Cordyceps sinensis ameliorates idiopathic pulmonary fibrosis in mice via inhibiting mitochondrion-mediated oxidative stress
Ying Zhang, Lirun Zhou, and Guangqing Cheng contributed equally to this work.
Abstract
Idiopathic pulmonary fibrosis (IPF) represents a chronic interstitial lung disease with an unclear underlying mechanism and currently lacks a definitive treatment. Cordyceps sinensis (CS), renowned for its pharmacological properties in traditional Chinese medicine and extensive use in lung disease treatment, holds promise as a therapeutic agent for IPF. However, the specific role of CS in treating IPF remains unclear. In this study, we aimed to assess the efficacy of CS in treating IPF and unravel potential underlying mechanisms. Our results demonstrate that CS treatment effectively mitigated pulmonary inflammation and collagen deposition in bleomycin-induced IPF mice. Proteomics analysis revealed that the regulation of mitochondrial oxidative phosphorylation may serve as a potential protective mechanism of CS against IPF in mice. Further investigation unveiled that CS could suppress the excessive production of mitochondrial reactive oxygen species in lung tissues induced by bleomycin through moderating the expression and activity of mitochondrial complexes, thus safeguarding the integrity and function of mitochondria. Overall, our findings not only underscore the effectiveness of CS in preventing bleomycin-induced IPF but also highlight mitochondrial-mediated oxidative stress as a promising therapeutic target for treating IPF.
1 INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) represents an unpredictable, progressive, and potentially fatal pulmonary fibrosis ailment characterized by a gradual decline in lung function, which can ultimately culminate in chronic respiratory failure and mortality without appropriate intervention.1-3 As one of the prevalent pulmonary conditions, IPF typically manifests in individuals of middle to advanced age.4, 5 The median survival duration for this condition ranges between 2 and 5 years, profoundly impacting patients' quality of life.1, 6 The pathogenesis of IPF entails progressive scarring within lung tissue, concomitant with aberrant activation of fibroblasts/myofibroblasts, persistent epithelial cell injury, and excessive deposition of extracellular matrix (ECM) proteins.7-9 Presently, the therapeutic approach to pulmonary fibrosis predominantly relies on antifibrotic medications such as Pirfenidone and Nintedanib.10, 11 Nevertheless, owing to the significant adverse effects associated with these antifibrotic agents, including allergenic reactions and gastrointestinal disturbances, there exists an exigent need to explore novel classes of antifibrotic medications.
The precise mechanism underlying IPF remains incompletely elucidated. However, literature indicates that redox imbalance plays a pivotal role in the onset and progression of various pulmonary disorders, including IPF.12 Notably, the accumulation of reactive oxygen species (ROS) has been implicated as a significant contributor to the pathogenesis of pulmonary fibrosis.13, 14 ROS encompasses a spectrum of molecular oxygen derivatives, such as hydroxyl radicals, superoxide, and hydrogen peroxide (H2O2), among others, and heightened ROS levels instigate molecular damage, commonly termed “oxidative stress.”15, 16 The mitochondrial respiratory chain, comprising five complexes (I–V) responsible for electron transfer within the inner mitochondrial membrane, serves as a principal contributor to mitochondrial ROS (MitROS) generation beyond adenosine triphosphate (ATP) synthesis in mammalian cells.17, 18 Complexes I and II facilitate the generation of oxygen-free radicals during the electron transfer process by releasing a limited number of electrons.19 Consequently, tissue and cellular damage trigger electron leakage from the mitochondrial respiratory chain, leading to the overproduction of ROS and subsequent impairment of mitochondrial function. This impairment manifests as an imbalance in the intracellular oxidation/antioxidant system, diminished MMP, and reduced ATP synthesis.20
Traditional Chinese medicine (TCM) boasts a rich history of alternative medical practices renowned for their efficacy and minimal adverse effects. Among these, Cordyceps sinensis (CS), a nourishing TCM characterized by its principal constituents including cordycepin, polysaccharides, sterols, and phenolic compounds, exhibits a myriad of pharmacological activities, encompassing antioxidative, antiaging, antitumor, antifibrotic, and anti-inflammatory properties.21, 22 For instance, CS has been shown to extend the lifespan of Drosophila by upregulating antioxidant-related genes such as catalase (CAT), superoxide dismutase 1 (SOD1), and methuselah (MTH).23 Widely utilized in clinical settings, CS has demonstrated efficacy in treating various ailments including cough, asthma, bronchitis, and nephritis.24, 25 However, the potential therapeutic implications of CS in the context of IPF and its associated mechanisms remain inadequately understood.
In this study, we established a mouse model of bleomycin-induced IPF and conducted preliminary investigations into the therapeutic potential of CS on IPF in mice. This assessment involved histopathological examination and evaluation of serum inflammatory factors. Subsequently, proteomics analysis of lung tissues unveiled a potential mechanism underlying the mitigation of IPF pathology by CS, involving the reprogramming of the oxidative phosphorylation pathway in the lungs. Additionally, our findings confirmed that CS exerts protective effects on lung mitochondria structure and function by inhibiting excessive ROS production, thus ameliorating IPF. Overall, our study offers novel insights into the clinical application of CS for the treatment of IPF.
2 RESULTS
2.1 CS ameliorated bleomycin-induced IPF in mice
Before investigating the potential of CS in treating IPF, the chemical constituents of the CS utilized in this study were analyzed using a mass spectrometer. The total ion chromatograms (TIC) of the test solution extracted from CS under positive and negative ionization modes are depicted in Supporting Information S1: Figure S1. A total of 58 compounds were identified, comprising 17 nucleosides, 15 amino acids, 5 purine alkaloids, 4 pyrimidine alkaloids, 8 organic acids, 2 nucleotides, 2 sugar alcohols, and 5 other compounds. Detailed mass spectrometry information is provided in Supporting Information S1: Table S1. These findings align well with previous reports concerning the active constituents of CS.21
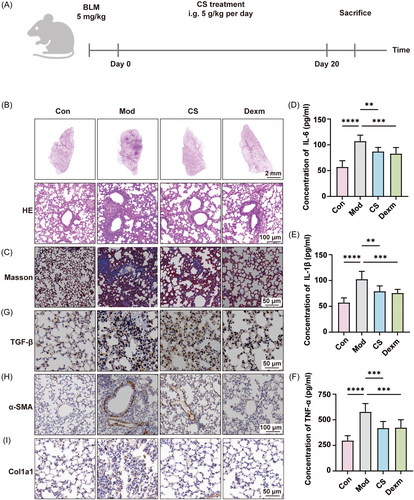
To investigate the potential therapeutic effects of CS on IPF in vivo, we conducted pharmacological experiments on mice as outlined in Figure 1A. Briefly, lung fibrosis was induced in mice via intratracheal instillation of bleomycin. Subsequently, the mice received daily intragastric administration of CS and dexamethasone for a duration of 3 weeks. Upon sacrifice, lung tissues and serum samples were collected for pathological and serum biochemical analyses. Hematoxylin and eosin (H&E) staining results (Figure 1B) revealed that bleomycin instillation led to severe alveolar hemorrhage, edema, thickening of alveolar septa, and infiltration of inflammatory cells. Treatment with dexamethasone (Dexm), a corticosteroid with anti-inflammatory properties, effectively mitigated these observed damages in lung pathology. Dexm is extensively utilized in clinical practice for the management of interstitial pneumonia and pulmonary fibrosis.26 Its protective mechanism involves the suppression of inflammatory mediator production, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).27 Moreover, previous studies have established dexamethasone as the positive control for pulmonary fibrosis treatment, owing to its notable efficacy, high biological potency, and minimal sodium retention.28, 29 Encouragingly, CS treatment demonstrated similar therapeutic effects to Dexm, markedly reducing pulmonary edema and inflammatory cell infiltration. Additionally, Masson's trichrome staining indicated that the administration of CS and Dexm alleviated pulmonary interstitial collagen deposition in the model group (Figure 1B). Serum analysis results revealed a significant elevation in the levels of inflammatory cytokines including IL-1β, IL-6, and TNF-α following bleomycin induction. However, administration of CS and Dexm efficiently suppressed the increase in these cytokine levels in the serum of mice with pulmonary fibrosis (Figure 1C–E). Furthermore, it has been reported that the increase in ROS levels can activate the transforming growth factor β (TGF-β) pathway and promote the onset of pulmonary fibrosis.30, 31 Additionally, the transformation of fibroblasts into myofibroblasts is a crucial indicator of IPF progression, with α-smooth muscle actin (α-SMA) and collagen type I alpha 1 (Col1a1) serving as specific marker proteins for myofibroblasts.32, 33 Immunohistochemical staining results demonstrated that the expressions of TGF-β, α-SMA, and Col1a1 in the model group were significantly elevated compared to the control group, whereas CS or Dexm treatment markedly suppressed their expression induced by bleomycin (Figure 1G–I). Taken together, these findings demonstrate that CS effectively mitigates bleomycin-induced IPF, similar to dexamethasone.
2.2 CS regulated mitochondrial-mediated oxidative phosphorylation
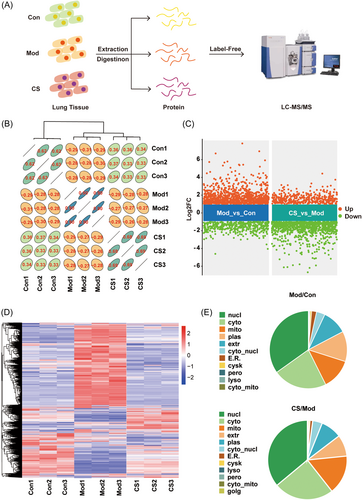
To elucidate the mechanistic insights into the action of CS against IPF, we conducted a label-free quantitative proteomic analysis of lung tissues, as depicted in Figure 2A. The correlogram illustrating the scaled abundance of proteins across the control (Con), model (Mod), and CS groups revealed a positive correlation coefficient between the CS and Con groups, whereas the Mod group exhibited negative correlation coefficients with both the CS and Con groups (Figure 2B). This suggests that protein expression levels in lung tissues of the Mod group tended to normalize following CS treatment. A total of 4862 proteins were identified across the three groups (Figure 2C,D), with differentially expressed proteins (DEPs) defined using a fold change >2 and p < 0.05, highlighted in scatter plots (Figure 2C). Specifically, 1074 proteins were upregulated and 824 proteins were downregulated when comparing the Mod group to the Con group, whereas in the CS group compared to the Mod group, there were 803 upregulated and 1263 downregulated proteins (Figure 2C). The changes in protein abundance for each group are depicted in a heat map (Figure 2D), wherein cluster analysis revealed a stronger tendency for the CS group to cluster with the Con group rather than the Mod group, consistent with the correlation analysis results (Figure 2B). Furthermore, we analyzed the cellular component of DEPs (Figure 2E). Comparing the cellular localization of differential proteins between the Mod and Con groups, we observed that the majority of differential proteins were localized in the nucleus, cytoplasm, mitochondria, and cell membrane. Notably, the differential proteins in the CS versus Mod group exhibited minimal changes in cellular localization compared to those in the Mod versus Con group.
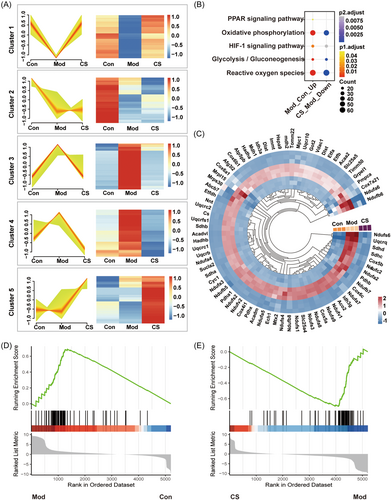
To further elucidate the changes at the proteomic level, the identified proteins were categorized into five clusters based on their variation patterns (Figure 3A). In cluster 1, proteins initially exhibited downregulation in the model group followed by upregulation in the CS group, whereas proteins in clusters 3 and 4 showed initial upregulation in the model group followed by downregulation in the CS group. Notably, proteins in these clusters displayed a consistent feature across the three groups, wherein CS reversed the alterations in protein abundance induced by bleomycin in IPF mice. Subsequently, proteins from clusters 1, 3, and 4 underwent Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis to elucidate the potential molecular pathways targeted by CS. Pathways including oxidative phosphorylation (OXPHOS), HIF-1 signaling, glycolysis, and ROS were concurrently enriched from the upregulated proteins in the Mod versus Con group and the downregulated proteins in the CS versus Mod group (Figure 3B). Mitochondria serve as pivotal energy metabolism centers wherein a significant array of proteins partake in the biological processes of oxidative phosphorylation and glycolysis.34 Furthermore, in line with the findings from KEGG analysis, bleomycin induction led to an augmentation in the expression of oxidative phosphorylation-related proteins, while CS treatment mitigated this upregulation, as illustrated by the circular heatmap (Figure 3C). Additionally, gene set enrichment analysis (GSEA) was conducted to specifically evaluate the enrichment score of oxidative phosphorylation. Here, CS treatment was observed to restore the gene signatures associated with oxidative phosphorylation in IPF mice towards those observed in healthy mice (Figure 3D,E). Collectively, these proteomic findings underscore mitochondrial-mediated oxidative phosphorylation as a potential molecular pathway through which CS confers protection against IPF.
2.3 CS restored the expression level and activity of mitochondrial complex I/II disturbed by bleomycin
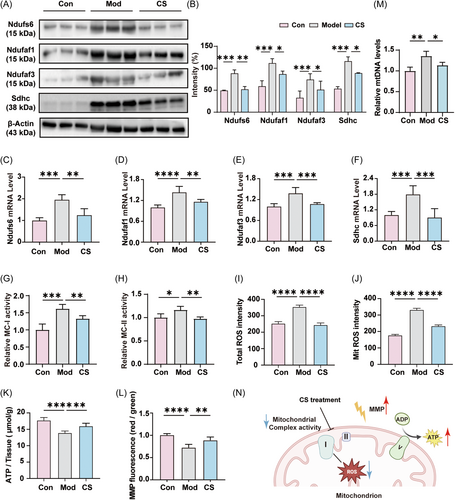
To validate the alterations in the abundance of proteins implicated in mitochondrial oxidative phosphorylation as elucidated by the proteomic analysis, we initially assessed the expression levels of mitochondrial complexes I and II (MC-I and MC-II) in lung tissues. Western blot analysis revealed a significant elevation in the expression levels of mitochondrial complex I components (Ndufs6, Ndufaf1, and Ndufaf3) and complex II component (Sdhc) in the Mod group, which markedly decreased following CS treatment (Figure 4A,B). Consistent with the protein quantification results, the messenger RNA (mRNA) levels of the four genes (Ndufs6, Ndufaf1, Ndufaf3, and Sdhc) assessed via quantitative real-time PCR (qPCR) exhibited similar trends of change across the three groups (Figure 4C–F), collectively indicating that CS partially counteracted the effect of bleomycin on the expression of mitochondrial complexes. Furthermore, the enzyme activity of mitochondrial complexes I and II in lung tissues was hyper-activated following bleomycin induction but returned to normal levels with CS intervention (Figure 4G,H). In summary, these findings suggest that the antifibrotic effect of CS may be closely linked to the expression and activity of mitochondrial complexes I and II.
2.4 CS alleviated mitochondrial dysfunction by reducing mitochondrial ROS production
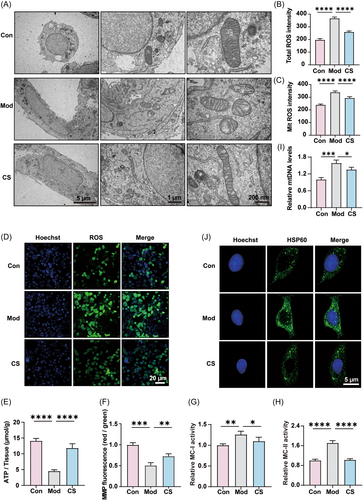
As crucial contributors to the respiratory electron-transport chain within mitochondria, mitochondrial complexes I and II have the capacity to generate excessive ROS, thereby instigating oxidative stress, which is deemed a pivotal factor in bleomycin-induced IPF.35, 36 Therefore, we postulated that a potential mechanism underlying the preventive action of CS against bleomycin-induced IPF may involve the inhibition of ROS release and mitigation of oxidative stress.35, 37 To assess the impact of CS on mitochondrial oxidative stress, the levels of total ROS and MitROS in lung tissue were measured. As depicted in Figure 4I,J, compared to the control group, the bleomycin-induced group exhibited elevated levels of both total ROS and MitROS, whereas CS administration mitigated the overproduction of ROS. Excessive ROS can impair mitochondrial function, often accompanied by a reduction in mitochondrial membrane potential (MMP) and ATP production. Consistently, CS effectively restored MMP levels and ATP production in lung tissue, even in the face of significant suppression induced by bleomycin treatment (Figure 4K,L). Similar results were observed in the levels of mtDNA, which were increased in the lung tissue of mice induced by bleomycin and significantly decreased after CS treatment. This indicates that CS can alleviate mitochondrial dysfunction by reducing mitochondrial over-replication caused by bleomycin (Figure 4M).38 In summary, CS modulates the expression and activity of mitochondrial complexes I and II, thereby alleviating oxidative stress and preserving normal mitochondrial functions (Figure 4N).
To further corroborate and extend the in vivo findings, we employed human lung epithelial BEAS-2B cells as an in vitro model. Transmission electron microscopy was utilized to directly visualize mitochondrial morphology in the three groups. The images revealed that bleomycin induced mitochondrial swelling, severe cristae damage, and membrane rupture, while CS treatment significantly alleviated these mitochondrial damages in the model group (Figure 5A). Consistent with the results observed in lung tissues, the elevated levels of total ROS and MitROS induced by bleomycin in BEAS-2B cells were reversed by CS treatment (Figure 5B,C). Furthermore, fluorescence imaging confirmed that CS markedly suppressed the excess ROS in bleomycin-induced BEAS-2B cells (Figure 5D). Moreover, with regard to the levels of ATP (Figure 5E), MMP (Figure 5F), mitochondrial complexes I and II (Figure 5G,H), and mtDNA (Figure 5I), the patterns of change in the in vitro model across the three groups closely mirrored the data observed in lung tissue, wherein CS alleviated the disturbances induced by bleomycin to a certain extent. Additionally, we detected changes in HSP60 expression using immunocytochemistry (ICC). The results confirmed that HSP60 expression was upregulated in the lung tissue of mice induced by bleomycin, while its expression significantly decreased after CS treatment (Figure 5J). As a typical mitochondrial molecular chaperone induced by cell stress, HSP60 causes a strong pro-inflammatory reaction in cells of innate immune system and serves as a danger signal for stressed or damaged cells.39, 40 Our findings indicated that CS can target HSP60 to regulate the levels of oxidative damage and inflammation. Furthermore, cordycepin, the principal ingredient of CS,41 could significantly reduce the activities of MC-I and MC-II as well as cellular ROS level, demonstrating its capacity to regulate oxidative phosphorylation and diminish cellular ROS release (Supporting Information S1: Figure S2). In summary, the congruence between the in vitro and in vivo findings consistently suggests that mitochondrial function, particularly the production of ATP and MitROS, may represent the critical targeted pathway responsible for the ameliorative effect of CS on bleomycin-induced pulmonary fibrosis (IPF).
2.5 CS alleviated oxidative damage in lung tissue of pulmonary fibrosis mice
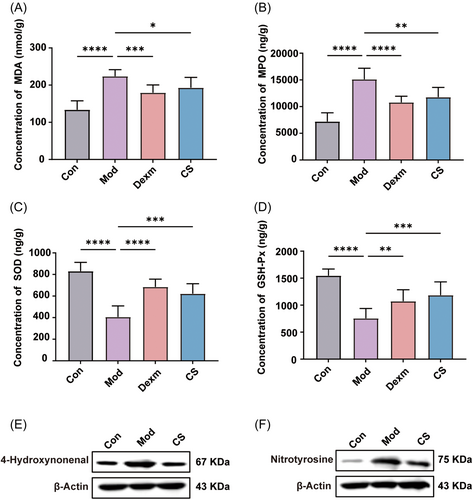
While the pathogenesis of pulmonary fibrosis has yet to be fully elucidated, oxidative stress is widely acknowledged as a prominent factor.42 This oxidative stress arises from a persistent imbalance between the generation of ROS and their clearance by the intracellular antioxidant system.43, 44 As mitochondrial dysfunction or impairment is known to trigger oxidative stress, we hypothesize that the attenuation of mitochondrial-mediated oxidative stress by CS may underlie its pharmacological activity in pulmonary fibrosis. The accumulation of ROS during oxidative stress leads to glycoxidation reactions and lipid peroxidation, with malondialdehyde (MDA) being one of the final products of polyunsaturated fatty acid peroxidation.45 Additionally, myeloperoxidase (MPO), a heme-containing peroxidase highly expressed in monocytes, neutrophils, and macrophages, catalyzes the production of ROS. Excessive MPO activity is closely associated with tissue damage in inflammation, fibrosis, and other diseases.45
As depicted in Figure 6A,B, administration of CS and Dexm significantly reduced the elevated levels of MDA and MPO in lung tissue from bleomycin-induced mice, indicating that CS has the capacity to decrease neutrophil release and alleviate oxidative stress. To further evaluate the impact of CS on the antioxidant system, we assessed the activities of two typical antioxidant enzymes, SOD and glutathione peroxidase (GSH-Px), known for their roles in scavenging free radicals and combating oxidative damage. Administration of CS and Dexm restored the activity of SOD and GSH-Px, which had been compromised by bleomycin in lung tissue, thereby enhancing the antioxidant capacity in the body (Figure 6C,D). Furthermore, it has been reported that lipid peroxidation induced by oxidative stress is closely related to the development of pulmonary fibrosis. Reactive lipid mediators, such as 4-hydroxynonenal (4-HNE) and nitrotyrosine, produced during oxidative stress, serve as the biomarkers of oxidative stress.46, 47 Western blot results showed that the expression of 4-hydroxynonenal and nitrotyrosine in the lung tissue of mice induced by bleomycin was upregulated, while their protein expression significantly decreased after CS treatment. This indicates that CS has the potential to regulate oxidative damage in mice with pulmonary fibrosis (Figure 6E,F). Hence, CS not only protects lung tissues against oxidative stress induced by hyper-activated mitochondrial-mediated oxidative phosphorylation but also promotes the normal function of the antioxidant system.
3 DISCUSSION
Natural products, particularly active ingredients derived from TCM, boast a millennia-old history of clinical utilization. Consequently, an expanding array of natural products, particularly those derived from plants, assume a significant role in the management of inflammatory diseases.48 IPF represents a progressive, chronic lung condition typified by compromised alveolar architecture and aberrant ECM deposition in lung tissues. These pathological alterations culminate in impaired gas exchange, reduced lung compliance, and ultimately respiratory failure and mortality.1-3 The primary treatment approach for pulmonary fibrosis revolves around preventing acute exacerbations, improving lung function, and alleviating oxygen deprivation. Prolonged use of bronchodilators and glucocorticoid medications in IPF treatment heightens the risk of adverse effects, particularly on the immune system.12 Consequently, TCM emerges as a potential alternative therapy for pulmonary fibrosis, owing to its advantages of reduced side effects and pleiotropic effects. CS is extensively documented in ancient pharmaceutical texts for its therapeutic efficacy in lung and kidney diseases.21 Furthermore, CS has been reported to exhibit beneficial effects on the respiratory system, including bronchodilation, asthma relief, phlegm elimination, and emphysema prevention.22 However, the therapeutic potential of CS in pulmonary interstitial fibrosis remains largely unexplored.
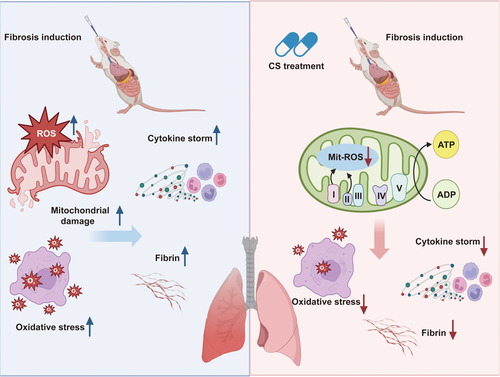
In this study, we discovered that CS effectively reduces airway inflammation and fibrosis in mice with early-stage pulmonary interstitial fibrosis. Notably, 3 weeks of CS treatment significantly improved inflammatory infiltration in alveolar cavities of lung tissue and decreased inflammatory factors in serum. To elucidate the antifibrotic mechanism of CS in pulmonary fibrosis model mice, we conducted proteomics analysis to identify differentially expressed proteins during IPF induction and treatment. Subsequent bioinformatic analysis and biochemical experiments confirmed that CS protects mitochondrial structure and function by suppressing the oxidative phosphorylation process and inhibiting excessive release of ROS. This mechanism helps maintain cellular redox balance and likely contributes to CS's ability to treat IPF (Figure 7).
Oxidative stress has long been implicated in the pathogenesis of IPF.35 This condition arises from an imbalance between oxidants and antioxidants within the body, particularly within the lung, which is particularly vulnerable to oxidative damage.36 The presence of ROS, generated by various oxidants, can inflict damage upon alveolar epithelial cells. Consequently, these cells release inflammatory cytokines and chemokines that promote fibrosis.49 Clinical evidence further substantiates this notion, with IPF patients exhibiting heightened oxidative activity in their bronchoalveolar lavage fluid (BALF). Additionally, a redox imbalance characterized by increased oxidants has been observed in the blood of IPF patients.50 Markers of oxidative stress have been demonstrated to correlate with disease severity in IPF patients.51 Inhibiting the oxidative stress process, such as the synthesis of H2O2 mediated by NADPH oxidase 4 (NOX4), can prevent the transformation of fibroblasts into myofibroblasts.52 Myofibroblasts, being resistant to apoptosis induced by oxidative stress, further exacerbate fibrosis by secreting angiotensinogen and interacting with TGF-β1 to perpetuate oxidative stress.31, 32 Additionally, our research indicates that the over-activation of oxidative phosphorylation in mitochondria, characterized by the high expression and activity of mitochondrial complexes I and II, contributes to ROS production and oxidative stress, thereby promoting the development of IPF. Overall, oxidative stress plays a significant role in driving the pathological processes involved in the development of IPF.
Previous studies have underscored CS's capability to mitigate ROS and treat various diseases. For instance, CS has been demonstrated to mitigate liver fibrosis by scavenging hydroxyl radicals and inhibiting lipid peroxidation.53 In patients with diabetic nephropathy, CS has exhibited efficacy in reducing serum creatinine levels, decreasing the rate of urinary protein excretion, and mitigating inflammation in the kidneys.54 The primary chemical constituents of CS encompass polysaccharides, amino acids, nucleosides, sterols, fatty acids, phenols, flavonoids, and volatile substances. Phenols and flavonoids are notable for their potent antioxidant properties, which entail reducing lipid peroxidation and augmenting the activity of antioxidant enzymes.55 Additionally, the presence of nucleosides in CS contributes to the elimination of hydroxyl radicals. These observations align with our findings, suggesting that CS attenuates IPF in mice through scavenging ROS and suppressing mitochondrial oxidative stress.
There are several limitations in this study that warrant attention in future research endeavors. First, the mechanism underlying IPF is intricate and multifaceted. Apart from oxidative stress, numerous potential factors influence the pathogenesis of IPF. Aging, for instance, has been identified as a significant risk factor for IPF.56 The presence of antiapoptotic aging fibroblasts in IPF leads to the aberrant production of ECM, disrupting cellular function, tissue homeostasis, and promoting the secretion of an aging-related secretory phenotype, ultimately driving the progression of pulmonary fibrosis.57 Targeting aging cells with small molecules has emerged as a promising strategy for alleviating pulmonary fibrosis. ABT263, a dual inhibitor of Bcl2 and BclxL, has shown promising therapeutic effects in both bleomycin-induced mouse models of pulmonary fibrosis and clinical trials.58
Additionally, autophagy represents another significant factor in the pathogenesis of IPF. Reports suggest that autophagy is diminished in lung tissue of IPF patients, leading to the death or apoptosis of autophagy-programmed cells.59 Furthermore, studies have highlighted the role of endoplasmic reticulum (ER) stress in IPF, which impairs alveolar epithelial cell function, promotes pulmonary fibrosis, and triggers the unfolded protein response (UPR).60 Therefore, future investigations should explore whether factors such as aging, autophagy, and ER stress contribute to the therapeutic efficacy of CS in IPF.
Undoubtedly, identifying the drug target holds innovative significance for the development of new drugs. Despite its therapeutic efficacy, the precise therapeutic target of CS remains elusive. Clarifying the therapeutic target of CS would not only elucidate its mechanism of action in treating IPF but also unveil new potential therapeutic avenues.48 Furthermore, it has been reported that combining CS with other drugs can enhance therapeutic outcomes. For example, the combined administration of CS with prednisone and cyclophosphamide has demonstrated improved protection for renal function.61 Therefore, the therapeutic effects with the combination of CS and dexamethasone and the underlying mechanism of the potential synergistic effect remains to be elucidated through further investigation.
4 CONCLUSION
In summary, our research demonstrates that CS effectively mitigates mitROS production, oxidative stress, and inflammation by targeting mitochondrial complexes I and II, thereby exerting therapeutic effects in pulmonary fibrosis. It is noteworthy that further exploration is required to identify the specific components in CS with pivotal roles and elucidate the detailed mechanism by which CS regulates mitochondrial function. Nevertheless, our findings underscore the potential therapeutic value of CS in treating IPF and offer novel insights into expanding its diverse pharmacological activities and understanding the involvement of mitochondria in the pathogenesis of IPF.
5 MATERIALS AND METHODS
5.1 Reagents and materials
The CS extract in Bailing capsules was supplied by Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd. Bleomycin hydrochloride (HY-K1053) and dexamethasone acetate (HY-14648A) were procured from MedChem Express. Iodoacetamide (IAA) and dithiothreitol (DTT) were sourced from Sigma. Trypsin (90057) and other LC-MS/MS-related reagents were acquired from Thermo Fisher. Specific primary antibodies against Ndufs6 (ab108208), Sdhc (ab92508), and HSP60 (ab110312) were obtained from Abcam. TNF-α assay kit (ab208348), IL-6 ELISA kit (ab222503), and IL-1β ELISA kit (ab197742) were sourced from Abcam. Ndufaf1 (H00051103) and Ndufaf3 (PAB22870) antibodies were procured from Abnova. The BCA assay (P0012S) was purchased from Beyotime Biotechnology. Activity kits for MDA, MPO, SOD, and GSH-Px were obtained from Shanghai Enzyme-linked Biotechnology Co., Ltd. Kits for assessing the level of total ROS, Mit ROS, ATP, and MMP were purchased from Nanjing Jiancheng Institute of Biological Engineering. Mitochondrial Complex I Activity Assay kit (ab287847) and Mitochondrial Complex II Activity Assay kit (ab287844) were obtained from Abcam. 4-Hydroxynonenal was purchased from Biosis (bs-6313R) and Nitrotyrosine was obtained from Abcam (ab314438). mtDNA fluorescence quantitative PCR kit was purchased from Biolabs (BTN14-141).
5.2 Analysis of the composition of CS by UHPLC-LTQ-Orbitrap mass spectrometry
First, CS powder form six different batches was accurately weighed and dissolved in 50 mL of 70% methanol. The mixture underwent sonication dispersion for 20 min and was subsequently filtered. Then, 25 mL of the filtered solution was vacuum-dried, yielding a dried powder. This powder was re-dissolved in 25 mL of deionized water, which served as the test solution after undergoing filtration once again. The ingredient analysis was conducted using an online linear ion trap (LTQ) Orbitrap Velos Pro (Thermo Fisher Scientific Inc.) coupled with ultrahigh pressure liquid chromatography (UHPLC) via an electrospray ionization (ESI) interface. Liquid chromatogram separation was performed on an ACQUITY UHPLC HSS T3 column (2.1 mm × 100 mm, 1.8 µm) maintained at a temperature of 30°C. The mobile phases consisted of solvent A (methanol) and solvent B (0.1% formic acid in water), with a gradient elution profile as follows: 0–20 min, 1% A; 20–36 min, 1%–15% A; 36–45 min, 15%–60% A; 45–45.1 min, 60%–95% A. The flow rate was set at 0.3 mL/min, with a sample volume of 10 µL for reference and 8 µL for testing. The ESI source parameters were configured for positive and negative ion scanning modes, with a capillary temperature of 350°C and a spray voltage of 3.5 kV in positive ion mode. The primary mass spectra of the samples were scanned in FT mode with a resolution of 30,000 and a m/z scanning range of 50 to 2000. Secondary and tertiary mass spectra were collected in the data-dependent mode. Data acquisition and analysis were performed using Xcalibur, Metworks, and Mass Frontier 7.0 software.
5.3 Animal experiments
Eight-week-old male Kunming mice weighing approximately 20 g were procured from Beijing Vital River Laboratory Animal Technology Co., Ltd. The animal experimental procedures were ethically approved by the Ethics Committee of the Experimental Animal Center of the Chinese Academy of Traditional Chinese Medicine (Approval No. 42020005322). Upon arrival, the mice were acclimatized for 3 days under controlled environmental conditions, including humidity (60%–80%), temperature (21 ± 3°C), and a 12-h light/dark cycle, with ad libitum access to standard diet and water. Following the acclimatization period, the mice were randomly allocated into four groups (n = 5 per group) as follow: control group (Con), bleomycin-treated group (BLM, 5 mg/kg), dexamethasone-treated group (Dexm, 5 mg/kg), Bailing capsule treated group (CS, 5 g/kg). The gavage doses of for CS in mice were determined as 1 and 5 g/kg/d referring to the recommend doses in previous reports.21 Dexamethasone was chosen as the positive control for pulmonary fibrosis treatment, aligning with previous studies citing its high efficacy, biological potency, and minimal sodium retention.28, 29 Anesthesia was induced in all mice via intraperitoneal injection of pentobarbital sodium before fixation on the operating table for tracheal instillation. A surgical incision was made in the skin near the neck, and 100 μL of bleomycin solution (5 mg/kg) or normal saline (control) was slowly injected into the detached trachea, followed by wound closure. Starting from the following day, the designated treatment drugs were administered to each group via oral gavage once daily for a duration of 3 weeks. On Day 21, all mice were euthanized, and lung tissue and serum samples were collected for subsequent analysis.
5.4 Histopathology and Masson's trichrome staining
The left lung tissue of mice was fixed in a 4% paraformaldehyde solution, followed by staining with hematoxylin-eosin solution (H&E) and Masson's Trichrome staining kits as per the manufacturer's instructions. For immunocytochemistry, tissue slices were incubated overnight at 4°C with primary antibodies against TGF-β, α-SMA, and Col1a1, followed by a 2-h incubation with a secondary antibody at room temperature. Subsequently, the sections were dehydrated, mounted, and imaged using a digital slide scanner (Nanozoomer-SQ; Hamamatsu Photonics). Analysis was performed using an inverted fluorescence microscope (Olympus) equipped with the MShot image analysis system.
5.5 Enzyme-linked immunosorbent assay assay
The proinflammatory cytokines (TNF-α, NO, IL-6, and IL-1β) in mice serum were quantified using commercially available kits following the manufacturer's instructions.
5.6 Evaluation of oxidative stress
We assessed the activity of antioxidant enzymes (GSH-Px, SOD, and MPO) and the level of MDA in lung tissues using kits in accordance with the manufacturer's instructions.
5.7 Cell culture
BEAS-2B cells were procured from the Chinese Academy of Medical Sciences (Beijing) and maintained in high-glucose Dulbecco's modified Eagle's medium (Corning) supplemented with 10% FBS (Corning) and 1% (v/v) penicillin/streptomycin (Thermo Fisher) at 37°C in a 5% CO2 atmosphere.
5.8 Assays for ATP and MMP measurement
BEAS-2B cells were seeded at a density of 4 × 103 cells per well in 96-well plates and incubated for 1 day. Following incubation, the cells were treated with bleomycin (40 μg/mL) or CS (250 μg/mL) for 24 h. The ATP and MMP levels were assessed using kits in accordance with the manufacturer's instructions.
5.9 RNA isolation and quantitative real-time reverse-transcription polymerase chain reaction
Total RNA was extracted from mouse lung tissues using TRIzol reagent (Invitrogen) and subsequently reverse-transcribed into cDNA using the TransScript® II Green Two-Step qRT-PCR SuperMix kit (Transgen Biotech). Amplification plots were analyzed, and dissociation curves were examined to ensure data accuracy. Invitrogen synthesized primers for PCR amplification, and their sequences were as follows: for mouse Ndufs6, forward: 5′-GGGGAAAAGATCACGCATACC-3′, reverse: 5′-CAAAACGAACCCTCCTGTAGTC-3′; for mouse Ndufaf1, forward: 5′-CTCTAATCGAGGAAGAATCCG-3′, reverse: 5′-AGAATGGACCATCCACTTT-ATC-3′; for mouse Ndufaf3, forward: 5′-GTGGTCCAGTGGAACGTGG-3′, reverse: 5′-CTCCTGTCACTCGACCTTCG-3′; for mouse Sdhc, forward: 5′-GGGGAATT-CATGGCTTTCTTGCTGAGACATGTCAGC-3′, reverse: 5′-GGGAAGCTTT-CACAGGGCGGCCAGCCC-3′. The relative mRNA levels were normalized to β-actin mRNA.
5.10 Total and mitochondrial ROS measurement
The level of total ROS in mouse lung tissues was evaluated using the ROS detection kit from Beyotime and a fluorescence microplate reader. MitROS levels in the lung tissues were determined after isolating mitochondria with the Beyotime mitochondria isolation kit. The MitROS levels were assessed using the mitochondrial ROS detection kit from Beyotime and were normalized by protein amount. Additionally, the total ROS and MitROS levels in BEAS-2B cells were quantified using the total ROS detection kit and the mitochondrial superoxide kit from Beyotime, respectively.
5.11 Assay for fluorescent staining of ROS
BEAS-2B cells (1 × 105/well) were plated in six-well plates and allowed to culture for 1 day. Subsequently, they were exposed to either bleomycin (40 μg/mL) or CS (250 μg/mL) for a duration of 24 h. After three washes with phosphate-buffered saline (PBS), the cells were sequentially treated with DCFH-DA probe (10 μM, 15 min) and DAPI (5 μg/mL, 15 min) at 37°C in a CO2 incubator. Following three additional PBS rinses, the cells were visualized under a fluorescence microscope (Olympus).
5.12 Western blot analysis
Mouse lung tissues were lysed using RIPA buffer supplemented with protease inhibitors (Sigma-Aldrich). The protein concentration was determined using the Pierce BCA Protein Assay Kit. Subsequently, 20 μg of proteins were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. After blocking, the membranes were incubated with primary antibodies against β-actin, Ndufs6, Ndufaf1, Ndufaf3, Sdhc, 4-hydroxynonenal and Nitrotyrosine at a dilution of 1:1000, followed by secondary antibody incubation. Protein bands were visualized using electrochemical luminescence (ECL)detection reagents, and Image J software (V1.8.0.112) was utilized for density analysis.
5.13 Transmission electron microscope
BEAS-2B cells were initially fixed in 2.5% glutaraldehyde for 2 h, followed by a 2 h fixation in 1% osmium tetroxide. Subsequently, the samples were sectioned into 50–60 nm slices, stained with 3% uranium acetate and lead citrate, and then analyzed and imaged using a transmission electron microscope (JEM-1011).
5.14 Activity of mitochondrial complex I and II in purified mitochondria from lung tissues and BEAS-2B cells
The level of mitochondrial Complex I and II in purified mitochondria from mice lung tissues were detected using the Mitochondrial Complex I and II Activity Assay kits (Abcam) following the manufacturer's instructions. Mitochondria were isolated using the Beyotime mitochondria isolation kit before the assay. For BEAS-2B cells, the cells were exposed to either bleomycin (40 μg/mL) or CS (250 μg/mL) for 24 h after seeding in six-well plates. Subsequently, the Mitochondrial Complex I and II Activity Assay kits were used according to the manufacturer's instructions.
5.15 Immunofluorescence images of HSP60
BEAS-2B cells cultured in a confocal dish, and treated with either bleomycin (40 μg/mL) or CS (250 μg/mL) for 24 h. Then cells were fixed and the exposed to anti-HSP60 (1:1000) at 4°C overnight, goat anti-rabbit IgG (1:1000) (Alexa Fluor 488) for 2 h, and DAPI (5 μg/mL) for 15 min at 37°C in a CO2 incubator. Following three additional PBS rinses, the cells were visualized under a fluorescence microscope (Olympus).
5.16 Statistical analysis
All data were presented as mean ± standard deviation (SD) and analyzed using Student's t-test and analysis of variance test. Statistical analysis was conducted using GraphPad Prism 8.0 software (GraphPad Prism). Statistical significance was determined at *p < 0.05, **p < 0.01, ***p < 0.001, or ****p < 0.0001.
AUTHOR CONTRIBUTIONS
Ying Zhang: Formal analysis (equal); investigation (equal); writing—original draft (equal). Lirun Zhou: Formal analysis (equal); investigation (equal). Guangqing Cheng: Formal analysis (equal); methodology (equal). Yanyan Zhou: Formal analysis (equal); methodology (equal). Qiuyan Guo: Formal analysis (equal). Jiangpeng Wu: Investigation (equal); methodology (equal). Yin K. Wong: Formal analysis (equal); writing—review and editing (equal). Junzhe Zhang: Investigation (equal); methodology (equal). Huan Tang: Conceptualization (equal); formal analysis (equal); supervision (equal); writing—review and editing (equal). Jigang Wang: Conceptualization (equal); funding acquisition (equal); supervision (equal); writing—review and editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from the CACMS Innovation Fund (CI2023E002-Y-30, CI2023E005TS01, CI2023D003, CI2021B014 and CI2021A05101), the National Key Research and Development Program of China (2020YFA0908000), the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202002), National Natural Science Foundation of China (32201177 and 82074098), the Fundamental Research Funds for the Central public welfare research institutes (ZZ14-YQ-061 and ZZ16-ND-1024), the Science and Technology Foundation of Shenzhen (Shenzhen Clinical Medical Research Center for Geriatric Diseases). Schematic illustrations presented in Figure 1A, Figure 2A, Figure 4M, Figure 7, and the Graphical Abstract were crafted using Adobe Illustrator and the online software BioRender. We extend our gratitude to the developers of these software tools for their valuable contributions.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The animal experiment was approved by the Ethics Committee of Experimental Animal Center of Chinese Academy of Traditional Chinese Medicine (Approval No. 42020005322).
Open Research
DATA AVAILABILITY STATEMENT
Original data will be provided upon request.




