Bivalent and bitopic ligands of the opioid receptors: The prospects of a dual approach
Abstract
Opioid receptors belonging to the class A G-protein coupled receptors (GPCRs) are the targets of choice in the treatment of acute and chronic pain. However, their on-target side effects such as respiratory depression, tolerance and addiction have led to the advent of the ‘opioid crisis’. In the search for safer analgesics, bivalent and more recently, bitopic ligands have emerged as valuable tool compounds to probe these receptors. The activity of bivalent and bitopic ligands rely greatly on the allosteric nature of the GPCRs. Bivalent ligands consist of two pharmacophores, each binding to the individual orthosteric binding site (OBS) of the monomers within a dimer. Bitopic or dualsteric ligands bridge the gap between the OBS and the spatially distinct, less conserved allosteric binding site (ABS) through the simultaneous occupation of these two sites. Bivalent and bitopic ligands stabilize distinct conformations of the receptors which ultimately translates into unique signalling and pharmacological profiles. Some of the interesting properties shown by these ligands include improved affinity and/or efficacy, subtype and/or functional selectivity and reduced side effects. This review aims at providing an overview of some of the bivalent and bitopic ligands of the opioid receptors and, their pharmacology in the hope of inspiring the design and discovery of the next generation of opioid analgesics.
1 INTRODUCTION
Opioids, such as morphine and codeine, are amongst the oldest drugrefs in the pharmacopeia; documented historical evidence of their use for recreational purposes or spiritual practices date from the Neolithic era.1 They act on their respective opioid receptors which are now known to belong to the Class A (Rhodopsin-like) G-protein coupled receptors (GPCRs). Till date, four different opioid receptor subtypes, sharing more than 60% sequence homology, have been characterized, termed the µ, δ and κ receptors (corresponding to MOR, DOR and KOR respectively) and the nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor. These receptors are widely distributed in the central and peripheral nervous systems (CNS and PNS) and mediate an array of physiological effects ranging from spinal/supraspinal analgesia to euphoria/dysphoria.2 Hence, they represent important therapeutic targets in the management of acute and chronic pain as well as in mood-related conditions such as depression and anxiety.
The opioid receptors mediate their effects through the heterotrimeric Gi/o protein which, upon activation of the receptor, dissociate into the subunits Gαi/o and Gβγ.2, 3 These subunits then interact with various intracellular effector systems. The results of these many interacting effector systems are a decrease in neurotransmitter release, hyperpolarisation, and a general inhibition of neuronal activity, ultimately leading to analgesia.2, 3 Opioid receptors, particularly the MOR and DOR, can also mediate excitatory activity on the CNS, leading to rewarding effects or euphoria.1, 2 Rapid desensitization ensues receptor activation as a result of phosphorylation of specific intracellular threonine and serine residues of opioid receptors by kinases such as G-protein-coupled kinases (GRKs).2 These phosphorylated residues then recruit β-arrestins (1 and 2), thereby arresting G-protein mediated signalling, inducing receptor internalization and desensitization.2 Studies have shown that β-arrestins can directly activate other cellular kinase pathways, for example, tyrosine kinase, protein kinase A and C (PKA and PKC), mitogen activated protein kinases (MAPKs), resulting in non-G-protein mediated cellular effects.2, 4, 5 In the early 2000s, reports that β-arrestin 2 knock out (KO) mice showed lower levels of constipation and respiratory depression while maintaining the analgesic properties were a major milestone in the field of opioid research.6-8 These studies proposed a new approach towards safer opioid analgesics based on the hypothesis that agonists which preferentially activate the G protein over the β-arrestin pathway would result in the desired antinociceptive but not the lethal side effects. This concept, referred to as biased signalling or functional selectivity, fuelled the next two decades of opioid research. One of the first ‘biased’ agonists reported in literature was TRV130 (1) (Figure 1), now approved by the FDA for the management of moderate to severe pain in adults. The works on TRV130 (1) have inspired the discovery of other so-called G protein-biased MOR agonists such as TRV734 (2) (now in clinical trials as an orally bioavailable analgesic)9 and SHR9352 (3) (Figure 1).10 While preclinical studies have indicated that TRV130 (1) exhibits analgesia with lesser respiratory depression compared to morphine, these results were not translated in clinical studies.11 Low incidence of respiratory depression was still observed with TRV130 (1) in phase III clinical trials in postsurgical pain patients.12 Similarly, PZM21 (4) also initially keyed as a G protein-biased agonist of the MOR with lower levels of respiratory depression compared to morphine,13 was later shown to still cause respiratory depression to the same level as the latter.14 Since, the improved side effects profile of TRV130 (1) and PZM21 (4) have been attributed to their low intrinsic efficacy, implying that it is rather their behavior as a partial agonist that leads to their improved safety profile in vivo rather than their G protein-bias.15
There is a general departure from biased agonists as a strategy to obtain safer opioid analgesics (see reviews by Ramos-Gonzalez et al.16 and Gillis et al.17). It has been suggested that G-protein signalling mechanisms such as G-protein-coupled inwardly rectifying potassium (GIRK) channels and Gαi signalling are responsible for opioid-mediated respiratory depression.18-22 Since the reports in the 2000s, the results by Bohn and colleagues6-8 could not be reproduced. Kliewer et al. observed dose-dependent respiratory depression in both β-arrestin 2 KO and wild type (WT) mice with morphine.23 Considerable constipation was also observed to a similar extent in β-arrestin 2 KO and WT mice.23 He et al. also showed that respiratory depression was comparable between β-arrestin 2 KO and WT mice.24 Moreover, they found that compared to WT, MROR mice (a mutant which engages both G protein and β-arrestin 2 upon activation by morphine) exhibited improved morphine analgesia, suggesting that β-arrestin 2 may, in fact, play an important role in mediating morphine-induced analgesia.24 They also observed a negative correlation between β-arrestin 2 recruitment and the development of analgesic tolerance, proposing a new strategy for safer opioids, that favors the engagement of β-arrestin 2.24 In phosphorylation-deficient mice which do not effectively recruit β-arrestin, opioid-induced analgesia and analgesic tolerance were significantly improved while side effects such as respiratory depression, constipation, and opioid withdrawal signs remained unaffected or worsened.25 These results suggest that G protein-biased agonists are likely to still cause the side effects and that β-arrestin 2 may be involved in opioid-induced tolerance.
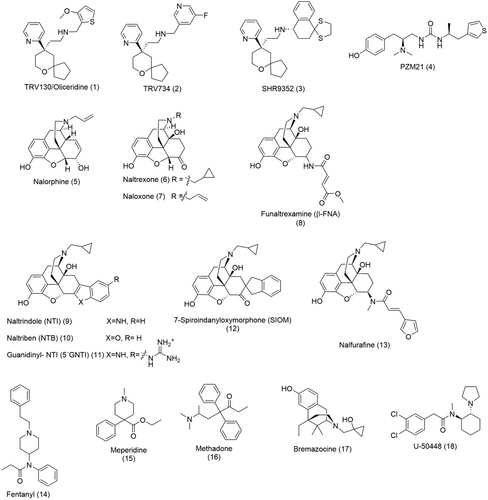
The opioid receptors are activated by a wide range of ligands from endogenous peptides such as enkephalins and endorphins to morphinan and non-morphinan derived small molecules.3 The on-going search for safer opioid analgesics has expanded the available pool of synthetic opioids (Figure 1). Early modifications of the morphinan scaffold yielded nonselective antagonists such as nalorphine (5), naltrexone (6) and naloxone (7) (Figure 1) with bulky substituents on the tertiary amine functional moiety.1 Later, the “message-address” concept led to the discovery of subtype selective ligands.26 Here, the amino and the aromatic functional moieties of the morphinan scaffold govern the activity (the “message”) of the opioids while the lipophilic core surrounding the allylic alcohol contributes to selectivity (the “address”). Some examples include the MOR-selective irreversible antagonist β-FNA (8), DOR-selective antagonists, NTI (9), NTB (10) and SIOM (12), the KOR selective agonist, nalfurafine (13), and antagonist 5'GNTI (11) (Figure 1).1 Another approach was the simplification of the three-dimensional pharmacophore of the morphine structure to afford smaller non-morphinans such as the MOR-selective agonist, fentanyl (14) (Figure 1), a very potent analgesic used in anesthesia. Other examples include the MOR agonists meperidine (15) and methadone (16) (Figure 1)1 used in acute and chronic pain therapy and the KOR agonists bremazocine (17) and U-50448 (18) (Figure 1).
Today, the quest for new ligands deprived of side effects, the ‘holy grail’ of opioid research, persists, especially in view of the advent of the ‘opioid epidemic’. According to the latest statistics by the Centre for Disease Control and Prevention (CDC), synthetic opioids were responsible for nearly 75% of deaths by overdose in the US in 2020.27 In the last few decades, synthetic efforts in the field of opioid analgesics and class A GPCRs in general have gravitated towards the design and synthesis of bivalent and bitopic ligands. Bivalent ligands consist of two pharmacophores, typically linked via a spacer, each targeting the OBS of receptor monomers within a dimer. On the other hand, the pharmacophores of bitopic ligands bridge the gap between the OBS and a less conserved ABS through the concomitant binding of the two sites. Such a departure from the conventional strategy of targeting primarily the OBS has been driven by substantial supporting evidence of opioid receptor oligomerisation and the discovery of allosteric modulators of the opioid receptors.28-32 The idea that opioid receptors can be effectively regulated allosterically either via another protomer or an allosteric binding pocket within the same receptor further expanded the ‘chemical space’ for these receptors. Bivalent and bitopic ligands do not only display interesting pharmacological profiles in comparison to the parent compounds from which they are derived but are also useful pharmacological tools in the investigation of the complex mechanisms of action of class A GPCRs. Here, we aim to provide an overview of currently available bivalent and bitopic ligands, their pharmacology, and their promising prospects in the field of opioid analgesia. We present some of the early works of Portoghese in the realm of bivalent ligands; how his research inspired others and led to the discovery of bivalent ligands derived from diverse scaffolds over the years. We also review some of the bivalent ligands of the opioid receptors and other class A GPCRs and the recently discovered bitopic ligands of the opioid receptors, substantially adding to the existing literature.33-36
2 STRUCTURE OF THE OPIOID RECEPTORS AND INSIGHT INTO THEIR ACTIVATION MECHANISM
Before going into the mechanistic details of allosteric regulation of the opioid receptors, it is perhaps noteworthy to consider the structural framework of the latter. X-ray crystallography paired with recent advances in cryo-electron microscopy (cryo-EM) have made it possible to study these receptors at the molecular level. Crystal structures of all four opioid receptor subtypes are now available in both their inactive and active states.37-43 Together with molecular dynamics (MD) and strategic mutagenesis studies, these three-dimensional structures have broadened our understanding of how ligand binding and activation trigger a series of structural rearrangements, ultimately resulting in the activation of the Gi/o-protein at the intracellular surface of the receptor.
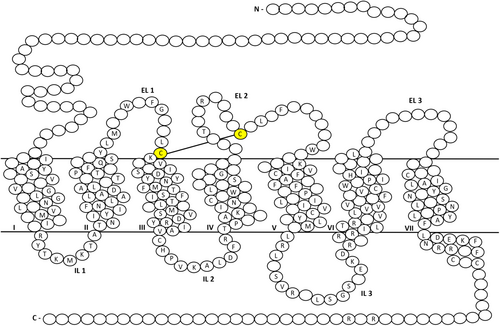
The opioid receptors adopt the general structure of other class A GPCRs which consists of 7 transmembrane (TM) helical domains (1 to VII) connected by 3 extracellular loops (ELs) and 3 intracellular loops (ILs) (Figure 2).39 Additionally, TM3 is connected to EL2 via a conserved disulfide bridge between C3.25 (Ballesteros-Weinstein numbering) and C217EL2 (Figure 2).39 All opioid receptors have an orthosteric binding pocket situated within the helical bundle delineated by TM helices 3, 4, 5, 6 and 7.39 This orthosteric binding pocket can be separated into two different regions. The lower part located deep within the helical bundle is highly conserved amongst the opioid receptors and is associated with ligand “message” while the upper part consists of non-conserved residues conferring receptor-subtype selectivity or ligand “address.”2
The notion that GPCRs are analogous to simple on-off switches, interchanging between their inactive and active states upon ligand binding, is now obsolete. GPCRs are rather flexible, capable of adopting multiple conformational states which then influence ligand affinity, efficacy, and intracellular signalling. Several structural motifs, also referred to as “microswitches” which play important roles in ligand binding, Gi/o coupling and receptor activation have been identified. These include the D3.32YYNM, the CWxP, the FPI, the NPxxY and the DRY motifs.37, 40, 42
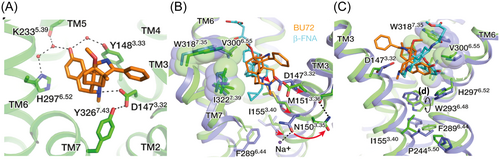
The D3.32YYNM motif is conserved in all opioid receptors and serve as an anchor for all known opioid receptor ligands. It is also thought to be at the center of the structural rearrangements occurring upon ligand activation of the opioid receptors. Though chemically diverse, many opioid receptor ligands share common features, particularly, a phenolic hydroxyl separated by six carbon atoms from a positive charge, usually a secondary/tertiary amine, analogous to the amino-terminal tyrosine of all endogenous opioid peptides. The amine moiety is usually anchored within the binding cavity via a salt bridge with D3.32 of TM3 (Figure 3A), a key stereotypic interaction for ligand recognition at opioid receptors.37, 39-42 Apart from the residues of the D3.32YYNM of TM3, other residues of TM helices 5, 6 and 7 also participate in interactions with both opioid agonists and antagonists within the binding cavity. These include polar water-mediated interactions with, K5.39, Y7.43, H6.52 (Figure 3A) and non-polar interactions with W6.48, I6.51, V6.55, W/L/Y7.35 and I7.39 (Figure 3B,C).37, 40, 41 The latter residues form the hydrophobic pocket which usually harbors the morphinan ring of conventional opioid ligands. The non-conserved leucine residue (L7.35) at the DOR (W7.35 and Y7.35 at the MOR and KOR respectively) is a significant feature responsible for naltrindole's (9, Figure 1) selectivity for the DOR.37, 39, 41
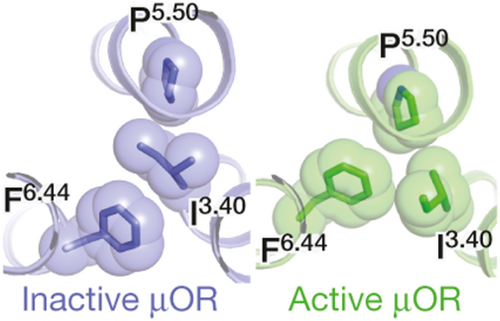
The conserved residue W6.48 of the CWxP motif, located within the hydrophobic pocket, makes hydrophobic contacts with F6.44 of the conserved core triad F6.44, P5.50 and I3.40 found just below the orthosteric binding pocket (Figure 4).37, 40 The FPI motif is also known to be a central “microswitch” for the activation of many class A GPCRs. In the active structures of the MOR and KOR, the residue W6.48 adopt a rotamer state different from the one observed in the inactive states of these receptors (Figure 3D), resulting in structural rearrangements in the core triad, linked to the TM6 swivel motion intracellularly.37, 40 Activation of the opioid receptors is associated with a large outward movement of TM6 at the cytoplasmic side for the accommodation of transducer molecules such as the Gi/o.37, 38, 40
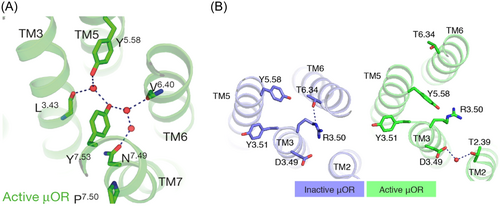
The NPxxY motif at the intracellular end of TM7 is said to propagate structural changes in the OBS through a series of conformational changes resulting in larger scale helical movements.44 Following activation, the NPxxY motif in TM7 moves inwards towards TM5 where N7.49 and Y7.53 participate in hydrogen bond interactions with Y5.58 in TM5, the backbone carbonyls of L3.43 in TM3 and V6.40 in TM6, and 3 ordered water molecules (Figure 5A). Activation of the opioid receptors also leads to structural rearrangements at the intracellular tip of TM3, namely within the conserved DRY motif. The characteristic salt bridge observed in the inactive MOR and KOR structures between the residues R3.50 and T6.34 is broken and R3.50 instead forms a hydrogen bond with Y5.58 within the receptor core in the active conformations (Figure 5B).37, 39, 40 This observation suggests that residues located at the intracellular end of TM3 play an important role in translating ligand-induced changes in the OBS to the transducer interface.
The latest member of the opioid receptor family, the NOP, has also been crystallized in both its active and inactive states with the NFQ peptide and the peptide mimetic, the antagonist C-24, respectively.43, 45 Despite having high sequence homology with the classical opioid receptors, the NOP shows distinct pharmacology. The NOP does not show affinity, both for the classical opioid receptor peptides and the small morphinan-like molecules, for example, naloxone (7, Figure 1).45 Moreover, its endogenous peptide, N/OFQ, is structurally similar to the other opioid peptides but does not interact with the MOR, KOR, and DOR.45 The inactive crystal structure along with some MD simulations of an active-state model of the NOP revealed the above-mentioned “microswitches” likely to be conserved and playing similar roles in receptor activation.45, 46
More recently, Wang et al. observed that the superimposed active structures of all four opioid receptors showed an overlap of the conformations of the residues of the conserved motifs important for receptor activation.43 The alignment of the intracellular parts of the receptors supports a similar consensus activation mechanism.43 However, key dissimilarities were also observed. When compared to the MOR, DOR and KOR, the NOP has an enlarged orthosteric binding pocket, capable of accommodating large endogenous peptides, notably, N/OFQ which is 17-residues long.45 The extracellular loop, ECL2, linking the helices TM4 and TM5, shows no sequence similarity with that of the MOR and DOR, but is similar to that of the KOR, albeit 3 residues shorter.46 The ECL2 of the NOP is highly acidic and seems to play an important role in binding its highly basic endogenous peptide N/OFQ; receptor chimera studies, involving a NOP-kappa chimera, indicate that the ECL2 of the NOP is a necessary requirement for activation of the NOP by N/OFQ unlike the KOR.47 Additionally, five non-conserved residues within the ligand binding pocket have been identified as epitopes affording the unique selectivity of N/OFQ for NOP, namely, the residues A5.39, V6.51, Q6.52, V6.53 and T7.39.29 Mutations of these specific residues to their MOR, DOR and KOR counterparts, A5.39K, V6.51I, Q6.52H, V6.53I and T7.39I respectively, effectively conferred high affinity binding to dynorphin (KOR endogenous peptide) and morphinans such as naltrexone (6) and naltrindole (9) (Figure 1).48
Structures of opioid receptors have also provided some insight into the molecular mechanism of biased signalling at these receptors. Ligand interactions with specific subpockets at the MOR and KOR have been found to result in biased signalling.49 In their study of different opioid structures, Uprety and colleagues found that specific ligand-receptor interactions at the TM5-ECL2 region of the OBS may diminish β-arrestin two recruitment at both the MOR and KOR and correlates with improved G protein agonism at these receptors.49 This is in line with the proposed hypothesis that the TM5-ECL2 region controls bias at other GPCRs such as the 5HT2B serotonin50 and D2 dopamine receptors51; however, the mechanisms behind the observed bias may be different between these receptors. In contrast, further interactions with a subpocket delineated by TM2 and TM3 may favor balanced agonism or a bias towards the β-arrestin 2 signalling pathway.49 Qu et al. also observed that interactions of lofentanil, a derivative of fentanyl (14, Figure 1), within a subpocket delineated by TM2-3 and ECL1-2 may stabilize an alternative conformation of the MOR.52 Notably, lofentanil stabilizes a conformation of Q1242.60 which then forms a hydrogen bond to Y3267.43, resulting in more intracellular counterclockwise rotations of TM7.52 Qu et al. suggest that this alternative intracellular conformation of TM7 may result in different transducer recruitment profiles, e.g., a narrower intracellular cavity due to the inward movement of TM7 may favor the binding of arrestins.52
The determination of the crystal structure of the G protein-biased agonist nalfurafine (13, Figure 1) at the KOR by El Daibani et al. revealed structural motifs potentially effecting G protein-bias at this receptor.53 The cyclopropyl methyl group of nalfurafine (13) fits deeper into a hydrophobic pocket. 53 Mutations of residues within this hydrophobic pocket led to a considerable decrease in the potency of nalfurafine (13) and a reduction or abolishment in the nalfurafine-mediated potency and efficacy of β-arrestin recruitment suggesting that these hydrophobic residues may play an important role in signalling bias at the KOR.53 The cyclopropyl methyl group of nalfurafine (13) mediates its agonist signal transduction through steric hindrance with the tryptophan residue W2876.48, resulting in the known “rotamer toggle switch.” 53 The W2876.48A mutation significantly reduces the β-arrestin recruitment of nalfurafine (13) without greatly affecting its G protein signalling.53 El Daibani et al. also suggest that the disruption of a salt bridge interaction between K2275.39 and E2976.58 by nalfurafine's amino methyl group results in decreased β-arrestin recruitment.53 The K2275.39A mutation led to reduced arrestin coupling but increased G protein coupling.53 Nalfurafine (13) also stabilized a specific conformation of TM7, namely a clockwise rotation and inward movement of TM7 which could potentially result in G protein-bias.53
Zhuang et al. determined the structures of the so-called G protein-biased agonists, TRV130 (1) and PZM21 (4) (Figure 1), bound to the MOR which they compared to MOR structures with the agonists morphine and fentanyl (14, Figure 1) to investigate some of the structural differences.54 They hypothesized that the lack of stable interactions with TM6/TM7 as observed with fentanyl (14), may explain the diminished arrestin recruitment of PZM21 (4) which formed more favorable interactions with TM2/TM3.54 Of note, mutations of residues near TM6 and TM7, especially of the residues W2956.48 and W3207.35, significantly reduced or eliminated arrestin signalling with limited effect on G protein activation.54 Extensive interactions with TM6/TM7 may result in an inward movement of TM6/TM7 towards the transmembrane core and to an improvement in β-arrestin coupling.54 Similarly, through their study of an analogue of PZM21 (4), FH210, Wang et al. showed that extended interactions with the extracellular parts of the MOR, a pocket formed by TM2, TM3 and ECL1, may confer G protein bias.55
3 THE ALLOSTERIC NATURE OF OPIOID RECEPTORS
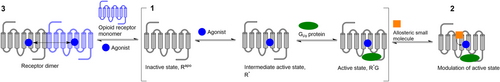
Allostery at the opioid receptors is a multifaceted concept. Like all GPCRs, opioid receptors are inherently allosteric, owing to their signalling mechanism which involves communication between spatially distinct but conformationally linked binding sites. The binding of an orthosteric ligand triggers a series of structural rearrangement, ultimately resulting in the binding of the intracellular transducer G protein, approximately 40 Å downstream from the OBS.56 The global conformational change between the inactive and active states is referred to as the “allosteric transition” (Figure 6–1).56 Additionally, opioid receptors consist of other functional binding pockets which can accommodate both endogenous or exogenous allosteric modulators such as small molecules, proteins, ions, or lipids (Figure 6–2).57-64 These are conformationally linked to the OBS and are thus able to influence the binding affinity and/or efficacy of orthosteric ligands. Finally, opioid receptors can mediate their effects as both monomers and dimers (Figure 6–3).65-69
3.1 Allosteric modulation of the opioid receptors via an allosteric binding site (ABS)
The discovery of small molecules, capable of modulating the response associated with orthosteric ligand binding, while targeting spatially distinct binding sites, opened up new avenues in the realm of GPCR signalling modulation. When druggable, these so-called allosteric binding sites (ABS) provide useful alternative approaches to target the GPCRs. The ABS is typically located towards the extracellular domains, the usually less conserved regions, of the receptor.70-75 It is thus an ideal target when aiming for subtype selectivity within the same GPCR family. A major advantage of allosteric ligands is that they are, in essence, modulators, which implies that most of them do not show any effect on receptor signalling on their own accord but influence that of an orthosteric ligand. This then allows for a fine-tuning of receptor response while maintaining spatial and temporal fidelity of endogenous GPCR agonist activity. Studies have also shown that allosteric ligands can show functional selectivity or signalling bias.76-78 Allosteric modulators can therefore favorably influence receptor response away from pathways mediating side effects while reinforcing the desired therapeutic effect.
While structural data on the ABS of the opioid receptors are currently not available, MD studies have identified a plausible allosteric binding pocket located towards the extracellular side of the DOR.79 The allosteric binding pocket is surrounded by TM1, TM2, TM7 helices, and part of TM6, according to metadynamics simulations results which were also supported by site directed mutagenesis studies of DOR residues deemed important for maintaining the allosteric response. This allosteric binding site is also comparable to that of the human M2 muscarinic receptor previously revealed by X-ray crystallography.80
Allosteric ligands can be classified into three principal categories: positive allosteric modulators (PAMs), negative allosteric modulators (NAMs) and silent allosteric modulators (SAMs). PAMs enhance the affinity and/or efficacy of an orthosteric ligand while NAMs show the opposite effect. SAMs or neutral allosteric modulators bind at the ABS without showing any influence on an orthosteric ligand's activity. They are thus useful tools in probing the activity of a PAM or a NAM.
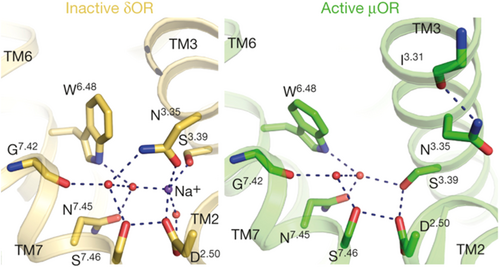
The earliest known NAM at the opioid receptors is the sodium ion. The first evidence of the negative allosteric nature of sodium ions was shown in in vitro assays involving mouse brain extracts. Sodium inhibited the binding of orthosteric agonists to the MOR while exhibiting no effect on orthosteric antagonist binding.81 It is now known that the sodium ion stabilizes the inactive conformation of opioid receptors or GPCRs.62, 82 The sodium ion occupies a binding site which is conserved across many GPCRs. Crystal structures of inactive GPCRs show that the Na+ binding site is buried within the 7-TM bundle and involves a coordination with an aspartate residue (D2.50) and a serine residue (S3.39) and an extensive water network across TM II, III, VI and VII (Figure 7).83 The same Na+ ion binding pocket is not present in the crystal structures of active GPCRs (Figure 7), likely due to movement of the transmembrane domains occurring during receptor activation which impairs the accommodation of sodium and its corresponding water molecules.37
Other putative NAMs of the opioid receptors include the cannabinoid type-1 receptor (CB1) agonist cannabidiol (19, Figure 8) and the potent KOR agonist salvinorin A (20, Figure 8). Cannabidiol (19) is a NAM of both MOR and DOR while Salvinorin A (20) elucidates its NAM activity only at the MOR.84, 85 In kinetic binding studies on rat cerebral cortex membrane homogenates, cannabidiol (19) accelerated the dissociation of the agonist 3H-DAMGO from the MOR by a factor of 12 and [3H]-naltrindole from the DOR by a factor of 2.84 Cannabidiol (19) also showed a Hill coefficient corresponding to positive homotropic cooperativity, indicating that it effectively binds to an allosteric site.84 However, it is important to note that the allosteric properties of cannabidiol (19) were observed at doses much higher than its activity in vivo. In assays of Chinese hamster ovary (CHO) cells expressing the cloned human MOR, the KOR agonist salvinorin A (20, Figure 8) partially inhibited both the binding efficacy and affinity of the MOR agonists [3H]-DAMGO and [3H]-diprenorphine, respectively.85 A right shift in their dose–response curves with a concomitant decrease in Emax were observed for both orthosteric ligands in the presence of increasing concentrations of salvinorin A (20), a typical profile for NAMs.85 In addition, saturation binding studies involving the same orthosteric ligands, showed that salvinorin A (20) lowered the MOR Bmax and increased the Kd in a dose-dependent nonlinear fashion.85 This is in sharp contrast with the dose-dependent linearity usually observed with competitive inhibitors. Results of kinetic binding experiments strongly suggest that salvinorin A (20) increased the dissociation rate of [3H]-DAMGO and [3H]-diprenorphine binding to the MOR.85 Salvinorin A (20) also behaved as a noncompetitive inhibitor of DAMGO when tested in the [35S] GTPγS functional assay.85
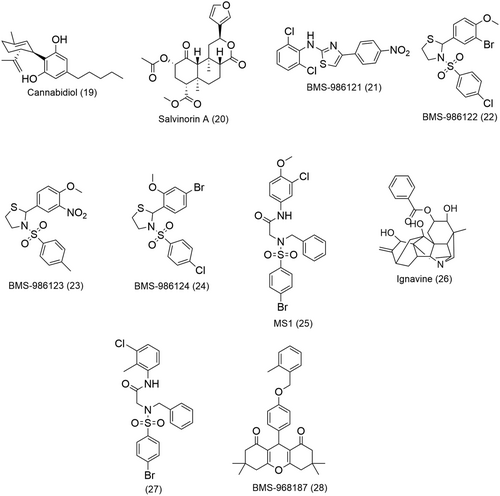
PAMs and SAMs of the opioid receptors have also been identified in a high throughput screen.28 Initial screening using a β-arrestin recruitment assay revealed two potential PAMs selective for the MOR, BMS-986121 (21) and BMS-986122 (22) (Figure 8), respectively. The two compounds did not exhibit significant β-arrestin recruitment on their own but significantly increased the β-arrestin response of the MOR agonist endormorphin-I (EM-1).28 BMS-986121 (21) augmented the β-arrestin response by 20 nM EM-1 to a maximal effect (Emax) of 76% of the response by a maximally effective concentration (1 µM) of EM-1 with an EC50 of 1.0 μM.28 BMS-986122 (22) also produced a similar potentiation of the effect EM-1 to 83% of the maximal observed EM-1 response with an EC50 of 3.0 µM.28 These compounds were further evaluated in two additional functional assays, namely, a cAMP-accumulation assay, and a G-protein activation assay using [35S]GTPγS binding. BMS-986121 (21) and BMS-986122 (22) considerably increased the inhibition of forskolin-induced adenyl cyclase activity produced by an EC10 (30 pM) concentration of EM-1 in CHO-MOR cells with EC50 values of 3.1 μM and 8.9 µM, respectively.28 In G-protein activation [35S]GTPγS-binding studies in membranes from C6 glioma cells stably expressing recombinant MOR, addition of BMS-986122 (22) and BMS-986121 (21) resulted in a leftward shift in the dose–response curve of the MOR agonist DAMGO by factor of 7 and 4, respectively.28 In an attempt to establish some SAR of the BMS chemical series, 2 analogues of BMS-986122 (22), namely, BMS-986123 (23) and BMS-986124 (24) (Figure 8), were identified as SAMs of the MOR. These two compounds were shown to inhibit the PAM activity of 12.5 μM BMS-986122 (22) in a β-arrestin recruitment assay in the presence of 30 nM EM-1.28
In light of the observed allosteric behavior of BMS-986122 (22) (Figure 8), MD studies were carried out to gain more insight into its mechanism of action.86 It was found that in the hypothetical ABS of the MOR, BMS-986122 (22) stabilizes the favorable interactions between the receptor and the full MOR agonist R-methadone while inhibiting the binding of the allosteric sodium ion.86
Kandasamy et al. investigated the antinociceptive effects of BMS-986122 (22, Figure 8) in mouse models of acute and inflammatory pain to test their hypothesis that an MOR agonist can potentiate the activity of endogenous opioids released during pain.87 In vitro, BMS-986122 (22) showed no agonist activity at endogenously expressed MOR; it did not stimulate G protein in mouse brain homogenates, including periaqueductal gray (PAG).*87 This is in agreement with the findings made in heterologous cell systems expressing MOR, where the PAM does not activate the heterotrimeric G protein or recruit β-arrestin.28 However, low activity (EC50 ~ 0.1 mM) of BMS-986122 (22) was observed at the more amplified pathway, adenyl cyclase, and in CHO cells overexpressing MOR.28, 87 In C57BL/6 mice, the PAM showed a dose-dependent antinociception when centrally administered.87 The duration of the antinociceptive response was short-lived but reversed by the orthosteric antagonist β-FNA (8, Figure 1).87 While this response could be due to BMS-986122's weak potency and efficacy, the PAM does not bind at the OBS.87 BMS-986122 (22) rather mediates its action by potentiating the activity of endogenous opioid peptides which are then hindered by β-FNA (8) binding to the OBS.87 This hypothesis was confirmed by Kandasamy et al. by using a low dose of enkephalinase inhibitor, RB-101, which prolongs the half-life of endogenous peptides in vivo, thereby increasing their available concentration.87 RB-101 showed minimal efficacy on its own accord but afforded strong antinociception in the presence of BMS-986122 (22).87
BMS-986122 (22, Figure 8) promoted swim-stress antinociception which was reversible with naloxone (7, Figure 1), further implying the involvement of endogenous opioid peptides and MOR.87 BMS-986122 (22) showed antinociceptive responses in mouse models of inflammatory pain.87 These observations suggest that a continuous peptide release is necessary for the effectiveness of BMS-986122 (22) and that endogenous opioid peptide levels in resting states are not sufficiently high to be influenced by allosteric modulators unless there is a nociceptive insult.87 This provides a basis for the therapeutic application of MOR PAMs whereby antinociception can be achieved by potentiating the activity of endogenous opioid peptides without the administration of the classical opioid agonists.87
More recently identified PAMs of the MOR are MS1 (25) and ignavine (26) (Figure 8).29, 88 MS1 (25) was the most active PAM in a β-arrestin recruitment assay in the search of new PAMs based on the chemical scaffold of BMS-986121 (21) and BMS-986122 (22) (Figure 8). In a competition assay, 10 μM MS1 (25) increased the binding affinity of R-methadone (16, Figure 1) by sevenfold and augmented the potency of the latter by over fourfold in a G-protein activation assay.29 MS1 (25) also displayed strong probe dependency; it had no effect on either the binding affinity or potency of other MOR agonists such as DAMGO and EM-1.29 Nonetheless, MS1 (25) could enhance the potency of morphine to that of a full MOR agonist while showing no influence on its binding affinity.29 Ignavine (26, Figure 8), a diterpene alkaloid and component of Aconitum tuber, affects the MOR activity in a unique manner. In vitro functional assays indicated that ignavine (26) exhibited bidirectional activity in a concentration-dependent manner. An increase in MOR activity produced in the presence of 1 μM of DAMGO was observed at low concentrations of ignavine (26) (1 μM) both in a receptor internalization assay and an intracellular cAMP assay.88 On the other hand, higher concentrations (>10 μM) of ignavine (26) led to an inhibition of MOR response in the same assays.88 Such results were obtained with other MOR agonists, including, morphine and the endogenous agonist EM-1 but not with the MOR antagonist naloxone (7, Figure 1).88 Similar observations were also made in an in vivo analgesia study of ignavine (26). The analgesic response of ignavine (26) was probed by the tail-flick and tail-pressure tests in normal mice. A maximum response was shown at a dose of 0.1 mg/kg which was lowered at higher doses of ignavine (26).88
Pryce et al. screened 21 derivatives of MS1 (25, Figure 8) for PAM activity in MOR/Gαi/o-mediated cAMP inhibition and β-arrestin recruitment assays, in the presence of increasing concentrations of MOR agonists endomorphin-1, DAMGO, or l-methadone.89 They identified compound 27 (Figure 8) which showed the most significant allosteric improvement of Gi/o activity when comparing EC50 values at 0 and 3 µM concentrations of allosteric modulators in the cAMP assay with all three MOR agonists.89 Of note, MS1 (25) and 27 did not exhibit a significant PAM effect on the endogenous endomorphin-1 but more pronounced effects on exogenous MOR agonists.89 In mice models of peripheral inflammation, Pryce et al. observed significant antinociceptive effects in the presence of MS1 (25) and 27 at opioid doses lower than those used to inhibit thermal hyperalgesia.89 In a model of postoperative pain whose induced thermal hypersensitivity can be treated with 3 mg/kg of morphine, MS1 (25) and 27 induced antinociception at lowered doses of opioids (2 mg/kg).89 Both MOR PAMs exhibited probe dependence which differed between in vivo and in vitro experiments. 27 enhanced the antinociceptive response to morphine and oxycodone in both female and male mice in different pain models which is also reflective of the results obtained in the BRET assays.89 However, contrary to the in vitro data, 27 did not potentiate the antinociceptive effects of methadone in the hot place assay.89 In vitro data suggest a more significant probe dependence for MS1 (25) than 27.89 Both 27 and MS1 (25) showed a rather balanced effect on the improvement of potency of the agonists l-methadone, oxycodone and morphine at the GαoA or β-arrestin 2 pathways in BRET assays.89 These results contradict the earlier observations that MS1 (25) behaved as a β-arresting-biased MOR PAM.29 However, the bias quantification methods employed by Pryce and colleagues are reported to be more accurate. 27 and MS1 (25) also showed promising side effect profiles as they did not worsen the development of somatic withdrawal symptoms or respiratory depression and did not favor analgesic tolerance.29
A structure–activity relationship study using a β-arrestin recruitment assay led to the first reported series of DOR PAMs, from which BMS-986187 (28, Figure 8) was the most potent PAM with a 100-fold selectivity for the DOR over the MOR.30 When evaluated in four different assays, namely, β-arrestin recruitment assays, forskolin-stimulated cAMP accumulation assays, [35S]GTPγS binding assay and ERK1/2 phosphorylation assays primarily using CHO cells, BMS-986187 (28) potentiated the activity of the DOR orthosteric agonists leu-enkephalin (Leu-Enk), SNC80 and TAN67 and completely inhibited the propagation of cAMP when administered on its own (at concentrations higher than 3 µM).30 The latter observation suggests that BMS-986187 (28) might be an effective DOR-selective PAM-agonist.
3.2 Intramolecular allosteric crosstalk within opioid receptor dimers
In the late 90 s and early 2000s, the idea that GPCRs exist as functional oligomers in the form of dimers and were the “actual targets” of neurotransmitters and small molecules binding at the OBS started to gain in popularity.90, 91 The DOR was the first opioid receptor for which dimerization was described, and the existence of the DOR dimers was later confirmed by time-resolved fluorescence resonance energy transfer (FRET) and bioluminescence resonance energy transfer (BRET).31, 32 The first structural evidence of opioid receptor dimerization was reported by Granier and Kobilka.39 They observed that MOR receptor molecules associated into pairs along the crystallographic two-fold axis through two distinct interfaces, one mediated by TM1, TM2 and helix 8 and another more extensive interface consisting of TM5 and TM6.39 More recently, Zhuang et al. also observed that the MOR-Gi complexes with the opioid agonists, morphine, fentanyl (14) and PZM21 (4) existed as anti-parallel dimers.54 However whether these dimeric complexes are merely artifacts of crystal structures remain unclear. Today, GPCR dimers and oligomers are well-characterized with a vast library of ligands, including peptides and small molecules, specifically designed to target these macromolecular agglomerations.92-97
Dimers can be further classified as homomers where the monomers are identical or heteromers where the monomers are of different subtypes or of different receptor classes. Dimerization naturally provides another level of allosteric modulation of these receptors, whereby a monomer, when bound by an agonist or an antagonist, can influence the binding properties of the partner monomer within a dimer.98 The additional level of three-dimensional molecular organization brought by dimerization confers different functional and pharmacological properties to the dimers in comparison to their monomeric counterparts.56, 65, 99 Over the years, dimers have established themselves as targets of choice when probing for subtype or functional selectivity unattainable by solely aiming for the OBS of monomers.
Opioid receptors have been reported to form both homomers and heteromers in which crosstalk is observed between 2 different receptor subtypes or between an opioid receptor subtype and another GPCR receptor.65, 100, 101 Homomers represent interesting targets when aiming for an increase in affinity and/or potency through positive allosteric cooperativity.11 Heteromers of the opioid receptors in various subtype combinations are described in the literature, e.g., MOR/DOR, MOR/KOR, or DOR/KOR, each showing distinct functional properties.65, 100 Additionally, opioid receptors have also been identified in dimeric complexes with other class A GPCRs such as the cannabinoid receptor (CB1), the neurokinin receptor (NK1), and the dopaminergic receptors (D2R-like).100 Such heteromers provide new avenues for multi-target approaches, particular useful when the aim is to avoid certain side effects.
However, the existence of heterodimers in vivo is still a subject of debate. The presence of opioid heterodimers has primarily been shown in vitro using co-immunoprecipitation, BRET and FRET assays with a few studies aiming to establish their existence and functional relevance in vivo (see reviews by Ugur et al.69 and Zhang et al.68). Heterologous systems usually make use of overexpression which does not exclude the possibility that the observed heterodimers could be mere artifacts. Research in the field of heterodimers of opioid receptors are still at the early stages. This further reinforces the need of heterodimer-selective ligands to investigate the translation potential of heterodimers as therapeutic targets.
3.3 Bivalent and bitopic ligands of the opioid receptors
Opioid receptors were the first ever GPCRs to be probed by bivalent ligands in 1982 by Philip Portoghese, even before the concept of GPCR oligomerization was well-accepted. At the time, Portoghese postulated that the sum effect of pharmacophores combined over a linker would exceed the efficacy of the individual units.102 His pioneering work provided the first evidence of opioid receptor dimers and has since paved the way for the design and synthesis of bivalent ligands of other GPCRs.102
Structurally, bivalent ligands consist of two primary pharmacophore units, connected over a linker, each aiming for the OBS of the monomers within a dimeric complex (Figure 9A). They may either be homobivalent ligands where the phamacophore units are identical or heterobivalent when the pharmacophore units are different. Bivalent ligands distinguish themselves from bifunctional ligands consisting of a single pharmacophore capable of interacting with multiple targets. One of the earliest examples of a bifunctional ligand of the opioid receptors is nalorphine (5), which is now known to be a MOR antagonist and KOR agonist.103, 104 Nalorphine (5) is able to exhibit antinociceptive effects when acting at the KOR but also can reverse those of morphine at the MOR (for a review of bifunctional ligands of opioid receptors see Rehrauer and Cunningham35).103, 104
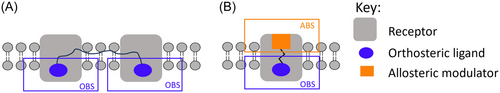
While Portoghese initially designed bivalent ligands with the aim to target the OBS of two receptors located in close proximity to each other, he did not preclude the possibility of the second pharmacophore subunit of these ligands binding to an accessory binding site within the same receptor. Ligands with the latter binding characteristics are now the so-called bitopic ligands. From a structural design perspective, bitopic ligands are a logical extension from bivalent ligands, in that they also consist of two pharmacophore units, linked over a spacer, one usually fitting within the conserved OBS and the other, the less-conserved ABS (Figure 9B).
In recent years, rationally designed bitopic ligands have been used to probe class A GPCRS like the dopaminergic receptors,105-107 the sphingosine-I-phosphate GPCRs108, 109 and the adrenergic receptor110, 111 providing useful insight into the intrinsic signalling mechanism of this class of receptors. In our research group, endeavors into the field of bitopic ligands have primarily been focused on the dualsteric targeting of the muscarinic acetylcholine receptors (mAChRs). The latter were “ideal” targets for rational bitopic ligand design considering their well-detailed allosteric pharmacology in literature and their significant physiological roles. We have designed and synthesized bitopic ligands showing a range of activities at mAChRs (from agonists to partial agonists112-114 to antagonists115) with improved affinities and receptor-subtype or functional selectivities.116
Bivalent and bitopic ligands deliver where traditional orthosteric ligands based solely on one pharmacophore have failed. The extensive work by Portoghese has shown how subtype selectivity can effectively be achieved using a bivalent targeting approach. Bitopic ligands with their concomitant occupation of both the OBS and less-conserved ABS also tend to show more subtype selectivity. Both bivalent and bitopic ligands may display improved affinity and/or efficacy. Bivalent and bitopic ligands, owing to their highly specific binding interactions, also show functional selectivity, positive or negative allosteric modulation or cooperativity.
3.4 Understanding bivalent and bitopic binding modes
When the two pharmacophores are linked through a spacer of appropriate length, bivalent ligands are expected to exhibit potencies higher than the combined effect of their individual monovalent ligands. This synergy occurs because of the thermodynamic advantage associated with sequential binding. Univalent binding of the first pharmacophore places the second pharmacophore, by virtue of being tethered, in close proximity to the neighboring unoccupied OBS (Figure 10B).26 Thus, the binding of the second pharmacophore resulting in a bivalent binding mode (Figure 10C) should be favored over the second univalent binding of a second ligand (Figure 10D).26 In situations where the monomers within a dimer are negatively allosterically coupled, synergy may not be observed as binding enhancement might then be hindered.26
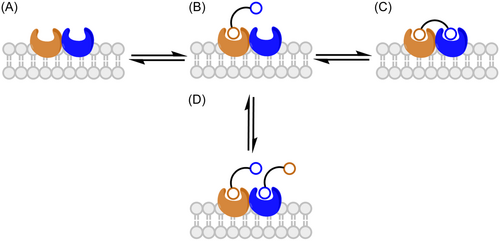
In his early studies of homobivalent ligands, Portoghese observed potencies and affinities higher than double those of the corresponding monovalent ligands, suggesting a bivalent binding mode.117, 118 Studies of more recent bivalent ligands of other GPCRs, including the serotonine,119 muscarinic,120 histamine121 and dopamine122 receptors indicate that homobivalent ligands generally show an increase in binding affinities compared to their monovalent pharmacophores. With heterobivalent ligands of mixed agonist/antagonist pharmacophores, an enhancement of binding affinity is not usually observed. Such a combination of pharmacophores usually requires careful investigation in multiple assays to obtain more information about their pharmacology. Another approach is to investigate the bivalent ligands in heterologous cell systems co-expressing the target GPCRs. If they efficiently bridge a dimer, bivalent ligands may show a higher selectivity for the co-expressed receptors than the singly expressed receptors.123-125
Careful analysis of Hill slopes of the binding curves of bivalent ligands in radioligand binding assays can confirm their bivalent binding modes. Kuhhorn et al. observed that the Hill slopes of the competitive binding curves of the monovalent antagonists are usually close to 1.126 Binding of bivalent antagonists showed an increased Hill slope of 2, suggesting a bivalent binding mode.126 Bivalent agonists displayed steeper binding curves in comparison to their monovalent pharmacophores.95, 127, 128 The displacement of the monovalent agonists by radiolabelled antagonists results in shallow curves of Hill slopes of 0.5 to 0.7.95, 127, 128 On the other hand, the Hill slopes of bivalent agonists significantly increased to 1.3 to 1.4.95, 127, 128 Another feature of bivalent ligands is that they show biphasic competition binding curves with two affinity values, Khigh and a Klow, corresponding to the bivalent and monovalent binding modes, respectively.129-131
When bitopic/dualsteric binding occurs (Figure 11A), the orthosteric moiety directs the binding affinity. In the presence of a radioligand, competition for the OBS will be observed and the bitopic ligand will show pronounced inhibitive action on radioligand binding.132-134 The bitopic ligand can however adopt a solely allosteric binding mode (Figure 11C). In that case, the binding of a radiolabelled orthosteric ligand might be favored through the formation of a ternary complex (Figure 11D).132 Provided the ternary complex is predominant in vitro, a concentration-dependent increase in radioligand binding may be observed.132 Bock et al. used the bitopic ligand iper-6-naph to illustrate how different ligand binding modes can control agonist efficacy at the muscarinic receptors.135 They were able to show that iper-6-naph can cause a concentration-dependent increase of the radioligand [3H] NMS in displacement experiments, direct evidence of an allosteric binding mode of iper-6-naph.135
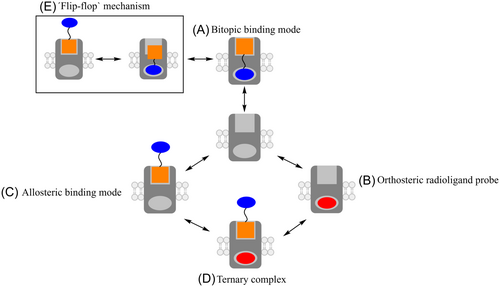
The bitopic ligand can also adopt a multiple binding mode, in a “flip-flop” manner between two or more receptor topographies, a purely allosteric and a purely orthosteric binding (Figure 11E).133, 134 This usually occurs where the pharmacophores possess low binding affinities or when the linker lengths are not optimal to afford bridging of the OBS and ABS. This “flip-flop” binding mode is experimentally indistinguishable from a bitopic binding mode.134 Mutagenesis studies can be used to validate the binding mode of the bitopic ligand.133, 134 Mutations of key residues important for binding within the OBS and ABS generally result in a decrease in the binding affinity of the bitopic ligand.133 In their study of bitopic ligands of the M2 muscarinic receptors, Antony et al. generated M2 muscarinic receptors with loss-of-function mutations in either the OBS or the ABS. They observed a loss in binding affinity for the bitopic ligand at the mutants.132 Moreover, mutations in the OBS led to a decreased inhibition of radioligand binding, suggesting that the bitopic ligand exhibited primarily allosteric behavior, enhancing the formation of the ternary complex.132 On the other hand, mutations in the ABS favored orthosteric behavior of the bitopic ligand, resulting in predominantly in the inhibition of radioligand binding.132
Molecular validation of the bitopic binding mode is not always possible, especially when the exact topography of the ABS is not known. In such cases, the effect on the binding affinity of the bitopic ligand in response to coaddition of an excess of the allosteric pharmacophore can be investigated.134, 136 When the bitopic ligand simultaneously occupies both the OBS and ABS, addition of the allosteric pharmacophore will have no pronounced effect on its binding affinity. On the contrary, if the ideal occupation of both binding sites is not afforded by the bitopic ligand, the excess allosteric pharmacophore might have an effect on the binding affinity.
3.5 Bivalent ligands of the opioid receptors
3.5.1 Homobivalent ligands of the opioid receptors
Philip Portoghese's extensive work on the bivalent ligands of the opioid receptors, culminating in the discovery of a KOR- and DOR-selective ligands, norbinaltorphimine (31, Figure 13) and naltrindole (9, Figure 1), respectively, is of remarkable significance. In an attempt to further knowledge of the effects mediated by each opioid receptor subtype in the late 80 s, Portoghese designed and synthesized bivalent ligands based on both agonist (oxymorphine) and antagonist (naltrexone, 6) pharmacophores (Figure 12).117 His rationale behind subtype-selective bivalent ligands derived from the assumption that spatial arrangement of proximate binding sites might be unique for each opioid receptor subtype based on their heterogeneity. Accordingly, Portoghese also hypothesized that the combined effects of bridging the gap between neighboring binding sites would be twice or more than that of a monovalent ligand.

Amongst his noteworthy findings at the time was the observation that homobivalent ligands (of linker lengths 0-4) based on the oxymorphine scaffold (compounds 29a-d, Figure 12) possessed agonist potencies and affinities more than twice of those of the monovalent agonists when tested in guinea pig ileum longitudinal muscle (GPI) and mouse vas deferens (MVD) preparations.118 Similar observations were made for bivalent antagonist ligands based on the naltrexone scaffold (compounds 30a-e, Figure 12) at both the MOR and KOR which strongly suggested concomitant engagement of two vicinal binding sites. Of note was the effect of linker length at the MOR and KOR. While peak potency was observed for the agonists when the spacer consisted of glycyl units (n = 2) at the MOR (Table 1, 29c) maximum binding selectivity was obtained for the antagonist with the shortest spacer (n = 0) at the KOR (Table 2, 30a).118 This was evidence of the differential molecular organization within different receptor subtypes. To further investigate whether the bivalent antagonist ligands bind two vicinal recognition sites or the OBS and an accessory binding pocket, Portoghese synthesized the corresponding meso-isomer of the most potent bivalent antagonist at the MOR (30c) by linking the active enantiomer (-) to the inactive enantiomer (+). Such an isomeric combination resulted in a 30-fold loss in antagonist potency (Table 2), supporting the hypothesis that the bivalent occupation of two vicinal binding sites occurred.118
| Compound no. | n | Morphinel C50, nM | Potency ratioa | Relative potencyb |
|---|---|---|---|---|
| 29a | 0 | 30.2 ± 8.7 | 4.6 ± 0.8 | 2.4 |
| 29b | 1 | 22.0 ± 5.0 | 4.0 ± 0.6 | 2.1 |
| 29c | 2 | 3.9 ± 0.6 | 36.0 ± 6.6 | 18.9 |
| 39d | 3 | 25.1 ± 5.2 | 5.5 ± 0.7 | 2.9 |
| Monomer | 66.8 ± 11.7 | 1.9 ± 0.3 | 1.0 |
- a Potency ratio is the ratio of morphine (MOR agonist) IC50 to compound IC50 on the same tissue
- b Relative potency ratio is the bivalent potency ratio divided by monovalent potency ratio.
| Compound no. | n | Morphine IC50 ratioa | Ethylketazocine (EK) IC50 ratioa | Selectivity ratiob |
|---|---|---|---|---|
| 30a | 0 | 27.6 ± 6.5 | 30.4 ± 9.6 | 1.1 |
| 30b | 1 | 57.4 ± 18.9 | 22.3 ± 4.7 | 0.4 |
| 30c | 2 | 181.0 ± 34.3 | 17.0 ± 2.4 | 0.2 |
| 30d | 3 | 61.4 ± 23.4 | 8.1 ± 2.4 | 0.1 |
| 30e | 4 | 40.7 ± 23.4 | 7.2 ± 1.2 | 0.2 |
| Monomer | 66.8 ± 11.7 | 1.2 ± 0.1 | 0.2 | |
| Meso-isomer of 30c | 2 | 7.8 ± 1.7 | - | - |
- a IC50 ratio is the IC50 of morphine or EK (KOR antagonist) in the presence of the antagonist (10 nM) divided by the control IC50 (same preparation without the antagonist).
- b EK IC50 ratio divided by morphine IC50 ratio.
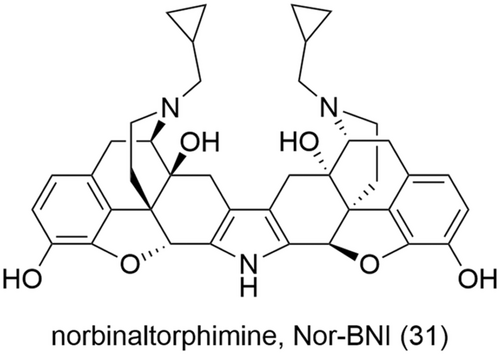
Based on the observation that the bivalent ligand with the shortest spacer was the most potent KOR antagonist, Portoghese went on to synthesize norbinaltorphimine (Nor-BNI) (31, Figure 13) which consists of two naltrexone pharmacophore subunits joined via ring fusion through a rigid pyrrole heterocycle.137 Nor-BNI (31) was the first reported selective antagonist of the KOR with no significant activity at the MOR and DOR. Nor-BNI (31) was later identified to be a monovalent ligand with parts of structure of the other naltrexone pharmacophore subunit binding a second subsite within the KOR which confers the subtype selectivity.138 This was the initial basis of the “message-address” concept later applied to aim for opioid receptor subtype selectivity. Further application of this concept led to the design and synthesis of naltrindole (9, Figure 1), a highly selective and potent non-peptide DOR antagonist.139, 140
In the early 2000s, the research group of Neumeyer used a similar approach as Portoghese to design and synthesize potent mixed KOR and MOR ligands based on new morphinan pharmacophores, cyclorphan (32) and its N-cyclobutylmethyl analogue butorphan (33) (Figure 14).141 Both ligands have shown high affinity for the two receptor subtypes, being full KOR agonists and partial MOR agonists.142, 143
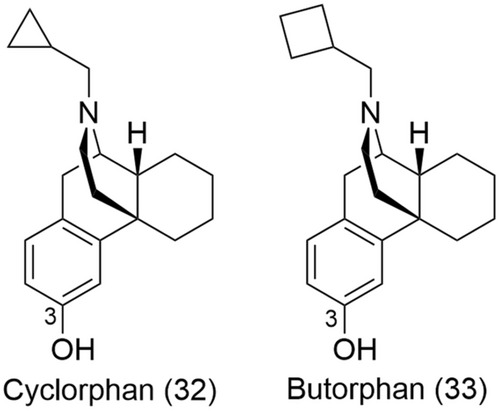
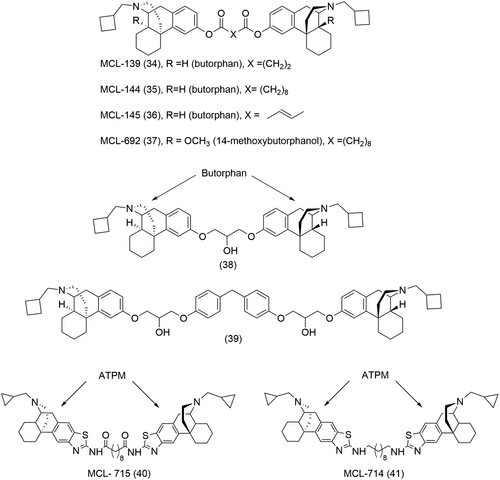
Dimeric ligands of cyclorphan (32) and butorphan (33) were synthesized with the introduction of varying length ether spacers between the two pharmacophores at the phenolic position 3 (Figure 14).141 Further modifications such as the incorporation of ester moieties or a conformationally restricted fumaryl group within the linker led to the identification of 3 potent ligands, namely MCL-139 (34), MCL-144 (35) and MCL-145 (36) (Figure 15).141 These dimeric ligands showed an increased selectivity for the MOR and KOR over the DOR (more than 90-fold), with MCL-139 (34) and MCL-144 (35) behaving as full KOR agonists and partial MOR agonists, respectively (Table 3).141 Further work by Neumeyer on this series of compounds included the introduction of methyl groups α to the ester moiety to improve hydrolytic stability; however, this also led to a subsequent decrease in binding affinities at both the MOR and KOR.145 An alternative approach to improve hydrolytic stability of the bivalent ligands was to introduce ether linkers with hydroxyl groups strategically placed beta to the ether moiety.146 This led to the discovery of two new bivalent ligands (38 and 39, Figure 15) with sub-nanomolar binding affinity at the MOR and KOR, respectively, and significant selectivity for the two receptor subtypes over the DOR.146
| Compound | % Emax (KOR) | EC50 in nM (KOR) | Ki in nM (KOR) | % Emax (MOR) | EC50 in nM (MOR) | Ki in nM (MOR) | Pharmacological properties |
|---|---|---|---|---|---|---|---|
| MCL-139 (34)141 | 70 ± 5.7 | 4.5 ± 1.7 | 0.076 ± 0.002 | 50 ± 4.4 | 7.4 ± 1.8 | 0.16 ± 0.01 | KOR full agonist MOR partial agonist |
| MCL-144 (35)141 | 60 ± 0.68 | 0.85 ± 0.053 | 0.049 ± 0.001 | 50 ± 6.6 | 1.3 ± 0.15 | 0.090 ± 0.004 | KOR full agonist MOR partial agonist |
| MCL-145 (36)141 | 90 ± 6.0 | 2.3 ± 0.34 | 0.078 ± 0.01 | 50 ± 2.5 | 2.9 ± 0.50 | 0.20 ± 0.032 | KOR and MOR partial agonist |
| MCL-692 (37)144 | 83 ± 0.95 | 2.4 ± 0.57 | 0.37 ± 0.040 | 42 ± 2.1 | 1.8 ± 0.36 | 0.26 ± 0.037 | KOR and MOR partial agonist |
| MCL-715 (40)144 | 130 ± 3.3 | 5.5 ± 0.31 | 0.37 ± 0.024 | 25 ± 1.8 | 130 ± 42 | 8.8 ± 0.66 | KOR full agonist |
| MCL-714 (41)144 | 130 ± 12 | 7.3 ± 0.81 | 0.27 ± 0.0023 | 38 ± 3.7 | 22 ± 5.3 | 2.5 ± 0.19 | KOR full agonist |
In a similar approach, homobivalent ligands were also derived from 14-methoxybutorphanol and ATPM pharmacophores.144 MCL-715 (40) and MCL-714 (41) (Figure 15) were found to be full agonists of the KOR, with a 24 and ninefold selectivity for the KOR over the MOR (Table 3).144 Both ligands also showed pronounced selectivity for the KOR over the DOR, 460- and 140-fold, respectively.144 The homobivalent ligand of 14-methoxybutorphanol, MCL-692 (37) (Figure 15) showed partial agonist activity at both the KOR and MOR and maintained selectivity for these 2 receptor subtypes over the DOR (Table 3).144
3.5.2 Heterobivalent ligands of the opioid receptors
Heterobivalent ligands can be useful pharmacological tools to study the physiological relevance of heterodimers as they can provide direct evidence of their existence in native tissues. The endeavors of many research groups have led to the design and synthesis of heterobivalent ligands targeting different opioid heteromers, namely the MOR/DOR, KOR/DOR, MOR/KOR and MOR/NOP (Table 4). Heterobivalent ligands behave as molecular yard sticks, providing valuable information about the distance between the heteromers and the pharmacological effects of simultaneously engaging the OBS of the monomers. This cannot be achieved with two individual agonists targeting each monomer as differing properties such as binding affinities and kinetics might not provide accurate understanding of the synergy or the lack of occurring as a result of the simultaneous occupation of two orthosteric binding pockets.35 However, due to their bivalent nature, it is difficult to determine whether heterobivalent ligands are simply engaging two different homomeric receptors or an actual heterodimer.156 Therefore, care is still needed when interpreting results obtained with these ligands.
| Heterobivalent ligand | Binding affinity/potency | Pharmacological properties |
|---|---|---|
| MOR/DOR heterobivalent ligands | ||
| TICP[Ψ]-DALDA (45)147 | KiMOR: 14.0 nM KiDOR: 4.79 nM |
MOR agonist DOR antagonist |
| 46148 | KiMOR: 1.03 nM KiDOR: 1.45 nM |
MOR agonist DOR antagonist |
| 47149 | KiMOR: 30.7 nM KiDOR: 1896 nM |
MOR and DOR agonist |
| 48149 | KiMOR: 26.1 nM KiDOR: 923 nM |
MOR and DOR agonist |
| D24M (49)150 | KiMOR/DOR; 0.63 nM IC50MOR/DOR: 0.85 nM |
MOR/DOR antagonist |
| MCL-450 (54)151 | KiDOR: 1.5 nM; IC50DOR: 3.9 nM KiMOR: 0.69 nM KiKOR: 0.28 nM |
DOR antagonist |
| 55152 | KiMOR: 14 nM KiDOR: 14 nM EC50MOR: 125 nM (GTPγS binding) EC50DOR: 510 nM (GTPγS binding) |
Mixed MOR and DOR agonist |
| 56152 | KiMOR: 23 nM KiDOR: 0.69 nM IC50MOR: 200 nM IC50DOR: 24 nM |
Mixed MOR and DOR agonist |
| 57152 | KiMOR: 0.38 nM KiDOR: 0.36 nM IC50MOR: 8.51 nM IC50DOR: 1.83 nM |
Mixed MOR and DOR agonist |
| 58153 | KiMOR: 0.14 nM; EC50MOR: 0.31 nM KiDOR: 0.14 nM; EC50DOR: 0.16 nM |
Mixed MOR and DOR agonist |
| 59154 | KiMOR: 0.1 nM KiDOR: 0.5 nM |
Mixed MOR and DOR agonist |
| KOR/DOR heterobivalent ligands | ||
| KDN-21 (60)123 | KiKOR+DORa : 64 nM KiKOR/DOR: 0.3 nM |
KOR/DOR antagonist |
| KDAN-18 (61)125 | ED50: 0.19 nmol/mouseb | KOR/DOR agonist |
| MOR/KOR heterobivalent ligands | ||
| KMN-21 (63)124 | MOR/KOR antagonist | |
| MOR/NOP heterobivalent ligands | ||
| DeNO (65)155 | In cAMP assay: pEC50MOR/NOP: 7.55 pEC50MOR: 8.77 pEC50NOP: 8.88 |
MOR and NOP agonist |
In GTPγS assay: pEC50MOR/NOP: 7.63 pEC50MOR: 8.25 pEC50NOP: 9.24 |
||
| De101 (66)155 | In cAMP assay: pEC50MOR/NOP: 8.81 pEC50MOR: 9.00 pEC50NOP: no activity |
MOR agonist and NOP antagonist |
In GTPγS assay: pEC50MOR/NOP: 8.67 pEC50MOR: 8.26 pEC50NOP: no activity |
||
- a KiKOR+DOR = Ki of singly expressed DOR or KOR; KiKOR/DOR = Ki of coexpressed DOR and KOR.
- b The ED50 were determined in a modified tail-flick assay upon i.t. administration in at least three groups of 10 male CDI mice weighing from 20 to 25 g. Antinociception was considered positive if the tail-flick latency was more than the control latency plus 3 SD of the mean of the reaction time.
3.6 MOR/DOR
The MOR and DOR subtypes both mediate analgesic effects, albeit to different extent. While DOR agonists possess fewer side effects, their analgesic properties are weaker than those of MOR agonists.157 Studies have shown that Coadministration of small amounts of a DOR antagonist, naltrindole (9, Figure 1), with a MOR agonist, leads to a slower development of tolerance.158 These observations, taken together with evidence of MOR and DOR oligomerisation,100 have provided the basis for the design and synthesis of bivalent ligands targeting MOR/DOR dimers with enhanced antinociceptive properties but possibly fewer side effects.
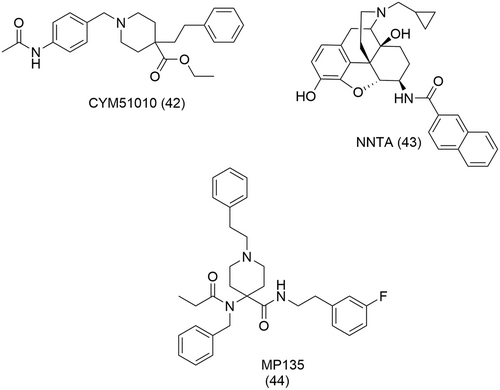
The MOR/DOR heterodimer is perhaps the best described heterodimer of the opioid receptors. Some of the approaches to confirm their presence and function in vivo include the use of antibodies that selectively recognize the endogenous heterodimers in the brain,159, 160 TAT peptides that disrupt the heterodimer161 and double fluorescent knock-in mice162 to investigate physical proximity of the MOR and the DOR. These studies suggest that the MOR/DOR heterodimer could behave as an anti-opioid negative feedback loop, triggered by chronic opioid exposure to mitigate the opioid system. These approaches, however, are indirect and thus provide no direct evidence of the physiological existence and role of the MOR/DOR heterodimer in vivo, therefore generating some controversy. Other studies suggest no functional MOR/DOR interaction in vivo.163 Moreover, the discovery of small molecules, namely, CYM51010 (42),164 NNTA (43)165 and, more recently, MP135 (44)156 (Figure 16) which selectively engage the MOR/DOR heterodimer over isolated MOR or DOR monomers challenged the notion that the concomitant binding of two pharmacophores at each opioid monomer are needed to probe heterodimers. Seemingly, the binding of a small molecule at one monomer could favor the activation of the other.
3.6.1 Peptidic heterobivalent ligands
Bivalent ligands of the MOR/DOR were synthesized by linking the DOR antagonists TICP-NH2[Ψ] and TIPP-OH to the highly potent MOR agonist [Dmt1]-DALDA via a tail-to-tail connection through a short spacer or the Lys side chain.147 Despite showing agonist and antagonist properties at the MOR and DOR, the binding affinities (Table 4) of TICP[Ψ]-DALDA (45, Figure 17) were considerably lower than that of its constituent monovalent pharmacophores (DALDA, KiMOR = 0.143 nM and TICP[Ψ] = 0.259 mM).147 Such a loss in affinity was attributed to the steric hindrance resulting from the introduction of the linker.147
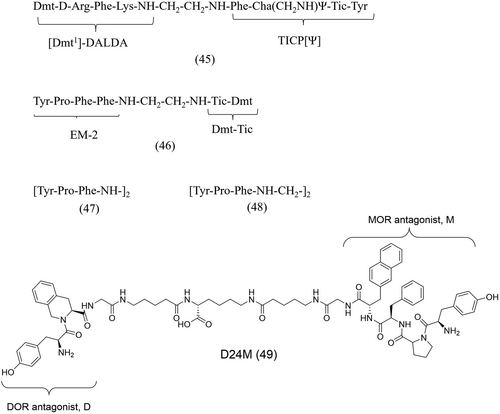
In another study, the bivalent pseudopeptide Tyr-Pro-Phe-Phe-NH-CH2-CH2-NH-Tic-Dmt (46, Figure 17) was synthesized by the tail-to-tail connection of the DOR antagonist Dmt-Tic and the MOR agonist endormorphin-2 (EM-2) via an ethylenediamine linker.148 The bivalent ligand showed comparable MOR and DOR affinities (Table 4) to that of their monovalent pharmacophores (EM-2, KiMOR = 0.69 nM, KiDOR = 1.45 nM; Dmt-Tic, KiDOR = 1.22 nM), with a mixed MOR agonist/DOR antagonist pharmacological profile.148
Gao et al. were able to improve the binding affinity of (EM-2), a MOR-selective agonist, to the DOR subtype.149 They synthesized “balanced agonists” which show affinity for a broad spectrum of opioid receptors, by linking two EM-2 analogue pharmacophore subunits in a tail-to-tail approach via diamine linkers of various lengths. Interestingly, two of the bivalent peptides, 47 and 48 (Figure 17), with the deleted Phe4 residue, showed improved binding affinity at both the MOR and DOR (Table 4) compared to their monomer, [Tyr-Pro-Phe-NH-] (KiMOR = 140 nM and KiDOR = >10000 nM).149 They were thus able to demonstrate that the affinity and selectivity profiles of a monovalent ligand can be altered by linking it to another pharmacophore.
More recently, Olson et al. designed a new series of bivalent ligands by linking the moderate/low affinity DOR (H-Tyr-Tic-OH, D) and the MOR (H-Tyr-Pro-Phe-DINal-NH2-, M) antagonists to develop MOR/DOR selective antagonists which could also be used as pharmacological tool compounds.150 The linker chain lengths employed ranged from 15 to 41 atoms resulting in the synthesis of six different ligands. One of the bivalent ligands, D24M (49) (Figure 17), emerged as a selective MOR/DOR antagonist (~ 90-fold when compared to the DOR) with sub-nanomolar affinity and potency (Table 4).150 D24M (49) also strongly reduced naloxone-mediated behavior in models of acute and chronic morphine dependence.150 These findings, combined with the observed MOR/DOR selectivity of D24M (49), support the implication of the MOR/DOR receptor in the modulation of analgesia, tolerance, and dependence.
3.6.2 Morphinan heterobivalent ligands
The compounds of the MDAN series were amongst the first bivalent ligands, derived from a morphinan-scaffold, targeting the MOR/DOR dimer. These bivalent ligands were designed and synthesized by Daniels et al. to determine if both receptor subtypes mediate their effects in a dimeric state.166 In this regard, the MOR agonist, oxymorphone, was linked to the DOR antagonist, naltrindole (9, Figure 1), by means of a variable length spacer. Chronic intracerebroventricular (i.c.v.) administration in the tail-flick test in mice revealed that the length of the linker could influence the development of tolerance. Compounds with shorter linkers (e.g., MDAN-16, 50, Figure 18) showed tolerance similar to that of morphine while those of longer linkers (e.g., MDAN-21, 51, Figure 18) showed no significant tolerance.166 In additional immunocytochemical trafficking and receptor activation studies, MDAN-21 (51) induced significantly less receptor MOR/DOR internalization than MDAN-16 (50), suggesting that these two ligands bind differently (bivalent vs. monovalent binding modes).166 These findings also confirmed the role of the MOR/DOR heteromer in mediating the underlying mechanisms of tolerance development.
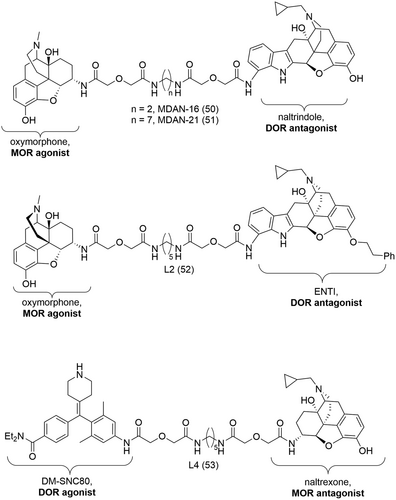
In a similar approach, Harvey et al. synthesized two bivalent ligands, L2 (52) and L4 (53) (Figure 18), by tethering high affinity MOR compounds to low affinity DOR compounds.167 The idea behind such a combination of pharmacophores was to develop ligands with tuned affinity for the MOR/DOR heteromer over the DOR subtype that could ultimately be used as selective pharmacological tool compounds to study the MOR/DOR heteromer. L2 (52) was designed from the MOR agonist oxymorphone and the DOR antagonist ENTI (Figure 18) and L4 (53), from the MOR antagonist naltrexone and the DOR agonist DM-SNC80 (Figure 18). L4 (53) showed high affinity at the MOR (pKiMOR > 9 nM), and improved affinity (~10-fold) for the MOR/DOR over the DOR.167 Harvey et al. were thus able to prove that it is possible to fine-tune affinity and specificity of bivalent ligands for a particular receptor heteromer.
3.6.3 Mixed peptidic/non-peptidic heterobivalent ligands
Several bivalent ligands were also designed from structurally different pharmacophore subunits, i.e., a peptide and a morphinan scaffold. The first bivalent ligand synthesized with such a combination of pharmacophores was MCL-450 (54, Figure 19) consisting of the DOR-selective peptide Dmt-Tic and the MOR/KOR mixed agonist butorphan.151 Esterification of the phenol moiety allowed the introduction of a two-methylene spacer which was then coupled via amide formation to Dmt-Tic. MCL-450 (54) was shown to be a DOR antagonist with high affinity and moderate potency at the DOR (Table 4).151 The antagonistic properties of MCL-450 (54) was attributed in part to the Dmt-Tic pharmacophore. On the other hand, a slight loss in both affinity and potency, ranging from 3- to 6-fold, were observed at both the MOR and KOR (Table 4) when compared to the monovalent ligand butorphan (KiMOR = 0.23 nM and KiKOR = 0.079 nM).151
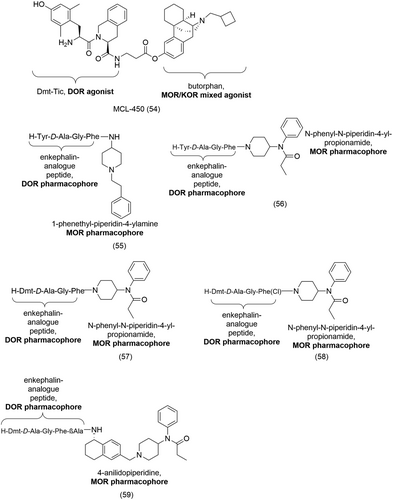
Lee et al. later studied bivalent ligands derived from enkephalin-like pharmacophores and parts of the fentanyl scaffold, namely, 1-phenethyl-piperidin-4-yl-phenyl-amine, 1-phenethyl-piperidin-4-ylamine and N-phenyl-N-piperidin-4-yl-propionamide.152 Their aim was to identify ligands with mixed MOR and DOR activities with analgesic properties but devoid of side effects. They reported a total of eight bivalent ligands showing broad biological activities at both the MOR and DOR. In general, improved binding affinities for the DOR was obtained by tethering enkephalin-like structures to parts of the fentanyl moieties.152 Interestingly, the selectivity profiles for the MOR or the DOR varied depending on the fentanyl moiety present at the C-terminal. For example, 55 (Figure 19), with the 1-phenethyl-piperidin-4-ylamine at the C-terminal displayed MOR-selectivity (DOR/MOR = 4.2) (Table 4) in the GTPγS binding assay while 56 (Figure 19), bearing the N-phenyl-N-piperidin-4-yl-propionamide at the same position, showed selective DOR binding affinity (DOR/MOR = 0.03) (Table 4).152 Further modification to 56 included the substitution of the Tyr residue, in the enkephalin analogues, for Dmt which is known to confer higher DOR affinity, resulting in 57 (Figure 19) which showed significantly improved binding affinities (Table 4) at both the MOR (60-fold increase) and DOR (twofold increase).152 57 also showed increased potency (Table 4) in the MVD (13-fold) and in the GPI (24-fold) assays, compared to 56.152
Additional work by Lee et al. involved structural modification of the bivalent ligands to improve lipophilicity.153 A series of SAR studies were done on the enkephalin analogue, 57 (Figure 19), while keeping the Dmt1 residue and the propionamide moiety constant. Substituting the d-Ala2 with d-Nle2 and para-halogenation of Phe4 led to improved opioid agonist activity and efficacy at both the MOR and DOR without significant alteration to their binding affinities.153 This could be due to the higher bioavailability of the ligands as a result of their increased lipophilicity.153 Truncating the Gly3 residue resulted in higher MOR/DOR selectivity and para-halogenation of Phe4 afforded MOR sub-nanomolar activity and affinity.153 58 (Figure 19), with tuned MOR and DOR affinities (Table 4), possessed potent antihyperalgesic and antiallodynic properties in in vivo assays.153
In a further study, bivalent ligands targeting the MOR and DOR subtypes were designed and synthesized by tethering enkephalin analogues to the 4-anilidopiperidine derivatives.154 The peptide pharmacophore subunit, an enkephalin moiety with either a N-terminal Tyr- or Dmt- were linked, with or without a spacer, to the non-peptide pharmacophore subunit consisting of a 5-amino substituted (tetrahydronaphthalene-2yl) methyl with 4-anilidopiperidine derivatives.154 Introduction of a short β-alanine spacer between the enkephalin analogue and the 4-anilidopiperidine moiety and substitution of the N-terminal Tyr- for Dmt- yielded the bivalent ligand 59 (Figure 19) with high affinity for the MOR and DOR (Table 4) but no activity at the KOR.154 However, 59 also showed short duration of action (< 15 min) and moderate activity in vivo.154
3.7 KOR/DOR
Spinally administered KOR and DOR agonists can potentiate antinociception168 and the KOR and DOR have been reported to be co-localized on spinal neurons.169 Moreover, the observation of KOR/DOR dimerization and oligomerisation in cultured cells65 and porcine ileum has prompted the design and synthesis of bivalent ligands specifically targeting the KOR/DOR heterodimer.170
Bhushan et al. investigated a series of KOR/DOR bivalent ligands by connecting the KOR-selective antagonist 5’-GNTI (11, Figure 1), over a linker, to the DOR-selective antagonist NTI (9, Figure 1).123 In intrathecal in vivo studies, the bivalent ligand, KDN-21 (60, Figure 20), was identified as a potent antagonist of the DOR-subtype δ1-selective agonist, [d-Pen] enkephalin (DPDPE) and the KOR-subtype κ2-agonist, bremazocine (17, Figure 1).123 Binding studies in human embryonic kidney (HEK) cells with singly expressed KOR and DOR or co-expressed KOR/DOR revealed that KDN-21 (60) has a 200-fold higher affinity for the latter (Table 4) suggesting that KDN-21 (60) effectively binds a KOR/DOR heterodimer.123 Another observation of significance was the different antagonism profiles of KDN-21 (60) in intrathecal (i.t.) and intraventricular (i.c.v.) in vivo studies. KDN-21 (60) did not antagonize intraventricularly administered agonists deltorphin-II (δ2- selective), U50448 (18, Figure 1, κ1-selective) or bremazocine (17, κ2-selective) which suggests differing population of KOR and DOR subtypes in the brain and in the spinal cord.123 These findings led to the conclusion that the bivalent antagonist KDN-21 (60) selectively bound a specific population of δ1-κ2 present in the spinal cord but not in the brain.123
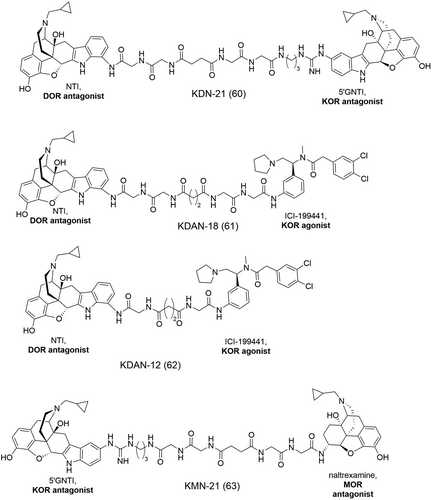
The studies by Daniels et al. on a series of bivalent ligands helped to gain more insight into the organization of DOR and KOR which is manifested as the δ2 and κ1 phenotypes.125 The KDAN series of bivalent ligands was designed by tethering together, via variable length oligoglycyl linkers, the high-affinity DOR-selective antagonist NTI (9, Figure 1) and the high-affinity KOR(κ1)-selective agonist ICI-199441 (61 and 62, Figure 20). The spacer length was varied from 12 atoms (KDAN-12, 62, Figure 20) to 20 atoms (KDAN-20). In intrathecal (i.t.) studies in mice, the bivalent ligands all showed antinociceptive activity with full agonism properties.125 The bivalent ligand, KDAN-18 (61, Figure 20), exhibiting the highest potency (Table 4) in the in vivo studies, was potently antagonized by both norBNI (31, Figure 13) (κ-selective) and naltriben, NTB (10, Figure 1, δ2-selective) but not by CTOP (µ-selective) which suggests that the MOR did not play a role in the antinociceptive activity mediated by KDAN-18 (61).125 It was observed that the length of the linker is of significance as bivalent ligands of shorter linker lengths, e.g., KDAN-12 (62, Figure 20), were not antagonized by NTB (10).125 This implied that KDAN-18 (61) likely binds neighboring δ2 and κ1 phenotypes with a linker length of 18 atoms facilitating their bridging. Studies on HEK cells which consist of singly expressed or co-expressed DOR and KOR showed that KDAN-18 (61) had a ~ 65-fold higher affinity for the co-expressed cell line in comparison to the mixed cell lines when the δ2-selective [³H]-deltorphin-2 was used.125 This provided further evidence of the binding of KDAN-18 (61) to associated KOR and DOR of the δ2 and κ1 phenotypes. Interestingly, the heterobivalent KDN-21 (60, Figure 20) which selectivity binds the δ1 and κ2 phenotype heterodimer, did not antagonize KDAN-18 (61), indicating that the latter bridges a different phenotypic heterodimer, namely, δ2 and κ1.125
3.8 MOR/KOR
Peng et al. studied a series of heterobivalent ligands containing KOR agonist and MOR agonist/antagonist pharmacophore subunits.171 The KOR agonist butorphan (33, Figure 14) and other N-substituted derivatives were linked via ester- or amide- linkers to the MOR mixed agonist/antagonist cyclorphan (32, Figure 14). A 10-carbon ester linker, the stereochemistry of the pharmacophores and the N-substituents were significant parameters contributing to affinity at the MOR/KOR heterodimer.171 The heterobivalent ligands were either full or partial agonists at the KOR and MOR with a few showing mixed agonist/antagonist properties at the MOR.124 While they all possessed relatively similar affinities at the MOR or KOR as their monomeric pharmacophores, they provided a good basis for the design and synthesis of selective pharmacological tool compounds for the MOR/KOR.171
The bivalent ligand KMN-21 (63, Figure 20) emerged as a selective MOR/KOR antagonist from a series of ligands designed by tethering the KOR antagonist 5’-GNTI (11, Figure 1) and the MOR-antagonist naltrexamine (β-NTX) via linkers of varying length.124 KMN-21 (63), with a 21-atom linker between the two pharmacophores, significantly antagonized both U69508 (KOR selective agonist)- and DAMGO (MOR selective agonist)- induced Ca2+ release in cells with co-expressed MOR and KOR.124
3.9 MOR/NOP
There is evidence pertaining to the heterodimerisation or the co-localization of the MOR and the nonclassical opioid receptor NOP.155, 172, 173 It has been suggested that probing the MOR/NOP heterodimer might provide new avenues for improved analgesia with reduced side effects.174-177 The possible heterodimeric pairing of the MOR and NOP has been observed in both in vitro and in vivo experiments with nonhuman primates.178 Ko and Naughton observed that N/OFQ, when combined with a single dose of morphine, potentiated intrathecal morphine-induced antinociception in monkeys.178 Their findings indicated that mixed MOR/NOP agonists might show potent antinociception with fewer side effects.178 Moreover, the mixed MOR/NOP peptide agonist, [Dmt1] N/OFQ (1-13)-NH2, showed a 30-fold increase in potency when compared to N/OFQ and exhibited robust and long-lasting antinociception in monkeys.179 More recently, the new opioid analgesic, cebranopadol (64, Figure 21), a mixed MOR/NOP ligand, now in phase III clinical trials,180-182 has shown favorable safety profile in preclinical trials.177 Additionally, an in vitro study by Evans et al. demonstrated the co-localization of the MOR and NOP via the use of tagged receptors.173 Administration of N/OFQ to the co-expressing cell lines resulted in the co-internalization of both the MOR and NOP indicating that these two receptor subtypes might exist either co-localized or as heterodimers.173 Studies in CHO cells showed the co-immunoprecipitation of NOP and MOR and the displacement of [3H]-N/OFQ which does not occur in single cell expression systems.172 While heterodimerisation of these two receptor subtypes can negatively impact MOR signalling,183 targeting the heterodimers may result in an improvement in side effect profiles, previously observed in studies with the 6TM MOR and NOP heterodimer.184
To study the dimerization of the MOR and NOP and the role of the latter on MOR signalling, Bird et al. designed two bivalent ligands, DeNO (65) and De101 (66) (Figure 21) and evaluated their activity in a human HEK co-expression system (HEKMOR/NOP).155 DeNO (65), synthesized from the MOR agonist dermorphin and N/OFQ (Figure 21), displays full agonist activity at both the MOR and NOP.175 De101 (66) was synthesized by tethering together dermorphin and the high-affinity antagonist analogue of N/OFQ, UFP-101 (Figure 21).155
DeNO (65) and De101 (66) (Figure 21) were shown to be able to displace 140% and 120% of [3H]-N/OFQ in HEKMOR/NOP membranes, which is significantly higher than that of their pharmacophore subunits which were unable to produce more than 100% displacement.155 In GTPγ[35S] and cAMP assays, DeNO (65) showed a decreased potency in the HEKMOR/NOP co-expression system compared to single cells, HEKMOR and HEKNOP (Table 4).155 De101 (66) showed similar potencies in both HEKMOR/NOP and single cells expression systems (Table 4). The bivalent ligands were also evaluated for their ERK1/2 activity, which is involved in a number of cellular functions, all mediated by the spatio-temporal activation of the receptor. Typically, activation at the MOR occurs within 2 to 5 min following drug administration with a peak observed from 7.5 to 10 min post administration.185 DeNO (65) and De101 (66) both showed a significant delay in the activation of the ERK1/2 pathway in the HEKMOR/NOP cell line.155 The data observed in these three assays suggest that NOP does impact MOR downstream signalling. However, when evaluated in vivo in rats, DeNO (65) showed similar effects as demorphin and N/OFQ upon spinal administration, with no improvement in potency or efficacy indicating that no synergistic antinociceptive effects are obtained with the simultaneous targeting of the MOR and NOP.175 In vivo characterization of De101 (66) has not been reported. While Bird et al. were not able to confirm heterodimerisation of the classical MOR and the nonclassical NOP, they were able to demonstrate their co-localization and their results now pave the way for further pharmacological investigation of the possible MOR/NOP heterodimer. It is important to note that like their peptidic parent compounds, the instability of DeNO (65) and De101 (66) might limit their applications in vivo.
3.10 Heterobivalent ligands of opioid and non-opioid heteromers
The dimerization of opioid receptors with other non-opioid receptors of the GPCR family involved in the mechanisms of pain has also been pursued in the search for safer, efficient analgesics. The heterodimerisation of the MOR with other GPCRs such as the cholecystokinin receptor (CCK2), the adrenergic receptor (A1AR), the cannabinoid receptor (CB1), the dopamine receptors (D2-like receptors) and neurokinin-1 receptor (NK1) amongst others is described in the literature.130, 186-189 Bivalent ligands addressing these heterodimers are important pharmacological tool compounds in the investigation of their functional roles and their significance in pain therapy. In this section, we aim to summarize some of the efforts in the development of bivalent ligands targeting dimeric associations of an opioid receptor with a non-opioid receptor (Table 5).
| Heterobivalent ligand | Activity | Binding affinity/Potency | Pharmacological properties |
|---|---|---|---|
| 68b189 | MOR/NK1 | KeNK1: 21 nM IC50MOR: 55 nM |
MOR agonist NK1 antagonist |
| 70187 | MOR/A1AR | KiMOR: 800 nM KiA1AR:728 nM | MOR and A1AR antagonist |
| 72b188 | MOR/CB1 | ED50: 189 pmol/mouse (i.c.v.) ED50: 19 pmol/mouse (i.t.) |
MOR agonist CB1 antagonist |
| 72c188 | MOR/CB1 | ED50: 9 pmol/mouse (i.c.v.) ED50: 5 pmol/mouse (i.t.) |
MOR agonist CB1 antagonist |
| 73a130 | D4R/MOR | KiD4R: 184 nM | D4R antagonist MOR agonist |
| 73b130 | D4R/MOR | KiD4R: 369 nM | D4R antagonist MOR agonist |
| 73c130 | D4R/MOR | KiD4R: 339 nM | D4R antagonist MOR agonist |
The cholecystokinin (CCK) receptors also belong to the class A GPCR family. The two CCK receptors, CCK1 and CCK2, share a 50% sequence homology190-194 with the CCK1 receptors located primarily in the peripheral alimentary tract, while the CCK2 receptors are distributed in the CNS.195 Activation of both CCK receptor subtypes recruits the effector molecule Gq which leads to the stimulation of phospholipase C and intracellular calcium.190-195 At physiological level, this translates into stimulation of gall bladder contraction, pancreatic exocrine excretion, enteric mobility, gastric secretion, and modulation of neurotransmission.190-195
CCK2 and opioid receptors are present in the parts of the CNS involved in the modulation of pain.196, 197 Activation of the MOR or the DOR triggers CCK release which then negatively impacts opioid receptor-induced analgesia by interacting at the CCK2 receptor. The interactive mechanisms of action of these two receptors have also been demonstrated in in vivo studies; CCK2 receptor knockout mice show increased opioid-mediated algesia and an upregulated endogenous opioid receptor system.198, 199 Antagonism of the CCK2 receptor has also been reported to block morphine tolerance.200 Considering their opposite roles in mediating analgesia, it is tempting to investigate the activity of a MOR/CCK2 bivalent ligand based on a MOR agonist and a CCK2 antagonist, assuming that these two receptors can form functional dimers.
A study by Zheng et al. investigated the possibility of the MOR and CCK2 forming heterodimers using the BRET technology.186 Bivalent ligands consisting of the MOR-agonist oxymorphone linked via a variable length spacer, to the CCK2 antagonist, L-365260, (67a-c, Figure 22) were studied. Their findings point to a heterodimerisation of the MOR and CCK2 which is non-constitutive but rather induced in the presence of bivalent ligands of appropriate linker lengths (16 to 22 atoms) sufficient to bridge the two receptors.186 The BRET signal for bivalent ligands with spacer lengths 18 and 22 atoms (67b and 67c, Figure 22), respectively, significantly increased and plateaued at approximately 2, which suggests specific saturable interaction between MOR and CCK2 receptors.186 Conversely, the BRET signal of the bivalent ligand with a linker length of 16 atoms (67a, Figure 22), were significantly lower, approximately half that of the signal obtained with the bivalent ligands of linker lengths 18 and 22 (67b and 67c, Figure 22), respectively.186 Introducing the CCK2 antagonist, L-365260, attached solely to a 22-atom linker resulted in a decrease in the BRET ratio, indicating the competition between the latter and the antagonist pharmacophore of the bivalent ligands.186 The CCK2 antagonist triggered a switch from bivalent binding mode to univalent, which resulted in the disruption of the non-constitutive heterodimers, which was then observed as a reduction of the BRET signal.186
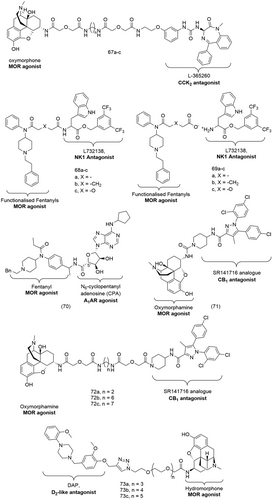
Based on the evidence that the heterodimerisation of the MOR and CCK2 receptor is non-constitutive, there seems to be no direct correlation between MOR/CCK2 receptor dimer formation and the development of tolerance.186 Zheng et al. determined the i.c.v ED50 of their bivalent ligands and observed no significant tolerance, independent of linker lengths, thereby reinforcing the notion that the underlying mechanism of tolerance is not mediated by a possible MOR/CCK2 receptor heterodimer.186
MOR is also co-expressed with neurokinin-1 (NK1) receptors in areas of the CNS involved in pain transmission that also controls opioid dependence and reward.201 The NK1 receptor is the target of tachykinin peptides such as substance P which has been reported to play an important role in the mediation of pain signals.189 Yamamoto et al. designed and synthesized a number of bifunctional ligands based on a peptide scaffold which showed excellent agonist activity for both the MOR and DOR and high antagonist activity at the NK1 receptor both in vitro and in vivo.202-206 Vardanyan et al. used a similar approach to develop non-peptide bivalent ligands of the MOR/NK1 heterodimer.189 Bivalent ligands based on the structures of the opioid agonist fentanyl and the NK1 antagonist L732138 were either tethered via a linker or synthesized as an ion pair (68a-c and 69a-c, Figure 22). These ligands were evaluated in competitive radioligand binding assays in recombinant cell lines expressing the human MOR or the human NK1 receptor and in functional assays using guinea pig isolated ileum/longitudinal muscle myenteric plexus (GPI/LMMP) for the MOR and NK1 receptor and mouse vas deferens (MVD) for the DOR.
In radioligand competition binding assays, the bivalent ligands, both covalently bonded and ion paired, showed a significant decrease in affinity for the MOR along with a dramatic loss of analgesic activity when compared to fentanyl.189 Such a loss of MOR activity was attributed to the introduction of a negatively charged carboxyl group on the fentanyl moiety which impairs MOR recognition.189 On the other hand, the activity of the bivalent ligands was unaltered at the NK1 receptor; the bivalent ligands showed high affinity in radioligand competition binding assays using [3H] Substance P.189 In functional assays, all bivalent ligands displayed antagonism of the NK1 receptor with ligand 68b (Figure 22) showing a 10-fold higher antagonist affinity (Table 5) than the other ligands.189 68b was also the most potent bivalent ligand at the MOR, making it a suitable lead compound for further development of non-peptide bivalent ligands of the MOR/NK1 heterodimer.189
There is evidence of a crosstalk between the A1 adenosine receptors (A1ARs) and the MOR. When activated this results in a decrease in cAMP levels in their target cells.207 Cross tolerance and cross withdrawal interactions between A1ARs and the MOR have been observed in the PNS suggesting that these receptors are co-localized on the same primary nociceptors and might even function as dimers.207 Targeting both the A1ARs and the MOR might be of significant therapeutic applications in some disease conditions; activation of A1ARs and the release of adenosine leads to hypotension during severe sepsis or septic shock, and ultimately tissue hypoperfusion and ischemia.208 Additionally, the MOR antagonist naloxone (7, Figure 1) has been employed against lowered blood pressure during septic or hypovolemic shock, implicating the participation of endogenous opioids in the severity of the latter syndromes.209, 210
Mathew et al. synthesized the heterobivalent ligand 70 (Figure 22), based on the MOR agonist fentanyl and the highly selective A1AR agonist N6-cyclopentyl adenosine (CPA) linked via an amide linker at the 5’-position of CPA.187 70 showed antagonistic properties at both the MOR and the A1AR when evaluated in nociceptive tests in mice and in cAMP production assays.187 70 was able to reverse the increased latency observed in both intra cerebro ventricular (icv) and intraperitoneal (ip) administration in mice.187 70 also activated cAMP production in a dose-dependent manner both in CHO K1 cells over expressing MORs and in CHO Chem 3 cells overexpressing A1ARs with a maximum stimulation of 29% and 17%, respectively.187 In comparison, the MOR antagonist naloxone (7, Figure 1) exhibited an increased cAMP production with a maximum stimulation of 50% while the A1AR antagonist, 8-cyclopentyl-1,3-dipropyl-xanthine (DPCPX, 74, Figure 23) enhanced cAMP production with a maximum stimulation of 45%.187 While its affinity was sufficient to trigger biological response at reasonable concentrations, 70 showed lower binding affinities (Table 5) than the antagonists, naloxone (7) (KiMOR = 1.5 nM) and DPCPX (74) (KiA1AR = 12.9 nM).187
The MOR and the cannabinoid receptor (CB1) have been reported to associate as heterodimers in cultured cells211, 212 and they are both present in overlapping regions of the CNS.213-217 Both receptor types share pharmacological properties and are involved in physiological mechanisms mediating analgesia, tolerance, and dependence after prolonged administration.218-220 It was suggested that both receptors have a significant role in antinociception and that the MOR/CB1 may be involved in the development of tolerance. Cross tolerance between tetrahydrocannabinol (THC, 75) (Figure 23) and morphine has been observed in studies where antagonism of antinociception was obtained with both naloxone (7, Figure 1) and CB1 antagonist SR141716 (76, Figure 23).221 Coadministration of morphine and CB1 antagonist prevented acute and chronic tolerance to morphine.222 Self-administration studies show that both receptors are involved in reward processes in which the CB1 antagonist SR141716 (76) and opioid antagonist naloxone (7, Figure 1) decreased self-administration of morphine or THC (75).223, 224 In CB1 knockout mice, dependence and tolerance were decreased for morphine but not for other drugs of abuse.225 Comparatively, in MOR and DOR knockout mice, the cannabinoid withdrawal syndrome is significantly inhibited.226
Le Naour et al. studied a series of heterobivalent ligands possibly targeting the MOR/cannabinoid heterodimer by tethering the MOR agonist oxymorphamine to the CB1 antagonist/inverse agonist SR 141716 (76, Figure 23) via a spacer of variable lengths (ranging from 2 to 20 atoms).188 Based on previous observations that the MOR/DOR heterobivalent ligand MDAN-21 (51, Figure 18) which effectively bridges the two receptors shows no internalization of co-expressed MOR and DOR in HEK-293 cells in imaging studies,227 similar experiments were carried out on co-expressed MOR and CB1 receptors. Interestingly, the MOR/CB1 heterobivalent ligand 72c (Figure 22) with a linker length of 20 atoms showed no co-internalisation of the MOR and CB1 receptors with both receptors remaining localized on the plasma membrane.188 These data strongly imply that 72c can bridge the two receptors.
The heterobivalent ligands were also assessed in in vivo assays using the mouse tail-flick test in which they were evaluated for antinociception and tolerance following intracerebroventricular (i.c.v.) or intrathethal (i.t.) injection. With the exception of the heterobivalent ligand 71 (Figure 22) with the shortest spacer length of two atoms, the bivalent ligands of linker lengths ranging from 6 to 20 atoms (72a-c, Figure 22) exhibited no tolerance.188 This observation suggests that the decrease or loss of tolerance associated with CB1 antagonism is not likely to be mediated via a MOR/CB1 heteromer. Le Naour et al. attributed the low potency and tolerance observed for heterobivalent compound 71 to its shorter spacer which places the two pharmacophores in close proximity to one another, hence compromising CB1-mediated antagonism and MOR-mediated antinociception.188 Of significance was the finding that 72c (Figure 22) with a 20-atom spacer is 20-fold more potent than its 19-atom counterpart, 72b (Figure 22) (Table 5) when administered supraspinally.188 Comparatively, 72c shows only a fourfold higher potency than 72b (Table 5) via the i.t. route.188 These data were indicative of a higher supraspinal distribution of MOR-CB1 heterodimers.
The dopaminergic D2-like receptor subtypes (namely, D2R and D4R) and the MOR are expressed in areas of the brain involved in schizophrenia, Parkinson's disease, addiction, and pain.130 They are co-localized in many nuclei of the brain which suggests intermolecular receptor interactions, thus making them relevant targets for the treatment of addiction and chronic pain.228, 229 In vivo studies support the presence of cross-regulation between the D2-like receptors and the MOR. Activation of the dopaminergic receptors leads to an intermediate reduction in MOR immunoreactivity and D2-like receptor/MOR interactions influence morphine-triggered upregulation of certain transcription factors like c-Fos, δFosB, and P-CREB.230-232
Qian et al. investigated the pharmacological properties of heterobivalent ligands of the D2R/MOR and the D4R/MOR heterodimers in mitogen-activated protein kinase (MAPK) and β-arrestin recruitment assays.130 The bivalent ligands were designed by linking either a MOR agonist (hydromorphone) or an antagonist (naltrexone, 6) to D2-like receptor agonist, 5-hydroxy-2-(dipropylamino) tetralin (DPAT, 77, Figure 23) or antagonist, 1,4-disubstituted aromatic piperazines (DAPs, 78, Figure 23) via a variable length PEG spacer. The heterobivalent ligands were evaluated in radioligand binding competition assays involving HEK293T cells with either singly expressed D2R, D4R and MOR cells or co-expressed D2R/MOR and D4R/MOR cells. The bivalent ligands based on the D2-like receptor antagonist DAP (78) and the MOR agonist hydromorphone (73a-c, Figure 22) showed a general decrease in binding affinities when compared to DAP (78) when tested in HEK293T cells expressing solely D4R.130 However, the heterobivalent ligand 73a (Figure 22) with a shorter spacer length (18 atoms) showed a twofold higher affinity than heterobivalent ligands 73b and 73c (Figure 22) with longer spacers (Table 5).130 Interestingly, when evaluated in HEK293T cells co-expressing both D4R and MOR, a biphasic competition curve was obtained for 73a with two distinct affinity constants (Kihigh = 1.2 nM and Kilow = 207 nM), which implies a bivalent binding mode.130 The high-affinity Ki value results from a bivalent receptor-bridging binding mode while the low affinity Ki value represents monovalent binding to only the D4R.130 When similar experiments were performed on HEK293T cells co-expressing D2R/MOR, a monophasic binding competition curve was obtained for bivalent ligand 73a which could be indicative that the latter is unable to simultaneously bind D2R and MOR.130
In a MAPK phosphorylation assay using HEK293 cells stably expressing D4R, all the bivalent ligands were able to activate the MAPK signalling pathway.130 The heterobivalent ligand 73a showed high potency (EC50 = 0.12 µM) and relatively high efficacy (Emax = 92%) in comparison to the monovalent ligand DAP (78, Figure 20, EC50 = 0.21 µM and Emax = 75%).130 The heterobivalent ligands were also tested for their MOR activity in a β-arrestin 2 recruitment assay. All the bivalent ligands possessed similar EC50 values as their parent monomer hydromorphone with 73a displaying excellent potency (EC50 = 12.73 nM) and efficacy (Emax = 85%).130
3.11 Bitopic ligands of the opioid receptors
The discovery of bitopic ligands of the MOR happened much by serendipity. In the search for novel selective antagonists of the MOR, Li et al. introduced heterocyclic substituents at position 6’ of naltrexone (6, Figure 1) via an amide linker.233 This led to the discovery of NAQ (79, Figure 24) which showed sub-nanomolar binding affinity (Table 6) to the MOR with over 200-fold selectivity for the MOR over the DOR and 50-fold selectivity over the KOR.233 NAQ (79) behaved as a competitive antagonist producing a right shift in DAMGO concentration-35GTP[γS] stimulation curve in CHO cell lines.239 NAQ (79) also exhibited significantly fewer opioid withdrawal effects compared to commonly used opioid antagonists such as naloxone (7, Figure 1) or naltrexone (6, Figure 1), even at 100-fold higher doses.239
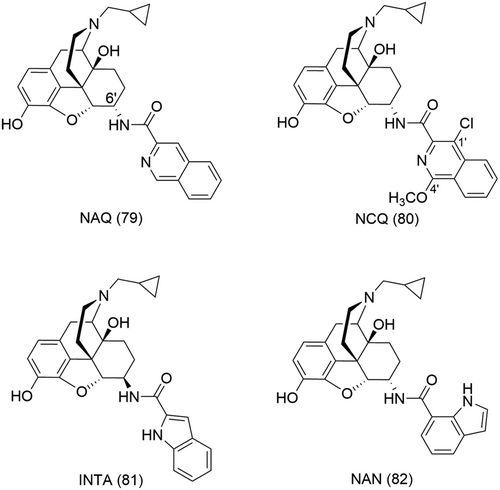
| Bitopic ligand | Activity | Binding affinity/potency | Pharmacological properties |
|---|---|---|---|
| NAQ (79)233 | MOR | Ki: 0.55 nM | MOR antagonist |
| NCQ (80)234 | MOR | Ki: 0.55 nM | MOR partial agonist |
| NAN (82)235 | MOR | Ke (mMOR-CHO cells): 2.08 nM | MOR antagonist |
| Ke (thalamus): 1.55 nM (with DAMGO) | |||
| INTA (81)236 | MOR, KOR and DOR | KiMOR: 0.29 nM KiKOR: 0.18 nM KiDOR: 1.54 nM |
Opioid agonist |
| 84a237 | MOR | KiMOR: 77 nM | |
| 84b237 | MOR | KiMOR: 4.6 nM Emax: 114% (cAMP assay) |
MOR agonist |
| EC50: 0.8 nM (cAMP assay) | |||
| Emax: 54% (β-arrestin 2 assay) | |||
| EC50: 4710 nM (β-arrestin 2 assay) | |||
| 84c237 | MOR | KiMOR: 4.1 nM ED50: 18.77 nmol (i.c.v.) |
MOR agonist |
| 84f237 | MOR | KiMOR:589 nM EC50: 1017 nM |
|
| DNCP-β-NalA-1 (85)238 | KOR and MOR | KiKOR: 3.9 nM (mKOR) | KOR agonist MOR antagonist |
cAMP assay at mKOR: EC50KOR:2.0 nM |
|||
GTγPS binding with CHO cells expressing hKOR EC50: 5.5 nM; Emax: 83% |
|||
β-Arrestin-2 EC50:22 nM Emax: 41% |
|||
β-Arrestin-1 EC50:28 nM Emax: 30% |
NAQ (79, Figure 24) was initially designed following the message-address concept in which the naltrexone core would confer the activity and the heterocyclic moiety at position 6’ selectivity for the MOR.233 The rationale behind the choice of aromatic substituents was to aim for π-π stacking interactions within a binding subdomain located extracellular at the MOR consisting of primarily aromatic residues, a subdomain absent at the DOR.233 The nitrogen atom on the isoquinoline ring was introduced to gain possible hydrogen bond interactions with Y210 (ECL2) or W3187.35 on the ELs of the MOR, which would then grant selectivity over the KOR.233 Subsequent structure–activity relationships on NAQ (79) led to the discovery of other selective ligands of the MOR like NCQ (80)234 or NAN (82) (Figure 24).236 Structurally, though initially designed as orthosteric ligands, NAQ (79), NCQ (80) and NAN (82) can also be perceived as bitopic ligands simultaneously targeting the OBS and a less-conserved allosteric binding subdomain. However, there is no structural evidence of this presumed bitopic binding mode till date.
Interestingly, NCQ (80, Figure 24) which is structurally similar to NAQ (79, Figure 24), albeit two additional substitutions at positions 1′ and 4′ of the isoquinoline ring, behaved as a partial MOR agonist in the [35S]GTPγS binding assay.234 NCQ (80) showed a binding affinity of 0.55 nM for the MOR with a 40-fold selectivity for the MOR over the KOR and a 62-fold selectivity over the DOR.234 Generally, SAR studies suggest that the presence of 1′- or 4′- substituents on the isoquinoline ring play a role on the binding affinities of the NAQ analogues.234
Wang et al. performed docking and MD studies to gain more insight into the differing pharmacology of NAQ (79) and NCQ (80) (Figure 24) at a molecular level.240 NAQ (79), being an antagonist, was docked into the crystal structure of the inactive MOR, MORinactive while NCQ (80) was docked into both the inactive and active MOR, MORactive, crytal structures. For both ligands, the epoxymorphinan core formed the stereotypical interactions observed in the OBS, hydrophobic interactions with M1513.36, W2936.48, and H2976.52, and the anchoring ionic interaction of the quarternary amine with D1473.32 (Figure 25).240 While the “address” portion of NAQ (79) and NCQ (80) occupied the same subdomain of the ABS in the MORinactive, in the MORactive, the 1′,4′-substituted isoquinoline ring of NCQ (80) bound within an alternative subdomain of the ABS (see arrows, Figure 25).240 The exact topography of the MOR ABS is yet to be confirmed; however, the two subdomains of the ABS which accommodated the “address” portions of NAQ (79) and NCQ (80) were identical to those of previously published allosteric sites of other class A GPCRs.240 In MORinactive, the binding subdomain was delineated by TMs 6, 7 and ECL3 (ABD1), the isoquinoline moiety of NAQ (79) and NCQ (80) finding interactions with V3006.55 and W3187.35, respectively (Figure 25A,B).240 In MORactive, the isoquinoline ring of NCQ (80) could form direct interactions with Q1242.60, W133ECL1, and I1443.29 within an allosteric binding subdomain formed by TMs 2,3, and ECL1 (ABD2) (Figure 25C).240
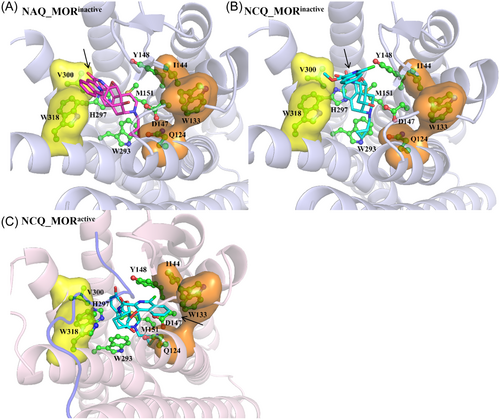
Similar interactions were observed in MD studies of the binding poses of NCQ (80) and NAQ (79) (Figure 24) in the MORinactive as in docking studies.240 However, following 100 ns of MD simulations, the “address” portion of NCQ (80) shifted from ABD1 to ABD2.240 Conversely, in MORactive, the isoquinoline moiety of NCQ (80) occupied ABD2 throughout the entire duration of the MD simulation.240 The “address” portion of NCQ (80) evidently preferred ABD2 in both the MORactive and MORinactive.240 When comparing the docking poses of NAQ (79) and NCQ (80) in MORinactive, the binding subdomain ABD1 was not spacious enough to accommodate the additional substituents at positions 1′ and 4′ of the isoquinoline ring of NCQ (80).240 Consequently, following 100 ns of MD simulations, the hydrophobic interactions between the “address” portion of NCQ (80) and residues of ABD1 decreased, at the expense of the gain in interactions within ABD2.240 The net effect of the electron withdrawing chloride substituent and the electron donating methoxyl substituent was an increase in the π-electron density of the isoquinoline ring, which further promoted π-π stacking interactions with W133ECL1 and CH-π interactions with I1443.29 within ABD2.240
Docking and MD studies indicate that the interactions of the “address” portion of NCQ (80, Figure 24) within ABD1 may bring the aromatic ring of the epoxymorphinan ring closer to W2936.48 resulting in a conformational change in the side chain of the latter.240 As previously described (see “2. Structure of the opioid receptors and insight into their activation mechanism” section), W2936.48 is a residue playing a pivotal role in receptor activation; it forms interactions with F2896.44, one of the residues of the FPI core triad which is a “microswitch” for MOR activation (Figure 3D). Additionally, average structures from 81 to 100 ns of MD simulations showed that due to the movement of the side chain of W293 in the MORinactive-NCQ bound system, TM5 moved inward while TM6 displayed an outward movement when compared to the docking poses.240 Thus, TM5 and TM6 in MD simulations of MORinactive-NCQ bound system adopted conformations similar to those of the MORactive-NCQ bound system. In summary, the “address” portion of NAQ (79, Figure 24) binding within ABD1 had no effect on the “message” portion fitting the conventional OBS, similar to a SAM. On the other hand, the “address” portion of NCQ (80, Figure 24), much like a PAM, modulates the function of the epoxymorphinan ring, when bound within ABD2, further stabilizing the active receptor conformation. The docking and MD studies by Wang et al.240 provide new insights to design bitopic ligands with positive allosteric modulation, for example, with the introduction of bulky electron-donating substituents so as to interfere with binding within ABD1 while favouring occupation of the allosteric subdomain ABD2.
NAN (82, Figure 24) is another putative bitopic ligand identified from SAR studies of NAQ (79, Figure 24).236 With an indole ring attached via an amide linker at position 6’ of naltrexone, NAN (82) behaved as a competitive MOR antagonist in [35S]-GTPγS functional assays.235 Co-incubation with 5 nM NAN (82) resulted in a parallel right shift in the DAMGO concentration-effect curve in both mMOR-CHO cells and thalamus (Ke= 2.08 nM and 1.55 nM, respectively).235 Moreover, NAN (82) inhibited DAMGO-stimulated Ca2+ flux in a Ca2+ flux assay in a concentration-dependent manner.235 Opioid withdrawal studies in morphine-pelleted mice revealed NAN to produce significantly less withdrawal symptoms than similar doses of naltrexone (6, Figure 1).235 NAN (82) is also unlikely to produce cellular MOR adaptations that may eventually lead to opioid tolerance.235
Obeng et al. also performed docking studies and MD simulations to identify the key epitopes contributing to NAN (82, Figure 24) antagonism despite it sharing structural similarities to the agonist INTA (81, Figure 24) which reportedly binds to KOR-DOR and KOR-MOR heterodimers.236 INTA (81) and NAN (82) both share an identical epoxymorphinan “message” portion with their “address” portion slightly differing in linker and substitution pattern. Similar to the results obtained from the docking studies of NAQ (79) and NCQ (80), it was observed that while the “message” moiety of both INTA (81) and NAN (82) maintained the stereotypical interactions observed with morphinan ligands, their “address” portion occupied different binding subdomains within the ABS.236 In both docking studies and MD simulations at MORactive, the indole moiety of INTA (81) found hydrophobic interactions with H54N-terminus and π-π stacking interactions with W3187.35.236 While NAN (82) did not form any interaction with H54N-terminus in the MORinactive, it did find similar interactions as INTA (81) with W3187.35, although less strong as estimated based on distance between the residue and the indole moiety.236 However, a 10 ns of MD simulations, the “address” portion of NAN (82) formed new hydrophobic interactions with L2325.38 and K2335.39, accommodating an alternative subdomain of the ABS.236 This switch in the binding poses of the “address” moiety of NAN (82) observed between docking and MD studies could be due to the α-configuration of the indole substituent which seems to favor the inactive conformation of the receptor.236 Hence, the indole moiety with a high π-electron density seems to stabilize an inactive receptor conformation, imitating the function of a NAM.236
More recently, Faouzi et al. aimed to target the sodium ion allosteric binding site (Na+-ABS) conserved in opioid receptors and class A GPCRs.237 The bitopic ligands were designed based on the fentanyl (14, Figure 1) scaffold where the benzene ring was replaced with alkyl chain linkers connected to either an amine or a guanidine moiety (Figure 26). The latter positively charged functional groups were introduced to gain interactions within the Na+ binding pocket, particularly with the carboxylate side chain of the residue D2.50 (Figure 7). Fentanyl (14) was first docked into an active state of the MOR so as to obtain an estimation of the distance between the OBS and the Na+-ABS. According to the models’ prediction, the amide nitrogen of fentanyl and the carboxylate group of D2.50 were separated by a distance of 13 Å.237 Hence, the aliphatic chain linker lengths were varied ranging from 3 to 11 methylene groups (n = 3, 5, 6, 7, 9, 11) (Figure 26). Faouzi and co-workers predicted that the linker length, n = 7, would be optimal for a direct interaction, via a salt bridge, between the amino functional group (83d) and D2.50 while a slightly shorter linker (n = 6) would allow for similar interactions for the guanidine moiety (84c).237
Surprisingly, the amino-fentanyl compounds (Figure 26, 83a-f) all showed decreased affinity for the MOR, indicating that the amine group did not engage in any interactions with the polar and negatively charged residues of the Na+- ABS.237 These include the residues N3.35, N7.45, S7.46 and S3.39 (Figure 7). Further extension of the aliphatic linker chains in an attempt to probe for possible interactions did not result in any notable effects on the binding affinities of these bitopic ligands.237
On the other hand, the guanidino counterparts showed moderate to high affinity for the MOR with Ki values ranging from 590 nM for 84f (Figure 26, N = 11) to 4.1 nM for 84c (Figure 26, N = 6, C6 guano).237 84c thus showed a slightly better affinity for the MOR than its parent compound fentanyl (Ki = 9 nM).237 The guanidine moiety seemed better than the corresponding amino moiety in terms of finding additional hydrogen bond interactions with the polar cavity usually harboring the Na+ ion. The Ki values of the bitopic ligands with the guanidine moiety were up to 1000-fold lower when compared to that of their amino counterparts.237 Linker lengths considerably affected the binding affinity of the bitopic ligands with 84b (Figure 26, N= 5, C5 guano) and 84c showing the highest affinities (Table 6).237 84a (Figure 2, N = 3) displayed a 16-fold lower affinity for the MOR (Table 6) possibly due to the shorter linker unable to reach into the Na+- ABS while 84f (Figure 26, N = 11) had the lowest affinity (Table 6) which could result from possible steric clashes with some residues in the Na+-ABS imposed by a longer linker.237
The bitopic ligands were also tested in functional assays, namely, the Tango assay for β-arrestin-2-recruitment and cAMP assays for Gi protein activation. As expected, the bitopic ligands with the amino group only weakly stimulated the Gi pathway, with EC50 values all above 293 nM.237 The bitopic ligand 84b (Figure 26, N = 5, C5 guano) showing the highest affinity for the MOR was a full agonist in cAMP assays with a subnanomlar potency (Table 6) comparable to that of the full MOR agonist DAMGO (EC50 = 0.9 nM).237 As observed in the determination of binding affinities, an increase in linker length resulted in a decrease in potency with 84 f (Figure 26, N = 11) showing the lowest potency (Table 6).237 Interestingly, 84b with the highest potency in cAMP assays, did not show a similar propensity to recruit β-arrestin-2 in the TANGO assays. In these assays, 84b had an EC50 of 4710 nM and an Emax of 54% which is significantly lower compared to the values obtained for the full agonists DAMGO (EC50 = 723 nM and Emax = 100%) and fentanyl (EC50 = 66 nM and Emax = 119%).237 Therefore, 84b displayed considerable functional bias for the Gi protein activation pathway over β-arrestin-2 recruitment.
Faouzi et al. were also able to obtain cryo-EM structures of the MOR-Gi complex bound to the bitopic ligands 84b (Figure 26, N = 5, C5 guano) and 84c (Figure 26, N = 6, C6 guano) at 3.2 and 3.3 Å, respectively. As predicted, the fentanyl scaffolds of 84b and 84c overlapped with that of the fentanyl model from docking studies in the OBS with the guanidine moiety reaching towards the Na+-ABS.237 In both structures, the stereotypical interaction between the anchoring residue D3.32 and the piperidine nitrogen atom was observed.237 The guanidine moiety of 84b was positioned within 4 Å of D2.50 in the Na+-ABS which indicated the formation of a possible interaction between the two, albeit weak considering the distance over 3 Å.237 However, this interaction could also be mediated by water molecules not resolved at a resolution of 3.2 Å. Faouzi et al. then employed MD to confirm that water molecules could play an important role in mediating the observed interaction between the basic guanidine moeity and acidic D2.50 residue.237 Conversely, the guanidine functional group of 84c was positioned slightly closer to D2.50, within 3 Å, thus allowing direct interaction via a salt bridge.237
The intrinsic efficacy of bitopic ligands 84a-f (Figure 26) was also investigated in BRET-based assays for arrestin recruitment and G-protein heterotrimer dissociation (TRUPATH) at all Gα subtypes (Gi1, Gi2, Gi3, GoA, GoB and Gz) through which the MOR signals. The physiological roles mediated by each of these Gα subtypes are yet to be completely understood. Some studies have tried to investigate the role of the Gα subtypes in mutant mice models. Mice lacking or deficient in Gαi2 have demonstrated inflammatory bowel disease241 with pronounced increases in pro-inflammatory TH1-type cytokines242 and increased basal production of IL-12 in some subsets of APC.243 The immune abnormalities in mouse deficient Gαi2 precede colitis.244 An increased incidence of colonic carcinomas arising from chronic inflammation was also observed in these mutant mice.245 While mice lacking Gz were viable and did not show any obvious neurological effects, Gz deficient mice displayed CNS defects.246, 247
If the on-target side effects of opioids are also associated with G-protein signalling, individual probing of each of these subtypes might provide new directions for the discovery of opioids deprived of side effects. Moreover, it was recently proposed by Gillis et al. that rather than G-protein over β-arrestin bias, low intrinsic G-protein efficacy results in reduced side effects.15 Therefore, more understanding into the G-protein signalling mechanism of opioids is highly valuable. Faouzi et al. observed differing efficacy and order of potency for diverse opioids, including fentanyl, DAMGO and morphine. Interestingly, despite their structural similarities, 84b and 84c (Figure 26) showed unique signalling profiles across the Gi, Go and Gz subtypes. 84b had a wide range of potencies (EC50 values ranging from 350 nM for Gi3 to 8.8 nM for Gz) but displayed a narrow efficacy range (Emax from 80% for Gi1 to 92% for GoA).237 In comparison, 84c exhibited a narrow potency range (EC50 from 133 nM for Gi1 to 25 nM for Gz) but wide range of efficacies (Emax from 50% for Gz to 79% for Gi2).237 Of note, 84c exhibited marginal decreases in G-protein potency and lower intrinsic efficacy compared to typical MOR agonists such as DAMGO, fentanyl, morphine and oxycodone, and showed the lowest efficacy for the Gz subtype.
To investigate how its unique signalling profile compared to other opioid agonists, especially its lower efficacy at the Gz subtype translates in vivo, 84c (Figure 26) was assessed with a standard warm water tail-withdrawal assay in mice. 84c displayed supraspinal MOR-mediated analgesia in various rodent pain models with reduced abuse potential.237 Following i.c.v administration, 84c had an average antinociceptive dose (ED50) of 18.77 nmol which was slightly higher than that of morphine (ED50 = 6.6 nmol).237 It was suggested that such a unique in vivo profile for 84c might be due to its lower Gz efficacy and/or at all Gα subtypes.237 The bitopic ligands synthesized by Faouzi et al. are the first aiming to target the Na+- ABS concomitantly with the classical MOR OBS. They successfully synthesized valuable pharmacological tool compounds to study the distinct signalling pathways of the MOR, the β-arrestin pathway or those mediated by the individual Gi, Go and Gz subtypes.
The KOR is an alternative therapeutic target in the development of nonaddictive pain therapeutics but it also mediates psychotomimesis, sedation and dysphoria which have limited the clinical application of KOR agonists as analgesic drugs.1-3 Muratspahic et al. used the available crystal structure of KOR bound to the dual KOR/DOR opioid agonist MP1104 and computational methods to design peptide–small molecule conjugates of the KOR.238 They aimed to exploit the advantages provided by both moieties; the small molecule would bury deeply into the OBS while the peptide would make extensive interactions, aiming for specificity. These peptide–small molecule conjugates therefore follow the design concept of bitopic ligands with pharmacophores simultaneously occupying the OBS and alternative secondary binding sites. The small molecule would provide affinity for the KOR while the peptide, modulate selectivity and efficacy.
Muratspahic et al. designed and synthesized four different cyclic peptide–small molecule conjugates (DNCP-β-NalA) by coupling 5-amino acid residues de novo cyclic peptides to β-NalA.238 All four DNCP-β-NalA (1-4) conjugates showed nanomolar affinities for the mouse KOR, with DNCP-β-NalA-1 (85, Figure 27) showing the highest binding affinity, Ki = 3.9 nM, an 18-fold increase compared to β-NalA.238 In cAMP assays, the conjugates behaved as full agonists (Emax ranging from 89% to 101%), a considerable improvement in efficacy in comparison to β-NalA which behaves only as a partial agonist (EC50 of 130 nM and Emax of 61%).238 DNCP-β-NalA-1 (85) and DNCP-β-NalA-4 were the most potent conjugates with EC50 of 2.0 and 1.0 nM, respectively.238 Similarly, in the [35S]GTPγS binding assays using CHO cells stably expressing the human KOR, DNCP-β-NalA-1 (85) was a full agonist (Table 6).238 In a BRET assay, DNCP-β-NalA-1 (85) only partially recruited β-arrestin-2 and β-arrestin-1 at KOR (Table 6).238 In TRUPATH assays to evaluate Gαi/o coupling, DNCP-β-NalA-1 (85) showed partial agonism at Gαi2, Gαi3, GαoA, GαoB and Gαgastducin subtypes with Emax values ranging from 48% to 77%, whereas it elicited full agonism at Gαi1 and Gαz with Emax values of 81% and 101%, respectively.238 These results indicate that DNCP-β-NalA-1 (85) shows preferential efficacy bias for G proteins over β-arrestins.
DNCP-β-NalA-1 (85, Figure 27) also exhibits an approximately 80-fold and 330-fold selectivity for KOR over DOR, and for KOR over NOR, respectively but was equipotent at the MOR.238 However, the conjugate is an antagonist at the MOR but an agonist at the KOR.238 This mix activity of biased agonism at the KOR and antagonism at the MOR might result in antinociception with a favorable side effect profile of DNCP-β-NalA-1 (85) observed in male mice.238 DNCP-β-NalA-1 (85) was shown to produce significant and potent antinociception in the formalin test and CFA-induced inflammatory hyperalgesia as well as anti-inflammatory properties, without KOR-mediated liability of motor dysfunction/sedation following subcutaneous (s.c.) administration in male mice.238
The cryo-EM structure of human KOR bound to DNCP-β-NalA-1 (85, Figure 27) and Gαi1/Gβ1/Gγ2 heterotrimer revealed that residues in ECL2/3 and TM6/7 controlled the functional selectivity of the human KOR.238 The mutations E209ECL2A, E2976.58A and L3097.32A resulted in an improvement in β-arrestin efficacy bias and Gi potency of DNCP-β-NalA-1 (85).238 Additionally, the small molecule part of the conjugate, βNalA, adopted a similar conformation as KOR agonists U50488 and MP1104 in the OBS while the cyclic peptide formed extensive interactions with ECL2/3 and TM6/7 residues.238 This supports the hypothesis that ECL2 can modulate bias at GPCRs.49-51
4 OUTLOOK
Portoghese's pioneering work along with the endeavors of many others in the realm of bivalent ligands are evidence that “the whole is better than the sum of its parts.”102 Bivalent ligands still represent valuable tool compounds to study receptor dimerization and its effects on receptor signalling. Heterobivalent ligands, in particular, of opioid and non-opioid receptors, offer alternative approaches to address certain therapeutic conditions with the aim of producing the desired physiological effect without associated on-target side effects. A multiple target approach is sometimes desirable in some disease conditions, and this can be elegantly achieved with the rational design of bivalent ligands. Portoghese's legacy has also translated into the dualsteric targeting of GPCRs through the design and synthesis of bitopic ligands. The work relating to bitopic ligands of the opioid receptors is still at its infancy stage; however, the SAR studies of NAQ (79) leading to the discovery of NCQ (80) and NAN (82) is reflective of the untapped potential of bitopic ligands of the MOR. Moreover, the discovery of the bitopic ligands C5 guano (84b), C6 guano (84c) and DNCP-β-NalA-1 (85) reflects the significant role of structural information in the design of these ligands. The increasing availability of X-ray crystal structures and advances in cryo-EM might provide more insight on the allosteric binding sites of the opioid receptors, therefore encouraging the design of bitopic ligands for this class A GPCRs. Due to their structural complexity, the pharmacological effects and functional selectivity of bitopic ligands might be challenging to study with the currently available pharmacological assays. This highlights the need for functional assays in which such ligands can be effectively tested for signalling bias. When X-ray crystallography and docking or MD studies support the rational design of bitopic ligands, which can be effectively evaluated in multiple parallel functional assays following their successful synthesis, the prospects of these ligands are vast.
ACKNOWLEDGMENTS
M.E.H gratefully acknowledges the Elite Network of Bavaria (ENB) for awarding a PhD position within the international doctoral program “Receptor dynamics Paradigms for Novel Drugs” and funding by the Bavarian State Ministery of Science and Art (K-BM-2013-247). Open Access funding enabled and organized by Projekt DEAL.
Biographies
Marie Emilie Hovah studied Pharmaceutical Chemistry at the International Medical University (Malaysia) and received her BSc (Hons) in 2015. She graduated from the University of Copenhagen with an MSc in Medicinal Chemistry in 2018. In 2019, she joined the Elite Network of Bavaria (ENB) “Receptor Dynamics” graduate program as a fellow PhD student under the supervision of Prof. Dr. Holzgrabe at the University of Wuerzburg. Her research focuses on the design and synthesis of bitopic ligands for the mu opioid Receptor (MOR).
Ulrike Holzgrabe studied Chemistry and Pharmacy at the University of Marburg and Kiel, respectively, receiving her doctorate under the supervision of Prof. Dr. R. Haller. In 1989, she finished her habilitation and, in 1990, she accepted an associate professorship at the University of Bonn where she served as a vice-rector from 1997 to 1999. Since 1999 she holds a full Professorship at the University of Würzburg where she served as Vice President, too. She was President of the German Pharmaceutical Society from 2003 to 2007. Her main research interests are drug discovery in the fields of muscarinic ligands and anti-infectives in addition to quality analysis of active pharmaceutical ingredients.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- * PAG homogenates are an important locus for opioid-mediated descending control of pain and express high levels of MOR.




