Severe brain calcification and migraine headache caused by SLC20A2 and PDGFRB heterozygous mutations in a five-year-old Chinese girl
Hao Sun and Zhijian Cao contributed equally to this work.
Funding information
National Natural Science Foundation of China grants (31871262, 31671301), National Key R&D Program of China grant (2016YFC1306000) and Shanghai Municipal Science and Technology Major Project (2018SHZDZX05).
Abstract
Background
Primary familial brain calcification (PFBC) is a rare inheritable neurodegenerative disease characterized by bilateral calcification in different brain regions and by a range of neuropsychiatric symptoms. Six causative genes of PFBC (SLC20A2, PDGFRB, PDGFB, XPR1, MYORG, and JAM2) have been identified.
Methods
Sanger sequencing was used to identify the causative genes associated with PFBC in this study.
Results
We describe the first PFBC case with both SLC20A2 and PDGFRB heterozygous mutations. Notably, this patient with the digenic mutation (who was only 5 years old) showed severe brain calcification and migraine, whereas the patient's parents, who each carried a heterozygous mutation in SLC20A2 or PDGFRB, exhibited varying degrees of brain calcification but were clinically asymptomatic.
Conclusion
This case highlights the digenic influences on the characteristics of PFBC patients.
1 INTRODUCTION
Primary familial brain calcification (PFBC) is a rare inheritable neurodegenerative disease mainly characterized by symmetrical calcification in the basal ganglia, thalamus, cerebellum, and brainstem (Wang et al., 2012). Patients with PFBC may experience movement disorders, cognitive impairment, psychiatric signs, or other associated manifestations with diverse severity and variable onset age or may be asymptomatic throughout their lives (Nicolas et al., 2015; Wang et al., 2015).
PFBC can be transmitted in an autosomal dominant or recessive manner. PFBC causative genes identified to date include four autosomal dominant (SLC20A2 [OMIM:158378], PDGFRB [OMIM:173410], PDGFB [OMIM:190040], and XPR1 [OMIM:605237]) and two autosomal recessive (MYORG [OMIM:618255] and JAM2 [OMIM:606870]) genes (Keller et al., 2013; Legati et al., 2015; Nicolas et al., 2013; Schottlaender et al., 2020; Wang et al., 2012; Yao et al., 2018). These pathogenic genes are thought to be associated with two main pathogenic mechanisms of PFBC. On the one hand, the inorganic phosphate (Pi) dyshomeostasis via dominant mutations in SLC20A2 and XPR1. Specifically, SLC20A2 and XPR1 encode Pi transmembrane transporters, which perform Pi absorption and efflux functions, respectively. Mutations in SLC20A2 or XPR1 probably disrupt cerebral Pi homeostasis, eventually resulting in the accumulation of hydroxyapatite in the brain (Legati et al., 2015; Wang et al., 2012). On the other hand, dysfunction of the neurovascular unit (NVU) or blood-brain barrier (BBB) occurs due to mutations in the other four causative genes. The NVU, which is mainly composed of pericytes, endothelial cells, astrocytes, and neurons, reportedly has an irreplaceable role in maintaining the integrity of the BBB (Sweeney et al., 2018). PDGFRB and PDGFB encode platelet-derived growth factor receptor β and its main ligand, which are associated with the function of pericytes and BBB integrity. Loss-of-function of PDGFRβ and PDGFB could lead to the impairment of BBB integrity, which might lead to abnormal calcium phosphate deposits in the brain (Keller et al., 2013; Nicolas et al., 2013). MYORG mutations might disturb the normal function of astrocytes, which may cause NVU dysfunction and further lead to brain calcification (Yao et al., 2018). JAM2 encodes junctional adhesion molecule 2, and loss-of-function mutations in JAM2 cause cell-to-cell adhesion impairment and failure of the NVU, which may result in brain calcification (Schottlaender et al., 2020). Interestingly, Slc20a2-knockout mice present calcified nodules in pericytes and astrocytes and increased BBB permeability, suggesting a possible link between the two pathogenic mechanisms (Jensen et al., 2018). However, further studies are needed to investigate the potential associations between Pi dyshomeostasis and disruption of the NVU or BBB in contributing to brain calcification.
Here, we report a family in which the members have various genetic states and manifestations of PFBC. Notably, the proband carrying the SLC20A2 and PDGFRB heterozygous mutations showed more severe brain calcification and earlier onset age of clinical symptoms than her family members, highlighting digenic influences on the characteristics of PFBC patients.
2 MATERIALS AND METHODS
2.1 Ethical compliance
This study was approved by the Ethics Committee of Huazhong University of Science and Technology. Informed consent was obtained from all the participants and the guardians of the child.
2.2 Patients, sample collection and Sanger sequencing
A Chinese family with PFBC was identified in Anhui Province. Their clinical information was obtained from the First Affiliated Hospital of Anhui Medical University. Genomic DNA was extracted from their peripheral blood by standard methods, and Sanger sequencing was used to identify the causative genes. Specific PCR amplification of the sample DNA was carried out, and PCR products were gel-purified and then sequenced by the ABI 3730XL sequencer. The gene reference sequence transcripts were NM_001257180.2 (SLC20A2) and NM_002609.4 (PDGFRB). The Ensembl Database (http://asia.ensembl.org) and Genome Aggregation Database (gnomAD, https://gnomad.broadinstitute.org) were used to evaluate the minor allele frequency (MAF) of the obtained variants, and the SNPs&GO (http://snps-and-go.biocomp.unibo.it/snps-and-go), Mutation Taster (http://www.mutationtaster.org), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2) and PROVEAN (http://provean.jcvi.org/index.php) were applied to predict the pathogenicity of the variants.
3 RESULTS
The proband (Figure 1a, III:1), a 5-year-old girl, was admitted to the hospital because of intermittent headache for over two days. She tended to experience headaches when she woke up and always felt pain in the left temporal region. Computed tomography (CT) scans of her brain showed bilateral calcifications in the basal ganglia, temporal lobe, and frontal lobe (Figure 1b, III:1). Magnetic resonance imaging of the brain also displayed symmetrical calcified lesions in the basal ganglia and frontal lobe, magnetic resonance angiography and venography showed no arterial or vein stenosis or other vascular abnormalities (data not shown), and her serum calcium, phosphate and parathyroid hormone (PTH) levels were normal (data not shown). The proband's father (Figure 1a, II:3) was 32 years old and clinically asymptomatic. Nevertheless, he showed obvious symmetrical calcification in the basal ganglia, thalamus and cerebellum (Figure 1b, II:3). The proband's mother (Figure 1a, II:4), who was 29 years old, displayed slight bilateral calcification in the globus pallidus (Figure 1b, II:4) but remained asymptomatic. In addition, two aunts of the proband (Figure 1a, II:1 and II:2) both showed obvious symmetric calcification in the basal ganglia (Figure 1b, II:1 and II:2) but no clinical symptoms at ages of 34 and 36 years.
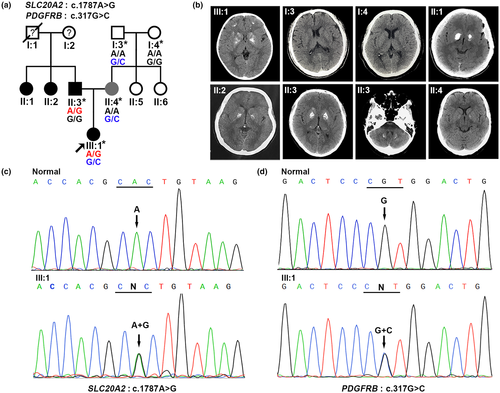
Genomic DNA was extracted from peripheral blood, and the DNA sample of the proband was subjected to screen the known causative genes for PFBC. Surprisingly, we identified two missense mutations in the proband: NM_001257180.2, exon10, c.1787A>G, p.His596Arg in SLC20A2 (Figure 1c) and NM_002609.4, exon3, c.317G>C, p.Arg106Pro, rs544478083 in PDGFRB (Figure 1d). Subsequently, we further detected the distribution of the two variants in this family and found that the proband's father carried the SLC20A2 mutation, the proband's mother and maternal grandfather carried the PDGFRB variant (Figure 1a). The c.1787A>G (p.His596Arg) mutation of SLC20A2 has been reported in a 66-year-old patient with sporadic primary familial brain calcification who was also clinically asymptomatic (Guo et al., 2019). The c.317G>C (p.Arg106Pro) variant of PDGFRB, a rare single nucleotide polymorphism (SNP, rs544478083), has not yet been shown to be related to PFBC and is likely benign predicted by Mutation Taster, PolyPhen-2, and PROVEAN (data not shown). However, this variant was also predicted to be disease-related polymorphism by SNPs&GO, and we have confirmed that the variant was absent in 100 unrelated healthy controls (data not shown), which was consistent with the variant with a MAF of <0.01 in the Ensembl Database and gnomAD. Taken together, we speculated that the variant was associated with PFBC in our study.
4 DISCUSSION
We present a Chinese family with PFBC in which the previously reported heterozygous mutation c.1787A>G (p.His596Arg) in SLC20A2 and the SNP (rs544478083) c.317G>C (p.Arg106Pro) in PDGFRB were identified. The proband's father with the SLC20A2 c.1787A>G (p.His596Arg) mutation showed obvious brain calcification but was clinically asymptomatic. The proband's mother with the PDGFRB c.317G>C (p.Arg106Pro) variant showed very slight calcification and was clinically asymptomatic. However, the proband, who carried the two variants, exhibited characteristics of PFBC at an early age, including extensive brain calcification and severe migraines. Therefore, the brain calcification in the proband might have primarily resulted from the SLC20A2 mutation and secondarily from the PDGFRB variant.
Currently, the genetic basis for the clinical heterogeneity of PFBC is not largely understood, and it cannot be explained only by a single variant. PFBC patients with biallelic variants in SLC20A2 have been reported. In 2012, Wang et al. reported that PFBC patients with compound heterozygous SLC20A2 mutations (c.362C>G, p.Ser121Cys and c.1802C>G, p.Ser601Trp) presented extremely severe brain calcification, accompanied by repetitive seizures, mental retardation, and developmental delay since infancy (Wang et al., 2012, 2015). Recently, Chen et al. showed that PFBC probands with homozygous pathogenic SLC20A2 variants displayed severe brain calcification and parkinsonism (Chen et al., 2019). All PFBC patients with biallelic SLC20A2 variants showed more severe phenotypes than their family members with heterozygous variants, which indicated that the second mutation might promote the development of PFBC. Coincidentally, PFBC patients with variants in both a PFBC pathogenic gene (SLC20A2 or PDGFRB) and another PFBC-unrelated gene (THAP1, CHRNB2, CASR, SCN2A or MEA6) were described as presenting more complex phenotypes, supporting the notion that a variant of a second gene may promote a heterogeneous phenotype in PFBC patients (Baker et al., 2014; Borges-Medeiros & de Oliveira, 2020; DeMeo et al., 2018; Fjaer et al., 2015; Fujioka et al., 2015; Knowles et al., 2018). The SLC20A2 c.1787A>G (p.His596Arg) variant detected in our study has been reported to cause brain calcification without clinical manifestations due to PiT2 dysfunction, which probably results in the accumulation of Pi in affected brain regions (Guo et al., 2019). In addition, the PDGFRB c.317G>C (p.Arg106Pro) variant, which may destroy the integrity of the BBB, leading to the transfer of Pi from blood vessels into the brain and further promote the accumulation of Pi in affected brain regions. Accordingly, the PDGFRB heterozygous mutation may have played an essential role in promoting the phenotypes of the proband, who showed more extensive brain calcification and headaches significantly ahead of the typical onset age between 30 and 60 years (Wang et al., 2015). To the best of our knowledge, the proband with both SLC20A2 and PDGFRB variants in this study is the first reported case resulting from two known pathogenic genes, providing new proof for the digenic effect on clinical heterogeneity among PFBC patients.
In conclusion, we present the first case in which heterozygous SLC20A2 and PDGFRB mutations are carried simultaneously by a member of a Chinese PFBC family. Our study indicates that mutations in SLC20A2 and PDGFRB can collectively promote the development of calcification and clinical manifestations, thereby providing new insights into the genotype-phenotype correlation of PFBC patients.
ACKNOWLEDGMENTS
We thank the patients and the family members for their enthusiastic participation. Without their support, this study would not have been possible. This study was mainly supported by the National Natural Science Foundation of China grants (31871262, 31671301), the National Key R&D Program of China grant (2016YFC1306000) and the Shanghai Municipal Science and Technology Major Project (2018SHZDZX05) to J.Y.L.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
J.Y.L. and X.X. conceived and supervised the study. J.Y.L. obtained financial support. J.Y.L. and J.W. recruited patients, and J.Y.L. evaluated the patients’ clinical manifestations. H.S., Z.C., R.G., Y.L., R.C., S.D., and T.M. carried out mutation screening for candidate genes. H.S. and Z.C. performed data collection and analysis. H.S., Z.C., and X.X. drafted the manuscript. J.Y.L. and X.X. revised the manuscript. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




