Meta-analysis of the use of Ofatumumab in the treatment of relapsing-remitting multiple sclerosis
Abstract
Background
Ofatumumab is the first monoclonal antibody developed specifically for treating relapsed multiple sclerosis (RMS). This disease (Multiple Sclerosis) includes relapsing–remitting multiple sclerosis (RRMS), a chronic autoimmune illness that affects the central nervous system (CNS), including the brain and spinal cord. The purpose of this study is to determine whether Ofatumumab is efficacious and safe in the treatment of relapsing–remitting multiple sclerosis.
Methods
An analysis of studies comparing Ofatumumab with placebo in people with relapsing-remitting multiple sclerosis was done in accordance with PRISMA guidelines. We looked up information in MEDLINE, SciSearch, BIOSIS Previews, Derwent Drug File, Embase, and International Pharmaceutical Abstracts. In patients with relapsing–remitting multiple sclerosis, randomized double-blind and non-randomized trials contrasting Ofatumumab with placebo were found by two independent investigators. Utilizing Review Manager 5.4.1, data were examined. The main results were total gadolinium-enhancing (Gd+) T1 lesions, annualized relapse rate, and new or expanding total T2 lesions. Secondary outcomes concentrated on general adverse events and adverse events related to infections.
Results
Three studies were included involving 334 patients, and the meta-analysis indicated that Ofatumumab showed good efficacy and safety in patients with relapsing forms of multiple sclerosis. The annual rate of relapse was significantly reduced by Ofatumumab (OR 0.51; 95% CI 0.27, 0.98; P = 0.04). Ofatumumab reduced the number of gadolinium-enhancing (Gd+) T1 lesions per scan (Mean difference −0.59; 95% CI −0.63, −0.55; P < 0.05). Ofatumumab treatment decreased new or enlarging total T2 lesions significantly (Mean difference −1.03; 95% CI −1.29, −0.76; P < 0.05). Infection-related adverse effects were seen more frequently with Ofatumumab shown by the odd ratio 0.48; 95% CI 0.22, 1.04; P = 0.06. Infection is, thus, a major limitation to its use.
Conclusion
The meta-analysis indicated that Ofatumumab is efficacious and safe for patients with relapsing forms of multiple sclerosis.
Abbreviations
-
- BIOSIS
-
- part of clarivate analytics web of science suite
-
- BMJ
-
- British medical journal
-
- CD
-
- cluster of differentiation
-
- CI
-
- confidence interval
-
- CNS
-
- central nervous system
-
- DMT
-
- disease-modifying therapy
-
- EDSS
-
- expanded disability status scale
-
- GD
-
- gadolinium-enhancing
-
- IO
-
- Ifeoluwa Olorunyomi
-
- MD
-
- mean difference
-
- MEDLINE
-
- medical literature analysis and retrieval system online
-
- MO
-
- Missouri
-
- MRI
-
- magnetic resonance imaging
-
- MS
-
- multiple sclerosis
-
- OO
-
- Olorunyomi Oluwatobi
-
- OR
-
- odd ratio
-
- PO
-
- Peter Odutola
-
- PRISMA
-
- preferred reporting items for systematic reviews and meta-analyses
-
- RCT
-
- randomized controlled trials
-
- RMS
-
- relapsed multiple sclerosis
-
- RRMS
-
- relapsing-remitting multiple sclerosis
-
- SSM
-
- sisters of St. Mary
-
- TX
-
- Texas
-
- USA
-
- United States of America
1 INTRODUCTION
In multiple sclerosis (MS), myelin, which is a fatty material that covers and shields nerve fibers in the central nervous system, is attacked by the immune system, causing inflammation and damage [1-4]. Young adults who suffer from multiple sclerosis are most likely to suffer permanent disability. MS is more common in females than in males with a ratio of 3:1 [5, 6]. In most cases, MS manifests between the ages of 20 and 40, but it can occur at any age. The commonest type of MS is relapsing-remitting MS (RRMS), with about 85% of patients manifesting the symptomatology of MS [7, 8]. It involves episodes of flare-ups or relapses during which new symptoms appear or existing symptoms get worse, followed by periods of improvement or remission [9-11]. Despite the fact that healthcare needs tend to rise with age, MS patients typically have extended lifespans [12, 13]. Ofatumumab is a recently approved medication that serves as a disease-modifying therapy (DMT). Disease-modifying therapy (DMT) refers to a class of drugs used to treat multiple sclerosis (MS) that alter the underlying disease process rather than just treating symptoms [14-18]. These medications are designed to limit the progression of disability, lessen the frequency and severity of relapses, and slow down the start of secondary progressive MS [16, 18]. Ofatumumab is the only monoclonal antibody that is available for patients to self-administer at home [15-17, 19]. This systematic review and meta-analysis use data from randomized clinical trials to assess the safety and effectiveness of ofatumumab in the treatment of relapsing-remitting multiple sclerosis.
2 METHODS
2.1 Eligibility criteria
Only papers that matched all the requirements for eligibility were allowed to be included in this meta-analysis and these include (1) randomized trials or observational cohorts; (2) studies comparing Ofatumumab to placebo; (3) patients diagnosed with relapsing-remitting multiple sclerosis; (4) Studies that reported results which focused on our objectives; (5) Studies whose articles are available for full review; and (6) Studies published in English. We excluded studies with (1) Studies without a control group; (2) Studies with patients overlapping populations; (3) case series or case reports; and (4) previous systematic reviews and meta-analyses.
2.2 Search strategy and screening
A systematic database search using the Preferred Instrument for Systematic Reviews and Meta-Analysis (PRISMA) was carried out in April 2023 with no date limit Figure 1 [20]. The search engines/databases BIOSIS Previews, Derwent Drug File, Embase, International Pharmaceutical Abstracts, MEDLINE, and SciSearch were used to find studies comparing ofatumumab with placebo. The following search phrases were used to look for papers that included them in their title or abstract: Ofatumumab OR arzerra OR “gsk 1841157” OR gsk1841157 OR “humac CD20” OR “HuMax CD20” OR HuMaxCD20 OR kesimpta OR “omb 157” OR omb157, “multiple sclerosis”. Only English-language texts were included, and there were no age limitations. Two authors/reviewers (PO, OO) independently evaluated the selected manuscripts once more for inclusion, evidence grading, and data extraction. A third reviewer (IO) handled any difference between the identified data. Also reviewed were additional items that were discovered through related articles.
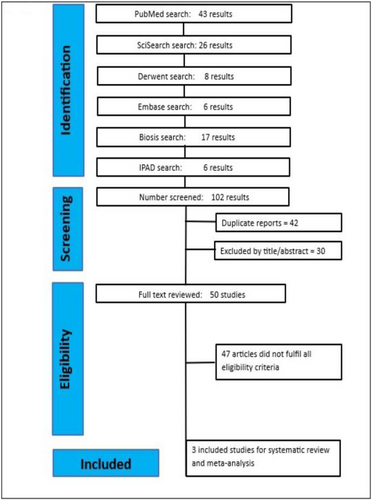
PRISMA2020 Flow diagram.
2.3 Endpoints of the systematic review
The major objectives of interest were the annualized relapse rate, total gadolinium-enhancing (Gd+) T1 lesions, and new or enlarging total T2 lesions. Secondary objectives focused on the overall adverse events and infection-related adverse events.
2.4 Data extraction
The inclusion and data extraction of each manuscript were evaluated separately by two reviewers. In addition to the author and the year of publication, the following information was pertinent to this meta-analysis: for clinical data, annualized relapse rate, adverse events overall, and infection–related adverse events were considered; for MRI parameters, the total gadolinium-enhancing (Gd+) T1 lesions, and new or enlarging total T2 lesions were included in the analysis. Data were extracted using the data extraction form. One of the authors copied the data from the articles, and the other author confirmed the data. Discussion amongst.
2.5 Quality assessment
Using Cochrane's technique for measuring bias in randomized trials [21], the included randomized controlled trials (RCTs) were evaluated for quality. Accordingly, studies are rated in five categories—selection, performance, detection, attrition, and reporting—as high, low, or unclear.
2.6 Statistical analysis
Review Manager V5.4.1 (Cochrane) was used to analyze the obtained data. The Higgins I2 was used to measure heterogeneity, and the studies underwent an analysis of the random effects model. If the studies that included the meta-analysis were less than 5, then a fixed-effect model was chosen. Continuous data including total gadolinium-enhancing (Gd+) T1 lesions, and new or enlarging total T2 lesions were estimated using mean difference with 95% confidence intervals (CI). Dichotomous data such as annualized relapse rate, overall adverse events, and infection–related adverse events were reported using odds ratios (OR) with 95% CI. Resulting values with associated p values < 0.05 were considered significant.
3 RESULTS
Three studies in total met the inclusion requirements for data extraction. A total of 334 individuals were included in the analysis, of whom 234 (or 70%) received ofatumumab treatment and 100 (30%) received a placebo. Between groups, baseline characteristics were comparable, as shown in Table 1. Table 2 shows the summary of the findings from each study.
| Study | Population | Intervention | Follow up (weeks) | No. of patients | Sex, female | Duration (years) | No. of relapses | EDSS score | No. of Gd + T1 |
|---|---|---|---|---|---|---|---|---|---|
| Kira 2022 | RRMS | Ofatumumab | 24 | 64 | 55 (85.9) | 5.4 ± 6.30 | 1.5 ± 0.85 | 2.21 ± 1.12 | 1.2 ± 2.29 |
| Sorensen 2014 | RRMS | Ofatumumab | 24 | 38 | 111.5 | 2.75 (2.9) | NA | 2.65 (1.15) | 1.2 (2.7) |
| Bar-or 2018 | RRMS | Ofatumumab | 24 | 231 | 155 (67) | 4.38 (5.530) | 1.3 (0.3) | NA | NA |
- Abbreviations: EDSS, Expanded Disability Status Scale; GD+, Gadolinium-enhancing; RRMS, Relapsing Remitting Multiple Sclerosis.
| Outcomes | Included studies | Ofatumumab | Placebo | MD/ORa (95% CI) | p value | Heterogeneity I2 | Heterogeneity p value |
|---|---|---|---|---|---|---|---|
| Annualized relapse Ratea | 3 | 132 | 100 | 0.51 (0.27, 0.98) | 0.04 | 0 | 0.69 |
| Total T1 GdE lesions | 3 | 132 | 100 | −0.59 (−0.63, −0.55) | <0.00001 | 94% | <0.00001 |
| New or enlarging total T2 lesion | 3 | 132 | 100 | −2.36 (−4.70, −0.01) | 0.05 | 97% | <0.00001 |
| Adverse events overall | 3 | 128 | 99 | −1.75 (−17.05, 13.54) | 0.82 | 0% | 0.48 |
| Infection related adverse event | 2 | 86 | 77 | −9.68 (−17.59, −1.78) | 0.02 | 0% | 0.63 |
- Abbreviations: MD, mean difference; OR, odds ratio.
- a signal variables of frequency analyzed through odds ratios.
3.1 Demographics
Age: Three studies with 133 patients taking ofatumumab and 100 receiving a placebo-described age. Meta-analysis of these data showed a mean difference of −0.93 years [−3.38, 1.51], p = 0.45. Figure 2a below displays the findings as analyzed.
Duration of Multiple Sclerosis diagnosis: Three studies described the time since multiple sclerosis diagnosis, totaling 132 patients who took Ofatumumab and 100 that took a placebo. Meta-analysis of these data revealed a mean difference of 1.12 years [0.25, 3.95], p = 0.07. Figure 2b shows the forest plot of the analysis.
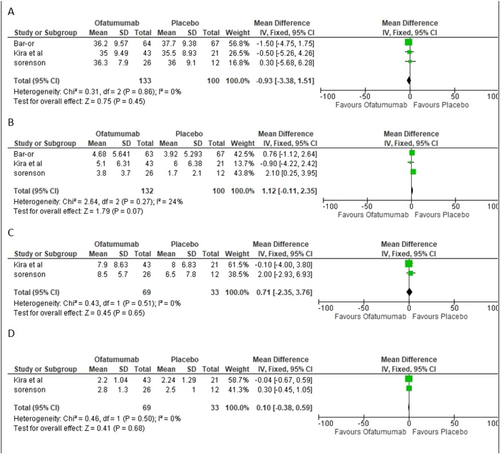
(a) Age. (b) Duration of Multiple Sclerosis diagnosis. (c) Duration of Multiple Sclerosis since first symptoms. (d) EDSS Score; EDSS, Expanded Disability Status Scale.
Duration of Multiple Sclerosis since first symptoms: Two studies described the time since multiple sclerosis first symptoms, totaling 69 patients who took Ofatumumab and 33 that took a placebo. Meta-analysis of these data revealed a mean difference of 0.71[−2.35, 3.76], p = 0.65. The forest plot of the analysis is shown in Figure 2c.
EDSS Score: Two studies described the EDSS baseline scores, 69 took Ofatumumab and 33 were given placebo. This data meta-analysis revealed a mean difference of 0.10 [−0.38, 0.59], p = 0.68. The Forest plot analysis of the data is shown in Figure 2d.
3.2 Clinical data
Analyzed clinical data included annualized relapse rate, overall adverse events, and infection–related adverse events. Three studies described the annualized rate of relapse as well as the overall adverse events, while only two studies compared infection-related adverse events. Forest plot analysis of the data is shown in Figure 3.
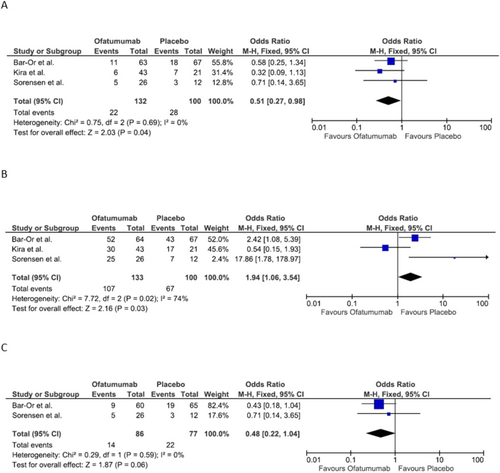
(a) Annualized relapse rate. (b) Adverse events overall. (c) Infection-related adverse event.
3.2.1 Annualized relapse rate
Three studies compared annualized relapse rates, totaling 133 patients who took Ofatumumab and 100 that took a placebo. This data meta-analysis found an Odd ratio of 0.51 [0.27, 0.98], p = 0.04. The three studies individually found Ofatumumab significantly reduced the annualized relapse rate. Figure 3a displays the forest plot.
3.2.2 Adverse events overall
Three studies also compared the adverse events overall, with a total of 133 patients taking Ofatumumab and 100 taking a placebo. Meta-analysis of these data showed an Odd ratio of 1.94 [1.06, 3.54], p = 0.03. There was a significant difference between Ofatumumab and placebo in terms of adverse events overall. Figure 3b shows the forest plot of this analysis.
3.2.3 Infection-related adverse events
Two studies representing 65% of the sample population determined Ofatumumab is not significantly linked with greater infection risk compared with placebo. The Odd ratio of 0.48 [0.22, 1.04], p = 0.06 is shown by the Forest Plot Figure 3c.
3.2.4 MRI parameters
The total gadolinium-enhancing (Gd+) T1 lesions and new or enlarging total T2 lesions were the two MRI parameters compared. Three studies participated in the analysis of these outcomes and the results are displayed in Figure 4.
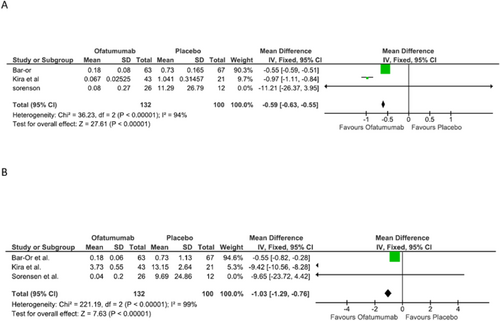
(a) Total gadolinium-enhancing (Gd+) T1 lesion. (b) New or enlarging total T2 lesions.
3.2.5 Total gadolinium-enhancing (Gd+) T1 lesion
Three studies compared Total gadolinium-enhancing (Gd+) T1 lesions, totaling 133 patients who took Ofatumumab and 100 that took a placebo. Meta-analysis of these data showed a mean difference of −0.59 [−0.63, −0.55], p = <0.00001. The three studies individually found that total gadolinium-enhancing (Gd+) T1 lesions were dramatically reduced. Figure 4a shows the results of this research.
3.2.6 New or enlarging total T2 lesions
Three studies compared new or enlarging total T2 lesions, totaling 133 patients who took Ofatumumab and 100 that took a placebo. Meta-analysis of these data showed a mean difference of −2.36 [−4.70, −0.01], p = 0.05. The three studies individually found Ofatumumab significantly reduced the new or enlarging total T2 lesions. Figure 4b shows the results of this research.
3.3 Quality assessment
The evaluation of RCTs is detailed in the Cochrane Collaboration's technique for evaluating the risk of bias in randomized trials, shown in Table 3.
4 DISCUSSION
- a.
Ofatumumab significantly reduced the annual rate of relapse (p = 0.04) and this met the expectation of a decrease in the rate of illness relapses;
- b.
The total gadolinium-enhancing (Gd+) T1 lesions were reduced (p = < 0.00001) leading to a slower accumulation of brain lesions preventing accelerated degeneration;
- c.
Compared to placebo, new or enlarging total T2 lesions were also reduced in keeping with the outcome expectation; The findings of this meta-analysis corroborate the findings of separate clinical trials that looked into the efficacy and safety of Ofatumumab in the treatment of relapsing-remitting multiple sclerosis. This statistical analysis indicates that Ofatumumab can be considered as a therapy option for relapsing-remitting multiple sclerosis patients [22, 23]. Ofatumumab as a disease-modifying treatment option in multiple sclerosis decreases the relapse rate as well as leads to slower accumulation of brain lesions on MRI [24]. Ofatumumab is a completely human anti-CD20 monoclonal antibody that appears to be a safe and effective disease-modifying medication for patients with relapsing-remitting multiple sclerosis, according to the findings of this meta-analysis. Ofatumumab is also unique in that patients can self-administer it at home, making it a convenient treatment option for patients [17, 18]. It may be preferable for patients with more active disease, for those who prioritize efficacy highly, and for those who can tolerate risk. Starting treatment early in the disease course has been shown to be significantly beneficial in preventing secondary progressive multiple sclerosis [25]. In our comprehensive study, the majority of patients improved following Ofatumumab administration, but it is worth noting that infection-related adverse events were more frequent with Ofatumumab, and this may limit its use in some patients. However, it is essential to recognize that the risk of infection may be offset by the potential benefits of treatment with Ofatumumab. Careful patient selection, appropriate dosing, and close monitoring for adverse events are essential when using Ofatumumab in the treatment of relapsing-remitting multiple sclerosis. Overall, this statistical analysis provides strong evidence that patients with relapsing-remitting multiple sclerosis have a safe and effective therapy option for ofatumumab. The rising amount of recent research demonstrating that monoclonal antibodies are the best disease-modifying treatments currently on the market is strengthened by this meta-analysis [26-28]. Additional research is required to examine the long-term safety and effectiveness of ofatumumab in this patient population and to evaluate its effectiveness in comparison to other disease-modifying medications.
5 STUDY LIMITATIONS
This study has limitations. The population of Americans affected by Multiple sclerosis is about 1 million and 85%–90% have relapsing-remitting multiple sclerosis. More research is required to examine the long-term effects of ofatumumab. More studies are also needed to compare Ofatumumab head-to-head with other monoclonal antibodies that are employed in treating complicated multiple sclerosis. The population in the studies utilized in this analysis might not have enough statistical power to identify certain Ofatumumab-related significant changes. This should be taken into consideration in future studies to enroll more people in the trials and increase the power.
6 CONCLUSION
The effectiveness and safety of ofatumumab for treating relapsing-remitting multiple sclerosis are highlighted in this meta-analysis. The rate of relapse was significantly low in patients who took Ofatumumab as well as the associated brain lesions associated with the disease. Infection is a negative effect seen with the use of Ofatumumab in keeping with this finding observed in other monoclonal antibodies. Ofatumumab's potential advantages outweigh the risk of infection observed in patients taking the monoclonal antibody. These results support the use of ofatumumab in patients with multiple sclerosis who are experiencing its crippling symptoms. Limitations on its further use and wide acceptability must be considered in light of the increased infection rate. The necessity for long-term follow-up studies is imperative to learn more about Ofatumumab and some other effects.
AUTHOR CONTRIBUTIONS
Peter Olujimi Odutola: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Methodology (lead); Resources (equal); Software (lead); Supervision (lead); Writing—original draft (lead); Writing—review and editing (supporting). Peter Oluwatobi Olorunyomi: Data curation (supporting); Formal analysis (supporting); Methodology (supporting); Project administration (equal); Resources (supporting); Writing—review and editing (supporting). Olanrewaju Olamide Olatawura: Data curation (supporting); Methodology (equal); Writing—review and editing (supporting). Ifeoluwapo Olorunyomi: Writing—review and editing (supporting). Olukayode Oluyinka Madojutimi: Data curation (supporting); Writing—review and editing (supporting). Ayomide O. Fatunsin: Writing—review and editing (supporting). Uju Okeke: Writing—review and editing (supporting). Victoria Eneh: Writing—review and editing (supporting).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no affiliations or associations which may show potential conflicts of interest and take full accountability for ensuring the accuracy, reliability, and impartiality of the data presented and the corresponding interpretations provided.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in Pubmed Medline (https://pubmed.ncbi.nlm.nih.gov/), Embase (embase.com), and Cochrane (https://www.cochranelibrary.com/).




