Variation at ACOT12 and CT62 locus represents susceptibility to psoriasis in Han population
Abstract
Background
Psoriasis is a chronic inflammatory disorder of the skin, and genetic factors are reported to be involved in the disease pathogenesis. Many studies have named psoriasis candidate genes.
Objective
In this study, we determined the mutation frequency of 7 variable genes in 1,027 psoriatic patients and investigated its possible mechanism associated with psoriasis.
Method
A total of 7 variable genes from 1,027 psoriatic patients were amplified and sequenced using the Sanger method. The mutation frequency was compared to that of non-psoriatic individuals in Asia using information from databases.
Results
Among the 7 investigated genes, the mutation frequency of ACOT12 (c.80A>G, 9.98% vs. 5.85%, p < .05) and CT62 (c.476C>T,15.8% vs. 9.93%, p < .05) was found to be significantly higher than among non-psoriatic Asian individuals. The mutation frequencies of CASZ1(c.599T>G), SPRED1(c.155A>G), and ACOT12 (c.80A>G) differed significantly between the groups organized by medical history, PASI, and family history. SPRED1 gene variants (17.25% vs. 7.78%, p < .01) showed a stronger association with the family history group at the onset of psoriasis than with the no family history group.
Conclusions
Our results provide a comprehensive correlation analysis of susceptibility genes in psoriasis patients. Clinical characteristics of patients play important roles in the development of psoriatic skin.
1 BACKGROUND
Psoriasis is a common immune-mediated chronic inflammatory disease characterized by hyperplasia, altered proliferation and differentiation of keratinocytes, vascular remodeling, and inflammation in the skin (Ellinghaus et al., 2010; O'Rielly, Jani, Rahman, & Elder, 2019). Its prevalence varies markedly by ethnicity and geographic location, affecting 2.5% of Europeans and 0.1%–0.5% of Asians (Bejaoui et al., 2019; Chandran & Raychaudhuri, 2010). Psoriasis has a high recurrence rate and is considered a complex disease attributable to both environmental and genetic factors (Tang et al., 2018). Psoriasis has a spectrum of clinical subtypes and can lead to a wide variety of specific diagnoses (Cid et al., 2009; Sarac, Koca, & Baglan, 2016).
Psoriasis has a strong genetic predisposition with estimated heritability of up to 80% (Zhang, Wang, Te-Shao, Yang, and Chen 2002). In the few past decades, many studies have confirmed a number of psoriasis susceptibility genes and loci that lie within the major histocompatibility complex (MHC) (Fan et al., 2019). It is reported that the associated SNPs rs28947206 and linkage disequilibrium with them could potentially affect the functionality of IL-36γ cytokine, which in turn may impact plaque psoriasis pathology (Traks et al., 2019). The study have found the TRAF3IP2-rs33980500 variant was associated with the susceptibility to psoriasis (Dębniak et al., 2014). However, few studies show the relationship between susceptibility loci and the medical history and family history (Wang et al., 2019).
Recently, we obtained the whole-genome sequences of 9 pairs of monozygotic twins with psoriasis discordance (Li et al., 2019). Notably, we found 7 locus mutations in the normal individuals but were not identical in patients with psoriasis. In order to further investigate the relationship between these mutations and the pathogenesis and clinical classification of psoriasis, we collected and tested gene mutations in 1,027 patients with psoriasis.
2 MATERIALS AND METHODS
2.1 Samples
A total of 1,027 Han Chinese individuals were enrolled in this study. All the patients were diagnosed with psoriasis vulgaris (PV) by at least two experienced dermatologists based on clinical and histopathological manifestations, and their clinical information was collected through a comprehensive clinical check-up by professional investigators. Self-reported information from a standard questionnaire was used to collect demographic and other characteristics from the patients (severity, medical history, and family history) and to exclude any other systemic, infectious, autoimmune, atopic, or malignant disease and history of systemic treatment in the six months prior to data collection. None of the patients had hypertension, gout, asthma, or multiple coffee spots. All participants provided written informed consent. The study protocol was approved by the ethics committee of the Taiyuan Central Hospital.
2.2 Characteristics of the study subjects
The clinical characteristics of these cases are shown in Table 1. In the study, we investigated 1,027 Chinese Han people with psoriasis vulgaris. The cases included 526 male and 501 female patients, age range from 9 to 90 years, medical history mainly distributed in 1 month to 65 years, and PASI concentrated in 0.3–48.
| Variable | Subtype | n | % |
|---|---|---|---|
| Gender | Male | 526 | 51.02 |
| Female | 501 | 48.78 | |
| Age | ≤40 | 630 | 61.34 |
| >40 | 391 | 38.07 | |
| Medical history | n < 20 | 637 | 62.02 |
| n ≥ 20 | 362 | 35.25 | |
| PASI | ≤10 | 755 | 73.51 |
| >10 | 271 | 26.38 | |
| Family history | Yes | 296 | 28.82 |
| No | 593 | 57.74 |
The participants were divided into two groups by medical history, less than 20 years (62.02%) and more than 20 years (35.25%). Patients were selected based on disease severity assessment using body surface area and the psoriasis area severity index (PASI), and the group as mild (PASI ≤ 10) was 755 (73.51%) and moderate-to-severe (PASI > 10) was 271 (26.38%). Patients were grouped by family history into positive (28.82%) and negative (57.74%) groups. In all of these categories, the number of each component is basic to 300.
2.3 DNA extraction
Genomic DNA was extracted from the peripheral whole blood of the patients using the Blood Genomic DNA Midi Kit (Cwbio Biotech, Beijing, China) according to the manufacturer's instructions. All DNA samples were dissolved in water and stored at −20°C until use.
2.4 Sanger sequencing
Sequencing primers were designed for the 7 SNPs using Primer Premier 5.0 (Table 2). Genomic DNA was amplified using the Bio-Rad PCR System (Bu, Liu, Hu, Tan, & Zhao, 2019). Thermal cycling was performed as follows: 5 min at 96°C for 10 cycles (20 s at 96°C; 30 s for touchdown at 52°C–62°C; and 60 s at 72°C), followed by 35 cycles (20 s at 96°C; 30 s at 52°C; and 60 s at 72°C), ending with 5 min at 72°C.
| gene | version number | OMIM | HGNC | SNP | Primer sequences-F | Primer sequences-R |
|---|---|---|---|---|---|---|
| SPRED1 | NM_152594.2 | 609,291 | 20,249 | rs2272105 | TGGTGATGACCCGAGAT | AGGGAAGGCAGGATGTT |
| CASZ1 | NM_001167674.1 | 609,895 | 26,002 | rs10511083 | TTCCCAGAACTCCTCCAA | CCTACCCACCCACCATC |
| ACOT12 | NM_130767.2 | 614,315 | 24,436 | rs7735423 | GGGACAGATCAGGACAGG | TGTAAGCCCAGCTACTCG |
| EXOC4 | NM_021807.3 | 608,185 | 30,389 | rs62470027 | CAGGTGGGTCAGAGTTT | TGGATCTAGCAGCATCA |
| CT62 | NM_001102658.1 | 27,286 | rs1343698 | AATGGGTGAAGGACAGG | AAGCCAACTATGAACGACT | |
| SORCS3 | NM_014978.1 | 606,285 | 16,699 | rs4259767 | AGAAATGGTGACTCTGTGCC | TGGAGTTGGGCAGGATTACA |
| DENND5B | NM_001039350.1 | 617,279 | 28,338 | rs35660473 | AGCGGAGATGGCATCAAAATC | GCTCAAGCGATCTTTCTATCCT |
2.5 Statistical analysis
Information on the mutation frequency in 8,624 normal Asian individuals was obtained from the Pubvar database (https://www.pubvar.com/) (Table S1). Amplicons were bidirectionally sequenced on an ABI 3,730 system. We performed sequence analysis using Mutation Surveyor software. Mutations included hybrid mutations and homozygous mutations. The mutation frequency was calculated with the following equation: (hybrid + homozygous × 2)/1027/2. Disease severity was scored using the Psoriasis Area and Severity Index (PASI) by the same dermatologist, and the patients were grouped as mild (PASI ≤ 10) and moderate-to-severe (PASI > 10). Demographic characteristics of the groups (stage, gender, age, severity, medical history, family history) were evaluated using SPSS version 18.0. The chi-square (χ2) test was used to test the relationship between psoriasis and investigated factors. Statistical significance was set at p < .05.
3 RESULTS
3.1 Mutation rates in patients with and without psoriasis
In 305 loci of 1,027 psoriatic patients, the variant c.80A>G in the Acyl-CoA Thioesterase 12 (ACOT12) gene was found, and the mutation frequency in psoriasis (9.98%) was significantly higher than that of non-psoriatic Asian individuals (5.85%, p < .05). Moreover, the variant c.476C>T in the cancer/testis associated 62 (CT62) gene was found, and the mutation frequency in psoriasis (15.8%) was significantly higher than that of non-psoriatic Asian individuals (9.93%, p < .05). In addition, the mutation frequency of Exocyst Complex Component (EXOC4, c.299G>A, 4.87% vs. 6.45%, p > .05) and DENN domain containing 5B (DENND5B,c.174G>T, 3.06% vs. 3.77%, p > .05) was lower than in non-psoriatic individuals. However, the mutation frequency of sprouty related EVH1 domain containing 1 (SPRED1, c.155A>G, 7.44% vs. 6.05%, p > .05), castor zinc finger 1 (CASZ1,c.599T>G, 12.56% vs. 11.71%, p > .05), and sortilin related VPS10 domain-containing receptor 3 (SORCS3, c.298G>C, 80.62% vs. 73.81%, p > .05) were higher than in non-psoriatic individuals (Figure 1).
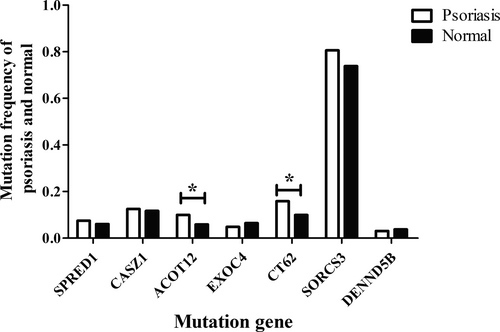
3.2 Occurrence of psoriasis and relationship with sociodemographic characteristics of participants
A total of 501 women and 524 men participated in the study. We analyzed the difference between men and women with mutations with respect to 7 genes, and we found there to be was no difference between male and female (Figure 2). In the age group, most patients with psoriasis were concentrated in the 18–40 age range (50.8%). But there was no significant difference across age groups.
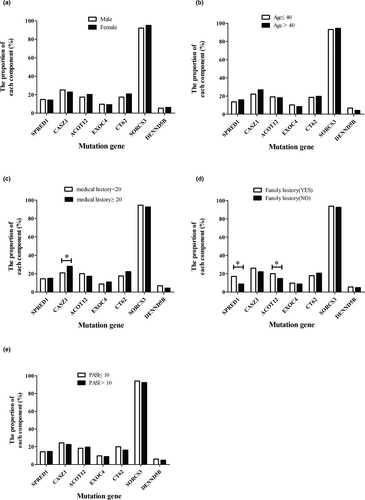
The medical history, PASI, and family history were used to clarify the different clinical subtypes. In this way, the relationship between medical history, PASI score, family history, and mutation gene was investigated. The results showed that CASZ1 (χ2 = 6.83, p < .01) had a significant difference across patients with different medical histories. Analysis of PASI score showed that mutations in genes had no significant association with PASI.
Next, we further analyzed the relationship between family history and genetic mutation. We found SPRED1 (χ2 = 12.67, p < .01) and ACOT12 (χ2 = 4.12, p < .05) to differ significantly across different groups. In comparison to patients with no family history, the mutation in SPRED1 (17.03% vs. 8.78%) and ACOT12 (20.06% vs. 14.86%) may be risk factors in individuals whose family members have psoriasis.
For research purposes, we rearranged the population data and divided individuals with psoriasis into two groups, as shown in Table 3. In the under 20 years medical history group, we analyzed the relationship between PASI, family history, and gene mutation, and we found that SPRED1 (χ2 = 9.358, p < .01) differed significantly between individuals with different family histories. In the over 20 years medical history group, we also analyzed the relationship between PASI, family history, and genetic mutation, and the results showed that SPRED1 (χ2 = 4.778, p < .05) differed significantly between individuals with different family histories.
| Medical history < 20 | Medical history ≥ 20 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PASI ≤ 10 | PASI > 10 | χ 2 | Family history (YES) | Family history (NO) | χ 2 | PASI ≤ 10 | PASI > 10 | χ 2 | Family history (YES) | Family history (NO) | χ 2 | |
| SPRED1 | 14.80% | 12.65% | 0.435 | 17.25% | 7.78% | 9.358 | 14.46% | 15.93% | 0.233 | 16.58% | 10.40% | 4.778 |
| CASZ1 | 21.80% | 21.50% | 0.005 | 21.00% | 23.95% | 0.675 | 27.71% | 28.32% | 0.025 | 26.42% | 29.60% | 0.765 |
| ACOT12 | 19.00% | 20.25% | 0.117 | 20.25% | 14.37% | 3.031 | 17.67% | 18.58% | 0.077 | 19.69% | 16.00% | 1.387 |
| EXOC4 | 9.40% | 6.30% | 1.385 | 9.75% | 6.58% | 1.647 | 10.04% | 12.39% | 0.785 | 9.84% | 11.20% | 0.3 |
| CT62 | 17.20% | 17.08% | 0.001 | 16.50% | 16.76% | 0.007 | 23.29% | 19.47% | 1.162 | 20.73% | 24.80% | 1.455 |
| SORCS3 | 93.60% | 95.56% | 0.809 | 94.00% | 93.41% | 0.079 | 92.77% | 92.04% | 0.107 | 93.26% | 91.20% | 0.928 |
| DENND5B | 7.20% | 5.06% | 0.85 | 6.50% | 4.70% | 0.686 | 3.61% | 5.31% | 0.99 | 3.63% | 4.80% | 0.533 |
4 DISCUSSION
To the best of our knowledge, this is the most important study to use next-generation sequencing technique to assess the status of 7 genes, and we finally found that ACOT12 and CT62 had mutated in psoriasis patients. The results of the study indicated that genetic mutation has a very close correlation with clinical features.
Acyl-CoA thioesterase 12 (ACOT12) is the major enzyme known to hydrolyze the thioester bond of acetyl-CoA in the cytosol in the liver (Horibata, Ando, Itoh, & Sugimoto, 2013). Acetyl-CoA plays a key role in many aspects of metabolism. In mammalian cells, ACOT12 is mainly generated by glycolysis, b-oxidation of fatty acids, and catabolism of glutamine (Harris, Joshi, Jeoung, & Obayashi, 2005; Patel, Nemeria, Furey, & Jordan, 2014; Rufer, Thoma, & Hennig, 2009). In cancer cells, acetate can be utilized as an alternative carbon source to produce acetyl-CoA (Comerford et al., 2014). This has been reported to play important roles in tumor growth and progression (Gao et al., 2016; Lin et al., 2013). In addition, ACOT12 plays a fundamental role in cell signaling and metabolic pathways, with its cellular levels tightly controlled through reciprocal regulation of enzymes that mediate its synthesis and catabolism (Swarbrick et al., 2014). The enzyme is regulated by ADP and ATP, and this regulation is believed to be mediated through steroidogenic acute regulatory protein-related lipid transfer (START) domain (Lu et al., 2019). CT62 (cancer/testis antigen 62) has been observed in breast cancer and lung cancer before (Liu et al., 2012). Moreover, CT62 is a potential immunotherapeutic target suitable for treating breast cancer (Gilmore et al., 2019). In this study, we found the rates of mutation of ACOT12 and CT62 to be significantly higher in psoriasis patients than in non-psoriatic Asians, indicating the described ACOT12 and CT62 variant may disrupt the microenvironment and normal metabolism of the skin. Our findings might contribute to the use of information on gene variants to improve diagnostic approaches to treating psoriasis vulgaris of different clinical phenotypes in Chinese Han populations, as suggested for other complex diseases (Han et al., 2014).
Medical history is commonly used in psoriasis clinical trials. Its use in scoring algorithms greatly expands options for quantifying treatment outcomes in cost-effectiveness analyses of psoriasis therapies (Matza et al., 2019). To further evaluate the relationship between gene mutation and clinical characteristics, we grouped psoriasis patients according to gender, age, medical history, PASI, and family history. The mutation rate differed distinctly across patients with different medical histories, PASI, and family histories. We found that medical history of psoriasis going back more than 20 years was related to CASZ1 mutation in individuals with psoriasis.
Several studies have shown the significant impact the onset of psoriasis in not only patients with previous medical histories of psoriasis but also those whose families and closest relatives have such histories (López-Estebaranz, Sánchez-Carazo, & Sulleiro, 2016). Chi-square analysis revealed that mutation frequency of SPRED1 was affected by family history of psoriasis. This is likely to reflect the genetic differences of the patients and their resulting different phenotypes. Several studies have shown the differences between familial and sporadic cases in psoriasis (Chandran et al., 2009). Differences in the strength of association with mutation genes and family history have been reported for the major histocompatibility complex (MHC), including a stronger association of HLA-C*06 and HLA-B*27 with psoriasis (Solmaz et al., 2019).
Overall, the results showed the mutation rate of ACOT12 and CT62 to be significantly higher than that of other genes and could be a novel susceptibility gene for psoriasis in the Chinese population. We also found pronounced differences in the relationship between clinical characteristics and genetic mutation. Our results suggest that additional genetic factors may contribute to the complex disease phenotypes. Further studies will be required to establish the functional mechanisms and etiology that affect the risk of psoriasis.
ACKNOWLEDGMENTS
The authors are grateful for funding from the National Natural Science Foundation of China(no. 81773336, 81602768, 81803146). The funding was attributed to the design of the study and the collection, analysis, and interpretation of data. We would like to thank LetPub [www.LutPub.com.cn] for English language editing.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.




