of the TME may not yield the desirable anti-tumor effects, thus the multi-target and multi-pathway targeting of TME could be a new therapeutic strategy. Overall, we think that flavonoids not only make the drug-resistant cancer cells sensitive but also exhibit a promising prospect as compounds for combination therapy. These studies provide crucial information for a new generation of treatment strategies based on flavonoids.
Acknowledgements
N.D. and X.H. contributed equally to this work and should be regarded as co-first authors. The work was supported by the Natural Science Foundation of Shanghai (No. 21ZR1427300), the National Natural Science Foundation of China (Grant No. 82173731), and the Shanghai Frontiers Research Center of the Hadal Biosphere.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Namin Duan a graduate student in the College of Food Science and Technology, Shanghai Ocean University. She focuses on identifying effective nutrients for cancer prevention and therapy.

Xiaohui Hu a graduate student in the College of Food Science and Technology, Shanghai Ocean University. She focuses on anti-tumor activity and molecular mechanisms of natural dietary compounds.

Ning Liu is an associate professor in the College of Food Science and Technology, Shanghai Ocean University. She earned her doctorate in Biomedical Sciences at East China Normal University in 2011. Now, she is a visiting scholar at Dana Faber Cancer Institute, Harvard Medical School. She focuses on investigating biological functions and molecular mechanisms of functional foods and their active ingredients, and identifying novel effective molecular agents from libraries of natural dietary compounds for cancer prevention and therapy.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




