Role of next-generation sequencing in revolutionizing healthcare for cancer management
Om Saswat Sahoo and Hiya Aidasani and Arnab Nayek Equal Contribution.
Abstract
Next-generation sequencing (NGS) has emerged as a transformative technology in oncology, revolutionizing cancer diagnostics and personalized treatment strategies. By providing comprehensive insights into the genetic landscape of tumors, NGS enables the identification of critical somatic and germline mutations, copy number variations (CNVs), and gene fusions. Over the past decade, advancements in NGS platforms have led to greater accuracy, speed, and cost-effectiveness, making it an integral part of cancer research and clinical diagnostics. Despite its widespread adoption, significant challenges remain, including the need for improved methods to detect minimal residual disease (MRD) and accurately profile tumor heterogeneity. This review explores the evolution of NGS technologies and their pivotal role in cancer biology, from early diagnostics to therapeutic guidance. It delves into the application of NGS in identifying CNVs and gene fusions, monitoring MRD, and the increasing relevance of targeted NGS and spatial genomics. Furthermore, the integration of spatial transcriptomics is highlighted as a frontier in understanding the tumor microenvironment. By addressing these critical aspects, this review provides a comprehensive overview of how NGS is shaping the future of cancer research and treatment, offering a complete overview of potential NGS applications in scientific and clinical oncology.
1 INTRODUCTION
Next-generation sequencing (NGS), also known as high-throughput sequencing, is a groundbreaking technology that has significantly advanced genomics and molecular biology. The development of NGS began in earnest between 2008 and 2010, though its foundational technologies were established in the early 2000s.1 The concept of NGS emerged as a response to the limitations of traditional Sanger sequencing, which was the prevailing method for DNA sequencing at that time. The development and commercialization of NGS technologies began around the mid-2000s with the introduction of the 454 Life Sciences system, which utilized pyrosequencing technology.2 This marked the initial phase of NGS as a practical and high-throughput tool for genomic research. Subsequently, other NGS platforms, such as Illumina's Genome Analyzer, were introduced shortly after. These developments in the early 2000s laid the foundation for the widespread adoption of NGS in genomics and various scientific disciplines.3 Before the advent of NGS, DNA sequencing relied primarily on the Sanger sequencing method, which Frederick Sanger developed in the late 1970s.4 This method involved labeling DNA fragments and separating them by size to determine the sequence. While highly accurate, it was time-consuming and low throughput. The development of other related NGS techniques like Pyrosequencing followed this.
Developed in the 1990s, it was one of the first techniques to be considered a precursor to NGS. It measured the release of pyrophosphate during DNA synthesis and allowed for faster sequencing.2 In 2005, 454 sequencing was developed by 454 Life Sciences.,5 and was one of the first commercially successful NGS platforms. Illumina's Solexa technology, introduced in 2006, marked a breakthrough in NGS.6 It utilized reversible dye-terminator sequencing, allowing for massively parallel sequencing of DNA fragments. Illumina's platform became the dominant technology in the NGS field and remains widely used today. While Illumina was developing technology for massively parallel sequencing, Ion (later acquired by Thermo Fisher Scientific), devised a different chemistry. It is based on detecting hydrogen ions released during DNA synthesis.7
While Illumina and other early technologies primarily utilized short read lengths, Pacific Biosciences (PacBio) introduced single-molecule real-time (SMRT) sequencing. This technology is distinguished by its long read lengths and its capability to observe DNA synthesis in real time. SMRT sequencing is particularly useful for detecting structural variants, exploring complex genomic regions, and performing de novo genome assembly.8 Following the success of PacBio, another long-read technology, Nanopore Sequencing, gained prominence. Introduced in 2014, Nanopore Sequencing utilizes nanopores to directly read DNA sequences as they pass through the pore.9 This method is known for its portability and the potential for real-time sequencing, making it useful in field applications and diagnostics.
While third-generation Sequencing (TGS) such as PacBio and Nanopore sequencing offer long read lengths, resolving complex genomic regions and detecting structural variations more accurately, they are also helpful in clinical research, clinical diagnostics, personalized medicine, metagenomics, epigenetics, and so on.10 They have enabled the study of genetic variation, gene expression, and the identification of disease-causing mutations.10 Over the years, NGS technologies have become more cost-effective, making them accessible to researchers and healthcare professionals.11 This accessibility has led to a proliferation of genomic data and accelerated advancements in genetics and genomics. Therefore, the history of NGS is marked by the gradual development and refinement of sequencing technologies, which have revolutionized our ability to read and analyze DNA at an unimaginable scale and speed.11 These advancements have profoundly impacted various fields of biology and medicine, enabling breakthroughs in our understanding of genetics and disease (summarized in Table 1).10, 11 Some vital clinical applications of NGS include but are not limited to Cancer Genomics NGS is used to analyze the genomic alterations in cancer cells, including mutations, copy number variations (CNVs), and gene fusions (explained below). This information helps in personalized cancer treatment, prognosis, and monitoring of treatment response.
| Application | Description | Refs |
|---|---|---|
| Clinical genomics | Diagnosis of genetic disorders | [12, 13] |
| Carrier screening for genetic diseases | [12] | |
| Pharmacogenomics for personalized medication selection and dosing | [14-17] | |
| Prenatal testing for chromosomal abnormalities and genetic disorders in fetuses | [18] | |
| Structural Variation Analysis to characterize large scale genomic alterations | [19, 20] | |
| Comparative genomics to study similarities and differences of clinical conditions across species | [21-23] | |
| Cancer genomics | Comprehensive tumor profiling for diagnosis, prognosis, and treatment selection | [24-28] |
| Liquid biopsy for noninvasive monitoring of cancer progression, treatment response, and minimal residual disease | [29, 30] | |
| Precision oncology for personalized cancer treatment based on molecular targets and actionable mutations | [30-32] | |
| Infectious disease genomics | Pathogen identification for rapid diagnosis, outbreak investigation, and antimicrobial resistance profiling | [33-37] |
| Microbiome analysis to study microbial communities and their role in health and disease | [38-40] | |
| Genetic counseling | Risk assessment for genetic conditions and informed decision-making in disease prevention and management | [41] |
| Rare disease research | Gene discovery in rare diseases through identification of novel genetic variants and genotype-phenotype correlations | [31, 42] |
| Forensic genomics | Human identification and kinship analysis in forensic investigations and criminal justice applications | [43] |
| Evolutionary biology | Phylogenomics to reconstruct evolutionary relationships across species | [44] |
| Molecular Ecology to study ecological interactions of organisms | [45] | |
| Population genetics to investigate genetic variations and evolutionary processes within populations | [44] | |
| Agricultural genomics | Crop improvement through marker-assisted selection | [46, 47] |
| Livestock breeding and management | [48] | |
| Pathogen detection in agricultural settings | [49] | |
| Neurogenomics | Investigation of genetic basis of neurological diseases | [50] |
| Brain development studies | [51] | |
| Identification of rare variants in neurodevelopmental disorders | [50] | |
| Astrobiology | Metagenomic analysis of extreme environments | [52] |
| Search for biomarkers of extraterrestrial life | [53] | |
| Study of microbial diversity in space analogs | [54] | |
| Detection of extremophiles in space-related conditions | [54] |
Despite significant advancements in NGS technology, its integration into clinical practice has not yet reached its full potential. This review explores the progress made in NGS within the field of oncology and examines the various ways it can be utilized to enhance patient care. It outlines potential applications of NGS across different aspects of cancer management, including early diagnosis, treatment selection, and monitoring of therapeutic responses. NGS is instrumental in identifying single nucleotide polymorphisms (SNPs), CNVs, and minimal residual disease (MRD). Additionally, different RNA sequencing and spatial transcriptomic tools are being developed to assess tumor heterogeneity and better understand the tumor microenvironment. By highlighting these advancements and their practical applications, the review aims to illustrate how NGS can revolutionize cancer care, enabling more precise diagnostics and targeted treatment strategies to improve patient outcomes.
2 NGS IN CLINICAL DIAGNOSTICS
In addition to its widespread usage in research and oncology care, NGS-based technology is also rapidly used in clinical and diagnostic setups. NGS enables the identification of genetic mutations underlying inherited diseases, facilitating early diagnosis and providing opportunities for genetic counseling. For instance, in Mendelian disorders, NGS can pinpoint specific gene mutations responsible for conditions such as cystic fibrosis, Huntington's disease, or sickle cell anemia, allowing for timely intervention and personalized patient care.55 For patients with rare genetic diseases, NGS can identify causative mutations, aiding in diagnosis and potential treatment strategies.12 NGS can also help to identify and characterize pathogens (viruses, bacteria, fungi) in clinical samples, assisting in the diagnosis and monitoring of infectious diseases like what we experienced in the recent COVID-19 pandemic.33 Ongoing research and technological advancements continue to expand the utility of NGS in healthcare and genomics. It has also profoundly impacted research across various fields of biology and genomics (Table 1) due to its ability to rapidly generate large volumes of sequencing data at a relatively low cost.56
NGS allows for the comprehensive sequencing of an organism's entire genome, aiding in discovering genetic variations, genes, and regulatory elements.57 It is also instrumental in sequencing the genomes of previously uncharacterized species (de-novo sequencing), which is crucial for biodiversity studies and evolutionary research.58, 59 It helps researchers study the genetic diversity of organisms in various ecosystems and assess their responses to environmental changes. Although we listed numerous applications of NGS, oncology and maternal care are the two dominant fronts where NGS technology forms the backbone.15, 18
3 ADVANCEMENT OF NGS
Over the past two decades, DNA sequencing technologies have undergone a dramatic evolution, driving substantial advancements in genomics and enabling precision medicine, particularly in cancer research (Figure 1). This progress is characterized by the development of three distinct generations of sequencing technologies, each offering significant improvements in speed, accuracy, and data throughput.
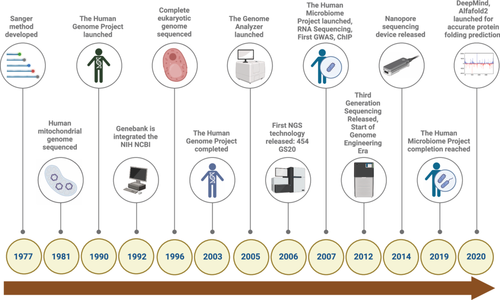
3.1 First-Generation Sequencing Technology
The foundation of modern sequencing technologies can be traced back to the mid-20th century. Early efforts at deciphering nucleic acid sequences relied on chemical degradation or enzymatic cleavage, fragmenting DNA and RNA molecules for subsequent analysis. A major milestone was achieved in 1964 when Robert Holley sequenced alanine tRNA using ribonuclease from S. cerevisiae.60 Soon after, Walter Gilbert and Allan Maxam developed a chemical degradation method that enabled the sequencing of the complete genome of bacteriophage PhiX174.61 However, the most transformative advance in first-generation sequencing came from Frederick Sanger, who introduced the chain termination method in the late 1970s.4 This approach, which employed dideoxynucleotides to terminate DNA replication, allowed for the generation of sequence reads up to several hundred nucleotides in length.
Sanger sequencing, as it became known, revolutionized molecular biology by enabling rapid and precise DNA and RNA sequencing. The method's impact deepened with the introduction of the Applied Biosystems ABI 370, the first automated sequencing machine, in 1987.62 This machine used fluorescently labeled dideoxynucleotides and capillary electrophoresis, streamlining the Sanger method to produce faster, more accurate results. The ABI 370 quickly became the industry standard for DNA sequencing and laid the groundwork for sequencing the human genome in 2001,14 marking a critical turning point in cancer genomics. While first-generation sequencing has since been eclipsed by more advanced technologies, its foundational role in the study of genetics and the development of cancer diagnostics and therapies cannot be overstated.
3.2 Second-generation sequencing technologies
The advent of second-generation sequencing (also known as NGS) marked a quantum leap in the field by introducing high-throughput, parallel sequencing. In contrast to Sanger sequencing, which could only process a single DNA fragment at a time, second-generation sequencing platforms introduced the capability to sequence thousands to hundreds of millions of DNA fragments simultaneously. This advancement revolutionized the field by dramatically boosting both the speed and scale of data generation, allowing for a massive increase in sequencing throughput and enabling large-scale genomic studies to be completed far more efficiently.
One of the earliest and most prominent platforms in this generation was Roche's 454 sequencing, which employed pyrosequencing, a method that detects pyrophosphate release during nucleotide incorporation.63 Another notable system is the Ion Torrent platform, which measures hydrogen ion release during DNA synthesis.64 Illumina sequencing, arguably the most widely used second-generation platform, introduced a sequencing-by-synthesis approach involving reversible dye terminators.39 Each of these technologies has enabled significant advances in cancer genomics, making it possible to analyze tumor heterogeneity, discover novel oncogenes, and perform comprehensive whole-genome and transcriptome analyses in cancer patients.
Another key player in this generation is the SOLiD (Sequencing by Oligonucleotide Ligation and Detection) platform,39 which uses ligation-based chemistry to achieve high sequencing accuracy. These second-generation platforms have empowered researchers to explore genetic mutations, epigenetic modifications, and gene expression changes at an unprecedented scale. In cancer research, this has translated into the identification of critical biomarkers, enabling early diagnosis, targeted therapies, and personalized treatment strategies for malignancies like breast, lung, and colon cancers.
3.3 Third-generation sequencing technologies
Third-generation sequencing technologies represent the cutting edge of DNA sequencing, overcoming many of the limitations of earlier methods, particularly in terms of read length and real-time analysis. These platforms can sequence much larger DNA fragments, sometimes spanning tens of kilobases, with single-molecule precision.
PacBio sequencing, which utilizes an approach of single-molecule real-time (SMRT), is a leading third-generation technology.65 By incorporating fluorescently labeled nucleotides and continuous real-time monitoring of DNA synthesis, PacBio can generate long-read sequences that provide insight into complex genomic regions previously inaccessible with second-generation methods. This is especially relevant in cancer research, where structural variations such as gene fusions, insertions, deletions, and CNVs play critical roles in oncogenesis and drug resistance, which have been explained later. Oxford Nanopore sequencing, another cutting-edge technology, relies on nanopore sensors to sequence single-stranded DNA molecules.64 As DNA molecules pass through a nanopore, variations in the electrical current are monitored and analyzed. The portability, speed, and long-read capacity of Oxford Nanopore make it a powerful tool for real-time analysis of cancer genomes, potentially enabling clinicians to monitor tumor evolution and therapeutic response in a near-instantaneous manner.
The application of third-generation sequencing technologies in cancer genomics has opened new avenues for understanding the full spectrum of genetic alterations driving cancer progression. Long-read sequencing is particularly beneficial for studying complex genomic regions such as telomeres, centromeres, and repetitive sequences, which are often altered in cancer cells. Additionally, third-generation methods allow for the direct sequencing of RNA molecules without the need for reverse transcription, providing a more comprehensive view of the cancer transcriptome, including the role of Noncoding RNAs, alternative splicing events, and fusion transcripts in tumorigenesis.
4 NGS APPLICATION IN CANCER BIOLOGY
NGS, which offers high-throughput and affordable tools for assessing the genetic and genomic changes linked to cancer, cancer biology research, and clinical practice, has been transformed over the years. Among the most essential uses of NGS in cancer biology is the fact that NGS has been a game-changing tool in the oncology domain. This sophisticated sequencing method has wholly transformed our comprehension of cancer biology, diagnosis, prognosis, and therapy (Figure 2).15, 39 NGS makes it possible to thoroughly investigate the cancer genome through the quick and affordable sequencing of billions of DNA fragments in parallel. Detecting somatic mutations in cancer genomes is one of its primary uses in oncology.66-68 NGS can identify genetic changes such as single nucleotide variants (SNVs), insertions/deletions (indels), and structural differences by comparing the DNA of malignant tissues to those of normal tissues.69 Finding driver mutations that start and encourage carcinogenesis, describing the mutational landscape of different cancer types, and discovering possible treatment targets depend on this information. Examining gene expression profiles is a crucial use of NGS in oncology. Researchers can measure gene expression levels, find fusion genes, and uncover alternative splicing events by RNA sequencing (RNA-seq) with NGS technology.70-72 The categorization of cancer subtypes, the identification of new biomarkers, and the creation of individualized treatment plans are all made possible by this knowledge. Additionally, NGS makes it easier to research Noncoding RNAs, which are essential for cancer development. Examples of these RNAs are long Noncoding RNAs and microRNAs. NGS enables precise, high-throughput sequencing, offering researchers the ability to perform comprehensive epigenomic profiling of cancer, with greater accuracy and efficiency.73 Comprehensive analyses of chromatin accessibility, histone modifications, and DNA methylation patterns can be performed with whole-genome bisulfite sequencing and chromatin immunoprecipitation sequencing (ChIP-seq).74, 75 These epigenetic changes are now considered crucial oncogenesis drivers that might offer important insights into tumor heterogeneity and possible treatment approaches. Moreover, NGS has improved our comprehension of tumor heterogeneity and evolution. By sequencing several locations within a single tumor and examining circulating tumor DNA (ctDNA) in the blood, scientists can detect intratumor heterogeneity and track clonal dynamics as cancer advances and is treated.30 This information immediately influences treatment plans, enabling more potent medications that target particular tumor subclones.
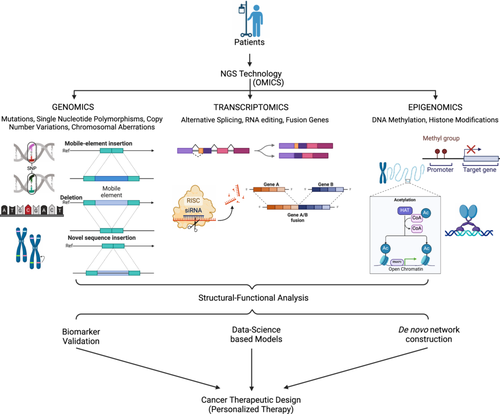
Moreover, germline testing for therapeutic applications has not yet been widely adopted but is anticipated to expand, partly driven by clinical trials like Pancreas Cancer Olaparib Ongoing and the Trial of PARP Inhibition in Prostate Cancer (TOPARP).76, 77 These trials have demonstrated clinical benefits of PARP (poly [ADP-ribose] polymerase) inhibitors in patients with specific germline mutations, such as BRCA1 or BRCA2 alterations.76 Moreover, compelling clinical evidence supports the role of germline pharmacogenetic testing in reducing the risk of chemotherapy-induced toxicity and optimizing the selection and dosing of supportive care medications. Comprehensive NGS platforms are now being developed to provide both somatic and germline genetic insights, with ongoing efforts to extract pharmacogenetic data from sequencing results.78 As a result, clinicians are increasingly confronted with vast amounts of clinically relevant genetic information that can influence the treatment and management of cancer patients. Oncologists now consider NGS to be a vital tool in the clinical environment. Because the mutations found by NGS can be used to inform the selection of medications that specifically target those mutations, it helps choose targeted therapies.15 NGS also helps with minimal residual illness surveillance and treatment response prediction. Liquid biopsies are a minimally invasive way to evaluate the effectiveness of treatment and the likelihood of illness recurrence. They rely on NGS to identify ctDNA. Below is an overview of some of the uses of NGS in oncology.
Using NGS, researchers can comprehensively profile point mutations, indels, CNVs, and structural variants, among other genomic abnormalities found in cancer cells (explained later).79, 80 This information facilitates the identification of carcinogenic pathways and driver mutations. NGS can also detect specific mutations in oncogenes (eg. EGFR, KRAS) and tumor suppressor genes (e.g., TP53) that lead to cancer development and progression.81, 82 This information is crucial for making personalized treatment decisions. NGS can discover new mutations and fusion genes in cancer genomes, allowing the identification of potential therapeutic targets and biomarkers. By revealing the diversity of mutations within a single tumor, NGS can assess intratumor heterogeneity.26, 27, 83 This information is important for understanding tumor development and therapy resistance. By analyzing ctDNA in blood samples, as described in Figure 3, NGS can allow noninvasive detection of cancer progression, treatment response, and MRD.15, 30 RNA-Seq enables the measurement of gene expression levels, alternative splicing events, and fusion transcripts in cancer cells. This will help identify dysregulated pathways and potential therapeutic targets. Further, individual treatment programs can be adopted to identify genetic variants that influence drug metabolism and response.
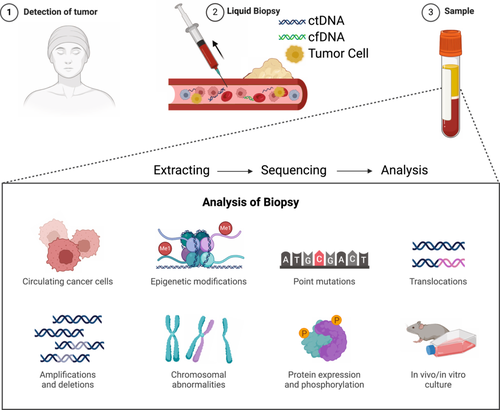
NGS allows tracking of clonal evolution and resistant subclones' emergence during cancer treatment. This information informs strategies for overcoming drug resistance. Combined with functional assays like CRISPR-Cas9 screening to identify genes and pathways essential for cancer cell survival and growth, NGS offers an additional overview of tumorigenesis.84-86 Characterizing the tumor microenvironment, including the study of infiltrating immune cells, NGS aids in developing immunotherapy strategies. NGS-based tests and targeted focused PCR panels are being developed for the early detection of cancer by identifying specific genetic or epigenetic alterations associated with precancerous lesions.87 In clinical settings, NGS helps to guide treatment decisions, such as selecting targeted therapies and immunotherapies based on the genetic profile of the patient's tumor like PTEN, MYC, EGFR, TP53, ALK, KRAS and many more.15, 88-90 Apart from these developments, NGS also enables the study of rare and understudied cancers, helping to uncover their genetic underpinnings and potential therapeutic vulnerabilities.
Once the molecular pathogenesis is known, NGS data can be used to stratify patients in clinical trials, identify biomarkers for drug response, and accelerate the development of novel cancer therapeutics. In summary, NGS has profoundly impacted the field of oncology by enabling comprehensive genomic and epigenomic analyses of cancer. It has provided insights into the molecular underpinnings of cancer, identified potential therapeutic targets, and facilitated the development of personalized treatment strategies. Moreover, NGS has improved our ability to monitor disease progression and treatment response in real-time, making it an invaluable tool in the fight against cancer. As technology advances, it is expected to play an even more significant role in oncology research and clinical practice, ultimately leading to improved patient outcomes.
4.1 Role of NGS in detecting MRD
MRD is the term used to describe cancer cells that remain in a patient's body after therapy, even if typical clinical evaluations are unable to identify them.31, 91 Residual cancers following treatment present a considerable challenge for oncologists. These cancers tend to be not only more resistant to therapeutic interventions but also often exhibit greater aggressiveness than the original tumor. Evaluating MRD after chemotherapy is crucial for planning subsequent treatment strategies. NGS plays a pivotal role in the detection of MRD, serving as an essential component in both cancer research and clinical management. NGS has transformed the field by enabling comprehensive genomic analyses, making it an invaluable tool for elucidating the genetic underpinnings of cancer, identifying mutations, and characterizing tumor heterogeneity. The detection of MRD is critical in oncology care, as it informs treatment decisions and provides insights into the likelihood of cancer recurrence, ultimately helping to tailor patient management strategies more effectively.31, 91 NGS is necessary for identifying MRD because of its excellent sensitivity and ability to identify even minute amounts of altered DNA or RNA.91, 92 Additionally, the sensitivity of MRD detection can be increased by using focused NGS panels that concentrate on known mutations or gene fusions linked with malignancy. Treatment response and the establishment of resistance mutations can be understood by tracking changes in the tumor's genetic profile with serial NGS assessments conducted both during and after treatment. NGS-MRD detection can provide helpful prognostic data, assisting physicians in assessing the possibility of novel drug delivery.
The use of NGS-MRD assessment as an endpoint in clinical studies to evaluate treatment effectiveness and guide medication development is growing in popularity. Physicians can assess the chance of a disease recurrence by using prognostic information obtained from NGS MRD detection. Several cancer types have been identified with NGS-based MRD detection, including solid tumors, lymphomas, leukemia, and multiple myeloma.29, 92-94
Several methods and markers may be used depending on the type of cancer and the genetic alterations taking place. Because NGS makes comprehensive genome analysis possible, it has changed clinical treatment and cancer research. When used for MRD detection, NGS exhibits exceptional sensitivity and specificity, facilitating the monitoring of cancer patients' prognosis and response to treatment.31, 94 Combining NGS with MRD evaluation is viable for integrating into one health approach.
4.2 Application of NGS to detect CNVs in cancer
The genomes of many species, including humans, have CNVs, which can be found and characterized using NGS, a potent and adaptable technique.79 DNA segments that range in size from kilobases to megabases are either deleted or duplicated in CNVs, a kind of structural variation, as compared to the reference genome.95 These changes to the genome may have significant ramifications for oncology and other genetics-related professions. NGS provides many methods, each with pros and cons, for identifying CNVs. Whole-genome sequencing (WGS) is a widely used technique in which all genomes is sequenced and read-depth variations are examined. A change in read depth within a specific area denotes the existence of duplications or deletions, respectively. WGS is valuable for identifying CNVs across the entire genome but can be computationally intensive and expensive.32, 96 Protein-coding portions of the genome are the focus of targeted sequencing techniques like exome sequencing.80 This method is frequently applied in clinical settings and is reasonably priced. It might, however, overlook CNVs outside of the designated areas. Targeted sequencing and genome-wide CNV analysis are used in hybrid capture-based approaches to balance specificity and genome coverage.
4.2.1 Challenges in CNV detection
Even though NGS has remarkably improved the detection of CNVs, some problems remain. There are bioinformatics tools and algorithms that were developed to solve it, but fine mapping may still be a trouble, especially in repetitive genomic regions.97 Further, it also entails differentiating between true CNVs and sequencing artifacts or systematic biases.98 Appropriate quality control measures and normalization methods should be used to minimize false positives and negatives. NGS can address these challenges.
4.2.2 Relevance in oncology
CNVs play a crucial role in oncology as they can lead to the activation of oncogenes or the inactivation of tumor suppressor genes. They are considered very important in oncology because they can result in the activation of oncogenes or the deactivation of tumor suppressor genes.99 NGS-based CNV analysis in cancer genomes has potential for identification of driver CNVs that cause tumorigenesis and progression. Besides, it can be utilized to distinguish cancer subtypes as well as identify possible therapeutic targets. Moreover, NGS-based CNV analysis is increasingly used in clinical oncology to determine treatment options. It allows the detection of particular CNVs that may make a tumor vulnerable to antitumor agents such as drugs against overexpression products of amplified oncogenes.100
4.3 Application of NGS in detecting gene fusion
NGS has revolutionized detecting and characterizing fusion genes, which are chimeric genes formed by the abnormal fusion of two genes.70, 100 These fusion events occur in various cancers and can serve as critical oncogenic drivers or therapeutic targets. In this academic discussion, we will explore how NGS is utilized in detecting fusion genes and its significance in cancer research and clinical practice.
4.3.1 RNA sequencing (RNA-Seq) for fusion gene detection
One of the primary applications of NGS in fusion gene detection is through RNA-Seq. RNA-Seq allows researchers to comprehensively analyze the transcriptome, including identifying gene fusions at the RNA level. The process involves sequencing RNA molecules, which can capture fusion transcripts resulting from chromosomal rearrangements. Fusion genes often arise from chromosomal translocations, inversions, or other structural variations.101, 102 These events lead to the formation of chimeric transcripts, where sequences from two distinct genes are fused. NGS enables the precise identification of these fusion breakpoints and the characterization of fusion partners.102
4.3.2 Advantages of NGS for fusion gene detection
NGS-based methodologies provide numerous advantages for the detection of fusion genes. One of the key benefits is their comprehensive detection capability: It can identify fusion genes without requiring prior knowledge of the specific fusion partners involved. This unbiased strategy is particularly valuable for uncovering novel fusion events in cancer, facilitating the discovery of previously unrecognized genetic alterations that may play critical roles in tumorigenesis. By enabling a more thorough exploration of the genomic landscape, NGS significantly enhances our ability to identify and characterize complex genetic rearrangements associated with various cancers.103, 104 NGS can detect fusion transcripts at low expression levels, enhancing sensitivity. Moreover, bioinformatics tools can help filter out false positives, ensuring specificity. It can also capture alternative splicing events, which may result in different isoforms of fusion genes.105, 106 This level of detail is crucial for understanding the functional consequences of fusion events.
4.3.3 Utility of detecting fusion genes in oncology
Fusion gene analysis using NGS has significantly contributed to cancer research. It has led to identifying driver fusion events promoting tumorigenesis and classifying cancer subtypes based on fusion gene signatures.81, 107, 108 Additionally, it has provided insights into the molecular mechanisms underlying cancer progression. Furthermore, NGS-based fusion gene detection has facilitated the development of targeted therapies. Fusion genes often produce abnormal fusion proteins that can be targeted with precision medicine approaches.109-111 Drugs inhibiting these fusion proteins have shown promise in various cancers, improving treatment outcomes.
4.3.4 Clinical utility
In clinical oncology, NGS is vital in identifying fusion genes to guide treatment decisions.70, 71 Molecular profiling of patient tumors using NGS can reveal actionable fusion events, enabling oncologists to select targeted therapies that specifically address the underlying genetic drivers of cancer. This personalized medicine approach can potentially enhance treatment response and minimize side effects.109, 111
As NGS technologies continue to evolve, the detection of fusion genes is becoming increasingly precise and cost-effective. Furthermore, the integration of fusion gene data with other genomic and clinical information is anticipated to enhance treatment strategies and improve patient outcomes. In summary, NGS has established itself as an essential tool for detecting and characterizing fusion genes within cancer research and clinical applications. Its capacity to provide a comprehensive analysis of the transcriptome and identify fusion events with high sensitivity has significantly advanced our understanding of the molecular mechanisms underlying cancer. This progress has facilitated the development of targeted therapies tailored to individual patients. As NGS technologies advance further, they are expected to play an even more crucial role in the discovery and treatment of cancers associated with fusion genes. However, despite its powerful capabilities, NGS does have limitations, particularly in its inability to provide information on the spatial expression of genes.112 This limitation becomes particularly pronounced in tissue-based gene expression profiling. NGS and spatial analysis techniques represent two powerful methodologies that, when used in conjunction, provide unparalleled insights into the spatial distribution of genetic material, gene expression, and various biological processes within tissues or cells. The synergy between NGS and spatial analysis is especially beneficial in the realms of genomics, transcriptomics, and spatial biology, enabling researchers to explore complex biological systems with greater depth and clarity. This integrated approach enhances our understanding of how genes are expressed in their native environments, paving the way for more comprehensive analyses of tissue functionality and disease mechanisms.112
5 TARGETED NGS
Targeted NGS platforms have been developed to expand the number of genetic targets that can be analyzed, with both amplicon-based and hybrid-capture-based methods available to sequence specific genetic regions and detect actionable alterations. Amplicon-based NGS uses primers to amplify select regions of circulating tumor DNA (ctDNA), which are then sequenced. This approach is advantageous due to its minimal requirement for starting material and lower cost compared to hybrid capture NGS.113, 114 However, amplicon-based NGS is limited to known mutation hotspots and targeted panels, restricting its ability to identify a broad range of genetic alterations.115, 116
Hybrid capture NGS, in contrast, involves using DNA or RNA fragments to selectively enrich ctDNA fragments from a larger pool of cell-free DNA (cfDNA). While this method offers more comprehensive coverage and consistent data, it comes with significant drawbacks—requiring larger DNA samples, a more labor-intensive workflow, longer turnaround times, and higher costs.113 Though technically more challenging, hybrid capture can detect a wider variety of genetic alterations, including complex rearrangements, which may be missed by amplicon-based approaches. Still, the cost and complexity can be prohibitive for routine clinical use without sufficient resources and bioinformatics infrastructure.
Despite advances in amplicon-based NGS improving sensitivity and reliability, it remains limited to predefined mutation sites and is less adept at detecting structural variations like gene fusions unless circulating tumor RNA (ctRNA) or multiplex PCR is incorporated.70 Hybrid capture NGS, while providing broader detection capabilities, requires more sophisticated systems and validations, making it less practical in some clinical settings compared to the relatively straightforward amplicon-based approach.113
In a direct comparison between amplicon NGS and droplet digital PCR (ddPCR), NGS demonstrated high sensitivity for detecting single nucleotide variants, indels, and specific rearrangements, with a positive percentage agreement of 95% and a positive predictive value of 100%.117 However, concordance between NGS findings in liquid biopsies and traditional tissue biopsies has shown variability.118 This discrepancy is often attributed to tumor heterogeneity, where mutations present in ctDNA may be missed in tissue samples, as seen in a case where the EGFR T790M mutation was detected in ctDNA but not in the corresponding tissue biopsy, yet the patient responded well to osimertinib therapy.119 Moreover, the low abundance of ctDNA can lead to false negatives when compared to tissue biopsies.120
NGS platforms provide a significant advantage over digital PCR techniques by enabling the detection of various genetic alterations occurring at the same locus. For example, Cancer Personalized Profiling by deep Sequencing (CAPP-Seq) uses probes derived from libraries of known genetic mutations in non-small cell lung cancer (NSCLC) to analyze ctDNA, amplifying multiple loci for sequencing simultaneously.121 This method was further enhanced by integrated digital error suppression, which significantly reduced background noise, resulting in a detection sensitivity of 0.004% (4 out of 105 cfDNA molecules) and high clinical sensitivity (90%) and specificity (96%).122
Overall, while NGS technologies offer powerful capabilities, their clinical application is limited by these technical challenges, need for larger starting material in some methods, higher costs, complex workflows, and the potential for false negatives due to low levels of ctDNA. Additionally, tumor heterogeneity and technical discrepancies between tissue and liquid biopsy analysis can affect the accuracy of results. Thus, while targeted NGS holds promise for comprehensive cancer profiling, its limitations must be carefully weighed against clinical resources, use cases, and downstream bioinformatics capabilities.
6 SPATIAL ANALYSIS IN GENOMICS USING NGS
Spatial analysis in genomics involves investigating the spatial distribution of genetic material or gene expression within tissues, cells, or even single molecules. It allows researchers to understand the spatial organization of biological structures, which is critical for understanding various biological processes, including development, disease pathology, and tissue microenvironments. Some methodologies for spatial analysis in genomics include the following contents.
6.1 Single-cell RNA sequencing (scRNA-seq)
While scRNA-seq is not strictly a spatial technique, it can provide insights into the gene expression profiles of individual cells, which can be integrated with spatial data to infer cellular interactions and relationships (Figure 4).123
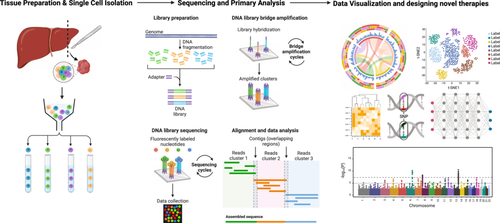
Integrating NGS and spatial analysis techniques allows researchers to Map Gene Expression spatially. Researchers can create spatial gene expression maps by combining spatial transcriptomics with NGS,25, 112 revealing how genes are expressed within specific tissue regions. Spatial analysis can also uncover the heterogeneity of tissues, showing variations in gene expression or genetic alterations across different areas. Further researchers gain insights into the spatial distribution of disease-associated markers or pathways within tissues, aiding in disease diagnosis and understanding. Integrating scRNA-seq with spatial data can elucidate cellular interactions and communication networks within tissues.124 Spatial genomics can guide the development of targeted therapies by identifying regions of interest for drug targeting.125, 126
In recent years, significant advancements have been made in the development of scRNA-seq techniques across both laboratory and computational domains. A key aspect of this methodology involves the meticulous isolation of individual cells from tissue samples. Following isolation, the cells are lysed using specialized reagents to extract the target RNA molecules, which are subsequently purified. To eliminate ribosomal RNA, the RNA molecules are polyadenylated using poly(T) primers, as analyzing non-polyadenylated mRNA poses challenges and necessitates specific protocols for effective analysis.127 The extracted total RNA is used to synthesize cDNA via reverse transcription, with specific adapter sequences (unique molecular identifiers) added for RNA detection by NGS platforms. The cDNA is then amplified using PCR or in vitro transcription, followed by another round of reverse transcription or nucleotide barcode-tagging amplification. Finally, the tagged cDNA from each cell is pooled and sequenced using NGS, employing library preparation techniques, sequencing platforms, and genomic-alignment tools similar to those used for bulk samples.128
Several methods are employed for single-cell transcriptome sequencing, with prominent techniques including SCRB-seq, CEL-seq. 2, MARS-seq, Drop-seq, Smart-seq. 1, Smart-seq. 2, and 10× Genomics. Among these, Smart-seq. 2 is notable for detecting the maximum number of transcripts in individual cells, while CEL-seq. 2, MARS-seq, Drop-seq, and SCRB-seq effectively quantify mRNA with minimal noise using unique molecular identifiers (UMIs). Drop-seq is the most cost-effective for analyzing a large number of cells, whereas SCRB-seq, MARS-seq, and Smart-seq. 2 are more effective for profiling the transcriptome of fewer cells.
6.1.1 Cell expression by linear amplification and sequencing (CEL-Seq)
Tang et al. introduced single-cell RNA-seq using poly(T) sequences for mRNA analysis from tissue samples.129 Initially suitable for RNA analysis, this technique could profile 75% more RNA analytes than microarrays but required higher RNA input concentrations. Hashimoshony et al. addressed this by developing the CEL-Seq method, which involved barcoding and pooling RNA from tissue samples.130 CEL-seq offers high strand specificity (over 98% of exonic reads from the sense strand) and efficient barcoding (> 96%).130 However, it has a high 3′-bias and low sensitivity for lowly expressed transcripts. To improve this, CEL-seq. 2 was developed, offering higher sensitivity, reduced costs, and faster analysis.131
6.1.2 Single-Cell RNA barcoding and sequencing (SCRB-Seq)
SCRB-seq was developed to analyze RNA from low input concentrations, using barcoding and sequencing with UMIs to reduce amplification bias.132 The advanced mcSCRB-seq version uses FACS sorting and molecular crowding conditions for improved reverse transcription and template switching.133 SCRB-seq is high-throughput, cost-efficient, and sensitive, but the template-switching reverse transcription introduces bias against full-length mRNA.133
6.1.3 Switching mechanism at the end of the 5′-end of the RNA transcript sequencing (Smart-Seq)
SMART-seq is a NGS method suitable for small transcripts and full-length gene sequencing, revealing complex structural variations. Ramskold et al. enhanced the analysis of small RNA samples, allowing the detection of 40% of transcripts from 10 pg of RNA, equivalent to a single cell's RNA content. This method uses the moloney murine leukemia virus for cDNA preparation, maintaining 5′-end transcript integrity.134 Although Smart-seq offers several advantages, it also has notable limitations. These include an absence of early multiplexing capabilities, a lack of strand specificity, and a bias towards longer transcripts, particularly those exceeding 4 Kb. Additionally, the method demands a high input of RNA, which can be a constraint in certain experimental contexts. Improvements led to Smart-seq. 2, which offers better sensitivity and uses standard reagents for full-length chromosomal DNA and sequencing libraries.135
6.1.4 Drop-sequencing (Drop-Seq)
Drop-seq is a cost-effective approach for analyzing single-cell transcriptomes, leveraging the encapsulation of individual cells with DNA barcoded microbeads to preserve the transcript origin information at the cellular level. These molecularly barcoded beads enable precise identification of the source of each mRNA, ensuring accurate tracking of gene expression. With an estimated cost of around USD 0.70 per cell, Drop-seq provides an efficient means of preparing libraries, capable of processing up to 10,000 cells in a single day. The use of unique molecular and cell barcodes simplifies the identification of mRNA transcripts, while reverse transcription combined with template-switching PCR generates a substantial amount of complementary DNA.136 It offers several key advantages, including the ability to assess single-cell sequences, the identification of gene-specific mRNA strands through the use of unique molecular and cell barcodes, and the generation of high read counts from individual cells. Additionally, it is a cost-effective method with rapid library preparation capabilities. However, Drop-seq does come with certain limitations; it necessitates a specialized microfluidic device for the separation of droplets, exhibits lower gene sensitivity per cell when compared to alternative methods, and is restricted to analyzing mRNA transcripts only.
6.1.5 Massively parallel RNA single-cell sequencing framework (MARS-Seq)
MARS-seq, or the automated massively parallel RNA sequencing framework, was developed for sampling thousands of in vivo cells with controlled bias during amplification and labeling. For instance, RNA from over 4000 mouse spleen single cells was sequenced to differentiate between spleen cell diversity and ductal carcinoma cells by focusing on the expression of surface marker CD11c. MARS-seq facilitates comprehensive genome-wide transcriptional profiling of tissues in both healthy and diseased conditions, providing valuable insights into the biological functions of cells in vivo. The methodology involves tagging polyadenylated RNA molecules with randomized molecular identifiers, followed by the pooling of these labeled samples. This process includes two rounds of amplification to generate material that is ready for sequencing, thereby enhancing the overall analysis of gene expression across diverse tissue types.137 It offers several advantages, such as the ability to perform in vivo sampling, high-throughput transcriptional profiling, and effective multiplexing through three distinct levels of barcoding: cellular, molecular, and plate-level. Despite these benefits, the technique encounters certain challenges, including potential biases during the purification process and the loss of strand-specific information that can occur during RNA fragmentation.
6.1.6 10× genomics single-cell RNA-Seq
10× Genomics has significantly advanced single-cell RNA sequencing, enabling the identification of various cell subpopulations in heterogeneous cancer tissues. Their GemCode Technology analyzes the transcriptome of thousands of cell populations by combining a microfluidic platform with molecular barcoding and custom bioinformatics software.138 Another significant advancement in the field is Chromium scRNA sequencing, which innovatively encapsulates reaction reagents, individual cells, and barcoded oligonucleotides within a single Gel Bead, forming nanoliter-sized Gel Bead Emulsions (GEMs). This approach allows for efficient processing and analysis of scRNA, enhancing the ability to study gene expression at an unprecedented resolution.139 Within GEMs, single cells are lysed, and polyadenylated mRNA undergoes barcoded reverse transcription, resulting in high-quality sequencing libraries of target transcripts. Chromium Software Suite then visualizes gene expression at a single-cell level. Chromium scRNA sequencing is high-throughput, profiling individual cell types in tissues, and identifying subpopulations of rare cell types in heterogeneous tissues.139 It is more efficient than droplet systems, processing over 80,000 cells in less than 10 min, offering a wider dynamic range.
6.2 Spatial Transcriptomics
Spatial transcriptome analysis is a significant breakthrough in medical biotechnology, enabling the mapping of analytes, such as RNA, within their physical locations in tissue sections. Various techniques, including in situ sequencing, fluorescent in situ hybridization (FISH), in situ capture, and in silico methods, have since been used for spatial RNA mapping.140
- 1.
NGS-based approaches.
- 2.
Imaging-based techniques.
6.2.1 NGS-based approaches
NGS-based approaches for spatial transcriptome analysis derive from single-cell RNA-seq methods, incorporating spatial barcodes before library preparation. Stahl et al. conducted the first NGS-based spatial transcriptomics study in 2016, analyzing tissue sections.141 10× Genomics has made substantial contributions to the field by developing innovative technologies that leverage microarray methods and barcoding techniques. Spatial transcriptomics facilitates an extensive analysis of the transcriptome across tissue sections, enabling the visualization of multiple genes within a single sample. This advancement allows researchers to gain deeper insights into gene expression patterns and spatial organization in biological tissues.140
The technique involves capturing RNA analytes on spatially barcoded microarray slides before reverse transcription, ensuring the mapping of each RNA molecule to its original tissue location using unique positional molecular barcodes. This method was first tested on mouse olfactory bulb tissue samples and has since been applied to various tissues.142, 143 10× Genomics' Visium technology has improved resolution and sensitivity.136
- -
Slide-Seq: Uses randomly barcoded beads on glass slides to capture mRNA, achieving 10 μm resolution and improved sensitivity, analyzing up to 500 transcripts per bead.
- -
High-Definition Spatial Transcriptomics (HDST): Enhances resolution by using beads deposited in wells instead of glass slides.
- -
DBiT-Seq: Employs microfluidic channels with polyT barcodes to capture RNA in tissues.
- -
Stereo-seq: Uses randomly barcoded DNA nanoballs for nanoscale RNA analysis.
- -
Seq-Scope: Maps RNA transcripts within the nucleus and cytoplasm using subcellular resolution spatial barcoding.
- -
Pixel-Seq: Uses a polony-derived gel oligo array for RNA capture, offering ~200-fold improved resolution compared to existing methods.
6.2.2 Imaging-based approaches
Imaging-based spatial transcriptomics can be divided into in situ hybridization and in situ sequencing methods.
MERFISH (multiplexed error-resistant fluorescence in situ hybridization) is capable of analyzing thousands of RNA molecules within cancer tissue samples. This technique utilizes uniquely designed encoding probes that contain complementary sequences along with flanking readout sequences, facilitating the detection of RNA through several rounds of hybridization. By employing this method, researchers can gain a detailed understanding of the spatial distribution and abundance of RNA in complex tissue environments.144 Initially, MERFISH faced sensitivity issues, which were mitigated by the Hamming distance error correction code, reducing the required hybridization rounds and improving sensitivity. Single-molecule fluorescence in situ hybridization (smFISH) provides quantitative RNA expression measurements and spatial localization by imaging individual RNA molecules in single cells.144 Multiplexed single-molecule fluorescence in situ hybridization (smFISH) and in situ sequencing methods have significantly improved detection limits and RNA sensitivity within tissue cells, allowing for the visualization of transcriptome organization at the single-cell level. MERFISH leverages fluorescent signals emitted by labeled probes that bind to RNA molecules. The intensity of these signals, which is essential for accurate RNA detection, can be enhanced by utilizing cameras with longer exposure times and powerful laser illuminations, thus facilitating more precise imaging of RNA distribution within cells.145 The technique's effectiveness may be reduced for smaller RNA molecules that bind fewer probes.
Fourth-generation RNA-seq platforms, such as in situ sequencing (ISS) and fluorescent ISS (FISSEQ), have significantly advanced RNA-seq objectives.146 ISS uses padlock probes and rolling circle amplification to generate targeted sequencing libraries, which are then sequenced using NGS techniques. ISS can sequence up to 256 RNA transcripts in a single hybridization round.146 FISSEQ, utilizing random hexamers and sequencing primer tags, performs unbiased RNA analysis and detects low RNA copy numbers. Despite its strengths, FISSEQ's sensitivity is lower than ISS.146 Both techniques are still developing, requiring improvements in sample preparation, efficiency, computational methods, and imaging scale.
Laser capture microdissection RNA sequencing (LCM-RNAseq) overcomes the limitations associated with bulk RNA sequencing techniques. This method involves the precise dissection of specific cells using laser capture, followed by RNA sequencing to analyze the extracted material. Recent advancements in LCM-RNAseq have made it possible to quantify low-input, degraded RNA molecules obtained from formalin-fixed paraffin-embedded (FFPE) tissues. For instance, Singh et al. employed LCM-RNAseq to investigate tumor heterogeneity by sequencing data derived from 10 isolated single cells, showcasing its capability to provide insights into the complexities of individual cellular environments.147 The LCM-Smart3seq technique, capable of analyzing minimal RNA quantities, was later developed.148 Further advancements, such as FFPEcap-seq, allowed accurate RNA detection from FFPE tissues by capping RNA 5′ ends.149 The first LCM-RNAseq study on cancer tissues focused on lung cancer, revealing genes involved in tumor growth.150 LCM-RNAseq has since elucidated the spatial organization of various cell types in cancer tissues, such as human glioblastoma, showing interconnected channels that facilitate cancer cell proliferation and migration through specific signals.151
6.3 Integration of spatial transcriptomics and scRNA-seq
ScRNA-seq has revolutionized the analysis of gene expression by preserving the histological context of tissues, allowing for the identification of thousands of genes in sampled tissues. This technology offers critical clinical tools for early cancer detection and the development of targeted treatment strategies.152 Moreover, scRNA-seq can pinpoint the locations of mutated or aggressive clones within tumors, differentiate between the tumor's core and its invasive edges, evaluate molecular changes in the stroma both inside and outside the tumor, and detect epithelial-mesenchymal transitions.153
Recent advancements demonstrate that scRNA-seq can analyze RNA from both fresh-frozen and fixed tumor cells without losing their spatial context.153 Additionally, it can integrate spatial features directly into specific genetic elements in organoids or native tissues using image analysis. This integration characterizes and maps sequences from tumor tissues, revealing the complex arrangement of various cell types regulated by single-cell interactions. The primary goal of spatial mapping techniques is to acquire comprehensive genomic or transcriptomic data from entire tissue slides, enhancing it with sequencing methods. Combining scRNA-seq with other omics technologies provides a more thorough analysis of RNA analytes within their spatial context.154 In situ visualization of the transcriptome through scRNA-seq offers multiplexed information on gene expression, cell types, and disease progression. For instance, ISS utilizes padlock probes and rolling circle amplification to spatially display RNA expressions across tissue sections.
In a study conducted by Gyllborg et al., hybridization-based in situ sequencing (HybISS) was utilized to investigate the spatial localization of RNA within human and mouse brain tissues. This enhanced version of the HybISS platform has significantly improved combinatorial barcoding and detection limits, enabling precise mapping of RNA transcripts in their respective spatial contexts. By leveraging this advanced methodology, researchers were able to obtain detailed insights into the distribution and organization of RNA within the complex architecture of brain tissues.155 Asp et al. integrated scRNA sequencing (scRNA-seq) with spatial transcriptomics and in situ sequencing (ISS) to provide a comprehensive overview of the cellular architecture of the human heart across different developmental stages. This innovative approach allowed for a detailed examination of the cellular components, while ISS offered insights into the subcellular features and their specific physical locations within the heart tissue. Through this combination of techniques, the study illuminated the intricate organization of cardiac cells and their developmental dynamics.156
Moncada et al. integrated scRNA-seq with microarray-based spatial transcriptomics to uncover spatial gene expression variations in pancreatic ductal adenocarcinomas, identifying various cell types and subsets, including cancer cells and immune cells, within the tumors.143 Another approach, geographical positional sequencing (Geo-seq), combined LCM techniques with scRNA-seq to analyze cellular heterogeneity in pancreatic ductal adenocarcinomas, differentiating various subpopulations of cells spatially.157 Further, spatial transcriptomics involves mounting tissue sections on glass slides fixed with arrays of transcription primers, followed by permeabilization and reverse transcription to synthesize cDNA. This process allows for the spatial mapping of RNA transcripts on a two-dimensional plane. Advances in spatial profiling of transcriptomes, particularly in cancer research, have been meticulously reviewed, detailing methods and applications, including liver histology analysis.141 In a significant investigation conducted by Ji et al., a combination of single-cell RNA sequencing (scRNA-seq), spatial transcriptomics, and multiplexed ion beam imaging techniques was employed to study the spatial localization of tumor-specific keratinocytes (TSK) in breast carcinoma. This integrated approach allowed for the detailed visualization of TSK populations, along with immune infiltrates, revealing the cellular heterogeneity present at the tumor margins. By utilizing these advanced techniques, the study provided valuable insights into the complex interactions between tumor cells and the immune environment in breast cancer.142 ScRNA-seq has identified distinct cancer cell populations within pancreatic ductal adenocarcinoma (PDAC-A), revealing significant histological differences. For example, PDAC-A sub-region 1 showed an abundance of endothelial cells, monocytes, and fibroblasts, but a notable scarcity of cancer cells.158
7 PROS AND CONS OF NGS
7.1 Pros of NGS
NGS technologies can sequence millions to billions of DNA fragments simultaneously, allowing for the analysis of entire genomes, transcriptomes, or metagenomes in a single run are much faster than traditional Sanger sequencing and produce results in hours to days instead of weeks to months.64 Although this may seem expensive, over time, the cost of sequencing has dropped tremendously, making NGS accessible to researchers and clinicians.64 NGS provides high coverage for genomic regions. Thus increasing detection rates for rare variant calls and expanding knowledge about genetic diversity across populations. Some long-read-length sequencing platforms like PacBio and Nanopore sequencing can resolve complex genomic regions, detect structural variations, and investigate epigenetic modifications.20, 159, 160 This means that NGS is a widely applicable research tool in different scientific spheres, such as genomics, transcriptomics, epigenomics, and metagenomics, among others, which makes it a versatile tool for studying scientists and clinicians use it (specificities given in Figure 5). It has changed the way that we find disease-associated genetic variation; it has become an essential part of clinical diagnostics for personalized medicine and early disease detection.161 It is also used to evaluate complicated microbial communities in environmental samples, with the human microbiome being one of those ecosystems.
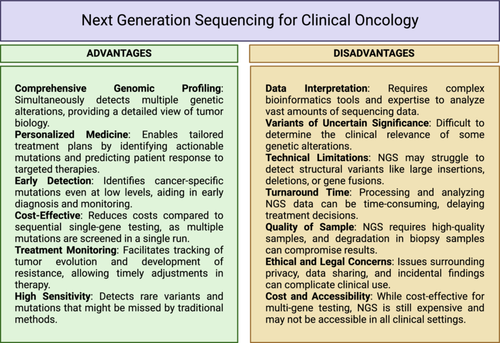
7.2 Cons of NGS
The massive amount of data generated by NGS requires substantial computational resources and expertize for data storage, processing, and analysis.14 Sample preparation for NGS can be time-consuming and sensitive to DNA quality and quantity. Inadequate sample preparation can lead to data artifacts.104 This can be a significant challenge for smaller research groups (Figure 5). Despite advances, NGS technologies can still produce sequencing errors, especially in regions with repetitive sequences or GC-rich regions. Careful data analysis and quality control are essential to mitigate these errors. NGS platforms are also technically demanding, requiring specialized equipment and highly trained personnel for operation and maintenance. Besides the technical challenges, the bioinformatics challenges are also significant, and manpower needs to be trained to harness the complete data information. NGS data requires bioinformatics expertize and specialized software tools, which may pose a barrier for researchers without a computational background.14 Data output often runs in several gigabytes and terabytes.162
While some NGS technologies offer long read lengths, many platforms produce short reads, making it challenging to assemble complex genomes or accurately characterize repetitive regions. While NGS has become more cost-effective in recent years, achieving deep genome coverage, and other specialized assay like single-cell genomics can still be quite expensive. The ability to generate extensive genomic information also raises ethical and privacy concerns related to the confidentiality and potential misuse of personal genetic data. The probability of widespread adoption in the future is heightened by the long-turn-around times and lower capital investment required for short-read sequencing technologies. However, the considerable costs and extended timelines of metagenomic research, coupled with its ambiguous correlation to conventional oncology methods, present major challenges to its routine or frequent application. Most commercial platforms currently rely on short-read sequencing, and since these tests are typically outsourced, the process of shipping specimens introduces further logistical delays, in addition to the runtime of the sequencing process itself and the subsequent bioinformatics analysis.163
These factors also complicate the timely reporting of data, making it difficult for clinicians—and, in some cases, even patients—to properly interpret the results. This issue could be mitigated by developing localized sequencing infrastructure, streamlining bioinformatics pipelines, and enhancing clinician education regarding metagenomic analysis, thereby improving both turnaround times and the clarity of results interpretation.
7.3 Future directions
Future research directions for NGS in oncology are expanding its precision and application to deepen our understanding of cancer's molecular underpinnings. Key areas of development include:
Single-cell NGS for Clonal Dynamics and Tumor Heterogeneity: Single-cell sequencing offers high-resolution insights into the clonal architecture of tumors, enabling the identification of subclonal populations and their roles in tumor evolution, immune escape, and therapeutic resistance. This will provide critical data for developing targeted therapies that account for clonal diversity within tumors.
Liquid Biopsy and MRD Detection: NGS applied to liquid biopsies—particularly in analyzing ctDNA and circulating tumor cells—is critical for early detection of MRD. Enhanced sensitivity will allow for the identification of ultra-low-frequency variants, crucial for predicting recurrence and guiding adjuvant therapies, even when tumors are not detectable via traditional imaging.
Immunogenomics and Neoantigen Discovery: NGS will continue to enhance immunogenomic profiling, allowing for the identification of tumor-specific neoantigens that inform personalized immunotherapy approaches. This includes leveraging tumor mutational burden and neoantigen prediction for optimizing immunotherapies, including checkpoint inhibitors and personalized cancer vaccines.
CNVs and single nucleotide polymorphisms (SNPs) Detection: Research will further enhance NGS for the detection of CNVs and SNPs, both critical in cancer development and progression. Accurate identification of CNVs will improve our understanding of gene dosage effects, such as oncogene amplification and tumor suppressor gene loss. Similarly, SNP profiling will reveal key germline and somatic mutations that influence cancer susceptibility, treatment response, and resistance mechanisms, with implications for risk stratification and therapeutic targeting.
Spatial Genomics and Transcriptomics for Tumor Microenvironment: Spatial NGS techniques are being developed to map gene expression and genomic alterations in situ within the tumor microenvironment. Understanding the spatial distribution of tumor cells, stromal elements, and immune infiltrates will provide crucial insights into localized interactions that drive metastasis, therapy resistance, and tumor immune evasion.
Noncoding RNA and Epigenomic Sequencing: Expanding NGS to noncoding RNA (ncRNA) profiling, including microRNAs and long noncoding RNAs (lncRNAs), will unveil regulatory networks that contribute to cancer progression. Epigenomic sequencing will provide additional insights into DNA methylation, histone modifications, and chromatin architecture, offering new therapeutic targets within the cancer epigenome.
AI-Enhanced NGS Data Analysis: Machine learning algorithms are poised to revolutionize NGS data interpretation, improving the accuracy of variant calling for SNPs, CNVs, and structural variants. AI-driven models will enable the integration of multi-dimensional genomic data, predicting therapeutic responses and identifying novel biomarkers, streamlining clinical decision-making in precision oncology.
These advances will further refine NGS's role in cancer biology, driving innovations in early detection, diagnostics, and tailored treatment strategies, cementing its importance in precision oncology.
8 CONCLUSION
To sum up, NGS has been essential in expanding our knowledge of genetics, genomics, and the complexities of the life sciences. It has made ground-breaking discoveries possible, including clarifying complicated genetic illnesses, discovering disease-causing mutations, and the characterization of genomic variances among various populations, especially cancer. With its many benefits in throughput, speed, and cost-effectiveness, NGS has significantly influenced genomics research and clinical diagnostics. However, it also has drawbacks, including technological complexity, data analysis difficulties, and the requirement for bioinformatics and computational skills. Researchers and physicians need to weigh these advantages and disadvantages when planning NGS studies and evaluating findings. In summary, NGS has brought about a revolutionary age in genetics and biomedical research. NGS technologies have advanced quickly since the early 2000s, providing high throughput and affordability. Since its inception in the early 2000s, NGS technologies have rapidly evolved, offering high-throughput, cost-effective, and versatile DNA and RNA sequencing approaches. This innovation has had profound implications across numerous scientific disciplines and clinical applications. Clinical applications of NGS have expanded, enabling personalized medicine approaches by tailoring medical treatments to an individual's genetic makeup. It has become a cornerstone in cancer genomics and pharmacogenomics, amongst others, revolutionizing patient care and diagnosis. The continued development of NGS technologies promises even more significant strides in genomics research and clinical practice. Third-generation sequencing and emerging synthetic biology approaches hold the potential to address existing limitations and open new frontiers in genomics. However, challenges remain, including managing and interpreting vast datasets, ethical considerations surrounding genetic information, and equitable access to NGS technologies worldwide.
In conclusion, NGS has redefined the landscape of biological research and oncology healthcare, empowering scientists and clinicians with unparalleled tools to unlock the genome's secrets. Its contributions to science, medicine, and our understanding of life's fundamental processes are a testament to the remarkable progress in genomics. As NGS continues to evolve, it promises to be at the forefront of future discoveries and innovations in the biological sciences and oncology.
AUTHOR CONTRIBUTIONS
Om Saswat Sahoo, Hiya Aidasani, and Arnab Nayek drafted the manuscript with assistance from Ruby Dhar. Joyeeta Talukdar, Anamta Gul, and Deepak Kumar corrected the proof, arranged references, and drafted the paper layout. Om Saswat Sahoo made the figures using BioRender. Smita Tripathi assisted in clinical interpretation. Ruby Dhar and Subhradip Karmakar conceptualized the entire work and guided the team. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Subhradip Karmakar thanks ICMR (Grant Number: I-1291) and AIIMS Interdepartmental (Grant Number: AC-61) grant for financial assistance.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.




