Mitochondrial Dysfunction in Aging: Future Therapies and Precision Medicine Approaches
Lanlan Jia and Ziyu Wei contributed equally to this study.
ABSTRACT
Mitochondria are the primary energy hubs of cells and are critical for maintaining cellular functions. However, aging leads to a decline in mitochondrial efficiency. This decline is marked by increased reactive oxygen species, accumulation of mitochondrial DNA mutations, impaired oxidative phosphorylation, and breakdown of mitochondrial quality control systems. Such changes are associated with the development of neurodegenerative, cardiovascular, and metabolic diseases. Although much research has been done, the precise connection between mitochondrial dysfunction and aging remains unclear. Furthermore, current literature exhibits a lack of systematic organization regarding the mitochondria-targeted therapeutic interventions. This review systematically explores the mechanisms underlying mitochondrial deterioration during aging. Key focuses include impaired biogenesis, disrupted dynamics, dysregulated stress responses, and defective clearance of damaged mitochondria. Additionally, this review explores innovative therapeutic strategies for these mitochondrial problems, including a combination of nanodelivery systems, artificially intelligent drug-screening techniques, and cutting-edge tools, such as CRISPR/Cas9 gene editing. By integrating recent advances in mitochondrial biology, this review provides a comprehensive framework that bridges basic mechanisms with clinical applications. The insights presented here underscore the potential of precision mitochondrial medicine as a novel approach to combating age-related disorders, enhancing our capacity to address age-related diseases, and foster healthy aging.
1 Introduction
The global population is aging rapidly. By 2050, over 1.5 billion people—about 16% of the world's population—are projected to be aged 65 or older [1]. Consequently, the prevalence of considerable mortality and disability stemming from age-related diseases is also anticipated to rise. Such diseases encompass neurodegenerative disorders, cardiovascular conditions, metabolic disorders, immune system dysfunctions, and various cancers [2]. However, the underlying mechanisms of these age-related diseases are not yet fully comprehended due to the complex interplay of multiple risk factors. Given the accelerating pace of population aging, it becomes imperative to develop effective therapeutic approaches in a timely manner. This is crucial not only for promoting human longevity but also for enhancing the overall quality of life for individuals worldwide.
Mitochondria are essential organelles in eukaryotic cells. They function as the primary sites for cellular energy conversion and are often termed the “energy factories” of the cell. However, with advancing age, mitochondrial functionality tends to decline. This decline involves accumulated mitochondrial DNA (mtDNA) mutations, impaired proteostasis that weakens respiratory chain complexes, reduced organelle turnover, and altered mitochondrial dynamics. These changes compromise the mitochondria's role in energy production, elevate the generation of reactive oxygen species (ROS), and may lead to unintended mitochondrial membrane permeabilization, potentially resulting in inflammation and cellular apoptosis [3]. The deterioration of mitochondrial function correlates with aging and a myriad of age-related diseases, including cancer, neurodegenerative disorders, metabolic conditions, and renal diseases [4-6]. Mitochondrial dysfunction and related ROS production have been observed in stress-induced senescence [7, 8], replicative senescence [9], oncogene-induced senescence [10], and senescence triggered by genetic telomere uncapping [8]. The link between mitochondrial dysfunction and cellular senescence remains complex. However, targeted ablation of mitochondria from senescent cells can reverse several features of the senescent phenotype [11].
Mitochondrial quality control (MQC) is essential for regulating ROS levels. Key MQC components include (1) mitochondrial proteases that degrade misfolded proteins [12], (2) the mitochondrial unfolded protein response (UPRmt), which refolds misfolded proteins [13], and (3) mitochondria-derived vesicles (MDVs) and mitophagy for removing damaged mitochondria [14]. Furthermore, the structural integrity of mitochondria is preserved through the balance of mitochondrial fission and fusion, which prevents either excessive elongation of the mitochondrial network or fragmentation [15]. Nowadays, MQC is gaining attention in antiaging research due to its close association with aging through functional deficiencies.
Recent advancements in aging research have increasingly demonstrated that addressing mitochondrial dysfunction through various treatments and interventions can effectively extend health span and delay the onset of age-related diseases [16]. For instance, calorie restriction interventions have been shown to reduce ROS produced by mitochondria, thereby preventing oxidative stress and potentially increasing lifespan [17]. Additionally, resveratrol has been found to enhance muscle performance in older individuals by promoting mitochondrial mass and elevating key factors, such as nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM) [18, 19]. Moreover, metformin has emerged as an inhibitor of complex I within the mitochondrial electron transport chain (ETC), leading to a reduction in ROS production and a mitigation of aging hallmarks [20, 21]. Rapamycin, a known inhibitor of the mechanistic target of rapamycin complex 1 (mTORC1), has also been associated with improved mitochondrial biogenesis and increased lifespan in the mice model [22].
This review systematically examines the role of mitochondrial homeostasis in aging processes. Declining mitochondrial function contributes to aging-related diseases. Excessive ROS production from dysfunctional mitochondria causes cellular damage, while accumulated mtDNA mutations and impaired MCQ exacerbate this harm. We discuss mitochondrial biogenesis, dynamics, stress responses, and the removal of damaged mitochondria during cellular senescence. We also review the current advances in mitochondria-targeted therapeutic interventions aimed at addressing age-related diseases. Meanwhile, with the rapid and ongoing development of artificially intelligent (AI) technology, we summarize the recent application of nanodelivery systems and CRISPR/Cas9 in disease treatment and explore the emerging prospect and potential of the combined application of the three. In summary, exploring the interplay between aberrant mitochondrial function and the mechanisms underlying various aging-related diseases holds significant potential for unraveling the complexities of aging. On this basis, it is expected that more targeted and efficient antiaging treatment strategies will be developed in the future to achieve precision and efficiency in the treatment of aging-related diseases.
2 The Mitochondrial Dysfunction in Aging
2.1 ROS and mtDNA Mutations in Aging
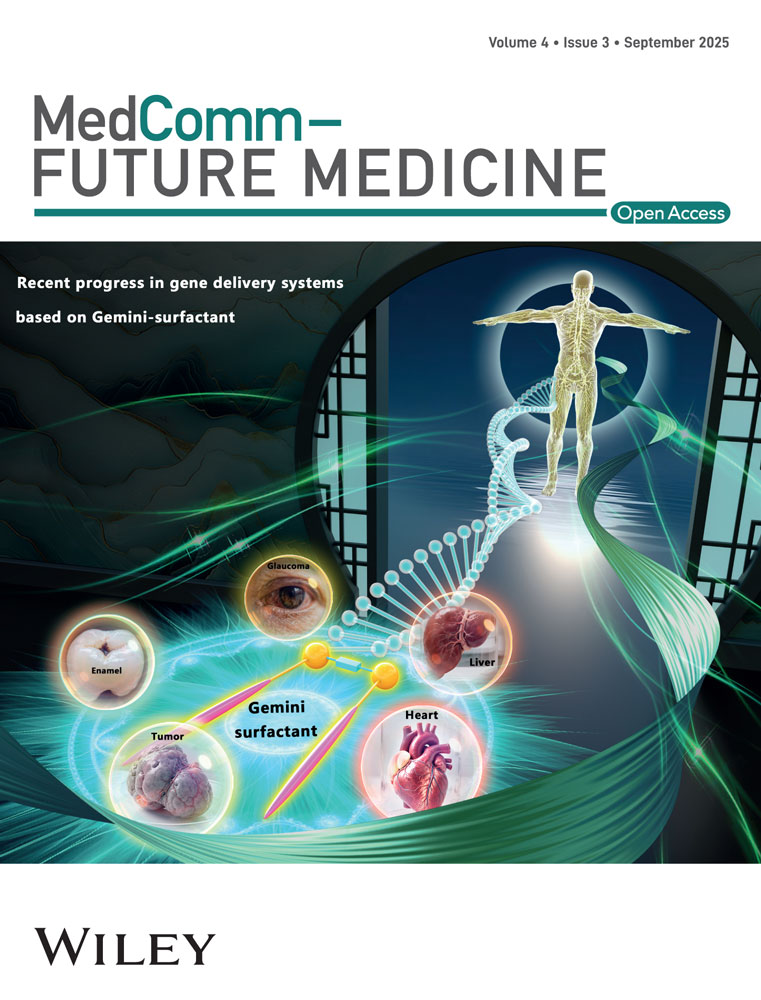
Aging is a multifaceted process driven by progressive damage accumulation, leading to declines in biological and metabolic functions. Beyond genetic and metabolic changes, elevated ROS levels are critical triggers of cellular senescence. ROS encompasses a group of highly reactive molecules, which include free radicals such as the superoxide anion (O2·−) and the hydroxyl radical (·OH), as well as nonradical derivatives like stable hydrogen peroxide (H2O2). Among these, the hydroxyl radical is recognized as the most deleterious form of ROS, as it exerts oxidative damage on virtually all classes of biomolecules within the cell, including lipids, proteins, and nucleic acids [23]. ETC is the primary source of cellular ROS (Figure 1). Additionally, endogenous factors such as phosphatases and cytochrome, along with exogenous agents like air pollution, food and nutrients, smoking, chemicals, and ultraviolet light, can also contribute to ROS production [24].
Unlike other organelles, mitochondria possess their own DNA (called mtDNA), which is required for producing respiratory chain proteins and adenosine triphosphate (ATP) synthases [25, 26]. Researches indicated that both the quantity of mitochondrial proteins and the activity of the respiratory chain decrease with age [27, 28]. The proximity of mtDNA to the respiratory chain renders it vulnerable to high levels of ROS, compounded by the absence of histone protection, making it particularly susceptible to oxidative damage. With an accumulation of ROS within the mitochondria, this can ultimately result in mtDNA mutations [29]. The buildup of such mutations in somatic progenitor cells is associated with early-onset sexual dysfunction, and the DNA repair mechanisms in these models frequently demonstrate inefficiencies, failing to address nucleotide anomalies in a timely manner, which leads to the emergence of aging-related phenotypes [30].
Mitochondria amplify oxidative stress through four mechanisms: (a) age-related ROS overproduction due to functional decline, (b) reduced ROS-scavenging enzyme activity, (c) accumulation of mtDNA mutations, and (d) a vicious cycle where mtDNA mutations impair the respiratory chain, exacerbating ROS production and oxidative damage [31-33]. When the balance between ROS production and neutralization is disrupted, oxidative stress will be accelerated. This oxidative stress can lead to excessive oxidation of proteins, resulting in the degradation of enzymes, which increases with aging and various pathological conditions [32]. Furthermore, oxidative stress induced by ROS contributes to the depletion of antioxidant enzymes, thereby accelerating the aging process [34]. Within mitochondria, compromised antioxidant enzymes increase mtDNA mutation frequencies and exacerbate oxidative stress, which diminishes the efficiency of oxidative phosphorylation (OXPHOS) and ATP synthesis [35]. And when the expression of related proteins involved in complex Ⅲ is reduced, such as tetratricopeptide repeat domain 19 (TTC19), it increases the risk of Parkinson's disease [36]. Thus, abnormal expressions of the respiratory chain complex and impaired energy metabolism lead to further production of ROS and acceleration of aging-related diseases.
2.2 Mitochondrial Quality Control in Aging
Although ROS production is unavoidable, MQC plays a crucial role in sustaining mitochondrial function and homeostasis [37]. MQC manages the disposal of damaged components within mitochondria through several mechanisms, including mitochondrial proteases, UPRmt, MDVs, and mitochondrial autophagy or mitophagy. Because of the presence of MQC, damaged or misfolded polypeptides are eliminated by mitochondrial proteases, while UPRmt facilitates the refolding and degradation of misfolded proteins within mitochondria [38]. Additionally, MDVs and mitophagy are responsible for the removal of impaired mitochondria and oxidized by-products [13, 39]. However, if MQC becomes unbalanced due to the excessive accumulation of ROS, mitochondria may be unable to expeditiously eliminate damaged components, ultimately leading to cellular senescence and apoptosis [40, 41]. Targeting MQC therapeutically is critical. Unraveling its mechanisms may identify novel targets to extend healthspan.
2.2.1 PGC-1α-Mediated Mitochondrial Biogenesis
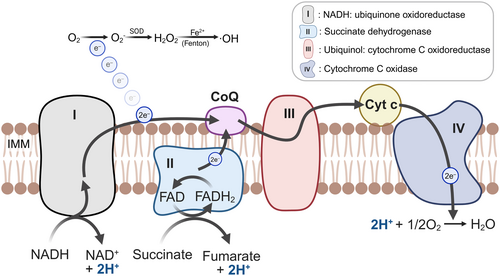
Mitochondrial biogenesis is the self-renewal process producing new mitochondria from existing ones, primarily regulated by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) through activating transcription factors for mitochondrial gene expression [42]. It plays a critical role in delaying aging by regulating mitochondrial gene expression, metabolic homeostasis, and antioxidant defense system [43]. Elevated ROS levels can activate PGC-1α via AMP, adenosine monophosphate (AMP)-activated protein kinase (AMPK) through two distinct pathways. First, AMPK directly phosphorylates PGC-1α, resulting in its activation. Second, AMPK enhances the levels of NAD+/NADH, facilitating the phosphorylation and activation of sirtuin 1 (SIRT1), which subsequently activates PGC-1α through its deacetylation [44]. Furthermore, SIRT1 may directly activate TSC2, an inhibitor of the mammalian target of rapamycin (mTOR), thereby inhibiting mTOR activity and promoting mitochondrial autophagy [45]. This process helps prevent the accumulation of damaged mitochondria and serves as an antiaging mechanism. PGC-1α activates nuclear factors, including NRF1 and -2, estrogen-related receptor-α, and peroxisome proliferator-activated receptors, which regulate TFAM [46]. Additionally, The phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway and calcium/calmodulin-dependent protein kinase II activate NRF1/2 and PGC-1α, respectively [47, 48]. Ultimately, it stimulates an increase in mtDNA in response to mitochondrial biogenesis (Figure 2) [49].
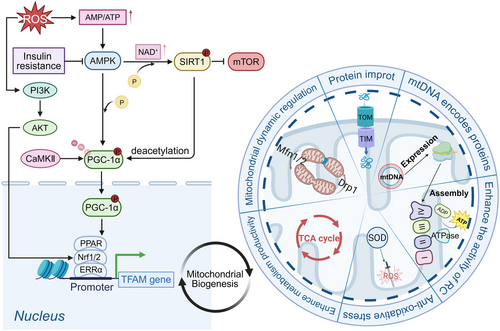
2.2.2 Mitochondrial Dynamics: Regulatory Mechanisms of Fusion and Fission in Aging
Mitochondrial dynamics encompass fission and fusion processes, both of which are essential for mitochondrial functionality and cellular metabolism. Mitochondrial fission enables the segregation of damaged mitochondria for timely phagocytosis, whereas mitochondrial fusion facilitates the exchange of contents between mitochondria. These processes are vital for sustaining normal mitochondrial function and morphology. An imbalance between fission and fusion can result in mitochondrial dysfunction and contribute to age-related diseases. Mitochondrial fission is predominantly regulated by dynamin-related protein 1 (Drp1), which is recruited by mitochondrial fission 1 (Fis1), mitochondrial fission factor, and mitochondrial dynamics protein of 49/51 kDa (Mid49/51) and needs assistance from the endoplasmic reticulum (ER) [50]. Mitochondrial fusion is primarily mediated by mitofusin 1 and 2 (Mfn1/2) in the outer mitochondrial membrane (OMM) and optic atrophy 1 (OPA1) in the inner mitochondrial membrane (IMM) [15, 51, 52] (Figure 3).
Various dietary habits and lifestyle choices exert distinct influences on mitochondrial fission and fusion. Intermittent fasting, characterized by reduced energy intake, leads to the activation of AMPK and the inhibition of mTOR, ultimately promoting mitochondrial fusion and inhibiting mitochondrial fission [53]. Furthermore, high-intensity intermittent exercise has been found to elicit comparable effects by enhancing PGC-1α and AMPK activity [54]. Mitochondrial fragmentation is frequently observed in insulin-resistant patients and may exacerbate insulin resistance and impair glucose metabolism, particularly in hybrid cells harboring mitochondrial haplogroup B4 [55]. This phenomenon potentially contributes to the onset and progression of diabetes. To this end, we summarized the abnormalities of mitochondrial dynamics in various aging-related diseases (Table 1).
| Diseases | Protein | Expression | Mechanism | Reference |
|---|---|---|---|---|
| Huntington's disease (HD) | Drp1 | Increased | Huntingtin protein interacts with Drp1 and enhances Drp1 enzyme activity. Oxidative damage to DNA was found in cortical specimens from patients. | [56, 57] |
| Fis1 | Increased | |||
| Mfn1/2 | Decreased | |||
| OPA1 | Decreased | |||
| Alzheimer's disease (AD) | Drp1 | Decreased | Mitochondrial Drp1 levels are comparable and show a high degree of phosphorylation at Ser616, resulting in a mitochondrial bias toward fission. Meanwhile, amyloid-β induces Drp1 S-nitrosylation, enhancing its activity. The distribution of mitochondria away from axons in pyramidal neurons of the patient's brain may also be associated with low levels of cytoplasmic Drp1. | [58, 59] |
| Fis1 | Increased | |||
| Mfn1/2 | Decreased | |||
| OPA1 | Decreased | |||
| Parkinson's disease (PD) | Drp1 | Decreased in CNS | Increased mitochondrial distance from Drp1 and significantly elevated phosphorylation levels at the Ser637 site inhibit Drp1 fission activity. Changes in the levels of Fis1 and Mfn1/2 can be considered as compensatory enhancement of fission. In addition, alpha-synuclein pathology further damages the central nervous system (CNS) by exacerbating Drp1 functional defects. | [60] |
| Fis1 | Increased in Cortex | |||
| Mfn1/2 | Decreased in CNS | |||
| Amyotrophic lateral sclerosis (ALS) | Drp1 | No change | ROS-induced CaMKII oxidation activates Drp1 phosphorylation at Ser616. The increased levels of Mfn1/2 might be a compensatory response to the organism. The above data were from mouse models. Similarly, Drp1 was detected in the motor cortex of postmortem ALS patients with the same results, but the expression of Mfn1/2 and OPA1 was not increased. | [61, 62] |
| Mfn1/2 | Increased | |||
| OPA1 | No change | |||
| Lung adenocarcinoma (LUAD) | Drp1 | Increased | Mitochondrial fusion and fission are accelerated in cancer cells. OPA1 plays a leading role in the development of LUAD, maintaining the mitochondrial membrane potential (MMP) stability and ATP supply of cancer cells and promoting immune escape and migration of cancer cells. | [63] |
| Fis1 | Decreased | |||
| Mfn1 | Increased | |||
| OPA1 | Increased | |||
| Nonsmall cell lung cancer (NSCLC) | Drp1 | Decreased | Increased level of phosphorylation of Drp1. Enhanced recruitment of Drp1 to the mitochondrial membrane by Fis1. | [64] |
| Fis1 | No change | |||
| Mfn1/2 | No change | |||
| OPA1 | No change | |||
| Heart failure (HF) | OPA1 | Decreased | OPA1 reduction induces apoptosis with DNA fragmentation, heightens ischemia sensitivity, and causes irreversible damage to cardiomyocytes. | [65] |
| Ischemia/reperfusion (I/R) hypoxia/reoxygenation (H/R) | Drp1 | Increased | Drp1 is transferred from the cytoplasm to the mitochondria. Mitophagy-related proteins are downregulated, such as the PTEN-induced kinase 1, Parkin, and microtubule-associated protein 1 light chain 3, which suggest enhanced mitochondrial fission and attenuated fusion and mitophagy. | [66] |
| OPA1 | Decreased | |||
| Diabetic cardiomyopathy | Mfn2 | Decreased | Increased oxidative stress, increased apoptotic index, and decreased SOD activity. | [67] |
| Drp1 | Late slight decline | |||
| Fis1/OPA1 | No change | |||
| Pulmonary arterial hypertension (PAH) | Drp1 | Increased | Enhanced mitochondrial fission increases the pulmonary artery smooth muscle cell proliferation and decreases apoptosis, and downregulation of PGC-1α indicates impaired mitochondrial biogenesis. | [68, 69] |
| Fis1 | Increased | |||
| Mfn1 | No change | |||
| Mfn2 | Decreased |
- Abbreviations: ATP, adenosine triphosphate; CaMKⅡ, calcium/calmodulin-dependent protein kinase II; Drp1, dynamin-related protein 1; Fis1, fission 1; Mfn1/2, mitofusin 1 and 2; OPA1, optic atrophy 1; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; ROS, reactive oxygen species; SOD, superoxide dismutase.
High levels of Drp1 and Fis1 were demonstrated in a mouse model of rapid aging, and after curcumin treatment, the levels of OPA1 and Mfn1 were significantly increased, and the levels of Fis1 were significantly reduced and improved the abnormal mitochondrial function in the model mice [70]. Unusually, however, Hong et al. found that miR-155-5p inhibited mitochondrial fission and increased mitochondrial fusion in mesenchymal stem cells through the low pDrp1 and high Mfn2, leading to cellular senescence [71]. Similarly, vascular progenitor cells in Mavan's synthesis show signs of aging with the consistent results described above [72]. These studies demonstrate that mitochondrial dynamics—governing structure and function—are regulated by diverse factors, including bioenergetic status, oxidative stress levels, and cellular differentiation. This complexity precludes a universal fission/fusion balance (Figure 4).
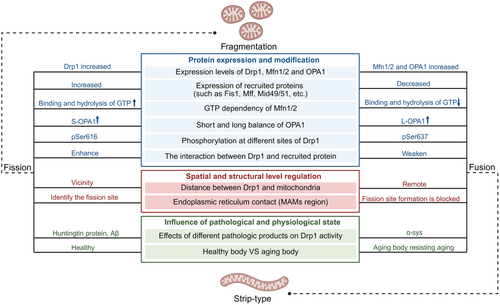
In healthy cells, frequent mitochondrial fission ensures energy supply, particularly in high-demand cells like neurons. Conversely, aged cells may favor mitochondrial fusion to protect functional mitochondria, serving as an antiaging mechanism. However, this compensatory mechanism will eventually disappear as the organism ages.
2.2.3 UPRmt: Adaptive Mechanisms to Aging-Related Stress
UPRmt is a protective mechanism. It activates when mitochondrial integrity and function are disrupted [73]. By enhancing cellular survival and restoring the mitochondrial network, UPRmt ensures optimal cellular homeostasis.
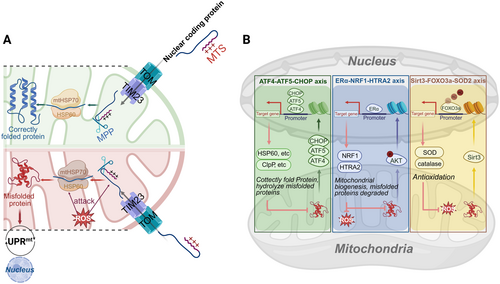
Most mitochondrial proteins are nucleus-encoded, synthesized in cytoplasmic ribosomes, and imported into mitochondria for folding and modification [74].
- 1.
Activating transcription factor 4 (ATF4)–activating transcription factor 5 (ATF5)–C/EBP homologous protein (CHOP) axis: Increased expression of ATF4/5 and CHOP in mammals initiates UPRmt [78], which increases the enhancement of mitochondrial chaperone proteins (e.g., HSP60 and mtHSP70) and protein hydrolases (e.g., caseinolytic protease P [ClpP] and ion protease 1) synthesis, thereby helping to correctly fold protein and degrade the misfolded protein, respectively [79].
- 2.
Estrogen receptor alpha (ERα)–NRF1–high temperature requirement A2 (HTRA2) axis: Phosphorylation of AKT during stress promotes ERα activity, which in turn upregulates the transcription of NRF1 and HTRA2 [80]. HTRA2 degrades misfolded proteins, especially those aggregated in the inner membrane space, and maintains MMP and ATP levels [80, 81].
- 3.
Sirtuin 3 (Sirt3)–forkhead box O3a (FOXO3a)–SOD2: Sirt3 reduces ROS accumulation by deacetylating the FOXO3a transcription factor, facilitating its translocation to the nucleus, and attenuating the accumulation of ROS by upregulating antioxidant enzymes (e.g., SOD2 and catalase [CAT]) [80].
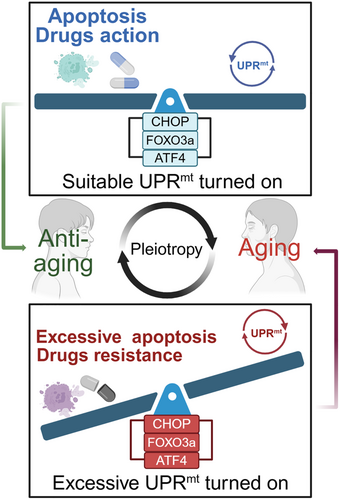
Recent advancements in understanding the regulation, mechanisms, and functions of UPRmt have elucidated significant and intricate connections to aging and age-related diseases. In dopaminergic neurons of alpha-synuclein (α-syn) A53T mice and postmortem brains of Parkinson's disease (PD) patients, overexpression of ClpP reduced αSyn-induced mitochondrial oxidative stress by increasing SOD2 levels [82]. But several studies have been published to indicate that the genes involved in UPRmt challenge cell survival: CHOP is aberrantly expressed in BioBreeding diabetes-prone mice, accompanied by activation of caspase-3 cleavage, which induces T-cell apoptosis, and these phenomena are dependent on a diabetic background [83]. After nuclear translocation of FOXO3a, B-cell lymphoma 2 (Bcl-2)-interacting mediator of cell death (Bim) and cleaved caspase-3 expression are induced which causes neuronal apoptosis [84]; ATF4 promotes cell survival and migration in the tumor microenvironment, and also enhances tumor resistance by inhibiting iron death, such as gemcitabine [85]. Thus, activation of UPRmt has a positive effect on aging-related diseases to a certain extent, but at the same time, overactivation of UPRmt can cause severe cellular damage and accelerate aging due to the pleiotropic nature of the genes involved (Figure 6). This also makes it difficult to develop UPRmt-targeted drugs.
2.2.4 Mitophagy: A Key Pathway for Mitochondrial Homeostasis and Antiaging
Mitophagy represents an evolutionarily conserved cellular process that eliminates dysfunctional or redundant mitochondria, thereby optimizing mitochondrial numbers and sustaining energy metabolism [86]. Recent proposals suggest that deficient mitophagy may constitute a hallmark of aging [87]. As observed in numerous MQC conditions, there is a decline in phagocytic efficiency with advancing age [35]. Mitophagy is a response to various types of stress; in addition to oxidative stress, nutritional stress also plays a contributory role in this process [88]. This mechanism can be mediated by PTEN-induced kinase 1 (PINK1)/Parkin pathway, mitophagy receptors, or alterations in mitochondrial lipid composition. PINK1/Parkin-dependent mitophagy is initiated following a reduction in MMP due to mitochondrial damage. This triggers the stabilization of PINK1 on the OMM, where it phosphorylates ubiquitin, subsequently recruiting the E3 ubiquitin ligase Parkin [89]. Phosphorylated and ubiquitinated Parkin translocates from the cytoplasm to the OMM, where it ubiquitinates OMM proteins, such as voltage-dependent anion channel (VDAC), mitochondrial Rho GTPase (MIRO), and Mfn1/2 [90]. Such polyubiquitinated mitochondria then interact with autophagy adapter proteins, which are mainly sequestosome 1, neighbor of BRCA1 gene 1, nuclear dot protein 52 kDa, and optineurin (OPTN) [91], which recognize and associate with the ubiquitinated mitochondria through their ubiquitin-binding domains and delivered to the autophagosome through a conserved amino acid motif (WXXL) of light chain 3 (LC3) [92, 93], which promotes mitochondrial degradation (Figure 7).
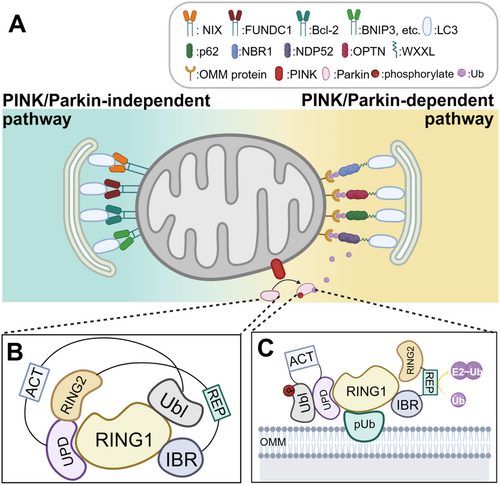
The PINK1/Parkin-independent mitochondrial phagocytosis pathway is dependent on the activity of a group of OMM-localized receptors that include B-cell lymphoma 2 interferon 3-like, FUN14 structural domain-containing 1, Bcl-2/adenovirus E1B 19 kDa interacting protein 3, autophagy and Beclin-1 regulator 1, and break-Schizophrenia-1 [94]. These receptors are like the PINK1/Parkin-dependent mitochondrial phagocytosis pathway, which eliminates damaged mitochondria by interacting with LC3 (Figure 7). Inadequate expression of these OMM receptors' mRNA may impair mitochondrial autophagy, leading to mitochondrial dysfunction and cellular senescence [95].
The mitochondrial precursor overaccumulation stress (mPOS) pathway also leads to the accumulation of damaged mitochondria, disrupting cytoplasmic protein homeostasis and accelerating degenerative diseases [96]. Mitophagy can clear low MMP, mitigating mPOS-induced damage and serving as a potential intervention strategy.
2.2.5 Elimination of Oxidized Proteins by MDVs
MDVs represent an emerging component of MQC, with implications ranging from mitochondrial biology to immune signaling [97]. MDVs are membranous protrusions originating from healthy mitochondria and are characterized as extracellular vesicles (EVs) with an approximate diameter of 100 nm. The production of ROS leads to the oxidation of lipid coenzyme Q, resulting in alterations to the mitochondrial membrane structure and the formation of membrane curvature. Through the activation of PINK1 and the recruitment of Parkin, MDVs encapsulate oxidatively damaged proteins, facilitating their eventual formation and release [98]. Both MDVs and mitophagy play pivotal roles in the clearance of oxidized proteins, thereby preventing more extensive protein loss [14]. However, the accumulation of oxidized proteins that surpass the scavenging capacity of MQC can adversely affect its functionality [99]. Furthermore, MDVs serve as a crucial link within MQC, contributing to mitochondrial autophagy and immunoregulatory processes. Researches indicate that mitochondrial antigen presentation in immune cells can proceed in the absence of PINK1 or Parkin, attributed to the production and transport of MDVs. Consequently, PINK1/Parkin deficiency associated with PD results in MQC disruption and neuroinflammation through MDV-mediated mitochondrial antigen presentation [100].
2.2.6 ER–Mitochondria–Lipid Droplet (LDs) Interactions: Calcium and Metabolic Crosstalk in Aging
Mitochondrial Ca²⁺ uptake occurs through two primary pathways: directly extract Ca²⁺ from local high calcium regions in the cytoplasm through the mitochondrial calcium uniporter [101], and from ER through mitochondria-associated membranes (MAMs)—a specialized contact site between ER and mitochondria consisted of a variety of proteins [102]: inositol 1,4,5-trisphosphate receptor (IP3R) and VDAC [103], Sigma-1 [104], glucose-regulated protein 75 (GRP75) [105], vesicle-associated membrane protein associated protein B (VAPB) and protein tyrosine phosphatase interacting protein 51 (PTPIP51) [106], and Mfn1/2 [107] (Figure 8).
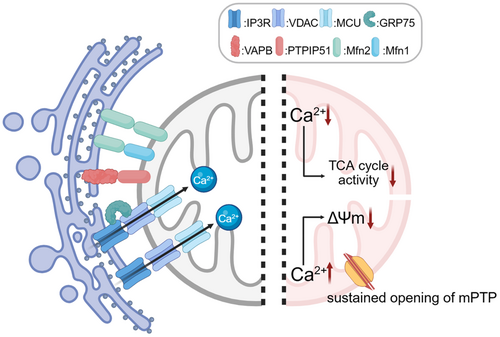
In neurodegenerative diseases, disease-specific pathogenic proteins disrupt the function of MAMs-associated proteins, resulting in dysregulated Ca²⁺ signaling between the ER and mitochondria. In Alzheimer's disease (AD), amyloid-β (Aβ) enhances ER calcium release to mitochondria by upregulating the expression of Sigma-1R and IP3R3, leading to a decrease in mitochondrial membrane potential and an increase in ROS [108]. Meanwhile, calcium release mediated by IP3R promoted Aβ production [109], which triggered the upregulation of ryanodine receptor (RyR) expression in mouse cortical neurons [110]. Fortunately, in the rat model, NAC can intervene in the aβ-induced upregulation of RyR levels and restore spatial memory capacity [111]. In Amyotrophic lateral sclerosis (ALS), overexpression of TDP-43 activates glycogen synthase kinase 3 beta (GSK3β), leading to disruption of the connection between VAPB and PTPIP51 [112]; The C9orf72 mutation disrupts the VAPB-PTPIP51 interaction, which impairs ER–mitochondrial contacts, leading to impaired mitochondrial Ca²⁺ uptake and reduced ATP production [113]. Early upregulation of GRP75 expression can partially counteract the negative effects of the C9orf72 mutation on ER–mitochondrial function [114]. Since Ca²⁺ in the mitochondrial matrix is important for activating the dehydrogenases of the tricarboxylic acid (TCA) cycle, their products NADH and FADH2 drive OXPHOS and ATP production [115]. Diseases such as ALS, which result in insufficient uptake of mitochondrial Ca²⁺, significantly reduce the activity of these enzymes, which in turn leads to impaired ATP synthesis. On the contrary, mitochondrial Ca2+ overload will cause mitochondrial permeability transition pore (mPTP) opening [116], leading to an increase in mitochondrial permeability accompanied by a decrease in the mitochondrial pH gradient and ΔΨm [117]. Turn-on of mPTP also induced a conformational change in complex I, leading to accelerated ROS production at complex I [118].
Mitochondrial fission is inextricably linked to ER [50]. Specifically, it can be divided into two processes, IMM and OMM contraction: the ER is localized precisely to the OMM mainly through a physical bolus system consisting of Mfn2 on the ER membrane and Mfn1/2 on the OMM [107]. Inverted formin 2 (INF2) on the ER membrane drives mitochondrial fission by promoting the polymerization of actin and myosin II to form a contractile ring [119, 120]. This process is a prerequisite for enhancing ER–mitochondrial calcium transfer [121]. The high calcium environment within the matrix may, by activating the ETC, lead to mitochondrial cristae remodeling or local conformational changes in the inner membrane, driving the physical contraction of the inner membrane [121]. At this point, the rupture of IMM is completed, and the rupture of OMM also requires the combined action of the contractile ring and the oligomerization of Drp1 [122]. Therefore, the contact between ER and mitochondria, as well as the intake of Ca²⁺, are crucial for mitochondrial fission. This also provides another perspective for observing mitochondrial dynamics: α-syn, a specific pathological product of PD, disrupts the interaction between VAPB and PTPIP51, thereby inhibiting the delivery of Ca2+ in MAMs, which leads to insufficient mitochondrial fission [123].
Mitochondria serve as an important site of oxidative catabolism for lipid metabolism. LDs serve as energy storage sites for the cell and have a neutral lipid interior, encapsulated by a monolayer of phospholipids [124]. In nutrient deficiency, LDs release fatty acids (FAs) for mitochondrial β-oxidation [124]. In lipid excess, to avoid lipid peroxidation, LDs store excess lipids to prevent cellular damage [125]. Recently, it has been found that there exists an ER–mitochondria–LDs three-way membrane contact site mediated by multiple proteins to coordinate lipid transfer, LD biogenesis, and mitochondrial dynamics [126-129].
Mitoguardin 2 (MIGA2) is an OMM-located protein that binds to VAPB proteins on the surface of LDs and in the ER through two structural domains at C-terminus, respectively [130]. In addition, MIGA2 maintains mitochondrial membrane integrity and avoids fragmentation by transporting glycerophospholipids [128]. Oxysterol-binding protein-related protein 5/8 (ORP5/8) localized to MAMs maintains mitochondrial morphology and function, as their knockout reduces mitochondrial matrix density and oxygen consumption, while PTPIP51 serves as a key factor mediating ORP5/8-dependent LDs synthesis [131, 132]. This gene may be involved in the regulation of aging or provide a new target for antiaging therapy, which needs to be studied. Seipin protein promotes mitochondrial calcium uptake by maintaining the localization and function of IP3R and VDAC, supporting TCA cycle activity and ATP production [133]. For cellular antiaging, LDs, in addition to their basic energy-supplying role, sequester polyunsaturated FAs and reduce lipid peroxidation [134]. In the nematode model, the accumulation of LDs near the nuclear membrane is now a newly identified indicator of senescence [135]. The role of LDs and ER–mitochondria–LDs contact sites in aging remains poorly understood. While proteins like MIGA2, ORP5/8, and Seipin regulate lipid metabolism, mitochondrial function, and stress responses, their impact on aging and age-related diseases requires deeper investigation. Future studies should focus on: (1) How LD dynamics influence cellular senescence. (2) Whether restoring LD–mitochondria–ER crosstalk can delay aging. (3) Develop therapies targeting these pathways.
3 Future Therapeutic Strategies Targeting Mitochondria in Age-Related Diseases
Aging is the most significant risk factor for age-related diseases, including neurodegenerative, cardiovascular, and metabolic disorders. In the subsequent sections, we will provide an overview of mitochondrial dysfunction in these diseases and focus on the mitochondria-targeted therapeutic interventions.
3.1 AD
AD is a progressive neurodegenerative disorder characterized by memory loss, cognitive decline, and behavioral changes. It predominantly affects individuals over 60 years old [136]. According to a WHO report, over 55 million people worldwide currently have dementia, with nearly 10 million new cases emerging each year. Pathologically, the presence of amyloid plaques and neurofibrillary tangles constitutes significant features of AD. Among these, atypical accumulation of Aβ is thought to be an initiating factor in AD progression [137]. Aβ and its precursor proteins can exacerbate the course of AD by disrupting mitochondrial function through altering typical mitochondrial physiology [138]. First, Aβ disrupts intracellular calcium homeostasis, leading to increased cell membrane excitability and neuronal dysfunction [139]. Second, the accumulation of Aβ adversely affects mitochondrial biogenesis by lowering the activity of AMPK and PGC-1α [140-142]. Thus, excessive aggregation of Aβ ultimately results in reductions in various mitochondrial biological activities, leading to further neuronal damage.
In addition to the mitochondrial abnormalities described above, mitochondrial oxidative damage—including abnormal OXPHOS, decreased ATP production, reduced cytochrome oxidase activity, and increased lipid and free radical peroxidation—represents an early event in AD pathogenesis [143]. Furthermore, Drp1-mediated mitochondrial fission is abnormal in the context of AD [144-147]. Notably, partial reduction of Drp1 lowered Aβ production, alleviated mitochondrial dysfunction, preserved mitochondrial dynamics, and enhanced mitochondrial biogenesis and synaptic activity [148]. Therefore, targeting Drp1 and its role in mitochondrial dynamics and motility may offer a promising therapeutic approach for AD.
Numerous interventions have been rigorously studied to enhance mitochondrial function to slow the advancement of AD (Table 2). Nicotinamide inhibits sirtuin activity by competing with NAD+ for binding to the enzyme, thereby reducing Thr231-Phosphotau levels [149]. In mouse studies, oral administration of nicotinamide mitigated both Aβ and tau pathologies and enhanced cognitive function [150]. Idebenone demonstrates therapeutic efficacy in AD patients by improving memory, attention, and behavioral deficits through enhanced OXPHOS [151]. Meanwhile, study in AD mouse models shows that Idebenone modulates amyloid pathology and tau pathology while suppressing associated neuroinflammation [152].
| Target | Therapy | Mechanism | Outcomes | FDA approval status | Reference |
|---|---|---|---|---|---|
| OXPHOS | Idebenone | NRF2 activation enhances mitochondrial antioxidation and blocks NLRP3-driven inflammation. | Restore mitochondrial function and improve cognitive impairment. | Unapproved drugs | [152] |
| Mitochondrial biogenesis | Pioglitazone | Upregulates PGC-1α/PPARγ. | Reduces oxidative stress. | Approved drugs | [153] |
| Bezafibrate | Activates PPAR family. | Reduce nerve damage and increase mitochondrial ATP levels. | Unapproved drugs | [155, 163] | |
| Resveratrol/pterostilbene | Activates SIRT1, NRF2 and SOD. | Improved MMP and neuroplasticity. | Unapproved drugs | [164] | |
| AICAR | Improve AMPK phosphorylation. | Increased the number of new DG cells but reversed after 14 days. | Unapproved drugs | [165] | |
| Nilotinib | PGC-1α, CaMKII, and NRF2 upregulation. | OCR and RC-related proteins were increased. | Approved drugs | [154] | |
| Echinacoside | PI3K/AKT/NRF2/PPARγ pathway was activated to upregulate the expression of SOD1 and SOD2. | Improved behavioral ability and neuroinflammatory response. | Unapproved drugs | [166] | |
| Mitochondrial dynamics | Mdivi-1 | Inhibit Drp1 (or Drp1 pSer616). | Restore the mitochondrial dynamics and memory. | Unapproved drugs | [167] |
| Anakinra | Decrease Mfn1/2 and prevent the decreased MMP induced by Aβ. | Approved drugs | |||
| ROS | Hydralazine | NRF2 was activated to improve SOD activity. Also improved OXPHOS and mitochondrial fragmentation. | Improve spatial ability and inhibit the negative effects of Aβ. | Approved drugs | [168] |
| Centella asiatica/araliadiol | Activate the antioxidant system through mild oxidation. | Inhibit lipid peroxidation. | Unapproved drugs | [169] | |
| Ca2+ homeostasis | Liquiritigenin | Inhibition of Ca2+ influx caused by glutamate. | Inhibition of glutamate excitotoxicity. | Unapproved drugs | [170] |
| Mitophagy | Kaem/Rhap | Activates the PINK1/Parkin pathway and reduces p-Tau. | Promotes paralysis resistance and has cholinergic protection. | Unapproved drugs | [162] |
- Abbreviations: AD, Alzheimer's disease; AICAR, aminoimidazole-4-carboxamide 1-β-D-ribofuranoside; AKT, protein kinase B; AMPK, adenosine monophosphate-activated protein kinase; ATP, adenosine triphosphate; CaMKII, calcium/calmodulin-dependent protein kinase II; DG, dentate gyrus; Drp1, dynamin-related protein 1; FDA, Food and Drug Administration; Kaem, Kaempferol; Mfn1/2, mitofusin 1/2; MMP, mitochondrial membrane potential; NLRP3, nucleotide-binding domain, leucine-rich repeat containing protein 3; NRF2, nuclear factor erythroid 2-related factor 2; OCR, oxygen consumption rate; OXPHOS, oxidative phosphorylation; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PI3K, phosphoinositide 3-kinase; PINK1, PTEN-induced putative kinase 1; PPAR, peroxisome proliferator-activated receptor; RC, respiratory chain; Rhap, Rhapontigenin; ROS, reactive oxygen species; SIRT1, sirtuin 1; SOD, superoxide dismutase.
An alternative strategy to combat mitochondrial impairment involves the generation of new mitochondria. Pioglitazone, classified as a thiazolidinedione, has been shown to confer protective effects in various neurological disorders by targeting transcription factors, such as PGC-1α [153]. Additionally, other compounds such as bezafibrate, resveratrol, 5-aminoimidazole-4-carboxamide ribonucleotide, and nilotinib exhibit protective effects in mitochondrial disorders by activating the process of mitochondrial biogenesis [154, 155].
Altered mitochondrial dynamics in AD have prompted exploration of pharmacological interventions targeting associated proteins. Echinacoside, a natural phenolic compound, induces mitochondrial fusion through enhanced Mfn2 gene transcription, showing neuroprotective effects [156]. Liquiritigenin, a flavonoid, also induces mitochondrial fusion and protects against Aβ cytotoxicity [157]. Hydralazine, a Food and Drug Administration (FDA)-approved hypertension drug, activates the nuclear factor erythroid 2-related factor 2 (NRF2) pathway, promoting antioxidant gene transcription and preventing oxidative stress [158]. It may also prevent Aβ misfolding [159]. Centella asiatica extract similarly activates the NRF2 pathway [160]. Inhibiting the mitochondrial fission pathway has also been associated with therapeutic benefits, such as with mitochondrial fission inhibitor-1, reduces mitochondrial fragmentation, maintains MMP, and prevents cytochrome C release [161].
Maintaining mitophagy can help remove damaged or dysfunctional mitochondria, which is beneficial. Using an AI-aided high-throughput workflow, two natural compounds—Kaempferol and Rhapontigenin—were identified and tested in nematodes and mouse models of AD. They showed potential to improve neuron survival and function, reduce Aβ and tau pathologies, and enhance memory performance [162].
3.2 PD
Aging is a significant risk factor for PD, with prevalence rates rising to 2% in the global population aged 65 and peaking at 5% among those aged 80 [171]. PD patients show reduced dopaminergic neurons in the substantia nigra pars compacta and Lewy body (LB) aggregates in these neurons [172]. Studies have shown that oxidative stress is a key driver of dopaminergic neurodegeneration in all forms of PD [173-175]. The oxidative stress resulting from ROS accumulation contributes to the aggregation and misfolding of α-syn, a primary constituent of LBs and a hallmark of PD [176]. The presence of α-syn disrupts the function of the ubiquitin-proteasome system. The dysfunction of this system leads to the accumulation of abnormal proteins, including α-syn, which adversely affects mitochondrial morphology function. This sequence of events ultimately culminates in neuronal cell death [177].
In patients with PD, there is a reduction in the levels of Parkin/PINK1, Mfn1/2, and OPA1, which caused a deficiency in mitophagy and an imbalance in mitochondrial dynamics [178]. In addition, lower Drp1 causes mitochondria to become dense and spherical, reducing their mobility in neurons. Decreased dendritic mitochondria lead to reduced energy supply, resulting in synapse loss and impaired neuronal development, learning, and memory [179]. Furthermore, Picca et al. identified a significantly higher concentration of small EVs in the serum of elderly patients with PD, which indicated the presence of MDVs in elderly PD patients [180].
While current PD therapies primarily target dopaminergic pathways—focusing on neurotransmitter release, receptor activation, and clearance [181]—emerging antioxidant therapies are gaining attention as complementary strategies to address oxidative stress. Coenzyme Q10, a lipid-soluble quinone critical for ETC function and mitochondrial antioxidant defense, demonstrates neuroprotective effects in dopaminergic neurons, supported by both preclinical mouse models and clinical evidence [182, 183]. In contrast, creatine supplementation has shown therapeutic potential only in preclinical studies to date [182]. N-acetyl cysteine (NAC) exerts antioxidant effects by boosting glutathione levels, and a pilot study has demonstrated its potential to alleviate motor symptoms in PD [184]. Ursodeoxycholic acid (UDCA) markedly increased the activity of all four complexes of the mitochondrial respiratory chain, showing the protective effects to dopaminergic neurons from oxidative stress [185, 186]. Nicotinamide adenine dinucleotide (NAD+), a key regulator of mitochondrial homeostasis, is notably depleted in PD. Restoring NAD+ levels through nicotinamide riboside (NR) supplementation may alleviate PD symptoms by rescuing mitochondrial respiratory dysfunction [187, 188]. Pioglitazone, an FDA-approved type 2 diabetes drug, shows promise as a disease-modifying therapy for PD by activating PGC-1α to boost mitochondrial biogenesis and strengthen antioxidant defenses [189]. Isradipine, a calcium channel blocker, shows promise as a neuroprotective agent for PD by acting on CAV-1 L-type calcium channels in the substantia nigra [190, 191].
Targeting the PINK1/Parkin pathway through drug intervention has emerged as another innovative strategy for the treatment of PD. An ATP analog known as kinetin triphosphate has been found to restore the kinase activity of mutant PINK1 while enhancing the activity of wild-type PINK1 in affected patients [192]. Furthermore, nanomedicine is a novel therapeutic approach. For instance, Mn3O4 nanoflowers have demonstrated effective cytoprotection in models of PD, mimicking the activity of three primary antioxidant enzymes: glutathione peroxidase, CAT, and SOD [193]. In addition to the scavenging metal oxide nanoparticle (NP) enzyme, CeO2 NPs. where Ce3+ and Ce4+ mimic, respectively, the SOD and CAT activities to protect cells from oxidative damage [194].
Iron deposition is a key pathological feature of PD. Our previous study demonstrated iron overload induces Parkinsonism phenotypes in mice, impairing locomotor and cognitive functions [195]. Subcortical iron accumulation in PD patients correlates with mitochondrial dysfunction [196], wherein iron-mediated oxidative stress via the Fenton reaction induces mitochondrial damage and disrupts dynamics. Elevated iron levels synergistically activate Drp1 through ROS generation [197] and Ca2+ overload [198], driving excessive mitochondrial fission. Iron chelation therapies show therapeutic potential, including deferoxamine [199], Clioquinol [200], and VK-28 [201]. Our unpublished data reveal dimercaprol, a clinical heavy metal chelator, rescues behavioral deficits in PD mice.
In recent years, several clinical studies on these drugs have been reported. Here, we summarize them in a table to highlight their clinical relevance (Table 3).
| Target | Therapy | Experimental and clinical results | FDA approval status | Reference |
|---|---|---|---|---|
| ROS | CoQ10 | Reduces electron leakage from complex Ⅰ. A large amount of oral administration in the human body also has a certain effect. | Unapproved drugs | [202] |
| ROS | NAC | Increased dopaminergic nerve viability and restoration of dopamine transporter levels. Intravenous NAC exhibits higher bioavailability and superior antioxidant efficacy in clinical studies. | Unapproved drugs | [203-205] |
| ROS, biogenesis, mitophagy | UDCA | Inhibition of mitochondrial membrane potential collapse and activation of AMPK/mTOR and PINK1/Parkin pathways. Clinical double-blind experiments show that the early curative effect is better. | Approved drugs | [206, 207] |
| Biogenesis, UPRmt | NR | The expression of NRF2, LonP1, and ClpP was increased. Long-term clinical use can worsen the disease, which is related to the rapid metabolism of NR and the reduction of the number of mitochondria in the damaged substantia nigra tissue. | Unapproved drugs | [208, 209] |
| Calcium exchange | Isradipine | The drug is neuroprotective in the laboratory. Clinically, it only stabilizes the condition and does not work well. | Approved drugs | [191] |
| Mitophagy | KTP | It promotes the phosphorylation of PINK1 and the recruitment of Parkin, and this process does not antagonize ATP. It is not currently being tested in clinical trials. | Unapproved drugs | [192] |
| ROS | Mnf | To some extent, it replaces antioxidant enzymes in the body. It has not been used clinically. | Unapproved drugs | [193, 194] |
| NPs | Unapproved drugs | |||
| OXPHOS | Creatine | Although it compensates for reduced ATP supply due to the block of complex Ⅰ, it has no clinical effect. | Unapproved drugs | [210] |
- Abbreviations: AMPK, adenosine monophosphate-activated protein kinase; ATP, adenosine triphosphate; ClpP, caseinolytic mitochondrial matrix peptidase proteolytic subunit; CoQ10, Coenzyme Q10; FDA, Food and Drug Administration; KTP, kinetin triphosphate; LonP1, lon peptidase 1; Mnf, Mn3O4 nanoflowers; mTOR, mechanistic target of rapamycin; NAC, N-acetyl cysteine; NP, nanoparticle; NR, nicotinamide riboside; NRF2, nuclear factor erythroid 2-related factor 2; OXPHOS, oxidative phosphorylation; PD, Parkinson's disease; PINK1, PTEN-induced putative kinase 1; ROS, reactive oxygen species; UDCA, ursodeoxycholic acid; UPRmt, mitochondrial unfolded protein response.
3.3 ALS
ALS is a severe and rapidly progressing disorder that affects motor neurons (MNs) in the brain, brainstem, and spinal cord. Predominantly affecting individuals in the later stages of life, most ALS patients are diagnosed between the ages of 40 and 70 [211]. Though the exact cause of ALS is unknown, evidence suggests that mitochondrial dysfunction plays a key role in its development.
The degeneration of MNs in the brain and spinal cord is the primary pathological hallmark of ALS, with mitochondrial abnormalities—including disrupted morphology and organelle aggregation—emerging as some of the earliest detectable changes in disease progression [212]. In ALS MNs, decreased MMP, impaired mitochondrial import, decreased OXPHOS, and altered cristae were observed [213, 214]. In the SOD1 mutant mouse model, abnormal mitochondrial morphology and dysfunction appear early in the nervous system, predisposing MNs to degeneration. Neuronal mitochondrial transport is progressively impaired before clinical symptoms, with retrograde transport affected earlier than anterograde transport [215]. Mitochondrial oxidative damage is a well-established driver of MNs degeneration in ALS [216]. The situation is exacerbated by the unique vulnerability of MNs to oxidative stress and the central nervous system's limited antioxidant defenses, including low protective enzyme activity [217].
In familial ALS, several pathogenic variants, such as SOD1, C9ORF72, TDP-43, FUS, ALSIN, VAPB, OPTN, SIGMAR1, SQSTM1, and VCP, are associated with a range of mitochondrial dysfunction [218].
Currently, only four drugs have been approved by the FDA for the treatment of ALS [219]. Riluzole, the first FDA-approved drug for ALS, is a benzothiazole and a glutamate antagonist. It exerts neuroprotective effects by inhibiting glutamate reuptake, inducing glutathione synthesis in MNs [220, 221], inhibiting Ca²⁺ efflux at synapses, and decreasing MMP [222, 223]. Edaravone (MCI-186 and 3-methyl-1-phenyl-2-pyrazolin-5-one), approved by the FDA in 2017, reduces oxidative stress in MNS and slows the decline of physical function by removing oxygen radicals and eliminating lipid peroxides in the central nervous system [224-228]. Sodium phenylbutyrate and Taurursodiol (PB/TURSO), approved in September 2022, reduce neuronal cell death by decreasing ER stress and mitochondrial dysfunction [229, 230]. Tofersen, approved in April 2023 for adults with SOD1 gene mutations [231], is an antisense oligonucleotide that degrades SOD1 mRNA and lowers SOD1 protein levels [232].
There have been numerous clinical trials over the last decades targeting mitochondrial functions. α-Lipoic acid has been shown to significantly increase the antioxidant capacity of the ER and mitochondria, reversing the redox state of glutathione and vitamin C [233]. PB/TURSO attenuates the hyperactivation of UPRmt by inhibiting eukaryotic initiation factor 2 alpha phosphatases, which increase ATF4 expression [234]. Dexpramipexole similarly attenuates UPRmt hyperactivation by inhibiting aberrant mitochondrial leakage channels, thereby maintaining ATP production and mitigating the toxic effects associated with C9ORF72 mutations [235]. Additionally, Lithium can activate mitophagy and increase the number of mitochondria in motor nerves in the treatment of ALS [236]. Rasagiline also protects mitochondria and stabilizes the MMP [237]. The development of specific pharmacological agents targeting proteins that enhance mitochondrial Ca2+ uptake, including sigma-1 receptor agonists and calreticulin, currently remains theoretical [238, 239]. But there is optimism regarding advancements in such drugs in the future. Here, we summarize the current clinical use of the therapies (Table 4).
| Target | Therapy | Clinic outcome | FDA approval status | Reference |
|---|---|---|---|---|
| UPRmt | PB/TURSO | The risk of death was reduced by about 44%, and survival was about 6.5 months longer than in the placebo group. In addition, 12- and 24-month survival rates were better. | Approved drugs | [240] |
| Calcium exchange | Riluzole | The rate of weight loss was significantly reduced in acute patients and slightly recovered in chronic patients. It also shows that the treatment effect is independent of the treatment time. | Approved drugs | [241, 242] |
| ROS | Edaravone | The drug improves survival by 50%, has a long-term effect lasting 64 months, and causes no serious adverse events such as deterioration of renal function. | Approved drugs | [243, 244] |
| SOD1 | Tofersen | Tofersen showed a significant reduction in serum neurofilament light chain (sNfL) in pathology regardless of severity. In addition, it can slow the progression of the disease, but it is not a cure. | Approved drugs | [231, 245] |
| ROS | α-Lipoic acid | It has not been used clinically, but given its ability to efficiently cross the blood–brain barrier, it should have great potential in the treatment of neurodegenerative diseases. | Unapproved drugs | [246] |
| Mitophagy | Lithium | Survival, ALS functional scores, and adverse event rates were all like placebo, so lithium did not show significant efficacy. | Approved drugs | [247] |
| OXPHOS | Rasagiline | In acute patients, there was a significant survival advantage in the first 12 months (82% vs. 69%), but no significant difference at 18 months, and in chronic patients, there was no difference from placebo throughout. | Approved drugs | [248] |
- Abbreviations: ALS, amyotrophic lateral sclerosis; OXPHOS, oxidative phosphorylation; PB/TURSO, sodium phenylbutyrate and taurursodiol; ROS, reactive oxygen species; SOD1, superoxide dismutase 1; UPRmt, mitochondrial unfolded protein response.
3.4 Coronary Heart Disease (CHD)
CHD is a form of heart disease characterized by atherosclerosis, which involves the narrowing or blockage of coronary arteries, ultimately resulting in myocardial ischemia, hypoxia, and potentially necrosis [249]. Atherosclerosis serves as the primary pathological basis for the onset of CHD. Research indicates that aging, particularly reproductive aging in women, correlates with an elevated risk of CHD [250, 251]. Emerging evidence highlights the relationship between mitochondrial dysfunction and the pathogenesis of CHD, encompassing genetic factors such as mtDNA damage or mutation, impaired functioning of the respiratory chain, and deficits in mitochondrial turnover [252]. Excessive ROS production is a key way mitochondria contribute to CHD development [252]. ROS plays a significant signaling role within vascular cells, including vascular endothelial cells (ECs), vascular smooth muscle cells (VSMCs), and fibroblasts [253]. Excessive ROS/RNS production drives inflammatory vascular responses that promote atherosclerotic plaque formation [254]. It also contributes to atherogenesis by causing EC dysfunction and apoptosis, activating matrix metalloproteinases, facilitating VSMC proliferation and migration, and oxidizing low-density lipoprotein [255]. In advanced stages of CHD, VSMC apoptosis, triggered in part by oxidative stress, may contribute to plaque instability and rupture [256]. Notably, as the severity of CHD progresses, there is a corresponding decrease in mtDNA copy number [257]. A specific deletion of 4977 bp occurring between 8470 and 13,447 within mtDNA results in OXPHOS abnormalities and mitochondrial dysfunction. The incidence of mtDNA4977 is significantly heightened in patients with CHD and exhibits a positive correlation with age [258, 259].
Recent decades have underscored the importance of dietary interventions as effective strategies to mitigate aging, enhance health, and prevent atherosclerosis [260]. Such interventions have been shown to reduce ROS production, mitigate the accumulation of nuclear DNA and mtDNA damage, and sustain mitochondrial homeostasis. In instances where CHD patients adhere to diets high in fats and cholesterol, sirtuin 3 (SIRT3) deficiency exacerbates EC damage and ischemia-reperfusion injury [261], further worsening atherosclerosis. Consequently, mutations or reduced expressions of SIRT3 may induce oxidative damage in conjunction with conditions, such as CHD or hypertension [262]. Restoration or enhancement of SIRT3 activity, along with the application of mitochondria-targeted hydrogen peroxide scavengers like ebselen, has demonstrated potential benefits for CHD management. Additionally, Amla, a plant in Indian traditional medicine, has been shown to alleviate oxidative stress caused by various factors. Its therapeutic effects include enhancing the residual respiratory capacity of mitochondria; stimulating mitochondrial biogenesis and antioxidant systems while activating the AMPK and NRF2 pathways; and providing protection against oxidative stress, thereby increasing oxygen consumption [263].
In the context of CHD, elevated levels of 7-ketocholesterol and advanced glycosylation end products lead to increased profilin [264, 265]. Profilin promotes lengthening and aggregation of actin filaments via INF2 [266], thereby bringing mitochondria and the ER into closer spatial proximity and facilitating Drp1-mediated mitochondrial fission [119]. This process ultimately contributes to coronary occlusion. For the restoration of abnormal mitochondrial structure, the compound mdivi-1 has been shown to inhibit Drp1-dependent mitochondrial fission and modulate complex I to prevent excessive ROS production [267]. Elamipretide (SS-31) preserves cristae architecture and mitigates mitochondrial swelling, while also inhibiting cytochrome C peroxidase activity to prevent cardiolipin (CL) peroxidation [268].
Significant reductions in OXPHOS and ETC functionality have been documented in CHD patients, with O2 flux per volume also showing marked decreases compared with healthy populations [269]. This situation results in increased electron leakage from the respiratory chain, contributing to oxidative stress. The use of monoamine oxidase inhibitors has proven effective in significantly reducing ROS production and alleviating oxidative stress, including compounds such as chalcones' derivatives and genistein [270, 271]. Furthermore, the accumulation and oxidation of mitochondrial CL within the respiratory chain complex are recognized as contributing factors to CHD. Beyond SS-31, artificial quinone amphiphilic antioxidants can target CL within the respiratory chain complex, thereby safeguarding the CL bilayer from oxidation through interactions in the aqueous phase [272].
Recent findings suggest that mitochondrial uncoupling agents may serve as therapeutic options for the treatment of CHD. For example, 2,4-dinitrophenol facilitates the release of energy from cellular respiration as heat by allowing protons to traverse back into the mitochondrial matrix. However, excessive heat production may lead to uncontrolled toxic hyperthermia, accompanied by significant morbidity and mortality. While these pharmacological agents exhibit therapeutic potential, their associated toxicity must be carefully considered, underscoring the importance of precise dosage control and targeted application [273].
3.5 Type 2 Diabetes Mellitus (T2DM)
Over the past 50 years, the aging population has grown, and the prevalence of T2DM has increased. Now, nearly half of all people with diabetes are older adults (aged ≥ 65 years) [274]. T2DM is an age-associated condition characterized by a reduction in pancreatic β-cell mass and function, coupled with insulin resistance in various tissues, ultimately leading to hyperglycemia [275].
During metabolic homeostasis, blood glucose fluctuations are efficiently regulated through subtle insulin secretion adjustments. In T2DM, however, chronic hyperglycemia and hyperlipidemia impair pancreatic β-cell function—a process driven largely by mitochondrial dysfunction involving structural abnormalities and compromised bioenergetics [276]. Sustained exposure to elevated levels of glucose and lipids results in proton leakage, an increase in MMP, and heightened oxidative stress, which in turn precipitates the accumulation of inflammatory factors and ROS, ER stress, and mitochondrial dysfunction of β-cells [277-280]. Furthermore, age-related declines in mtDNA content are correlated with a reduction in insulin secretion [281, 282].
The liver and adipose tissue, pivotal in maintaining the homeostasis of glucose and lipid metabolism, assume the role of essential energy reservoirs and endocrine organs. In the setting of T2DM, these tissues experience notable mitochondrial dysfunction. In T2DM, hepatic mitochondria show reduced respiratory capacity, increased uncoupling and proton leakage, and lower ATP content and turnover [283-285]. T2DM patients also exhibit higher mitochondrial ROS, reduced antioxidant enzyme activity, and decreased OXPHOS gene expression in adipose tissue [286, 287]. Additionally, many ETC components are downregulated in visceral adipose mitochondria of women with T2DM [288].
Exercise improves metabolic health and insulin sensitivity in T2DM patients [289], mitochondria are central to many of these processes [290, 291]. Exercise enhances insulin sensitivity in T2DM by boosting energy metabolism. It stimulates mitochondrial turnover and biogenesis, increases Ca²⁺ concentration and the ratios of AMP/ATP and NAD⁺/NADH, and enhances respiration and electron transport capacity [292].
Numerous pharmacological agents used in diabetes therapy have been shown to impact mitochondria directly or indirectly. Some drugs are specially designed to target mitochondria. We summarize the mitochondria-targeting treatment of nowadays (Table 5).
| Target | Therapy | Mechanism | Reference |
|---|---|---|---|
| ROS | SkQ1 | Mitigates oxidative stress and reduces blood glucose levels by increasing the expression of antioxidant enzymes (SDO1/2 and Gpx) and NRF1-mediated mitochondrial biogenesis. | [293] |
| Szeto–Schiller | The drug splits into the inner mitochondrial membrane, reducing ROS production and preventing electron leakage on the RC. It is also effective in treating both diabetes and CHD. | [294] | |
| Mitochondrial biogenesis | Resveratrol | The drug modulates PGC-1α by promoting the interaction of PGC-1α and SIRT1. Promotes mitochondrial biogenesis and insulin sensitivity. | [295] |
| AICAR | Activates AMPK and restores the normal hypoglycemic function of insulin. | [296] | |
| Mitochondrial biogenesis and mtDNA | Aerobic exercise training (AET) | AET activates SIRT1 and SIRT3 to activate mitochondrial biogenesis and antioxidant effects. In addition to this, AET can promote mtDNA transcription and protein expression by increasing the copy number of mtDNA. | [269, 297] |
| Mitophagy | Metformin | Restoration of mitophagy mediated by PRKA and SIRT1 rescues β-cell apoptosis. | [298, 299] |
| Oligomycin | Translocation of TFEB to mitochondria promotes mitochondrial autophagy and beta cell mitosis. | [300] | |
| ROS, biogenesis, and mitophagy | Urolithin A | Increased SIRT1 and Elf2α-mediated biogenesis, SOD1/2-mediated antioxidant effects. Increased LC3 expression promotes mitochondrial autophagy. Ultimately, avoid IR and lipotoxicity. | [301] |
| Mitochondrial respiratory | UK5099 | Restoration of mitochondrial respiratory response to nutrients. | [302] |
| Mitochondrial Ca2+ | CDN1163 | Prevented the overaccumulation of Ca2+ in the cytoplasm and mitochondria. | [303] |
- Abbreviations: AICAR, aminoimidazole-4-carboxamide 1-β-D-ribofuranoside; AMP, adenosine monophosphate; AMPK, adenosine monophosphate-activated protein kinase; CHD, coronary heart disease; Elf2a, elF2alpha; Gpx, glutathione peroxidase; IR, insulin resistant; LC3, microtubule-associated proteins 1a/1b light chain 3; mtDNA, mitochondrial DNA; NRF1, nuclear respiratory factor 1; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PRKA, AMP-activated protein kinase; RC, respiratory chain; ROS, reactive oxygen species; SIRT, sirtuin; SOD, superoxide dismutase; T2DM, Type 2 diabetes mellitus; TFEB, transcription factor eb.
3.6 Cancer
As of 2022, about 70% of cancer cases occur in people over the age of 50, and the incidence rate in people over 60 is more than 10 times that of younger people [304]. It is evident that aging is inevitably linked to the cancer process.
3.6.1 Metabolic Re-Editing
Based on the Warburg effect, cancer cells still preferentially produce energy through glycolysis over the more efficient OXPHOS. This phenomenon is called “aerobic glycolysis” and is a biochemical feature that distinguishes cancer cells from normal cells [305]. It has been found that most cancer cells actively choose glycolysis to meet their energy needs through metabolic reprogramming, and that mitochondria play an important role in this process [306]. Altered fate of acetyl CoA, an important bridge between glycolysis and the TCA cycle, is an important feature of cancer cells. In nasopharyngeal carcinoma, acetyl CoA is hijacked for lipid synthesis and histone acetylation, and similar results have been found in endometrial carcinoma [307, 308]. In this process, ATP citrate lyase (ACLY) is an important factor involved in metabolic re-editing in cancer cells. Citric acid in the TCA cycle traverses into the cytoplasm, where it is catabolized by ACLY into acetyl CoA, which maintains the raw material level for lipid synthesis [309] and facilitates cancer cell metastasis and invasion, as in the case of rectal and gastric cancers [310, 311]. Currently, the development of ACLY inhibitors is an important direction to target metabolic reprogramming. For example, SB-204990 not only promotes cancer cell apoptosis but also restores the chemosensitivity of ovarian cancer cells to cisplatin by inhibiting the PI3K-AKT and AMPK-ROS signaling pathways [312]. Similarly, hydroxycitric acid has also been shown to reverse tamoxifen resistance [313]. In terms of immune escape, Bempedoic acid enhances antitumor immunity by inhibiting ACLY, inducing mitochondrial damage and leading to mtDNA leakage, activating cyclic GMP-AMP synthase–stimulator of interferon genes (cGAS-STING) pathway, and promoting immune cell recruitment [314, 315]. Combined with anti-PD-L1 therapy (e.g., Atezolizumab) can further inhibit immune escape and synergistically enhance cancer cell clearance [314, 316]. Here we propose to accelerate the in vitro cellular and in vivo animal studies of ortho-dihydroxyphenyl flavonoids [317], NDI-091143 [318], styraxlignolide D [319], and their derivatives, and to assess whether these ACLY inhibitors can achieve inhibition of tumor spread and immune escape by targeting mitochondria.
Unlike the Warburg effect, cancer stem cells (CSCs), commonly found in leukemia and digestive system cancers, are more dependent on OXPHOS [320], and they maintain low cellular ROS levels by enhancing PGC-1α [321]. In addition, lactate, a product of glycolysis, not only aids cancer cell immune escape [322], but also promotes the metastasis of normoxic CSCs in response to PGC-1α-mediated OXPHOS [323]. Therefore, inhibiting OXPHOS or inducing the conversion of OXPHOS to aerobic glycolysis is the main therapeutic direction. A modified ruthenium complex (Ru1) targets mtDNA and inhibits transcription of the OXPHOS complex, exhibiting a low-toxicity and safe antipancreatic cancer profile [324]. In cholangiocarcinoma, metformin inhibits complex I, leading to energy starvation, possibly by arresting cells in the G0/G1 phase and slowing the spread of CSCs [325, 326]. Atorvastatin inhibits OXPHOS and reduces the burden of acute myeloid leukemia (AML), making it an ideal concomitant therapy for AML [327].
3.6.2 The Dual Role of ROS
Similar to normal cells, appropriate concentrations of ROS act as signaling molecules to enhance the secretion of immunosuppressive signaling molecules in cancer cells [328]; conversely excessive ROS disrupt mitochondrial function, which can both induce apoptosis and autophagy in cancer cells [329, 330], and at the same time may accelerate organismal aging through cumulative oxidative damage.
It has now been shown that the use of antioxidants in cancer patients accelerates tumor growth by disrupting the ROS-p53 axis [331]. Mainstream therapeutic options are more likely to target cancer cell death through overoxidation. Brusatol, an NRF2 inhibitor, induces oxidative stress and death of cancer cells in T-cell acute lymphoblastic leukemia [332]. Simultaneous enhancement of the therapeutic effect of lapatinib in breast cancer [333]. In hepatocellular carcinoma (HCC), Fangchinoline promotes ROS accumulation through activation of NRF2, and its anti-HCC effect is reversed by ROS scavenging by NAC [334].
Currently, nanodelivery systems are new strategies in the fight against cancer to improve drug stability, enhance efficacy, and reduce toxicity to normal cells. Sodium hyaluronate-modified CaO2 nanoparticles (SH-CaO2 NPs), which are stable in the internal environment, rapidly decompose in cancer cells to release Ca2+ and H2O2, leading to mitochondrial structural abnormalities and a membrane potential, and through calcium overload and oxidative stress, eventually inducing cancer cell death [335]. Normal cells are not susceptible to this method due to their high antioxidant capacity and well-established calcium regulatory mechanisms. A polyethylene glycol-modified, curcumin-containing calcium carbonate nanoparticles (PEGCaCUR NPs), in combination with ultrasound, triggers mitochondrial Ca2+ overload, and ROS-mediated immunogenic cell death [336]. Curcumin inhibits Ca2+ efflux from cancer cells, and when coupled with a polyvalent iron component, this would further deplete antioxidant enzymes and exacerbate cellular damage from oxidative stress [337].
4 Conclusion and Future Perspectives
The establishment and enhancement of the public health system have led to a significant increase in human life expectancy; however, this progress confronts the challenge of a rising incidence of aging-related diseases. Aging constitutes an inevitable pathophysiological process, and the pursuit of healthy longevity free from aging-related diseases has been a longstanding aspiration of humanity. Numerous mechanisms underlie aging, and variations exist in the development of different aging-related diseases. This review focuses on mitochondrial dysfunction in the context of aging and related diseases.
Mitochondrial dysfunction is a prevalent characteristic of aging-related diseases. In the initial stages of such diseases, mitochondria employ measures dominated by MQC to counter the disease's progression. However, in the later stages, an accelerated imbalance in mitochondrial function often occurs. ROS serves as both a source of mitochondrial dysfunction and an informational molecule that activates AMPK. Mutations in mtDNA are frequently observed in conditions such as CHD and diabetes, manifesting as decreased OXPHOS function and abnormal energy metabolism, often inherited through the maternal lineage. Anomalies characterized by excessive mitochondrial fission and insufficient fusion are common across various diseases; notably, in PD, inadequate mitochondrial fission can lead to complications, such as axonal dysplasia. Both mitophagy and MDVs contribute to the removal of damaged mitochondria, thereby partially preventing mitochondrial depletion and restoring mitochondrial function. In diabetes, reduced mitophagy often coincides with increased apoptosis, leading to parenchymal cell loss. In ALS and diabetes, ER stress and the activation of UPRmt have a beneficial impact on disease resistance at early stages through the activation of multiple signaling pathways that mediate the clearance of misfolded proteins. However, overactivation of UPRmt can ultimately impair mitochondrial function. These mitochondrial alterations disrupt cellular homeostasis and exacerbate the susceptibility of tissues and organs to age-related decline.
Therapeutic interventions targeting mitochondrial dysfunction offer promising strategies to mitigate age-related diseases. Emerging approaches, including mitochondrial replacement, enhancement of mitophagy, antioxidant supplementation, and modulation of mitochondrial biogenesis, have demonstrated potential in both preclinical and clinical studies. Notably, interventions targeting metabolic reprogramming of tumor mitochondria are an emerging and highly promising research direction that not only shows potential in antitumor therapy but also provides new perspectives for understanding the role of mitochondria in cellular metabolism regulation. Ultimately, lifestyle modifications, including regular physical exercise and dietary changes, can effectively preserve mitochondrial function and delay the aging process.
However, mitochondria-targeted therapies still face several challenges. First, delivery efficiency remains low. For example, CoQ requires 1200 mg/day to cross the blood–brain barrier. Although nanocarrier-based Liquiritigenin improves penetration, scaling up for clinical use is still difficult. Second, the duration of efficacy is too short due to rapid metabolism, as seen with AICAR and NR. Third, clinical translation is challenging. Drugs must overcome enzymatic breakdown, mesenteric absorption, and targeted distribution, all complicated by individual variability. For instance, oral creatine faces absorption and targeting issues, and NR may have opposite effects in healthy individuals versus Parkinson's disease patients. All FDA-approved drugs share some key features. They often act on multiple targets, as seen with Hydralazine and UDCA. Some, like Tofersen, show strong specificity by using antisense oligonucleotides to precisely target SOD1 mRNA. Additionally, they demonstrate high safety, as with Riluzole and PB/TURSO, which have passed cardiotoxicity and hepatic and renal metabolism assessments.
Nanodelivery systems are widely used due to their good targeting properties and improved drug bioavailability [338, 339]. Lactoferrin-modified gold-bismuth selenide nanodots (Lf-Au-Bi₂Se₃ NDs) provide triple-action innovative therapies for the treatment of PD by breaching the blood–brain barrier, mimicking the function of multiple antioxidant enzymes, and precisely protecting mitochondria [340]. NP-packed cyclosporin A (CsA@PLGA-PEG-SS31), utilizing its small molecular weight and positive charge properties, targets mitochondria in H/R-injured cells to inhibit mPTP opening and ROS generation [341]. MTSNP balances antioxidant and vascular regeneration functions by modeling mitochondrial structure and switching treatment modes according to ischemic stage [342]. Loading two mRNAs encoding propionyl-CoA carboxylase subunits into lipid NPs restored the function of the mitochondrial enzyme in the liver [343], which provides important insights for the treatment of genetic aging-related diseases. These breakthroughs no longer merely view NPs as simple drug carriers but rather involve them in the treatment of diseases through their inherent active ingredients, achieving a leap from point-to-point treatment to macroscopic intervention.
With the onset of AI, the use of AI in precision medicine is predictable [344]. It has the advantage of mimicking drug molecular structure by AlphaFold [345] and cost savings compared with the high cost and failure rate of traditional drugs [346]. AI has improved the efficiency and precision of drug candidate discovery with its powerful deep learning capabilities and efficient large-scale data processing [347]. QuickVina 2 and ePharmaLib driven by AI have optimized drug-target interactions and predicted side effects, improving treatment strategies [348]. In antiaging research, the PandaOmics platform can be utilized to screen for therapeutic targets with both antiaging and anticancer effects, such as mTOR, GSK3β, and AKT1/2 [349]. Machine learning models based on both Morgan fingerprinting and graph convolutional neural networks were constructed for screening drugs with the potential to treat Parkinson's disease. For example, the screened Nitazoxanide was experimentally demonstrated to activate PINK1 and enhance mitochondrial respiration [350]. Hence, AI shows efficient and precise application value in potential drug development, multitarget drug screening, and safety assurance.
CRISPR/Cas9 is a revolutionary gene editing technology capable of precisely modifying target DNA sequences. It shows great potential in disease treatment, providing tools for correcting disease-causing gene mutations and developing personalized therapies [351]. A Cas9/guide RNA (gRNA) transgenic fragment was introduced into mouse fertilized eggs by microinjection for lifelong expression, which targets and edits hSOD1-G93A. After crossbreeding with ALS model mice, the progeny completely avoided ALS symptoms, and there were no side effects such as tumors in the long term, which indicates the highly effective treatment and safety [352]. Reducing Aβ production and enhancing neuroprotection by truncating amyloid precursor protein C-terminal amino acids using CRISPR/Cas9 provides a new strategy for AD treatment [353]. Screening for drug-resistant genes is an important part of precision medicine: Genome-wide CRISPR screening revealed that inhibition of the mTORC1 pathway promotes cancer cell resistance to inhibitor of mitochondrial transcription 1 by maintaining mtDNA transcription and mitochondrial protein synthesis. Fortunately, resistance can be reversed if paired with TFAM siRNA can be reversed [354]. To improve the targeting of the CRISPR/Cas9 system, Hussain et al. efficiently entered the mitochondria by adding a mitochondrial localization signal to the Cas9 protein and RNA transport-derived stem loop modification of the gRNA [355]. The CRISPR/Cas9 system allows us to treat diseases at the genetic level. However, it still has challenges in targeting and safety. In the future, combining CRISPR technology with the efficient screening capability of AI and the targeted delivery advantage of nanodelivery systems will provide a safer and more efficient solution for the precision treatment of complex diseases and drive precision medicine forward.
Mitochondria engage dynamically in combating disease progression, and their functionality does not merely decline linearly throughout the disease spectrum. It is imperative to maintain a delicate equilibrium among various mitochondrial processes to promote disease resistance and overall cellular health. Therefore, therapeutic strategies should prioritize precision and specificity by targeting the root causes of mitochondrial dysfunction, rather than indiscriminately enhancing or suppressing individual functions. Future integration of emerging technologies with mitochondrial research has the potential to yield new insights into the mechanisms of aging and disease progression. A thorough understanding of mitochondrial dysfunction in aging will enable targeted therapies to combat age-related diseases effectively.
Author Contributions
Lanlan Jia: conceptualization, writing – original draft, funding acquisition. Ziyu Wei: conceptualization, writing – original draft. Jinyuan Luoqian: conceptualization, writing – review and editing. Xi Wang: conceptualization, writing – review and editing. Chao Huang: supervision, project administration, writing – review and editing. All authors have read and approved the final manuscript.
Acknowledgments
We sincerely acknowledge the use of BioRender (https://BioRender.com) for the creation of the graphical abstract figure in this manuscript. This study was supported by the National Natural Science Foundation of China (grant no. 32300648), the Sichuan Science and Technology Program (grant no. 2023NSFSC1182), the Sichuan Province Science and Technology Activities Funding for Returned Overseas Scholars, and the Sichuan Postdoctoral Research Special Funding.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.