Acute effect of whey peptides upon blood pressure of hypertensive rats, and relationship with their angiotensin-converting enzyme inhibitory activity
Abstract
Scope: The aim of this study was to investigate the antihypertensive effect of a peptide fraction (PepC) obtained from a whey protein concentrate following hydrolysis by Cynara cardunculus, as well as of its fraction with MW below 3 kDa (PepCF). Both these concentrates encompassed peptides that exhibited potent in vitro inhibition of angiotensin-converting enzyme (ACE): two were released from α-lactalbumin – KGYGGVSLPEW and DKVGINYW, and one from β-lactoglobulin – DAQSAPLRVY.
Methods and results: Upon oral administration, by gastric intubation, of 400 mg/kg body weight (bw) of those peptide concentrates, or 5 mg/kg bw of the corresponding synthetic peptides, to 12 wk-old spontaneously hypertensive rats (SHR), the systolic and diastolic blood pressures were monitored by the tail-cuff method – before, and 2, 4, 6, 8 and 24 h afterwards. Water and zofenopril (5 mg/kg bw) – a known ACE-inhibitor, were used as negative and positive controls, respectively. Acute administration of PepC, PepCF, KGYGGVSLPEW, DKVGINYW and DAQSAPLRVY caused antihypertensive effects in SHR; the maximum effect occurred by 4 h and 6 h after administration of the peptide concentrates and the synthetic peptides, respectively. PepC and KGYGGVSLPEW also showed ACE-inhibitory activity in vivo: the pressor effect of angiotensin I was significantly lower, and the response to bradykinin increased when the rats were pre-treated with either product.
Conclusion: Our results strongly suggest that PepC will be effective as nutraceutical ingredient for the formulation of functional foods aimed at hypertension control.
Abbreviations
-
- ACE
-
- angiotensin-converting enzyme
-
- PepC
-
- peptide concentrate
-
- PepCF
-
- fraction of peptide concentrate with MW < 3kDa
-
- bw
-
- body weight
-
- DBP
-
- diastolic blood pressure
-
- IC50
-
- concentration necessary to inhibit 50% of ACE activity
-
- MBP
-
- mean blood pressure
-
- SBP
-
- systolic blood pressure
-
- SHR
-
- spontaneously hypertensive rats
1 Introduction
Hypertension is a major public health problem worldwide, usually associated with such other disorders as obesity, prediabetes, renal disease, atherosclerosis and heart stroke 1-4. Its specific treatment will reduce the risk of incidence of cardiac diseases, which currently account for 30% of all deaths 5. Blood pressure is regulated by distinct pathways – neurotransmitters, hormones and local factors; and its control is associated with the renin-angiotensin metabolic route 6, 7. Captopril, enalapril and lisinopril have been classically used as antihypertensive drugs acting essentially as angiotensin-converting enzyme (ACE)-inhibitors; they found a widespread use in treatment of patients with hypertension, heart failure or diabetic nephropathies 2, 8, 9. However, they bring about undesirable side effects, so safer (and, hopefully, less expensive) alternatives are urged 10, 11. Since diet has a direct relationship to hypertension, the food industry, in association with research and public health institutions, have focused on the development of novel functional ingredients that aid in maintaining a normal blood pressure – thus avoiding the need to take antihypertensive drugs 12-14.
There is indeed scientific evidence that certain peptides are able to reduce blood pressure 15-17. The underlying mechanisms do apparently relate to the interaction with opioid receptors, with concomitant release of nitric oxide – which causes vasodilatation, and so affects blood pressure; those peptides hold the advantage of no side effects, unlike happens with such other opiates as morphine 18. One example is α-lactorfin, a tetrapeptide derived from α-lactalbumin 19, 20, which may even chelate minerals, thus facilitating calcium absorption 21. It has been claimed that a diet rich in foods containing antihypertensive peptides is effective toward prevention and treatment of hypertension 15, 19.
ACE-inhibitory peptides may, in general, be obtained from precursor proteins in food by enzymatic hydrolysis, using viable or lysed microorganisms, or simply specific proteases 6, 12, 22, 23. Although in vitro studies are useful at screening stages, the efficacy and safety of such peptides always require in vivo testing – first in animals, and eventually in human volunteers 7. This issue is relevant because in vitro ACE-inhibition does not necessarily correlate with in vivo antihypertensive features, since peptides often undergo breakdown during gastrointestinal digestion that may hamper their physiological function. Conversely, antihypertensive activity may be promoted after a long-chain peptide precursor releases peptide fragments via the action of gastrointestinal enzymes 12. In the latest two decades, several ACE-inhibitor peptides – including some with antihypertensive effects in animals (e.g. spontaneously hypertensive rats, SHR, which are the animal model normally accepted to study human hypertension) and even in humans, have been found in such food products as eggs, plants, fish, meat and milk 6, 7, 11-13, 15, 19, 22, 23. However, milk proteins remain to date the chief source of ACE-inhibitor peptides.
ACE is an enzyme ubiquitous in tissues and biological fluids; it plays an important physiological role upon regulation of the cardiovascular function, including basic regulation of the peripheral blood pressure via the renin-angiotensin system 24-27. Consequently, ACE-inhibitors and angiotensin II receptor blockers 28-30 have represented important therapeutical developments, since they act as efficient drugs and cause very few collateral effects. ACE removes the dipeptide His-Leu of the carboxy terminus of angiotensin I, thus leading to formation of a powerful vasoconstrictor – the octapeptide angiotensin II 10, 31, 32. ACE also acts in the kinin-kallikrein system, by catalyzing cleavage of the dipeptide Phe-Arg carboxy terminal of the nonapeptide bradykinin – which is, in turn, a vasoactive agent 7, 10, 32. ACE-inhibitory drugs exert an antihypertensive effect by blocking the activation cascade of the renin-angiotensin system, so hindering formation of angiotensin II and efficiently preventing bradykinin degradation.
Our study was performed with SHR – one of the best known experimental models for antihypertensive studies, because those animals exhibit vascular reactivity and renal function similar to those in human beings 33. Several results with SHR have indeed been described, which attained successful control of their arterial blood pressure following oral administration of hydrolyzates or/and plain peptides derived from milk proteins 6, 13, 16, 17, 19, 20, 32, 34, 25, 36-38. Hence, a deeper study of these peptides appeared interesting, in attempts to find alternative agents for hypertension treatment and control 10, 39.
The major objective of this work was accordingly to investigate the antihypertensive effects in SHR of a single oral administration of two peptide concentrates, obtained from hydrolysis of whey brought about by cardosins. These are aspartic proteases of the flowers of Cynara cardunculus – a plant related to the common globe artichoke, which can cleave whey proteins at specific and less usual sites, i.e. next to hydrophobic amino acid residues (especially Phe, Leu, Thr and Tyr 40). Another objective was to check a similar effect of three peptides present therein, but obtained by chemical synthesis – and previously proven in vitro to be ACE-inhibitors: α-lactalbumin f(16–26), KGYGGVSLPEW; α-lactalbumin f(97–104), DKVGINYW; and β-lactoglobulin f(33–42), DAQSAPLRVY. The in vivo effects of peptide concentrate (PepC) and KGYGGVSLPEW upon blood pressure changes induced by angiotensin I and bradykinin were also investigated.
2 Materials and methods
2.1 Feedstocks and chemicals
PepC (containing ca. 73% w/w protein) and its fraction below 3 kDa, PepCF (fraction of peptide concentrate with MW <3 kDa, containing ca. 43% w/w protein) was obtained from hydrolysis of a bovine whey protein concentrate, via a commercial crude aqueous extract of C. cardunculus (Formulab, Maia, Portugal). DKVGINYW, DAQSAPLRVY and KGYGGVSLPEW, previously found in these peptide concentrates, were chemically synthesized on demand. Both were dissolved in ultra-pure water; a detailed account of their preparation and composition is available elsewhere 41. Angiotensin I and bradykinin were purchased from Sigma Chemical (St. Louis, MO, USA). Stock solutions used ultra-pure water as the vehicle, and were stored at −20°C; appropriate dilutions were made on a daily basis. Zofenopril was kindly supplied by Menarini Ricerche (Florence, Italy).
2.2 Animals and feed
All procedures were carried out according to the European Union guidelines for ethical care and use of laboratory animals, under the supervision of author R. Carrón (an officially accredited expert in animal experimentation – ref. 20060460006955, by Consejeria de Agricultura y Ganadería of Junta de Castilla y Léon, Spain). Male SHR (Elevage Janvier, Le Genest Saint Isle, France) were housed in groups of four rats per cage, and kept under controlled environmental conditions (24°C, and 12 h light/dark cycle) – in a standard experimental laboratory of the Animal Experimentation Service of Salamanca University (NPAE SA001). Rats were supplied with a solid standard diet (Global Diet 2014, Harlan, France) and tap water ad libitum, during the whole duration of the experiment.
2.3 Measurement of in vivo blood pressure
Male, 12 wk-old SHR – weighing 320±15 g (n=6–10 per experiment) were employed. The antihypertensive activity brought about by PepC and PepCF (administered at 400 mg/kg body weight (bw)) and by three synthetic peptides – DKVGINYW, DAQSAPLRVY and KGYGGVSLPEW (at 5 mg/kg bw), were all assessed. Rats fasted for 12 h before the experiment, but received a solution of 8% w/v sucrose in 0.2% w/v NaCl to avoid excessive dehydration. The rats were then given single doses of the aforementioned peptide concentrates or pure peptides (as appropriate), dissolved in ultra-pure water, by gastric intubation. The same plain vehicle was used as the negative control, whereas a known ACE-inhibitor – zofenopril (at 5 mg/kg bw), dissolved in ultra-pure water, was used as the positive control.
Both systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in awake rats, using the CODA tail-cuff blood pressure system (Kent Scientific, USA) – before, and by 2, 4, 6, 8 and 24 h post-administration. This is a non-invasive blood pressure measuring system, which was clinically validated to assess blood pressure in mice and rats; it determines the tail blood volume, using a volume pressure recording sensor and an occlusion tail-cuff. Before taking any measurement, the rats were kept at 38°C for 15–20 min, to make the pulsations of the tail artery detectable. The values of SBP and DBP were taken as the average of 8–12 readings; the results were expressed as the difference between the value of these variables before and after product administration, measured at the various experimental times. To minimize stress-induced variations in blood pressure, the same person took all measurements under the same conditions – using a stable room temperature, and avoiding loud noise and extraneous odors. The rats underwent a training period of 1 wk, to assure reliability of the measurements.
2.4 Measurement of in vivo ACE-inhibitory activity
Male, 14–16 wk-old SHR – weighing 350±15 g, were used in this study. The in vivo ACE-inhibitory activities of PepC and KGYGGVSLPEW were assessed following Fuglsang et al. 42, with modifications. Rats were divided into four groups (n=6–8 per group) – which received PepC (at 400 mg/kg bw), KGYGGVSLPEW (at 5 mg/kg bw), zofenopril (at 5 mg/kg bw) and ultra-pure water (negative control); zofenopril was used to confirm that the model responds well to ACE-inhibitors.
The tail-cuff blood pressure was measured before and after 3 h (in the case of PepC, zofenopril and negative control) and after 5 h (in the case of KGYGGVSLPEW) post-administration. Afterwards, rats were anaesthetized with sodium pentobarbital (at 60 mg/kg bw, i.p.), and fixed in dorsal decubitus; the right jugular vein and the left carotid artery were cannulated for administration of the drugs and for recording of blood pressure, respectively. Typically, the animals were allowed to stabilize for 10–20 min prior to injection. Dose–response curves of both angiotensin I (at 0.02, 0.04, 0.075 and 0.150 μg/kg bw) and bradykinin (at 2.5, 5.0 and 10 μg/kg bw) were obtained with intervals of 3–5 min between injections. The blood pressure was measured using a pressure transducer (Abbot, Ireland), and recorded on a PC computer using the LabChart v. 3.4 software and a PowerLab/800 data acquisition system (AD Instruments, Oxford, UK). The results were expressed as differences of mean blood pressure (MBP) before and after product administration.
2.5 Statistical analyses
The changes in the measurement of SBP, DBP and MBP were expressed as mean±SEM, before and after administration of the various test compounds. The results produced by a minimum of six experiments were then analyzed using the Prism v. 5.0 software (GraphPad, CA, USA). Statistical significance of the differences found between control and treated groups was ascertained via Student's t-tests; a p-value of less than 0.05 was considered as statistically significant.
3 Results and discussion
3.1 In vivo blood pressure
The antihypertensive effect of the products analyzed was measured during a 24-h period, by tail-cuff pressure measurements taken after a single oral administration. Any putative errors arising from the stress of heating and restraint associated with the tail-cuff pressure device were eliminated by careful control of the equipment performance, coupled with a standard pattern of interaction researcher/animals.
As shown elsewhere 41, the peptide concentrates – both obtained via C. cardunculus-mediated hydrolysis of whey proteins (PepC and PepCF), were present at protein concentrations sufficient to inhibit 50% of ACE activity (IC50): 52.9±2.9 μg/mL and 23.6±1.1 μg/mL, respectively. Three potent ACE-inhibiting peptides, previously found in both PepC and PepCF – DKVGINYW, DAQSAPLRVY and KGYGGVSLPEW, exhibited IC50 values of 25.2±1.0 μg/mL (25.4 μM), 13.0±1.0 μg/mL (12.2 μM) and 0.8±0.1 μg/mL (0.7 μM), respectively. The ACE-inhibitory activity of these peptides can be rationalized by the substrate specificity for ACE; Tavares et al. 41 claimed that the strongest ACE-inhibiting effect is associated with the three C-terminal amino acid residues, which should be hydrophobic 15, 43, 44 – or, more specifically, with Pro, Trp, Tyr, Phe and Leu as the final residues 45-47, and/or Pro as the second before the final one 48.
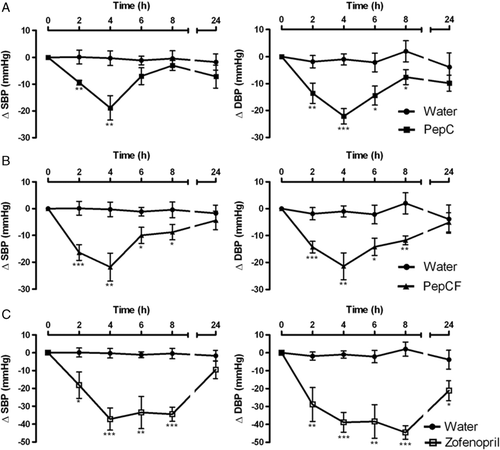
Just prior to oral administration of the various active agents, all pressure values were consistent with SHR, and the basal SBP and DBP were similar for all groups (Table 1). Before and throughout the whole experiment, oral administration of water (used as the negative control) did not affect the values of SBP and DBP (Figs. 1 and 2); however, administration of PepC and PepCF (at 400 mg/kg bw) caused a clear decrease in SHR blood pressure (Table 1, Fig. 1A and B). The consequences of the treatments throughout experimental time were similar in pattern; the largest decreases were attained, in both cases, by 4 h after administration, with blood pressure falling to ca. 21 mmHg. These results were apparently not in agreement with what would be expected from a comparison of PepC with its fraction below 3 kDa. Note, however, that the protein content of PepCF (43% w/w) was almost one half of that of PepC (73% w/w); if the effects on SBP had been ascertained for the same protein dose, the SBP decrease induced by PepCF should actually be higher than that by PepC. (Although the protein contents of PepC and PepCF were known in advance, the same overall amount rather than a protein level-based dose was used because the largest amount that can be added in industrial formulation of functional foods is more relevant than its specific activity on a protein basis.) It is well known that small peptides – more concentrated in the lower MW fractions, are likely responsible for ACE inhibition, and are also easily absorbed through the intestine in their intact form 7, 49, 50. Therefore, the similarity of blood pressure decrease caused by both concentrates could be accounted for by different protein contents – yet it cannot be fully ruled out that PepC contains other peptides than PepCF, also bearing some biological activity that might directly or indirectly modulate blood pressure. In any case, the maximum decreases in SPB and DBP are of the same order of magnitude as the maximum decrease of SBP reported by other researchers using also dairy mixtures 16, 20, 34, 36, 51.
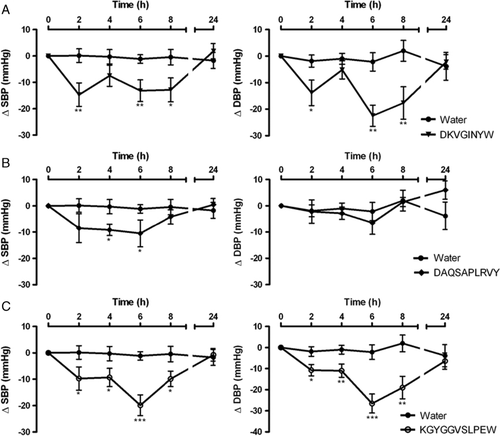
| Treatment | n | Before administration | After administration | |||
|---|---|---|---|---|---|---|
| SBP (mmHg) | DBP (mmHg) | Time (h) | SBP (mmHg) | DBP (mmHg) | ||
| Water | 7 | 176±3 | 128±2 | 4 | 175±3 | 127±3 |
| PepC | 7 | 169±5 | 132±5 | 4 | 150±7* | 110±6** |
| PepCF | 10 | 178±3 | 136±3 | 4 | 156±6** | 115±5** |
| Zofenopril | 6 | 179±5 | 138±6 | 4 | 142±4*** | 100±2*** |
| DKVGINYW | 6 | 166±3 | 125±4 | 6 | 151±4** | 102±6** |
| DAQSAPLRVY | 6 | 168±3 | 118±4 | 6 | 158±3* | 111±4 |
| KGYGGVSLPEW | 7 | 173±4 | 125±5 | 6 | 153±4** | 98±6** |
- Unpaired Student's t-test was used to compare groups: *p<0.05, **p<0.01, ***p<0.001 versus control.
The decrease in SPB and DBP observed when PepC or PepCF were administered was less pronounced than that caused by 5 mg/kg bw zofenopril – known as a potent ACE-inhibitor 52, which was accordingly used as the positive control. The time-course changes of SBP and DBP, after oral administration of zofenopril, are depicted in Fig. 1C: a statistically significant effect was noted by 2 h after administration. In all experiments, the maximum decrease of ca. 40 mmHg remained for at least 8 h post-administration.
As mentioned previously, the time-course changes in SBP and DBP after oral administration of DKVGINYW, DAQSAPLRVY and KGYGGVSLPEW were also monitored (Fig. 2); these peptides were obtained by chemical synthesis for the sake of purity (and to circumvent the complexity in composition of their source hydrolyzates), and had already been found in PepC and PepCF 41. The maximum decrease in SBP and DBP was observed with peptides DKVGINYW and KGYGGVSLPEW, by 6 h following administration; their effect lasted for at least 8 h. The performance of DKVGINYW and KGYGGVSLPEW upon decreasing arterial blood pressure was greater than that of DAQSAPLRVY – which exhibited a slight, but significant decrease in SBP (Fig. 2 and Table 1). A number of authors 6, 13, 19 reported antihypertensive effects of milk protein-derived ACE-inhibitors, showing a maximum SBP decrease in the range 3–34 mmHg. Two particularly potent ACE-inhibitory peptides, IPP and VPP, were found a few years ago, which are characterized by IC50 values of 5 and 9 μM 37, 38 – so they were meanwhile included in commercial products (Calpis® and Evolus®, respectively). Contreras et al. 34 reported that, under the same experimental conditions, the maximum SBP decrease in SHR was obtained after a 5 mg/kg bw single oral administration of VPP; this was similar to our results pertaining to the same level of KGYGGVSLPEW. ACE-inhibitory peptides originally present in our peptide concentrates, or shorter forms generated via gastrointestinal digestion can be efficiently absorbed through the animal intestine, thus remaining systemically active. Comparing these results with those obtained elsewhere relating to simulated gastrointestinal digestion 41, our in vivo results fully confirm them – which pointed at KGYGGVSLPEW as the best of the three peptides at stake. Although partially hydrolyzed, the fragments released via digestion of such peptides maintained its in vitro original ACE-inhibitory activity. For the other two peptides, in vitro digestion led to partial and full hydrolysis, of DKVGINYW and DAQSAPLRVY, respectively; DKVGINYW decreased, while DAQSAPLRVY kept its ACE-inhibitory activity. Therefore, it was expected that the in vivo antihypertensive effect of DAQSAPLRVY would be better than that of DKVGINYW – but this was not observed. Once again, in vitro studies provide a preliminary picture of the in vivo results to be expected, but the latter should always be obtained to validate beyond doubt the bioactivity under scrutiny.
As already noted with the peptide concentrates, the synthetic peptides yielded a clear decrease in blood pressure – which was, nevertheless, less pronounced than that associated with zofenopril (see Figs. 1 and 2). This is not at all surprising, since that drug is a potent ACE-inhibitor – with an IC50 value of 6–80 nM 52. However, when compared with zofenopril, our peptides possess higher in vivo activities than those expected based solely on their in vitro ACE-inhibitory activity. This might be rationalized by their higher affinity to the various tissues and their slower elimination – possibly complemented by supplementary mechanisms, including opiate-like, vasorelaxant or antioxidant routes 14, 18. For all products tested, SBP and DBP returned to the basal values by 24 h after administration.
Therefore, our work showed that both peptide concentrates and synthetic peptides possess antihypertensive activity in vivo; hence, the most promising peptide concentrate (PepC) and its active peptide (KGYGGVSLPEW, in synthetic form) were selected for further studies.
3.2 In vivo ACE-inhibitory activity
Recall that the antihypertensive effect of food-derived peptides has been frequently explained based on their ACE-inhibition mechanism – but only a few reports have demonstrated an equivalent in vivo ACE-inhibitory activity thereof 6, 10, 42, as other mechanisms of antihypertensive action rather than ACE inhibition may come into play 51. Therefore, animal models are crucial to assess the actual bioactivity of peptides. As stated above, this may be explained by the physiological transformations undergone by peptides from ingestion and until they reach the target organ: peptides may be digested, thus releasing smaller fragments that can be devoid of activity unlike their precursor peptides; conversely, they may exhibit an even higher ACE-inhibitory activity.
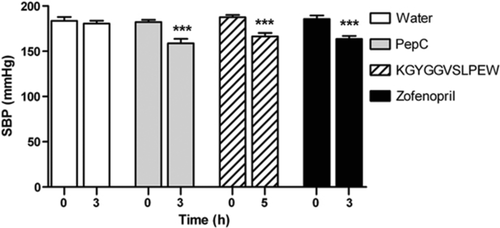
The method of recording blood pressure in anesthetized animals is convenient and simple, and allows quantification of action of the major mediators and antagonists in the autonomous nervous system, in the renin-angiotensin system, and upon substances derived from the endothelium. If the effect on blood pressure occurs through the central nervous system, our method presents some limitations. Previous to anaesthesia, the blood pressure was measured in awake SHR by the tail-cuff method. All animals showed similar values before administration; PepC and zofenopril after 3 h, as well as KGYGGVSLPEW after 5 h of administration then produced significant decreases in SBP – i.e. −20±2.9 mmHg for PepC, −22±3.8 mmHg for KGYGGVSLPEW, and −20±3.7 mmHg for zofenopril (Fig. 3). After anesthesia, the blood pressure measured in the carotid artery did not convey differences between control and treated groups (data not shown). This might raise a problem in attempts to assess the effects on the systemic arterial pressure; however, this was not the case here, because changes produced by angiotensin I and bradykinin can be evaluated under similar conditions of basal pressure.
A representative record illustrating the dose–response effect of angiotensin I and bradykinin upon blood pressure in anaesthetized rats is shown in Fig. 4. Exogenous administration of angiotensin I produces a hypertensive response, because it is converted to angiotensin II via ACE-mediated cleavage – which then constricts arteries, thus elevating blood pressure. Conversely, bradykinin is a potent vasodilator that is inactivated by ACE, thus resulting in a transient hypotensive effect. The rat group that had received zofenopril experienced lower responses to angiotensin I and higher responses to bradykinin than the control (Fig. 5). Since ACE is responsible for bradykinin degradation, and is involved in formation of angiotensin II from angiotensin I, this indicates that our model can be used to anticipate the effects on ACE inhibition in vivo. The pressor responses to different doses of angiotensin I in animals treated with PepC was similar to those in the control group, whereas KGYGGVSLPEW was able to significantly inhibit the response of animals to injection of angiotensin I (Fig. 5A).

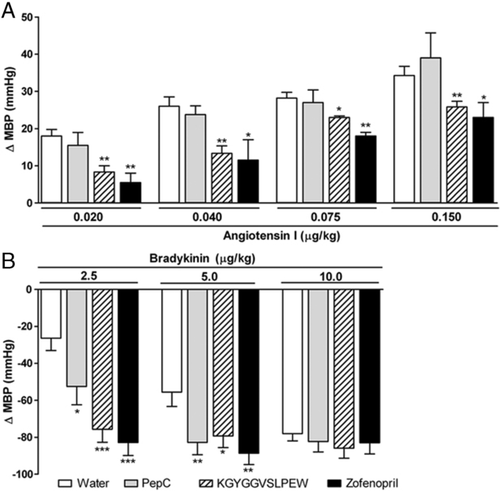
Both PepC and KGYGGVSLPEW enhanced the hypotensive response of bradykinin, with the latter proving essentially similar in potency to zofenopril (Fig. 5B). It should be emphasized that the pressor response to angiotensin I requires a much higher dose of ACE inhibitor than required to potentiate the depressor effect of bradykinin 53. Therefore, it is logical that PepC – a less potent ACE inhibitor, will only be able to potentiate the vasodilating response to bradykinin. The ACE protein has two catalytic domains, and some peptides (viz. bradykinin) are hydrolyzed at approximately the same rate by said catalytic domains – whereas in others (e.g. angiotensin I) the C-domain undergoes hydrolysis at a rate threefold that by the N-domain; hence, the former accounts for most angiotensin II production 54. Since zofenopril and KGYGGVSLPEW exhibited an effect upon angiotensin I and bradykinin responses, this might indicate inhibition of both domains – whereas PepC (which was only effective against bradykinin) would be more efficient in blocking the N-domain.
Despite the lack of inhibition of angiotensin I, the effect on bradykinin – supported by the PepC antihypertensive activity, leads one to conclude that ACE-inhibitory activity is (at least) one of the mechanisms of antihypertensive action of the peptide concentrates studied.
4 Concluding remarks
It was demonstrated here, for the first time, that PepC and PepCF, as well as the peptides KGYGGVSLPEW and DKVGINYW entail an antihypertensive effect in vivo, upon oral administration to SHR by gavage. Those peptide concentrates caused a similar decrease in SBP (ca. −20 mmHg); however, PepC offers more added value than its fraction below 3 kDa in terms of tentative commercial product, owing to lower production costs. The ACE-inhibitory activity of PepC was confirmed in vivo; it potentiates the bradykinin hypotensive effect. Although the observed bioactivity was contributed by three peptides found in such peptide concentrate, KGYGGVSLPEW exerted a particularly significant antihypertensive activity – even though it did not remain intact during gastrointestinal digestion; the inhibition of ACE was confirmed in vivo. Furthermore, the antihypertensive effect associated with peptides DKVGINYW and KGYGGVSLPEW is comparable to that exhibited by VPP – an antihypertensive peptide already included in functional foods.
Therefore, the aforementioned peptide concentrates are promising candidates for nutraceuticals – and have a major potential as ingredients in functional foods specifically designed for treatment of hypertension and related diseases.
Funding for author T. Tavares was via a Ph.D. fellowship, administered by Fundação para a Ciência e a Tecnologia – Portugal (ref. SFRH/BD/31604/2006), and supervised by author F. X. Malcata.
The authors have declared no conflict of interest.




