Elaidic acid (EA) generates dysfunctional high-density lipoproteins and consumption of EA exacerbates hyperlipidemia and fatty liver change in zebrafish
Abstract
Scope
It is well known that trans-fatty acids have proatherogenic properties while HDL has antiatherogenic activities in plasma. However, there has been no report on the effects of trans-fat on the functional and structural properties of HDL.
Methods and results
To compare physiological properties, we synthesized reconstituted HDL (rHDL) containing stearic acid (18:0), oleic acid (18:1, cis), or elaidic acid (EA, 18:1, trans). An rHDL containing EA (EA-rHDL) showed loss of antioxidant ability and induced the highest uptake of oxidized LDL into human macrophages. EA-rHDL caused the strongest cellular senescence in human dermal fibroblast cells along with the highest production of inflammatory species in macrophages co-treated with fructose. Injection of EA-rHDL into zebrafish embryos resulted in acute embryonic toxicity with the lowest survivability. Consumption of trans-fat for 20 weeks resulted in remarkable hyperlipidemia, elevation of serum cholesteryl ester transfer protein activity, hepatic inflammation, and fatty liver changes.
Conclusion
Incorporation of EA impaired the beneficial effects of rHDL against atherogenesis. In zebrafish, EA-rHDL resulted in acute embryonic toxicity, and consumption of EA caused remarkable hyperlipidemia, inflammation, and fatty liver changes.
Abbreviations
-
- acLDL
-
- acetylation of LDL
-
- CETP
-
- cholesteryl ester transfer protein
-
- EA
-
- elaidic acid
-
- EA-rHDL
-
- rHDL containing EA
-
- FA
-
- fatty acid
-
- GOT
-
- glutamic oxaloacetic transaminase
-
- GPT
-
- glutamic pyruvic transaminase
-
- HCD
-
- high-cholesterol diets
-
- HDFs
-
- human dermal fibroblasts
-
- NBD-cholesterol
-
- 22-(N-7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino-23,24-bisnor-5-cholen-3beta-ol
-
- OA
-
- oleic acid
-
- PAGGE
-
- polyacrylamide gradient gel electrophoresis
-
- POPC
-
- palmitoyloleoyl phosphatidylcholine
-
- rHDL
-
- reconstituted HDL
-
- SA
-
- stearic acid
-
- TC
-
- total cholesterol
-
- TFA
-
- trans-fatty acid
-
- TG
-
- triglycerides
1 Introduction
Production of trans-fatty acid (TFA) containing foods are increased with fast speed in developed countries 1, 2. Many reports concerned that TFA has deleterious effects on human health in various aspects, mainly in cardiovascular disease 3, 4. The increase of TFA is directly related with acceleration of atherogenesis via high levels of LDL-cholesterol and low levels of HDL-cholesterol by increasing cholesteryl ester transfer protein (CETP) activity 5, 6. As well as risk of cardiovascular disease, TFA is related with metabolic disorders such as insulin resistance and diabetes 7, 8. High content of TFA is also associated with an increased risk of cancers 9, such as human breast and colorectal cancers 10, and teratogenicity 11, 12. As described above, the accumulating evidences indicate TFA has a detrimental functions for human health. World Health Organization recommended that daily TFA intake should not exceed 1% of the total energy intake 13. A meta-analysis revealed that 2% increase in energy intake from TFAs was associated with a 23% increase in the incidence of coronary heart disease 3. Elaidic acid (EA) is a common TFA (trans-9, C18:1) in related with oleic acid (OA, cis-9, C18:1). Although OA is enriched in monounsaturated fatty acid (FA) and known to have beneficial functions of human health, it has been known that EA have deleterious effect of cholesterol removal 14 and HDL-cholesterol 15.
It has been well known that HDL-cholesterol is inversely related with incidence of coronary heart disease. HDL is a protein and lipid complex in plasma, which exerts potent antioxidant, anti-inflammatory, and antiatherosclerotic activity. ApoA-I, major protein of HDL, has also several beneficial activity in HDL. Many researchers including our group reported that the quality of HDL highly depends on structural and functional correlations of apoA-I during aging process 16, 17. Modification of apoA-I is directly related with production of dysfunctional HDL, which has more atherogenic and inflammatory properties to exacerbate cellular senescence 18. Taken together, many reports from our research group and other group strongly suggest that the functionality of HDL is highly affected by its composition.
Although it has been well known that TFA is unhealthy and should be avoided, its influence to the lipoprotein metabolism is still remained unclear. Since the dietary TFA should be transported via blood stream, the interaction of TFA with lipoprotein should be investigated. Especially HDL in functionality and structure can be modified by the foreign molecules, such as TFA.
In this study, we compared the physiological functions of three types of 18 carbon FAs, stearic acid (SA, saturated FA), OA, and EA. Because the FAs are nonsoluble in water, we synthesized reconstituted HDL (rHDL) containing each FA with different molar ratio. Although they have the same carbon number with very similar structure, there is a possibility that their physiological roles in lipoprotein metabolism and embryo development are different.
2 Materials and methods
2.1 Materials
SA (18:0, cat no. S4751), OA (18:1, cis-9-Octadecenoic acid, O1008), and EA (18:1 trans-9-Octadecenoic acid, E4637) were purchased from Sigma (St. Louis, MO, USA).
2.2 Purification of plasma apoA-I
ApoA-I was purified from human plasma using ultracentrifugation and column chromatography following the method described previously 19.
2.3 Synthesis of rHDL containing FA
Discoidal rHDL containing the FA was prepared by the sodium cholate dialysis method 20, 21 using initial molar ratios of palmitoyloleoyl phosphatidylcholine (POPC):free cholesterol:apoA-I:FA:sodium cholate of 95:5:1:x:150, where x represents 1, 5, or 10. For example, the SA-rHDL (1:10) indicates the molar ratio of 95:5:1:10:150 for POPC:free cholesterol:apoA-I:SA:sodium cholate. The rHDL particles were used without further purification because they showed high homogeneity. Their sizes were determined using 8–25% native polyacrylamide gradient gel electrophoresis (PAGGE; Pharmacia Phast system, GE Healthcare, Uppsala, Sweden) to compare them with standard globular proteins (AmershamPharmacia, Uppsala, Sweden). Particle sizes of rHDLs were determined by native 8–25% PAGGE comparison with standard globular proteins (cat no. 17–0445–01, AmershamPharmacia). The relative migrations were compared via densitometric scanning analysis using a Gel Doc® XR (Bio-Rad, Hercules, CA, USA) with Quantity One software, version 4.5.2. The number of apoA-I molecules per rHDL particle, as well as the self-association properties of lipid-free proteins, was determined by cross-linking with BS3 using the method described previously 22 and then analyzing the products of the reaction by SDS-PAGGE on precasted 8–25% gradient gels (AmershamPharmacia).
2.4 Circular dichroism
The average α-helix content of proteins in lipid-free and lipid-bound states was measured by circular dichroism spectroscopy using a J-715 Spectropolarimeter (Jasco, Tokyo, Japan) as described in our previous report 16 and the α-helical content was calculated from the molar ellipticity at 222 nm 23.
2.5 Characterization of tryptophan fluorescence
The wavelengths of the maximum fluorescence of tryptophan residues in apoA-I protein was determined from uncorrected spectra obtained using an LS55 spectrofluorometer (Perkin-Elmer, Norwalk, CT, USA) in conjunction with the WinLab software package 4.00 (Perkin-Elmer) and a 1-cm path-length Suprasil quartz cuvette (Fisher Scientific, Pittsburgh, PA, USA) as in our previous report 16.
2.6 Inhibition of LDL oxidation
Fresh LDL was incubated with lipid-free or rHDL-associated apoA-I at a concentration of 2 μM in the presence of 10 μM CuSO4 for up to 3 h. During incubation, the quantity of conjugated dienes formed was monitored by following the absorbance at 234 nm (Abs234) and 37°C 24 using a Beckman DU 800 spectrophotometer (Fullerton, CA, USA) equipped with a MultiTemp III thermocirculator (Amersham, Uppsala, Sweden).
2.7 Acetylation of LDL
The acetylation of LDL (acLDL) was performed using saturated sodium acetate and acetic anhydride according to a previously described method 25. After acetylation and subsequent dialysis, the acLDL protein content was determined and filtered through a 0.22-μm filter (Millex; Millipore, Bedford, MA, USA). To visualize phagocytosis of acLDL, a fluorescent cholesterol derivative (22-(N-7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino-23,24-bisnor-5-cholen-3beta-ol (NBD-cholesterol), Molecular Probes N-1148; 70 μg of NBD-cholesterol/mg of LDL) was added to acLDL particles.
2.8 Cholesteryl ester transfer assay
An rHDL containing apoA-I and cholesteryl oleate was synthesized and the CETP reaction was measured as previously described method 26 using trace amounts of [3H]-cholesteryl oleate (TRK886, 3.5 μCi/mg of apoA-I; GE Healthcare).
2.9 LDL phagocytosis assay
Differentiated and adherent macrophages from THP-1 cells were incubated with 400 μL of fresh RPMI-1640 medium containing 1% FBS, 50 μL of the acetylated LDL (acLDL, 1 mg of protein/mL in PBS), and 50 μL of PBS or each rHDL (final 2 μM of apoA-I in PBS) for 48 h at 37°C in a humidified incubator. In prior to treatment, apoA-I protein concentration in the rHDL and lipid-free states was determined by Lowry–Markwell assay 27 and our group 28 with BSA as a standard. The uptaken LDL was visualized and THP-1 macrophage-derived foam cells were then observed and photographed using a Nikon Eclipse TE2000 microscope (Tokyo, Japan). The NBD fluorescence (Ex = 485 nm, Em = 535 nm) and dihydroethidium (DHE) fluorescence (Ex = 485 nm, Em = 616 nm) were quantified by Victor X4 multimode plate reader (Perkin-Elmer Inc., Waltham, MA, USA).
2.10 Cellular senescence assay
Primary human dermal fibroblasts (HDFs) were cultured in DMEM (Life Technologies, Gaithersburg, MD, USA). HDFs were plated in DMEM at 1 × 105 cells per 100-mm culture plate and cultured at 37°C in a 5% CO2 humidified incubator as described in our previous report 17. HDFs were exposed at passage 11 (approximately 40% confluence) to the indicated concentrations of trans-fat (0.1, 1, 10, or 100 μM) for 30 days with subculture to passage 18. The extent of aging and cellular SA-β-gal activity was compared as previously described 29 by comparing intensity and area of stained extent.
2.11 Western blotting
In order to compare delivery efficiency and stability of each rHDL containing apoA-I and FA, equal amounts (22 μg of protein) of cell lysate were loaded and electrophoresed on 15% SDS-PAGE gels and detected by anti-human full-length apoA-I goat antibody (ab7613; Abcam, Cambridge, UK) and donkey anti-goat IgG-horseradish peroxidase (SC2020; Santa Cruz Biotechnology, Santa Cruz, CA, USA) as secondary antibody (1:2000 diluted). Anti-human GAPDH antibody (AAS79585C; Antibody Verify, Las Vegas, NV, USA) and secondary antibody was goat anti-rabbit IgG-horseradish peroxidase (SC2004; Santa Cruz, Dallas, TX, USA).
In order to normalize the expressional level, anti GAPDH antibody was employed as internal standard. The same amount of protein (25 μg/lane) from each lysate was loaded after protein quantification using the Bradford reagent (Bio-Rad). The relative band intensity was compared via band scanning with a Gel Doc XR (Bio-Rad) using Quantity One software (version 4.5.2) after normalization to the band intensity of the GAPDH.
2.12 Zebrafish and embryos
Wildtype zebrafish (Linebrass, AB strain) and embryos were maintained according to standard protocols 30. Maintenance and experimental procedures for zebrafish were approved (YUHS 01–13–004) by the Committee of Animal Care and Use of Yeungnam University (Gyeongsan, Korea). The fish were maintained in a system cage at 28°C during treatment under a 14:10 h light:dark cycle.
2.13 Microinjection of zebrafish embryo
Embryos at 2 days postfertilization were individually microinjected using a pneumatic picopump (PV820; World Precision Instruments, Sarasota, FL, USA) equipped with a magnetic manipulator (MM33; Kantec, Bensenville, IL, USA) with a pulled microcapillary pipette (PC-10; Narishigen, Tokyo, Japan). To minimize bias, injections were performed at the same position on the yolk abdomen. Just prior to injection, each rHDL containing FA (1.4 ng of FA) was mixed with 50 nL of PBS in the presence of 13 ng of oxLDL. Following injection, live embryos were observed at 48 h under a stereomicroscope (Motic SMZ 168; Hong Kong) and imaged using a Motic Cam2300 CCD camera 31.
2.14 Consumption of TFA
High-cholesterol diets (HCD) containing cholesterol (final 4%, wt/wt) and each FA (final 0.58%, wt/wt in tetrabit) was prepared as our previous report 32. Each diet was made by soaking Tetrabit (47.5% crude protein; 6.5% crude fat; 2.0% crude fiber; 10.5% crude ash; vitamin A, 29 770 IU/kg; vitamin D3, 1860 IU/kg; vitamin E, 200 mg/kg; and vitamin C, 137 mg/kg; Tetrabit Gmbh D49304, Melle, Germany) in a diethyl ether solution of cholesterol (Sigma #C-3045) and each FA. We calculated the amount of FA in the diet (0.53 cal per 0.058 mg of FA) should be 2% of calorie in the total calorie of diet (25.65 cal/10 mg of tetrabit) in according to the formula.
Each group (n = 80) consumed the designated diet (20 mg of diet/day/fish), as shown in Table 1. The zebrafish were maintained at 28 ± 1°C under a 14:10 h light:dark cycle. After feeding for 20 weeks, blood (2 μL) was drawn from the hearts of adult fish and combined with 5 μL of PBS-EDTA (final concentration, 1 mM), then collected in EDTA-treated tubes. Plasma and histologic analysis was carried out as described in Supporting Information—supplemental methods.
| Groups (n = 40) | Weight (mg) | Weight (mg)/height (mm) | TC a (mg/dL) | TG b (mg/dL) | GOT c (Karmen/mL) | GPT d (Karmen/mL) | CETP e activity (% CE-transfer/4 h) | |
|---|---|---|---|---|---|---|---|---|
| ND f | Plain diet | 531 ± 49 | 13.5 ± 1.5 | 137 ± 3 | 246 ± 1 | 79 ± 3 | 59 ± 1 | 15 ± 1 |
| SA h | 572 ± 58 | 16.4 ± 1.2 | 183 ± 3 | 350 ± 9 | 99 ± 1 | 57 ± 1 | 23 ± 1 | |
| OA i | 563 ± 81 | 12.3 ± 0.9* | 138 ± 4* | 228 ± 1** | 135 ± 1** | 65 ± 1 | 36 ± 1** | |
| EA j | 582 ± 114 | 13.6 ± 1.8 | 157 ± 2 | 308 ± 1 | 169 ± 1 | 64 ± 2 | 47 ± 1 | |
| HCD g | Plain diet | 564 ± 75 | 14.1 ± 1.4 | 551 ± 5 | 449 ± 16 | 147 ± 4 | 67 ± 1 | 41 ± 2 |
| SA | 653 ± 123 | 15.0 ± 2.1 | 714 ± 19 | 527 ± 5 | 152 ± 2 | 65 ± 2 | 39 ± 1 | |
| OA | 542 ± 65* | 13.2 ± 1.3* | 423 ± 11** | 375 ± 6** | 157 ± 7** | 65 ± 1 | 26 ± 2* | |
| EA | 621 ± 93 | 14.9 ± 1.6 | 775 ± 2 | 642 ± 13 | 340 ± 17 | 67 ± 1 | 33 ± 2 | |
- Tetrabit ®: Tetrabit (47.5% crude protein, 6.5% crude fat, 2.0% crude fiber, 10.5% crude ash, containing vitamin A (29 770 IU/kg), vitamin D3 (1860 IU/kg), vitamin E (200 mg/kg), and vitamin C (137 mg/kg)).
- a Total cholesterol.
- b Triglycerides.
- c Glutamic oxaloacetic transaminase.
- d Glutamic pyruvic transaminase.
- e Cholesteryl ester transfer protein.
- f Normal diet.
- g High-cholesterol diet (4% cholesterol).
- h Stearic acid (0.58% in diet, wt/wt).
- i Oleic acid (0.58% in diet, wt/wt).
- j Elaidic acid (0.58% in diet, wt/wt).
- *p < 0.05; **p < 0.01 versus EA.
2.15 Statistical analysis
All data are expressed as the mean ± SD of at least three independent experiments with duplicate samples. Comparisons between results were made by Student's t-test using the SPSS program (version 12.0; SPSS, Inc., Chicago, IL, USA). Statistical significance was defined as p < 0.05.
3 Results
3.1 Synthesis of rHDL
All rHDLs containing FA were synthesized well without precipitation with particle size of 89–94 Å as shown in Fig. 1. Incorporation of each FA in the particle size induced decreased size of rHDL and alpha-helix content of apoA-I with dosage-dependent manner as shown in Supporting Information Table 1. With increasing molar ratio of FA, rHDL containing EA (EA-rHDL) showed the highest increase of smaller band (around 78 Å) as indicated by arrow head. The FA incorporation induced smaller particle size with increasing content of FA especially EA. Furthermore, cross-linking ability of apoA-I in rHDL by the BS3 cross-linker was much impaired under the highest molar ratio of apoA-I:FA, 1:10 (Fig. 1 and Supporting Information Table 1).

3.2 LDL oxidation and more uptake of oxLDL by EA-rHDL
SA-rHDL and OA-rHDL showed potent inhibition abilities against cupric ion-mediated LDL oxidation until 80 min of incubation, whereas EA-rHDL exacerbated LDL oxidation induced by cupric ion after 60 min (Supporting Information Fig. 1).
Native rHDL could inhibit uptake of oxLDL into macrophages from observation of NBD fluorescence as previously described (Supporting Information Fig. 2). EA-rHDL caused the most extent of oxLDL uptake with increasing FA content, dosage-dependent manner, while OA-rHDL showed the least uptake of oxLDL (Supporting Information Fig. 2A). This result suggests that SA-rHDL and OA-rHDL could inhibit uptake of oxLDL with the least production of reactive oxygen species (ROS).
DHE staining, in order to visualize ROS in the cell as red fluorescence, revealed that EA-rHDL-treated cell showed the strongest red intensity with dosage-dependent manner, while OA-rHDL-treated cell showed the least red intensity. Merged image showed co-incidental existence of uptaken NBD-cholesterol and ROS in the cell. These results suggest that EA caused more atherogenic and proinflammatory response, while OA showed antiatherogenic and anti-inflammatory properties (Supporting Information Fig. 2A and B).
3.3 EA-rHDL suppressed cellular uptake of apoA-I in the rHDL
After 48-h incubation with rHDL, immunodetection of apoA-I in the treated cell revealed that EA-rHDL-treated cell showed the least detection of apoA-I detection in all dosage as shown in Supporting Information Fig. 2C. Under 1:1 ratio, the apoA-I detection level of EA-rHDL treatment was decreased only 70% level in comparison with native rHDL treatment (lane N), while SA-rHDL- and OA-rHDL-treated cell showed 120 and 140% based on densitometric quantification. OA-rHDL- and SA-rHDL-treated cell showed higher level of apoA-I in cell lysate than EA-rHDL, indicating that EA might interfere uptake of HDL into the cell. Since PBS-treated cell showed no band of apoA-I, the detected apoA-I band was appeared from exogenously treated rHDL containing each FA.
3.4 EA-rHDL caused more ROS generation and cellular senescence
Fructose treatment caused more ROS production in THP-1 cell (photos in Fig. 2) via inflammatory response as our previous report, however, native rHDL suppressed it (graph in Fig. 2). Among the rHDL containing FA, OA-rHDL-treated cell showed the least ROS production, while OA-rHDL-treated cell showed the least ROS. This result indicates that anti-inflammatory activity of rHDL could be impaired by trans-fat (EA), while unsaturated FA (OA) did not.
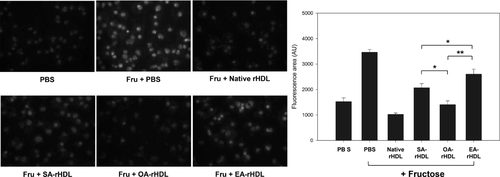
As shown in Fig. 3, EA-rHDL (final 2 μM of apoA-I and 17 μM of FA) caused the highest cellular senescence in the HDF cells at passage 13, while OA-rHDL caused the lowest senescence from SA-β-gal staining.

3.5 Survivability of zebrafish embryo
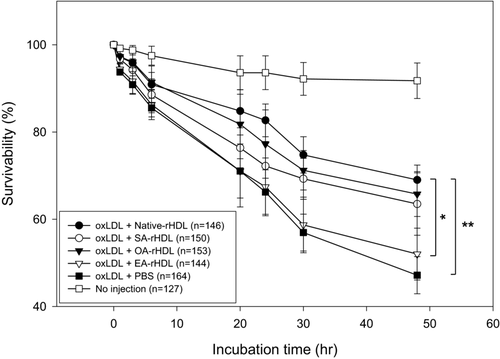
As shown in Fig. 4, injection of oxLDL caused the most severe embryo death around 47% survivability as similar with our previous report 31. Among rHDL, EA-rHDL showed the least survivability around 52%, while OA-rHDL-injected embryo showed 65% of survivability. As a control, native rHDL-treated embryo showed the highest survivability around 69%. There was no significant difference of survivability between OA-rHDL and SA-rHDL.
3.6 Serum profiles of zebrafish
After 20 weeks feeding of each FA (0.58% in diet, wt/wt), SA-fed group and EA-fed group showed 8 and 10% more body weight than control under normal diet (ND) supplementation, while OA-fed group showed 6% more body weight than control (Table 1). Under HCD supplementation, SA-fed and EA-fed groups showed 15 and 10% more gaining of body weight, respectively. Interestingly, OA-fed group showed even 4% smaller body weight than that of control (HCD alone diet), indicating that OA might exert antiobese effect.
Serum cholesterol and triglycerides (TG) were elevated by the HCD feeding around 4.0- and 1.8-fold more than ND (tetrabit) control as shown in Table 1. Under ND, SA-fed group showed the highest total cholesterol (TC) and TG levels with 33 and 45% more increase than control, while EA-fed group showed 14 and 25% more increase of TC and TG control. Under HCD, EA-fed group showed the highest increase of TC and TG levels although extent of body weight gaining was less than SA-fed group. The EA group showed 41 and 42% more increased serum cholesterol and TG level, respectively, compared with HCD control. SA-fed group showed the most increase of body weight and second highest TC level around 30% more than HCD control. Interestingly, OA-fed group exhibited the lowest level of serum TC level under both ND and HCD, indicating that OA exerted hypolipidemic effect. The OA-fed group showed 23 and 17% lower TC and TG levels, respectively, than control under HCD, while SA-fed group showed 1.3- and 1.2-fold higher serum TC and TG levels, respectively.
In hepatic inflammation parameters, EA-fed group showed the highest serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels, around twofold higher than control under either ND or HCD. Under HCD, all group showed similar GOT level around 150 Karmen/mL except EA group around 340 Karmen/mL, while GPT level was similar in around 65–67 Karmen/mL.
Under ND, serum CETP activity was around threefold increased by consumption of FA especially in EA-fed group, around 47% of CE-transfer activity. OA-fed group showed second highest CETP activity around 36%. Under HCD, control and SA-fed group showed higher CETP activity than OA-fed and EA-fed group, indicating that the serum CETP activity might be influenced by different FA consumption.
3.7 Fatty liver change
As shown in Fig. 5, histological analysis with hepatic tissue revealed that EA-fed group showed severe fatty liver change by oil-red O and hematoxylin staining under both ND and HCD, while OA-fed group showed the least oil-stained area. Among HCD group, OA-fed group showed the lowest oil-red O stained area, indicating that OA consumption can ameliorate the fatty liver change by the high cholesterol consumption.
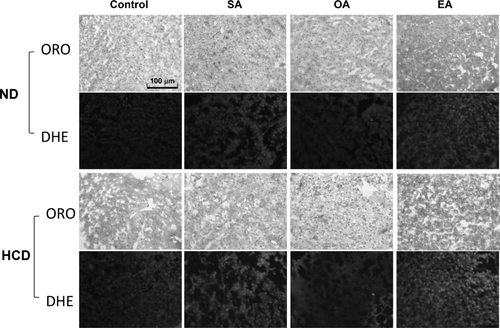
Malondialdehyde determination with the extract of hepatic tissue revealed that EA-fed group showed the highest production of MDA 39 and 32 μmol/mg of protein under ND and HCD consumption, respectively. However, OA-fed group showed the lowest MDA production among the FA group with 11 and 19 μmol/mg of protein (Supporting Information Fig. 3). These results suggest that EA consumption caused the most impairment of antioxidant activity in hepatic tissue with fatty liver change.
4 Discussion
Although detrimental effects of the dietary TFA in human health are well known, its effect on lipoprotein functions has not been fully understood. This study was designed to investigate interaction of the EA and apoA-I in rHDL. In contrary with SA and OA, incorporation of the EA into rHDL caused more production of smaller particle size of (78 Å) rHDL (Fig. 1 and Supporting Information Table 1). The smaller HDL is prone to be change into dysfunctional HDL with aging as our previous report 29. Interestingly, among the FA, EA-rHDL showed the weakest inhibition ability against the cupric ion-mediated LDL oxidation (Supporting Information Fig. 1). EA-rHDL caused more oxLDL uptake and acute inflammation in human macrophages (Supporting Information Fig. 2) with the highest production of intracellular ROS (Supporting Information Fig. 2 and Fig. 2) and the most extent of senescence (Fig. 3). EA-rHDL-injected zebrafish embryo showed the least survivability, indicating embryotoxicity (Fig. 4). Twenty weeks consumption of EA caused increase of serum TC, TG, and CETP activity (Table 1). EA-fed group showed elevated CETP activity and fatty liver change and increase of ROS (Fig. 5).
The elevated in vivo CETP activity (Table 1) in EA-fed zebrafish for 20 weeks makes a good agreement with the previous reports that the consumption of dietary TFAs enhanced plasma CETP activity in rat 33, monkey 34, and human 35. Interestingly, however, the OA-fed group showed the lowest serum CETP activity especially under HCD consumption. Similarly, Morton group reported that oleate showed inhibitory activity against CETP via perturbation of the lipoprotein surface 36.
Although deleterious effect of the TFA by feeding in animal model is well known, there has been no report about embryotoxicity by injection. As our previous report, dysfunctional HDL caused more rapid death with inflammation, while native apoA-I and rHDL exerted anti-inflammatory activity against lipopolysaccharide 31. As far as our knowledge, this is the first report that the TFA has embryotoxicity in vertebrates via direct injection into embryo.
After 20 weeks consumption, there was no induction of severe obesity in the EA group compared with SA group, while OA-fed group showed significant reduction of body weight compared with other group. Even though the EA group showed less increase of body weight than SA group, EA group showed the highest serum TG and GOT levels and fatty liver change. This result suggests that trans-fat consumption caused chronic inflammation and hepatic damage without remarkable increase of body weight. In the current study, our results also make a good agreement that the consumption of TFA induced elevation of CETP activity in zebrafish model (Table 1) with adverse effect of lipoprotein metabolism.
In conclusion, encapsulation of EA in rHDL induced smaller particle size with proinflammatory changes. In zebrafish, EA-rHDL showed acute embryo toxicity and consumption of EA caused remarkable hyperlipidemia, inflammation, and fatty liver change.
Acknowledgment
This work was supported by the Mid-carreer Researcher Program (2011-0015529) and Basic Science Research Program (2010-020910) through the National Research Foundation of Korea (NRF).
The authors have declared no conflict of interest.




