Role of dietary pro-oxidants in the maintenance of health and resilience to oxidative stress
Abstract
The average length of human life is increasing, but so does the incidence of age- and lifestyle-related diseases. Improving diet and lifestyle is a key strategy for lifelong health and underlying mechanisms may well include increasing resilience pathways. The purpose of this review is to highlight and evaluate novel mechanisms by which dietary pro-oxidants, including bioactive phytochemicals and fatty acids, increase reactive oxygen species (ROS) concentrations just enough to activate transcription factor activation of nuclear factor erythroid 2 related factor 2 (Nrf-2) and heat shock factor (HSF), leading to an increase in levels of antioxidant enzymes and heat shock proteins that protect against the damaging effects of ROS. An increasing number of in vivo studies have now shown that dietary pro-oxidant compounds can increase the production of such resilience products. In most studies, dietary pro-oxidants normalized levels of antioxidant enzymes that were decreased by a range of different challenges, rather than raising levels of resilience products per se. Also, it is important to consider that the antioxidant response can be different for different organs. For future studies, however, the measurement of resilience markers may significantly improve our ability to prove the efficacy by which dietary bioactives with pro-oxidant capacities improve lifelong health.
Abbreviations
-
- ARE
-
- antioxidant response element
-
- HSF
-
- heat shock factor
-
- HSP
-
- heat shock protein
-
- Keap1
-
- Kelch-like ECH-associated protein 1
-
- Nrf-2
-
- nuclear factor erythroid 2 related factor 2
-
- ROS
-
- reactive oxygen species
1 Health and resilience
The ageing process occurs across the entire life course. It begins at conception and continues throughout infancy, childhood, adolescence, and maturity. Hence, early intervention to promote good health can take place at any stage in the life course. The key focus of early intervention is to slow down or prevent the development of debilitating health problems. Each stage in ageing has implications for the individual and for society, and we need to understand how problems can be prevented or mitigated. There are many potentially remedial factors and the components of an early intervention strategy could include, e.g., changing the behavior of individuals to make healthier choices and/or nutritional treatments and strategies. However, for this we would need improved information on the efficacy by which certain diets and their active components can improve health. There are three important questions that need to be addressed in considering health and the key motivators influencing healthy behavior. First of all, what is good health and how should it be measured? Second, what determines our resilience to disease? And finally, how can we intervene to improve resilience and maximize health?
2 Measuring health
Currently, most of us may argue that good health is merely being “free from disease or illness,” which we can only assess by measuring biomarkers of disease. The World Health Organization (WHO) defined health in 1948 as “a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity” 1. At the time, this definition was groundbreaking because of its breadth and ambition. However, in 1948 acute diseases presented the main burden of illness and chronic diseases led to early death. But disease patterns have changed. Chronic noncommunicable diseases, which include cardiovascular conditions, some cancers, chronic respiratory conditions, and type-2 diabetes, affect people of all ages, nationalities, and classes, and are now reaching epidemic proportions worldwide 2, 3. Indeed, ageing with chronic illnesses has become the norm. Such an increased prevalence of age-related diseases suggests that the WHO definition is outdated as it would leave most of us unhealthy most of the time 4.
Just as environmental scientists describe the health of the earth as the capacity of a complex system to maintain a stable environment within a relatively narrow range 5, it has been proposed that the formulation of health should be the “ability to adapt and to self-manage” 4, 6. In the physical domain, this would mean that when confronted with physiological stress, a healthy organism is able to mount a protective response, to reduce the potential for harm, and restore an (adapted) equilibrium 7. The instruments to measure health should then not only be, as is currently the case, assessment of the main prognostic and diagnostic biomarkers for chronic diseases, such as blood pressure, plasma lipids, and blood glucose 8. Such biomarkers are indicators for a disorder that has already developed. Instead, markers to measure health should relate to resilience, i.e. the ability to cope with stress, the ability to produce a protective response, and the ability to return to homeostasis after a stress challenge. In this case, resilience relates to prevent disease from happening in the first place, rather than being “free of disease.” Yet, there are currently few instruments for measuring aspects of health relating to an individual's capacity to cope and adapt, or to measure the strength of a person's physiological resilience 4.
3 Resilience pathways
Declining resilience to endogenous and exogenous stresses is considered a key aspect of aging-related disease development, causing a diminished ability to bounce back to homeostasis after being exposed to an acute challenge, such as illness, injury, or exertion. A growing body of evidence points toward reactive oxygen species (ROS) as one of the primary determinants of resilience and human pathologies. High levels of ROS are known to function as harmful products of aerobic metabolism, being an important cause of mitochondrial dysfunction 9. On the other hand, low levels of pro-oxidant molecules have been discovered to be beneficial to health by modulating transcription factor activation 10 (Fig. 1). This process is referred to as hormesis, an adaptive response of cells and organisms to a moderate (usually intermittent) stress. In the human body, ROS are metabolized by a series of antioxidant defense mechanisms using dietary derived or endogenously synthesized compounds. The purpose of these mechanisms is not to remove all ROS, but to control their levels to allow useful functions (i.e. defense and redox signaling) while minimizing oxidative damage 11. Two main cellular signaling pathways and molecular mechanisms that mediate hormetic responses involve the transcription factors nuclear factor erythroid 2 related factor 2 (Nrf-2) and heat shock factor (HSF).
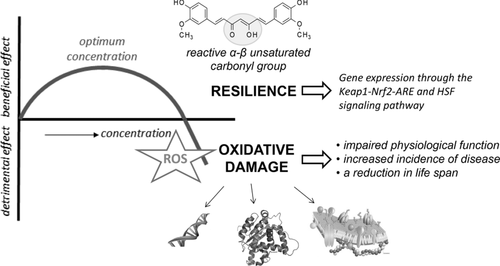
3.1 The Nrf-2 pathway
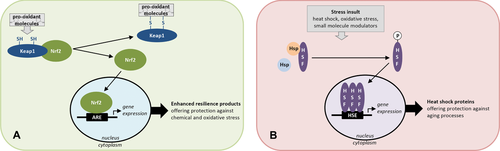
One of the main signaling pathways involved in the oxidative stress response is the Keap1-Nrf2-ARE (where Keap1 is Kelch-like ECH-associated protein 1 and ARE is antioxidant response element). Indeed, Nrf2 signaling is the major cellular defense to relieve oxidative and electrophilic stresses 12. In the absence of oxidative stress, Nrf2 protein is continually degraded in the cytoplasm by an E3 ubiquitin ligase complex containing the regulatory protein Keap1. Keap1 contains multiple cysteine residues that, when modified by oxidation or electrophiles, accelerate its dissociation from Nrf2. This change allows Nrf2 protein to accumulate in the cytoplasm and translocate into the nucleus where it binds to the ARE present in target gene promoters and enhancers, driving their expression (Fig. 2) 12. Upon binding, Nrf2-ARE regulates the expression of key protective enzymes, including xenobiotic metabolizing enzymes (glutathione-S-transferases and NAD(P)H:quinone oxidoreductase-1), antioxidant enzymes (heme oxygenase-1, superoxide dismutase, glutathione peroxidase, and catalase), and enzymes involved in glutathione metabolism (such as glutamate cysteine ligase). Furthermore, Nrf2 activates proteasomal and chaperone proteins, indicating that Nrf2 has an important role also in the regulation of reparation and removal of damaged proteins 13. The Nrf2 pathway may be the most sensitive pathway for the presence of thiol-modifying molecules such as ROS or electrophilic small molecules, as a result of the presence of multiple, highly reactive, functionally important cysteine residues in Keap1. Recent findings indicate that different electrophiles modify distinct cysteine residues within Keap1, referred to as the “cysteine code.” Many of the oxidized products that are able to bind and activate Keap1, contain an α,β-unsaturated carbonyl group that can form Michael adducts with the SH-groups of the Keap1 signaling protein 13. In addition, isothiocyanates and sulfhydryl reactive metals were found to be the strongest activators of Nrf2 in a reporter assay system 12.
An increasing number of in vivo studies have provided evidence that various dietary bioactive phytochemicals and fatty acids can increase the production of protective enzymes products, or resilience products, likely through the Nrf2 pathway (Table 1) 14. These dietary compounds are believed to increase ROS concentrations just enough to activate relevant transcription factor activation, such as the ARE-regulated response and/or the heat shock response (HSR), but not cell death pathways 15. In that respect it is important to note that the treatment doses in a number of the animal studies were high and not necessarily dietary relevant. For such pharmacological doses, it could be argued that the induced response is toxicological, rather than an induced resilience response (Fig. 1). An example of this was presented by Bak et al., where the higher dose of 100 mg 6-shogaol-rich extract per kilogram failed to restore reduced antioxidant enzyme expression induced by diethylnitrosamine (Table 1) 16. It is also important to consider that regulation of, e.g., the antioxidant response can be different—and sometimes in an opposite direction—for different organs, and may well depend on mode of administration (Table 1) 17, 18.
| Bioactive food or food component | Model | Design, dose, and exposure | Effects on resilience outcomes compared with control group | References |
|---|---|---|---|---|
| Polyphenols and phenolics | ||||
| Quercetin | Prostate cancer induced (MNU and testosterone treated) rats | Vehicle control, cancer-induced control, cancer-induced + quercetin (200 mg/kg BW/orally) and quercetin (200 mg/kg BW) three times a week for 16 wk |
|
43 |
| Quercetin and catechin | Wistar Unilever rats | Control flavonoid free diet or this diet enriched with quercetin or catechin (2 g/kg diet) |
|
45 |
| Catechin | Swiss albino mice | Tamoxifen treatment with or without pretreatment with catechin (40 mg/kg) | ↓ SOD production and TBARS in hepatic mitochondria | 80 |
| Curcumin | Inbred male Swiss albino mice | Control diet or this diet supplemented with 0.01 or 0.05% curcumin, for 16 days. Mice were given 1 mg B[a]P in corn oil or corn oil as vehicle by gavage on 15th day of dietary pretreatment |
|
81 |
| Curcumin | Kunming mice—arsenic toxicity model | Control mice or mice with arsenic (10, 50, and 100 mg/L drinking water) with or without curcumin (200 mg/kg) administrated by gavage, twice 1 wk for 6 wk |
|
23 |
| Curcumin | C57BL/6J/Nrf2−/− mice and WT C57BL/6J mice | Curcumin (1000 mg/kg, dissolved in 50% PEG 400 solution) or vehicle only by gavage. Mice were sacrificed 3 and 12 h after curcumin treatment or 3 h after vehicle treatment | Regulation of Nrf2 genes (involved in ubiquitination and proteolysis, electron transport, detoxification, transport, apoptosis and cell cycle control, cell adhesion, kinase and phosphatase, and many phase II detoxification/antioxidant enzyme genes) by curcumin in liver and small intestine of WT mice but not in knockout mice | 54 |
| EGCG | C57BL/6J/Nrf2−/− mice and WT C57BL/6J mice | EGCG (200 mg/kg, dissolved in 50% PEG 400 solution) or vehicle only by gavage. Mice were sacrificed 3 and 12 h after curcumin treatment or 3 h after vehicle treatment | Regulation of Nrf2 genes (involved in ubiquitination and proteolysis, electron transport, detoxification, transport, apoptosis and cell cycle control, cell adhesion, kinase and phosphatase) by EGCG in liver and small intestine of WT mice but not in knockout mice | 53 |
| Genistein | Wistar Unilever rats | Control flavonoid free diet or this diet enriched with genistein (2 g/kg), for 3 wk |
|
33 |
| Naringin | High fat diet—streptozotocin-induced type 2 diabetic rats | Control diet, high-fat control diet with naringin (0, 25, 50, or 100 mg/kg/day, by mouth) |
|
32 |
| Resveratrol | C57 BL6 mice—hyperlipidemia model | Chow or high-fat diet with or without resveratrol (0.1% w/w), minipump administration of vehicle or resveratrol at a flow rate of 0.25 μL/h to give a dosage of 100 mg/kg/day |
|
33 |
| Plants, plant extracts, or freeze-dried plant powder | ||||
| Anthocyanins from strawberry | Wistar rat—acute toxicity model | Control diet or this diet with a doxorubicin injection, a doxorubicin injection and freeze-dried cultivar Adria supplementation or a doxorubicin injection, and cultivar Sveva supplementation (both 10 g/100 g of strawberry lyophilized extract) for 16 wk |
|
24 |
| Green tea extract | C57BL/6 mice—PCB-induced toxicity model | PCB challenge (control) or this challenge with 1% w/w green tea extract |
|
25 |
| Welsh onion (Allium fistulosum L., Alliaceae) green leaves | ICR mice—inflammation model | Carrageenan-induced hind paw edema (control), intraperitoneally injected indomethacin (10 mg/kg), or Welch onion extract administered orally (0.25, 0.5, and 1 g/kg) for 2 h before the injection of carrageenan |
|
28 |
| Humulus lupulus L. (Cannabaceae, hops) | Sprague-Dawley rats | Control diet plus vehicle injection with sesame oil, control diet plus injection of pure xanthohumol (100 mg/kg BW/day); experimental diet containing 4′-bromoflavone (150 mg/kg BW/day) plus vehicle control, experimental diet containing powdered hop extract (7.5 g/kg BW/day) plus vehicle control injection |
|
82 |
| Seaweed extracts and unsaturated fatty acids from Ulva lactuca | Transgenic (B6C3-ARE-Tg) mice | Single dose of 200 μL (50 mg seaweed fraction/kg) administered by gavage |
|
83 |
| Bilberry (Vaccinium myrtillius L.) pomace extract | Healthy subjects and ileostomy patients | Single dose of 10 g bilberry extract corresponding to a total of 2.5 g anthocyanins |
|
84 |
| Green tea (Camellia sinensis, Theaceae) | ICR mice—oxidative damage model | Control group receiving orally distilled water daily with intraperitoneally administered olive oil (1 mL/kg BW) twice per week, five treatment groups receiving i.p. administered CCl4 (20% in olive oil) (1 mL/kg BW) twice per week of which one group received distilled water daily, one group received silymarin daily (200 mg/kg), and three groups were orally administered green tea powder dissolved in distilled water at doses of 125, 625, and 1250 mg/kg daily, for 8 wk |
|
20 |
| Phenolic compounds from Rosemary (Rosmarinus officinalis L.) | Wistar rats—hypercholesterolemia model | Control diet group, five hypercholesterolemic diet groups receiving water, aqueous rosemary extract (7 or 140 mg/kg BW), or nonesterified phenolic rosemary fraction (7 and 14 mg/kg BW) by gavage for 4 wk |
|
34 |
| Broccoli extract and the essential oils of turmeric, thyme, and rosemary | Wistar rats—model of inflammatory bowel disease | Control diet, or this diet supplemented with broccoli extract (8750 mg/kg broccoli sprouts extract), Curcuma longa oil (1494 mg/kg diet), Thymus vulgaris oil (618 mg/kg) or Rosmarinus officinalis oil (680 mg/kg) at pretreatment phase for 7 days, followed by DSS treatment of 6 days, and recovery of 6 days |
|
29 |
| 6-Shogaol-rich extract from ginger | Balb/c mice | Treatment with 6-shogaol-rich extract (10 and 100 mg/kg BW) or positive control silymarin (100 mg/kg BW), and challenged with diethylnitrosoamine (30 mg/kg BW) 3 days per week for 3 wk |
|
16 |
| Alperujo extract, hydroxytyrosol, and 3,4-dihydroxyphenylglycol | Vitamin E deficient Rowett Hooded Lister rats | Five groups on a vitamin E deficient diet for 10 wk and then this diet supplemented with either alperujo extract, hydroxytyrosol and dα-tocopherol (vitamin E) at a concentration of 100 mg/kg diet, or DHPG at a concentration of 10 mg/kg diet, or no additional supplement, for a further 2 wk. One intervention group was maintained on a vitamin E adequate diet (100 mg dα-tocopherol/kg) |
|
21 |
| Commercial preparation of phenols from olives, olive oil, and olive mill wastewater | Healthy subjects | Ninety-eight healthy subjects ingested 2 mL of a commercially available OMWW preparation and blood was drawn just before and 1 h after ingestion of the preparation |
|
50 |
| Turmeric and carrot seed extracts | Wistar rats | Control group and control group receiving 0.2 mL DMSO by gavages as vehicle. Four treatment groups receiving different doses of turmeric extract (100, 200 mg/kg BW) and carrot seed extract (200, 400 mg/kg BW) by gavage |
|
85 |
| Blackberry extract | Sprague-Dawley rats—oxidative damage model | Control group, control receiving CCl4 (1 g/kg), groups receiving CCl4 plus blackberry extract (100, 200, or 400 mg/kg, or blackberry extract (400 mg/kg) only. Oral administration every other day for 15 days |
|
22 |
| Strawberry extracts were obtained from Adria, Sveva, and Alba cultivars | Wistar rats | Control group, ethanol group receiving PEG 400 and then 1 mL of ethanol, positive control group received 100 mg/kg of BW of quercetin dissolved in 10% PEG 400, intervention groups received 40 mg/kg BW of strawberry crude extract dissolved in 10% PEG 400 per day, by gavage, for 10 days |
|
86 |
| Syzygium gratum (S. gratum) | C57BL/6J mice | Distilled water control, S. gratum aqueous extract (0.25 or 1 g/kg/day) once a day for 30 days |
|
35 |
| Grape pomace | New Zealand white rabbits—model of hyperlipidemia | Control diet, high cholesterol diet, or this diet supplemented with 0.2% grape seed extract, 0.2% grape peel extract, 10% grape seed powder, 10% grape peel powder, 0.1% grape seed extract, 0.1% grape peel extract, 5% grape seed powder, or 5% grape peel powder |
|
46 |
| Garlic and aged black garlic | db/db (+/+) C57BL/KsL mice—model of hyperlipidemia | Control diet or this diet containing 5% freeze-dried garlic or aged black garlic for 7 wk |
|
36 |
| Garlic extract | Sprague-Dawley albino rats—acute toxicity model | Arsenic-free distilled water, NaAsO2 in de-ionized water (5 mg/kg BW/day), and intervention group receiving NaAsO2 (5 mg/kg BW/day) immediately followed by garlic extract (20 mg/kg BW/day) for 5 days |
|
26 |
| Selenium and green tea extract | Kunming mice | Daily intragastric administration (0.6 mL) of saline (control); regular tea extract, selenium green tea extract (0.167, 0.333, and 0.669 μg Se/mL), regular tea plus selenite (0.333 μg Se/mL), selenite solution (0.333 μg of Se/mL), or 8, 5-fluorouracil, for 8 days |
|
87 |
| Edible artichoke (Cynara scolymus L.) | Wistar rats | Control diet or this diet supplemented with artichoke (138 g/kg of diet) |
|
88 |
| Broccoli seeds and isothiocyanates | Nrf2+/+ and Nrf2−/− mice | Control diet with broccoli seeds at 15% (by weight) for 7 days |
|
89 |
| Soy isoflavone | Wistar rats | Soy-deficient or soy protein diet for 12–16 months, or a soy-deficient diet for 10 months, switched to soy protein diet for 2 or 6 months |
|
90 |
| Isoflavone-rich soy isolate | C57BL6 | Control diet or this diet supplemented with 1.08 g isoflavone-rich soy isolate/kg diet for 60 days. The soy isolate contained 400 mg/g isoflavone aglycones (226 mg/g genistein and 174 mg/g daidzein) |
|
91 |
| Flaxseed | Wistar albino rats—acute toxicity model | Control diet with and without CCL4 challenge, and control diet supplemented with flaxseed (5 and 10%) for 14 days followed by single oral dose of CCl4 (2.0 g / kg BW) |
|
27 |
| White, brown, and germinated brown rice | New Zealand white rabbits—hyperlipidemia model | Control diet, high cholesterol diet (0.5 g/100 g) or this diet with 19.8% white rice powder, 19.0% brown rice powder, or 19.5% germinated brown rice powder |
|
37 |
| Fermented cowpea flour (Vigna unguiculata) | Albino Wistar rats | Casein–methionine control diet, raw or fermented V. unguiculata diets prepared with cowpea seed flour (75 g/kg BW) |
|
92 |
| Fructan from roots of Arctium lappa L. | d-Galactose (d-Gal) induced aging ICR mice model | Intraperitoneal injection of d-galactose (500 mg/kg/day) combined with oral administered of ascorbic acid (100 mg/kg/day) or Arctium lappa L. polysaccharide (100, 200, and 400 mg/kg/day) for 8 wk |
|
19 |
| Organosulfur compounds | ||||
| Diallyl trisulfide | C57BL/6 mice | 0.1 mL of sodium carboxymethyl cellulose vehicle by gavage or diallyl trisulfide (0.5 or 2 mg in sodium carboxymethyl cellulose per mouse) by gavage every other day for 2 wk |
|
93 |
| Brassica-derived isothiocyanate sulforaphane | C57BL/6 mice—DSS-induced acute colitis model | PBS (control) or 25 mg/kg BW of sulforaphane per os for 7 days. Subsequently, acute colitis was induced by administering 4% DSS via drinking water for 5 days |
|
30 |
| Sulforaphane | CD-1 mice—alcohol intolerance model | Control diet or this diet with sulforaphane (20 μmol per 3 g diet) for 7 days. After fasting overnight, they were gavaged with 35% v/v ethanol (2.0 g ethanol/kg BW) |
|
31 |
| Sulforaphane | Nrf2+/+ and Nrf2−/− mice | Exposure to 4.8 mg/m3 of the synthetic arsenic dust for 30 min/day. Sulforaphane was intraperitoneally injected (10 mg/kg) every other day for 14 days |
|
55 |
| Allyl-isothiocyanate and sulforaphane | C57BL/6 mice—hyperlipidemia model | High-fat diet and administration of allyl-isothiocyanate or PBS by gavage for 7 days |
|
39 |
| Diallyl sulfide | SD rats | Diallyl sulfide was given daily by gavage (100 or 500 mg/kg BW/day) for 7 consecutive days The positive control group was treated with NAC (500 mg/kg BW). The vehicle group was treated with soybean oil, and the group treated with ddH2O was the blank |
|
94 |
| Sulforaphane and sulforaphane–glutathione conjugate | TRAMP mice—model of prostate cancer | Control diet or experimental diet containing 2% or 8% broccoli sprouts for 16 wk |
|
44 |
| Fatty acids | ||||
| Dietary fatty acids | Metabolic syndrome patients | High-saturated fatty acid diet, high-monounsaturated fatty acid diet, and two low-fat, high-complex carbohydrate diets supplemented with n-3 polyunsaturated fatty acids or placebo for 12 wk, or given as fat challenge |
|
95 |
| Dietary fatty acids | Metabolic syndrome patients | High-saturated fatty acid diet, high-monounsaturated fatty acid diet, and two low-fat, high-complex carbohydrate diets supplemented with n-3 polyunsaturated fatty acids or placebo for 12 wk, or given as fat challenge |
|
96 |
| Fish oil | C57BL/6 mice | Control diet, safflower oil control diet, and fish oil diet for 3 wk. |
|
97 |
| Fish oil | ApoE–/– mice—hyperlipidemia model | Control diet and high-fat diet rich in fish oil or corn oil (200 g/kg) for 10 wk |
|
38 |
| Fish oil | Sprague-Dawley rats | Diets supplemented with saturated fat, fish oil, or control oil (17% by weight) for 4 wk |
|
98 |
| Oxidized fish oil | Healthy subjects |
|
99 | |
| Lycopene or fish oil | Healthy subjects | Three-month intervention with two 15 mg lycopene soft gel capsules daily, 1 g fish oil (1098 mg EPA + 549 mg DHA) capsule daily, or placebo capsules |
|
100 |
| cis9, trans11-conjugated linoleic acid | ApoE–/– mice—hyperlipidemia model | High-fat, high-cholesterol diet with 7% of fat w/w as linoleic acid (control, 7% of fat w/w as cis9, trans11-CLA, or 7% of fat w/w as trans10, cis12-CLA) |
|
40 |
| Extra virgin olive oil | Healthy subjects | Control group or consumption of extra virgin olive oil as only added fat plus an extra daily dose of 50 mL for 6 wk |
|
101 |
| 10-Nitro-oleic acid | WT angiotensin II-induced hypertension model and Cys521/ser sEH redox-dead knock in mice | Daily gavage with conjugated linoleic acid and sodium nitrate in 200 μL PEG400 for 5 days |
|
60 |
| Juices and alcohol | ||||
| Tropical fruit juices | Albino Wistar rats | Control diet with water or 100, 200, or 400 mg of tropical fruit juices (FA or FB) per kg BW for 4 wk |
|
102 |
| Fruit juices | Wistar rats | Control diet with tap water or fruit juices (grapefruit, apple, blackcurrant, apple/blackcurrant, orange) for 7 days, or quercetin control (0.1 g/kg BW) during last for days of study |
|
103 |
| Açaí juice | ApoE−/− mice—hyperlipidemia model | Control diet with or without 5% freeze-dried açaí juice powder |
|
41 |
| Red wine | Wistar rats—hyperlipidemia model | Low-fat diet control group, four high-fat diet groups receiving 770–1360 μL water, 800–1380 μL red wine with low antioxidant activity, 790–1170 μL red wine with intermediate antioxidant activity, or 820–1340 μL red wine with high antioxidant activity daily, by gavage, for 4 wk |
|
42 |
| Combination interventions | ||||
| Coffee, thyme, broccoli, rosemary, turmeric and red onion | EpRE-luc reporter mice | Vehicle with or without extract by gavage |
|
56 |
| EGCG plus sulforaphane | Nrf2−/− or C57BL/6J mice | Vehicle with or without sulforaphane (45 mg/kg) and EGCG (100 mg/kg) by gavage |
|
104 |
| Antioxidant supplements | Healthy subjects | Intake of two capsules per day, for 4 wk, with a 4-wk washout period in between active dose or placebo |
|
57 |
| Diet rich in various antioxidant foods, or kiwifruit diet | Healthy subjects | Diet rich in various antioxidant foods, or three kiwifruits per day, or a control diet, for 8 wk |
|
58 |
| Mixture of vitamins and minerals | C57BL/6 mice | Control diet with or without an antioxidant mixture containing β-carotene, vitamins C and E, selenium, and zinc |
|
18 |
- Summary excludes studies assessing the effects of herbal medicine, medicinal plants, or probiotics, or studies without an appropriate control group. These studies were extracted from a PubMed search on the August 5, 2014 using the search term (diet or food) and (antioxidant enzyme* or HSP70 or Nrf2 OR heme oxygenase or aldehyde dehydrogenase or soluble epoxide hydrolase) and “in vivo.” ALDH, aldehyde dehydrognase; B[a]P, benzo[a]pyrene; BW, body weight; CCL4, carbon tetrachloride; CAT, catalase; DSS, dextran sodium sulfate; GR, glutathione reductase; GST, glutathione-S-transferase; EGCG, epigallocatechin-3-gallate; EpRE, electrophile response element; γ-GCL, gamma-glutamylcysteine ligase; Gpx, glutathione peroxidase; HO-1, heme oxygenase 1; HSP70, heat shock protein 70; Keap1, kelch-like ECH-associated protein 1; NQO1, NAD(P)H dehydrogenase, quinone 1; Nrf2, nuclear factor (erythroid-derived 2)-like 2; PBMC, peripheral blood mononuclear cells; PBS, phosphate buffered saline; RBC, red blood cells; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substance; TRAMP, the transgenic adenocarcinoma of the mouse prostate; TXNRD1, thioredoxin reductase 1.
In most studies, dietary bioactives did not raise levels of these resilience products per se, but rather enhanced homeostasis and recovery by normalizing levels of antioxidant enzymes that were decreased by a range of different challenges, including the induction of aging 19, oxidative stress 20-22, acute toxicity 23-27, acute inflammation 28-30, alcohol intolerance 31, hyperlipidemia 32-42, or carcinogenesis 43, 44 (Table 1). Supplementation with bioactives often did not significantly modulate an antioxidant response in the absence of a challenge (Table 1). Inclusion of challenge tests in a study design has been a promising concept in the substantiation of efficacy 4 to test “the ability to adapt” to measurable oxidative damage.
A number of studies have indicated upregulation of the antioxidant response as well as direct antioxidant action as evidenced by lower levels of thiobarbituric acid reactive substances. However, others report modulation of the antioxidant response only (Table 1) 21, 33, 38, 45, 46. It has been suggested that the impact of a direct antioxidant action of polyphenols in humans may be minimal, and that the role of polyphenols in targeted transcriptional gene regulation, such as the Nrf2/Keap1 pathway, could be more important 47. For example, we found that the in vivo free radical scavenging properties of olive phenolics in alperujo, an olive production by-product, appeared relatively modest in a vitamin E deficient rat model. But proteomics and subsequent network analysis revealed that the olive phenolics regulated protein and activity levels of hepatic mitochondrial aldehyde dehydrogenase (ALDH2), a key enzyme of cardioprotection (Table 1) 48. Expression of many aldehyde dehydrogenases is regulated by Nrf2, but it is currently uncertain whether this is also the case for ALDH2 49. Another study by Visioli et al. found that a commercial extract containing phenolics from olives, olive oil, and olive mill wastewater did not affect plasma antioxidant capacity, whereas it did significantly increase total plasma glutathione concentrations. The authors suggested that such an effect could be modulated through the antioxidant response element (ARE) mediated increase in phase II enzyme expression, including that of gamma-glutamylcysteine ligase and glutathione synthetase (Table 1) 50.
The use of transgenic mice models has provided new insights in the role of Nrf2 in the antioxidant response. Nrf2–/– mice are seemingly normal, but show a decreased capacity to cope with oxidative insults 51. Genes that are lower in Nrf2-null mice compared with wild-type (WT) mice are those important in the detoxification of ROS, such as superoxide dismutase 1 and 2, catalase, and peroxiredoxin 1, as well as the genes for epoxide hydrolase-1, UDP-glucuronosyltransferases, and aldehyde dehydrogenases 52. Treatment of Nrf2–/– mice and WT mice with the green tea component epigallocatechin gallate, and the flavonoid curcumin revealed the capacity of these polyphenols to activate phase II detoxifying enzymes through the Nrf2 pathway 53, 54. Also, curcumin supplementation in WT mice resulted in increased expression of the detoxification enzymes glutathione-S-transferase, glutathione reductase, epoxide hydrolase, HO-1, catalase, and NQO1 in the liver, small intestine, and kidney tissues, which did not occur in Nrf2–/– mice (Table 1) 54. Likewise, exposure to arsenic-containing dust and sulforaphane resulted in increased expression of Nrf2, as well as its target genes NQO1, γGCS, and HO-1 in lung tissue of WT mice but not Nrf2 knockout mice (Table 1) 55. An elegant study in EpRE-luc reporter mice indicated that a combination extract made of coffee, thyme, broccoli, rosemary, turmeric, and red onion induced EpRE-mediated luciferase in lung and adipose tissue, indicating the important role of these dietary bioactives with respect to their NRf2/EpRE-inducing properties in an intact organism 56. This study also showed that treatment with dietary plant extracts led to a significant higher induction of the Nrf2 pathway as compared with pure compounds, indicating combinatorial effects of compounds found in whole foods 56. It is interesting to see, therefore, that significant effects on the antioxidant response in human intervention studies are found in studies applying interventions with combined bioactives foods or food components (Table 1) 57, 58.
Interesting new research has revealed that a combination of dietary unsaturated fatty acids and nitrates may give rise to a range of electrophilic nitro-fatty acids. These compounds can covalently bind to Keap1, leading to an induced expression of phase II gene expression, as well as inhibiting the enzyme soluble epoxide hydrolase 59, 60. Inhibition of the sEH enzyme prevents hydrolysis of the enzymes’ substrate epoxyeicosatrienoic acids, leading to accumulation of these potent anti-inflammatory and vasodilatory compounds. In Cys521Ser sEH redox-dead knock in mice, which are resistant to inhibition of sEH activity by electrophilic lipids, dietary intervention with linoleic acid and nitrite did not affect sEH activity, whereas the same diet reduced sEH activity in WT mice 60.
3.2 The HSR
Another important integrated stress signaling pathway triggered by endogenous stimuli or environmental stresses is the HSR. This is an ordered response to a loss of proteostatic control due to a wide array of acute and chronic stress conditions including heat, metabolic dysregulation, electrophiles, and exposure to inflammatory derived reactive species 61. HSR is regulated at the transcriptional level by HSFs, primarily by HSF1. The principal mechanism of activation of HSF1 is incompletely understood, but it is known that HSF1 is tethered by heat shock protein (HSP)90 in the cytoplasm in an inactive state. HSP70 and its co-chaperone HSP40 also suppress HSF1 activity. Upon activation, HSF1 undergoes multistep processing involving posttranslational modifications, nuclear enrichment, trimerization, and binding to heat shock elements (HSEs). This results in the induction of a number of HSPs each identified by molecular mass, e.g. HSP27, HSP40, HSP60, HSP70/HSP72, and HSP90 (Fig. 2). Many of the proteins function as chaperones, proteases, or other proteins essential for protection of the cell against proteotoxic stress 62, 63.
HSPs are stress sensors that are believed to play a critical role in the development of cardiovascular disease, insulin sensitivity, and longevity. Indeed, transgenic mice overexpressing HSP70 are protected against the damaging effects of ischemia 64, and high levels of human HSP70 have been associated with a low coronary artery disease risk, independent of traditional risk factors 65. Also, experimentally elevated levels of HSP72 specifically in muscle or globally in mice by genetic or pharmacologic means conferred protection against diet- and leptin-induced obesity and insulin resistance 66, while mice lacking HSP72 display glucose intolerance and skeletal muscle insulin resistance 67. Cellular stress resistance against inflammatory and metabolic stresses appeared critical for disease prevention and longevity in certain models. For example, upregulating proteostasis activities by the HSF-1 transcription factors resulted in an increased longevity of worms harboring misfolding-prone proteins 68. Although regulation of HSP proteins may not affect longevity in humans, it is believed that restoring or maintaining proteostasis should increase quality of life by delaying the onset or decreasing the impact of cardiovascular disease or type 2 diabetes 61.
Dietary compounds may have the potential to upregulate the HSR, although evidence is only available from a limited amount of in vivo studies (Table 1). Structure–activity relationships have shown that HSP induction requires the presence of a reactive α,β-unsaturated carbonyl group that can form Michael adducts with cellular nucleophilics, and to covalently bind to cysteine residues of proteins 69. We showed that cis9, trans11-conjugated linoleic acid, a fatty acid present in the lipid fraction of meat, milk, and dairy products or other foods derived from ruminant animals, significantly increased levels of five different posttranslationally modified forms of hepatic HSP70 in Apoe–/– mice. This increase coincided with a reduction in atherosclerotic plaque formation, a reduced inflammatory response, and improved insulin sensitivity 40. In the rat insulinoma cell line INS 1E, the plant phenolic, caffeic acid, the flavanone naringenin from citrus fruits, and the flavonol quercetin from fruits and vegetables induced Hsp70 gene expression, indicating a possible role of these phenolics in β cell survival during glucotoxicity 70. In high-fat diet streptozotocin induced type 2 diabetic rats, dietary naringin, a flavonoid from grapefruit, significantly decreased insulin resistance and dyslipidemia, in addition to increasing protein levels of HSP27 and HSP72 in the pancreas, liver, and kidney 32. It has been proposed that activation of HSP72 protects against obesity-induced hyperglycemia, hyperinsulinemia, and insulin resistance by inhibition of c-jun amino terminal kinase activation and κΒ kinase, which are critical inflammatory kinases in the development of insulin resistance and type 2 diabetes 66.
4 How can we intervene to improve resilience and maximize health?
Consumption of plant-based foods and beverages rich in phytochemicals is epidemiologically associated with a decreased risk of chronic and age-related disease development 71-73, although mechanisms of protection remain unclear. Much attention has focused on polyphenols that have powerful antioxidant activities in vitro as they scavenge a wide range of reactive oxygen, nitrogen and chlorine species, and chelate metal ions capable of promoting oxidation 11. However, many of these in vitro results may be artifactual due to the rapid oxidation of polyphenolic compounds in cell culture media, generating H2O2 and quinones/semiquinones 11, 74. Furthermore, some of the observed antioxidant effects in vitro may be less relevant in vivo, simply because plasma concentrations of polyphenols are usually very low, rarely exceeding 1 μmol/L. Moreover, polyphenols are rapidly metabolized in the liver and by colonic bacteria, and many of the metabolites are believed to have decreased antioxidant activity 75.
A large number of human intervention studies have been performed to assess the antioxidant effects of dietary polyphenols in vivo. Perhaps, the most important outcomes to consider would be urinary F2-isoprostanes and oxidized LDL, which are currently the most reliable in vivo disease biomarkers of lipid peroxidation 76. However, there is limited evidence that polyphenol-rich products modify these biomarkers in humans 47, 74, and furthermore, a causal relationship between these biomarkers and cardiovascular health has not yet been established 47. In addition, there is no substantive evidence that increased intakes of the classic dietary antioxidants vitamin C, vitamin E, and β-carotene decrease levels of oxidative damage in well-nourished people 11, despite their well-known antioxidant capacity preventing either polyunsaturated fatty acid oxidation or other events driven by free radicals 9. Beneficial effects of antioxidant vitamin supplementation on primary or secondary prevention of mortality in healthy participants and patients with various diseases also are equivocal 77. Indeed, β-carotene and vitamin E seem to increase mortality, although it should be said that many studies used pharmacological, rather than dietary doses 78. Consequently, it is unlikely that any potentially beneficial bioactive effects of polyphenols and certain vitamins are ascribed to direct antioxidant effects.
Instead, as we show in this review, transcriptional gene regulation may be more important in vivo, and the action of dietary bioactives, especially polyphenols, may indeed go beyond their antioxidant capacity 14. Indeed, enhancing resilience through moderate exposure to pro-oxidants, which can be obtained through the diet, may confer protection in a much more effective way by increasing the production of protective enzymes and proteins through regulation of the ARE-regulated and/or the HSRs. The resilience response would offer enhanced endogenous protection against any subsequent chemical and/or oxidative stress that may arise. Indeed, the resilience response is not just the intrinsic capacity of a system to respond to a stress. It is a system that can be “tuned” by external factors, such as habitual intake of moderate concentrations of dietary pro-oxidant molecules. In this respect, it is interesting to note that new therapeutic drugs based on a resilience response, such as SOD and GPx1 mimetics, are being developed to alleviate complications in diabetic patients who suffer from a decline in cellular antioxidant defenses 79.
5 Conclusion
Emerging new research suggests that exposure to the right dietary pro-oxidant molecules, in the right concentrations, and on a regular basis, can cause a sustained increase in cellular levels of protective enzymes, or enhanced resilience products. Increasing levels of such products by dietary means may protect against the damage induced by oxidative stress challenges, and increase health, although current evidence obtained from human intervention studies is not sufficient to design dietary strategies based on pro-oxidant capacity. Thus, analyzing levels of enhanced resilience products, such as enzyme activity levels of superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and glutathione-S-transferase, plasma levels of HSPs, urinary levels of epoxyeicosatrienoic acids, and expression of Nrf2 genes, as a measure of the strength of a person's physiological resilience, may be more effective than conventional disease biomarkers to establish the efficacy by which dietary compounds affect the “health” of an individual. Assessing the role of dietary pro-oxidants (rather than antioxidants) in the maintenance of health (rather than reduction of disease) may therefore improve our understanding of the protective mechanisms that underlie the beneficial effects of specific food bioactives. Ultimately, the use of novel resilience markers and signatures could significantly improve our ability to prove the efficacy by which pro-oxidant dietary bioactives improve lifelong health, as well as targeting those with lowest resilience as a way to make best use of limited healthcare resources.
Acknowledgments
This research is funded by the Scottish Government's Rural and Environment Science and Analytical Services Division.
The authors have declared no conflict of interest.




