Dedicator of cytokinesis 8 (DOCK8) mutation impairs the differentiation of helper T cells by regulating the glycolytic pathway of CD4+ T cells
Abstract
Dedicator of cytokinesis 8 (DOCK8) deficiency is a primary immunodeficiency disease caused by mutations in exon 45 of the DOCK8 gene. The clinical signs primarily consist of increased serum IgE levels, eczema, repeated skin infections, allergies, and upper respiratory tract infections. Using CRISPR/Cas9 technology, we generated a DOCK8 exon 45 mutation in mice, mirroring the mutation found in patients. The results indicated that DOCK8 mutation impairs peripheral T cell homeostasis, disrupts regulatory T cells (Tregs) development, increases ICOS expression in Tregs within peripheral lymph nodes (pLn), and promotes Th17 cell differentiation within the spleen and pLn. Upon virus infection, DOCK8 mutation CD4+ T cells have a Th2 effector fate. RNA-bulk sequencing data revealed alternations in the mTOR pathway of DOCK8 mutant CD4+ T cells. We observed that DOCK8 mutation upregulates the glycolysis levels in CD4+ T cells, which is related to the Akt/mTOR/S6/HIF-1α pathway. In summary, our research elucidates that DOCK8 regulates the differentiation of helper T cells by modulating the glycolytic pathway in CD4+ T cells, thereby advancing the comprehension and offering potential treatment of diseases in DOCK8-deficient patients.
1 INTRODUCTION
Dedicator of cytokinesis 8 (DOCK8) is a protein belonging to the DOCK family, encoded by the DOCK8 gene. It functions as a guanine nucleotide exchange factor (GEF), regulating the activity of the GTP enzymes of the Rho family.1 DOCK8 is crucial in the regulation of the actin cytoskeleton.2 It is predominantly expressed in immune cells and hematopoietic stem cells. DOCK8 is also expressed in nonimmune tissues including the kidney, lung, and pancreas.3 DOCK8 deficiency broadly affects the development, migration, growth, and adhesion of immune cells,4-6 thus impacting innate and adaptive immune responses.7-11 On the human level, DOCK8 mutations give rise to autosomal recessive (AR) Hyper-IgE Syndrome (AR-HIES) accompanied by combined immunodeficiency (CID), which was first reported in 2004.12 This finding identified DOCK8 immunodeficiency syndrome (DIDS) as a clinical entity.13 Mutations in DOCK8 lead to severe allergic reactions, refractory viral skin infections in AR-HIES, and leukemia.14-16 Furthermore, there is no specific treatment for DIDS, and the main treatments are early diagnosis and immunological assessments.17
Numerous studies have shown that DOCK8 deficiency affects the survival, differentiation, and function of various T cell subsets. For instance, DOCK8 deficient mice and patients have a strong Th2 bias.18, 19 Th2 cells, which produce IL-4, IL-5, and IL-13, are essential in combating extracellular parasites and facilitating allergic responses.20 DOCK8 deficiency also seriously affects Th17 and Th1 cell differentiation.21 Th1 cells, characterized by their production of IFN-γ, are key to defending intracellular pathogens. Th17 cells, known for secreting IL-17 and IL-22, are critical in protecting the body from extracellular bacteria and fungi while contributing to autoimmune diseases. DOCK8 mutant mice have reduced numbers and shorter lifespans in naive CD8 T cells. After antigen stimulation, synaptic polarization was poorly displayed and cell division was delayed.8
Previous research has shown that helper T cell development and differentiation are influenced by metabolism,22 which acts as the regulator of T cell function and survival. Different T cell subsets need different energy and biosynthetic pathways to maintain unique functional requirements. For example, effector T cells are prone to using aerobic glycolysis, while naive T cells and Tregs rely on oxidative phosphorylation to supply energy needs.23 Upon antigen stimulation, naive T cells rapidly initiate metabolic pathways, upregulated glycolysis, and mitochondrial metabolism to enter the synthetic metabolic phase.24 Th17 cells exhibit high glycolytic activity and depend on pathways activated through mTOR and HIF-1α. Additionally, Th17 cell development is hindered by HIF-1α depletion or glycolysis inhibition.25 Therefore, understanding the metabolism of T cells might reveal insight into T cell subset differentiation in DOCK8 mutant mice.
To develop DOCK8 mutant mice that mimic the pathological state of DOCK8 patients, CRISPR/cas9 technology was used to construct a DOCK8 point mutation in exon 45. This DOCK8 mutation destabilized the homeostasis of peripheral T cells, impeded the growth and ICOS expression of Treg cells within pLn, and promoted Th17 cell differentiation. RNA-bulk sequencing data indicated that cell metabolism was crucial in the impact on CD4+ T cells with DOCK8 mutation. Western blotting and seahorse experiments found that DOCK8 mutation upregulated the level of glycolysis in CD4+ T cells, which was associated with the Akt/mTOR/S6/HIF-1α pathway. In summary, our study elucidates that DOCK8 modulates helper T cell differentiation by altering glycolytic pathways in CD4⁺ T cells, providing insights into the molecular mechanisms underlying DOCK8 deficiency.
2 RESULTS
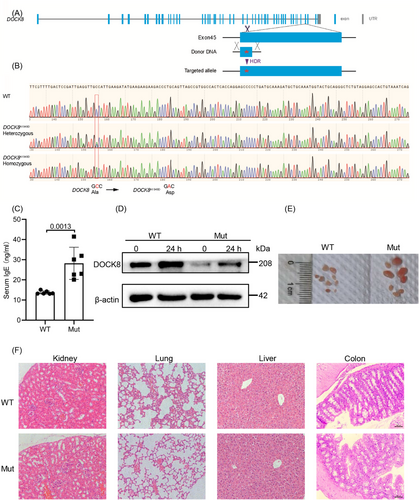
2.1 DOCK8 mutation increases serum IgE levels and decreases DOCK8 protein in T cells
To substitute the alanine residue (encoded by GCC) with an aspartic acid residue (encoded by GAC) at position 1949 of the DOCK8 gene (Transcript: ENSMUST00000025831.8 DOCK8-201), we developed a repair oligonucleotide in conjunction with two single guide RNAs (sgRNAs) specifically targeting exon 45 of the DOCK8 gene. A silent mutation was incorporated at amino acid position 1947 to prevent re-cleavage by Cas9 following homologous recombination. To confirm that the sequence of DOCK8 was changed in exon 45, PCR and sanger sequencing were employed. Sequence alignments were conducted using the basic local alignment search tool (BLAST) search on the NCBI website (Figure 1A,B). To verify that our constructed DOCK8 mutation mouse model could mimic AR-HIES, we examined serum IgE levels by enzyme linked immunosorbent assay (ELISA), which revealed that DOCK8 mutant mice had increased levels of serum IgE (Figure 1C), suggesting that the patient's high IgE clinical phenotype was reproduced in this mouse model. AR loss-of-function mutations in the DOCK8 cause DIDS and the mutations commonly lead to a lack of DOCK8 protein expression.13 We found that the DOCK8 mutant mice had a significant decrease in DOCK8 protein expression in CD4+ and CD8+ T cells (Figure 1D and S1A,B). Therefore, the DOCK8 mutation mouse model was successfully constructed. Finally, although no significant lymphocytic infiltration was observed in DOCK8 mutant mice, we did find that they had larger pLn (Figure 1E,F), which is consistent with observations in DOCK8 Treg-specific deletion mice.26 In summary, the DOCK8 mutation increases serum IgE levels and decreases DOCK8 protein in T cells.
2.2 DOCK8 mutation impairs peripheral T cell homeostasis without affecting thymus T cell development
Reports indicate that DOCK8-deficient patients have reduced numbers of peripheral blood CD4+ and CD8+ T cells.14 To further validate the T cell immunophenotype of DOCK8 mutant mice, we analyzed T cell expression in the thymus, spleen, pLn, and mesenteric lymph nodes (mLn) of DOCK8 mutant mice. The results indicated that DOCK8 mutant mice had significantly lower proportions of CD4+ and CD8+ T cells in the spleen, pLn, and mLn, along with a depletion of splenic CD4+ and CD8+ T cells, but the pLn and mLn CD4+ and CD8+ T cell numbers showed no significant difference (Figure 2A–E and Figure S2A). In contrast, no difference was found in the proportion and number of thymus in DOCK8 mutant mice (Figure 2A and Figure S2B,C), suggesting that DOCK8 mutation did not affect the development of thymic T cell.
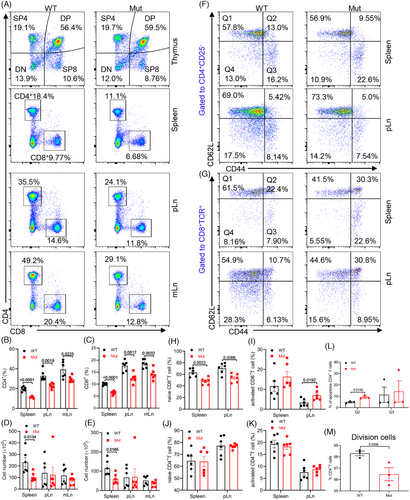
Subsequently, we examined T cell activation in the DOCK8 mutant mice. The findings revealed that, in DOCK8 mutant mice, the proportion of naive CD44lowCD62Lhigh CD8+ T cells in the spleen and pLn was distinctly lower, whereas notably higher in the thymus (Figure 2F–H and Figure S2D–I). However, activated CD44highCD62Llow CD8+ T cells in the pLn were significantly increased, whereas no significant difference was seen in the spleen, thymus, and mLn (Figure 2I and S2D–I). Moreover, no significant differences were detected in the proportion of naive and activated CD4+ T cells in the spleen and pLn (Figure 2J,K). Additionally, we noted an increase in late apoptotic CD4+ T cells in the spleen of DOCK8 mutant mice (Figure 2L and Figure S2J). Finally, we examined the proliferation of CD4+ T cells. The results demonstrated reduced proliferation of CD4+ T cells in the spleen of DOCK8 mutant mice (Figure 2M and Figure S2K). These results demonstrate that more peripheral T lymphocytes in DOCK8 mutant mice are in an activated state and affect peripheral T cell homeostasis.
To test whether the reduced ratio of CD4+ and CD8+ T cells in DOCK8 mutant mice was not influenced by the microenvironment of the mice and was intrinsic to the T cells, we constructed a mouse bone marrow chimeric model. To do this, the donor (CD45.2) bone marrow cells were mixed with the bone marrow cells of the CD45.1 recipient mice in a ratio of 1:1. The mixed cells were injected via the tail vein into the irradiated CD45.1 recipient mice (Figure S3A). Furthermore, the proportion of CD4+ and CD8+ T cells in the spleen and pLn of CD45.2+ DOCK8 mutation donor mice was markedly reduced compared to CD45.2+ wild type (WT) donor mice (Figure S3B–H), which indicates that the DOCK8 mutation affects the proportion of peripheral T cells intrinsically within T cells, rather than due to abnormal thymic T cell development.
2.3 DOCK8 mutation disturbs the development and ICOS expression of Tregs in the pLn
Tregs are an essential subpopulation of T cells that mediate immune tolerance and regulate immune system homeostasis by maintaining peripheral T cell homeostasis, which inhibits the activation of auto-reactive T cells. Studies have reported that Tregs are reduced and exhibited impaired suppressive activity in DOCK8-deficient patients.27 Therefore, we investigated the effects of DOCK8 mutation on Tregs subpopulations in the DOCK8 mutant mice. The findings revealed that DOCK8 mutant mice showed a significantly lower proportion of CD4+CD25+ and CD4+Foxp3+ Tregs in the pLn and a reduced proportion of CD4+Foxp3+ Tregs in the spleen. However, the number of CD4+CD25+ and CD4+Foxp3+ Tregs was not significantly changed, which might be due to the enlargement of the pLn (Figure 3A–F). These studies indicate a possible link between elevated activated T cells and a reduction in Tregs in DOCK8 mutant.
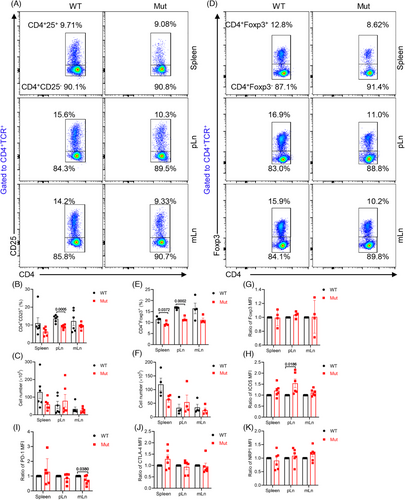
To further investigate the mechanism of Tregs reduction, we first examined Foxp3, a key transcription factor specifically expressed by Tregs, which determines the development and function of Tregs as well as maintains homeostasis and suppresses the immune response effectively.28 The results showed that in the CD4+Foxp3+ Tregs of DOCK8 mutant mice, there was no distinct difference in the mean fluorescence intensity (MFI) of Foxp3 (Figure 3G), suggesting that the reduction in Tregs was not due to a functional defect in Foxp3. Next, we detected the inhibitory molecules CTLA-4, ICOS, PD-1, and NRP1 that are expressed on the surface of Tregs and enhance their inhibitory function. The results showed that DOCK8 mutant mice exhibited increased MFI of ICOS in pLn CD4+CD25+ Tregs, decreased MFI of PD-1 in mLn CD4+CD25+ Tregs, and no significant difference in CTLA-4 and NRP1 in CD4+CD25+ Tregs (Figure 3H–K). It has been shown that Tregs high expression of ICOS facilitates the maintenance of immune homeostasis.29 These results suggest that DOCK8 mutations disrupt Tregs development and ICOS expression in the pLn, thereby affecting immune system homeostasis.
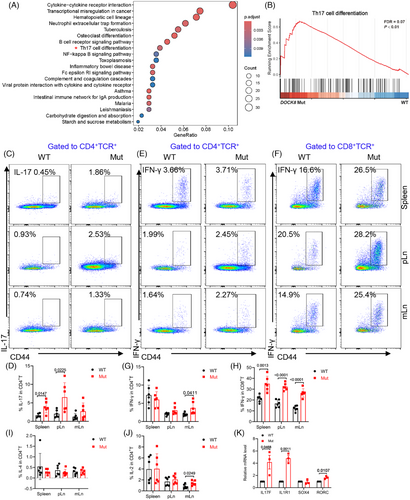
2.4 DOCK8 mutation promotes Th17 cell differentiation
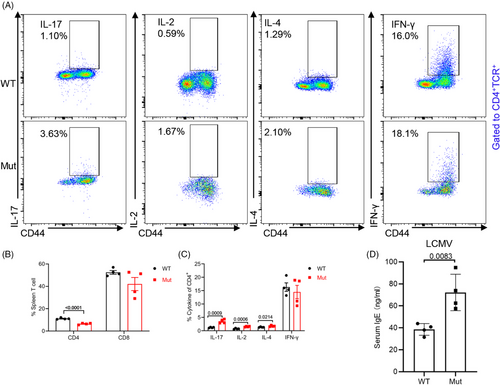
To investigate the transcriptome changes in DOCK8 mutation, we isolated CD4+ T cells for RNA-bulk sequencing. We found that Th17 cell differentiation was significantly enriched in DOCK8 mutant mice (Figure 4A). Also, gene enrichment analysis (GSEA) showed that genes on Th17 cell differentiation were significantly enhanced in CD4+ T cells from DOCK8 mutant mice (Figure 4B), suggesting that DOCK8 mutation might promote Th17 cell differentiation. To validate the hypothesis, we detected the levels of IFN-γ, IL-4, and IL-17, which demonstrated that the proportion of IL-17 by CD4+ T cells was increased in the spleen and pLn of DOCK8 mutant mice (Figure 4C,D). The results were consistent with those of RNA-bulk sequencing data. Elevated IFN-γ by CD4+ T cells in mLn and by CD8+ T cells was also observed in DOCK8 mutant mice (Figure 4E–H), whereas no significant difference was detected in IL-4 and IL-2 by CD4+ T cells in the spleen and pLn (Figure 4I,J). Furthermore, we also analyzed the RNA-bulk sequencing data for IL-17 producing related genes such as RORC, SOX4, IL1R1, and IL-17F. We detected a significant elevated mRNA level of RORC, IL1R1, and IL-17F in DOCK8 mutant mice by RT-PCR (Figure 4K). These results suggest that DOCK8 mutation promotes Th1 and Th17 cell differentiation.
2.5 DOCK8 mutation CD4+ T cells have a Th2 effector fate after lymphocytic choriomeningitis virus (LCMV) infection
Viral infections are frequently observed in DOCK8-deficient patients.14 To find out the role of DOCK8 mutation in viral pathogenesis, the level of cytokines in CD4+ T was examined in the spleens of WT or DOCK8 mutant mice following LCMV infection. The findings revealed that DOCK8 mutant mice had markedly lower proportions of CD4+ T cells after LCMV infection (Figure 5A,B). Unsurprisingly, IL-2, IL-4, and IL-17 secreted by DOCK8 mutation CD4+ T cells were increased after LCMV infection but IFN-γ levels remained unchanged (Figure 5C). Meanwhile, ELISA experiments demonstrated that the expression of IgE in serum also increased after LCMV infection (Figure 5D). Together, our findings indicate that DOCK8 mutation CD4+ T cells are biased toward Th2 effector fate after LCMV infection.
2.6 DOCK8 mutation upregulates the glycolysis level of CD4+ T cells associated with Akt/mTOR/S6/HIF-1α pathway
Th1 and Th17 cell differentiation is regulated by mTOR signaling.30 Transcriptomic data showed that GSEA-enriched genes to the mTOR signaling pathway were enriched in CD4+ T cells from DOCK8 mutant mice (Figure 6A). Furthermore, we detected the expression of mTOR pathway related molecules. The findings showed that protein levels of pAkt, pmTOR, and pS6 were also markedly elevated in CD4+ T cells of DOCK8 mutant mice after TCR activation (Figure 6B), indicating that the DOCK8 mutation upregulated mTOR signaling. Transcriptomic data suggest that starch and sucrose metabolism may be crucial in DOCK8 mutant mice (Figure 4A). mTOR can upregulate HIF-1α to promote glycolysis and induce Th17 cell differentiation.31 Furthermore, the protein expression of HIF-1α and pyruvate kinase M2 (PKM2, an important enzyme in the glycolysis process) was significantly elevated in DOCK8 mutation CD4+ T cells (Figure 6B). The above results indicate that DOCK8 mutation affects CD4+ T cell metabolism.
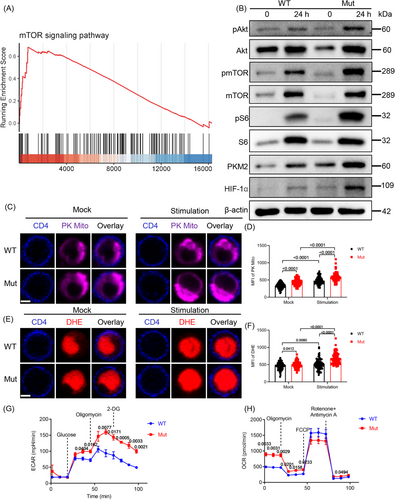
To examine the role of DOCK8 mutation in CD4+ T cell metabolism, the mitochondria morphology and reactive oxygen species (ROS) were observed by staining the PK Mito and dihydroethidium (DHE), respectively. DOCK8 mutation CD4+ T cells showed remarkably increased MFI of PK Mito and DHE in both anti-CD3 plus anti-CD28 stimulated and unstimulated (Figure 6C–F). Additionally, extracellular acidification rate (ECAR) revealed that the basal glycolytic capacity of DOCK8 mutation mice in CD4+ T cells was elevated by the addition of glucose, the maximal glycolytic capacity of the cells was elevated by the addition of oligomycin, and the glycolytic reserve and non-glycolytic acidification were elevated by the addition of 2-Deoxy-D-glucose (2-DG) (Figure 6G). This suggests that DOCK8 inhibits the CD4+ T cell glycolytic pathway. The results of oxygen consumption rate (OCR) showed that basal respiration of DOCK8 mutation CD4+ T cells was significantly elevated, the oxygen consumption rate of oxidative phosphorylation was elevated by the addition of oligomycin, and maximum respiration was not significantly different by the addition of FCCP, but spare respiratory capacity and non-mitochondrial oxygen consumption were elevated after the addition of rotenone and antimycin A, indicating that DOCK8 inhibits the oxidative phosphorylation of CD4+ T cells (Figure 6H). In conclusion, these results imply that DOCK8 mutation upregulates the glycolysis level of CD4+ T cells associated with the Akt/mTOR/S6/HIF-1α pathway.
3 DISCUSSION
In this study, we constructed a mouse model with the same exon 45 mutation as DOCK8 patients using CRISPR/Cas9 technology to investigate the impact of DOCK8 mutation on T cell differentiation. The proportions of CD4+ and CD8+ T cells in the spleen, pLn, and mLn of DOCK8 mutant mice were markedly decreased, while T cells in the thymus were not affected. DOCK8-deficient patients and mice had significantly reduced frequencies of T cells.32 Also, the spleen and pLn of DOCK8 mutant mice exhibited decreased naive (CD44lowCD62Lhigh) and increased activated (CD44highCD62Llow) T cell proportions, indicating that DOCK8 mutation disrupts murine peripheral T cells homeostasis.
We discovered that the DOCK8 mutation mice led to a decreased scale of both central and peripheral Treg cells, which was related to the increased expression of the inhibitory molecule ICOS. Studies report that DOCK8 maintains the inhibition function of Treg cells by regulating the IL-2 signal and can also enhance immune tolerance by forming immune synapses.26 Treg cells in DOCK8-deficient patients show impaired suppressive activity.27 This inconsistency may be due to the maintenance of IL-2 signaling. Therefore, whether DOCK8 regulates the function of Treg cells requires further investigation.
DOCK8-deficient patients demonstrate hyper IgE and a lower proportion of CD4+ T cells, which secretes type-2 cytokines. Similarly, DOCK8-deficient mice have a type 2 differentiation bias in CD4+ T cells and elevated Th17 cells in the periphery.18, 19, 32, 33 Our study pointed out that CD4+ T cells in DOCK8 mutant mice expressed more IFN-γ and IL-17, which are produced by Th1 and Th17, respectively, and participate in the antiviral immune response.34, 35 They also serve as significant pro-inflammatory cytokines that facilitate the release of inflammatory mediators and are pivotal in various inflammatory and autoimmune conditions.36 After LCMV infection, DOCK8 mutant mice had remarkably lower proportions of CD4+ T cells in the spleen, higher proportions of IL-17, IL-2 and IL-4 produced by DOCK8 mutation CD4+ T cells and elevated IgE levels in serum, indicating a shift of DOCK8 mutation CD4+ T cells toward Th2 effector fate post-LCMV infection.
Th17 and Treg cells generally maintain a dynamic balance of the immune system. Our study shows that the increase in Th17 cells and the decrease in Tregs further illustrate the impact of DOCK8 mutation on immune homeostasis in mice. Also, Th17 cell differentiation was found to be very prominent by KEGG pathway enrichment analysis, further indicating that DOCK8 mutation affects Th17 cell differentiation. The imbalance of the Treg/Th17 ratio is also a major pathogenic factor in infections and autoimmune diseases. The significant differential gene enrichment in CD4+ T cells in pathways such as asthma and inflammatory bowel disease is consistent with some clinical symptoms of DOCK8-deficient patients, suggesting that the DOCK8 mutation is related to these diseases. Therefore, DOCK8 mutation may increase susceptibility to autoimmune disease in mice through upregulation of Th17 differentiation pathway.
Immunometabolism changes in cells have been shown to regulate the differentiation of CD4+ T cells into specific functional helper T cell subsets.22 Th1 and Th17 cell differentiation is mainly regulated by the mTOR pathway and the activation level of its downstream signaling molecule S6 is increased in the CD4+ T cells of DOCK8 mutant mice. 29 In our study, the mTOR signaling pathway in CD4+ T cells of DOCK8 mutant mice was significantly increased. Since mTOR can upregulate glycolytic metabolism pathway via HIF-1α and PKM2,30 and Th17 cells have high glycolytic activity, we examined the metabolic pathway of DOCK8 mutation T cells. We detected elevated levels of HIF-1α and PKM2 in CD4+ T cells of DOCK8 mutant mice. Also, the increase in ECAR further indicates that the glycolysis level of CD4+ T cells in DOCK8 mutant mice is increased. Because the overexpression of HIF-1α can inhibit mitochondrial respiration, we also tested this by detecting the OCR of cells. The oxidative phosphorylation level of CD4+ T cells in DOCK8 mutant mice was increased. Therefore, DOCK8 regulates the mTOR/S6/HIF-1α signaling to differentiate CD4+ T cells into subsets.
The current study had some limitations. Studies have indicated the critical role of DOCK8 in the survival and function of mouse and human CD8+ T cells.8 In our study, the DOCK8 protein expression in CD8+ T cells of DOCK8 mutant mice was significantly reduced. However, the effect of DOCK8 mutation on CD8+ T cell metabolism has not been reported yet. In a future study, we will explore the role of DOCK8 mutation on glucose metabolism in CD8+ T cells. Additionally, no metabolic abnormalities have been reported in DIDS, while metabolic inhibitors have been reported widely.37, 38 Currently, small molecule inhibitors against metabolic targets are widely studied in anti-tumor, cancer and anti-viral.39-42 The next aim of our in-depth research project is to perform more metabolism-related experiments on clinical samples to find metabolic inhibitors to treat DIDS.
In summary, the DOCK8 mutation mouse model is suitable for studying DOCK8 deficiency, as it can replicate some of the patient's clinical manifestations and thus simulate the environment of the disease for potential molecular mechanism research. We also found that DOCK8 can affect the proliferation ability of CD4+ T cells and regulate their glucose metabolism. Taken together, these data show that DOCK8 can regulate the levels of glycolysis and oxidative phosphorylation by stabilizing the mTOR/HIF-1α signaling pathway, thereby inhibiting the abnormal differentiation of Th17 cell and autoimmune disease development.
4 METHODS AND MATERIALS
4.1 Mice
All mice were fed under specific pathogen-free (SPF) conditions with a 12 h light/dark cycle. All experiments were approved by the Animal Experiment Ethics Committee of Tongji Medical College (approval number, 3954).
4.2 Generation of DOCK8 mutation
To convert the GCC codon in exon 45 of the DOCK8 gene (Transcript: ENSMUST00000025831.8 DOCK8-201) from coding for an alanine residue to coding for aspartic acid at position 1949 of DOCK8, we designed a repair oligonucleotide along with two sgRNAs that target exon 45 of the DOCK8 gene. The repair oligonucleotide sequence, with differences from the wild-type C57BL/6 sequence indicated in lowercase and the protospacer sequences of the sgRNAs underlined, is as follows: TGGCCTCACTCTGCGGTTGTCTTCTGTTCAGTTCGTTTTGACTCCGATTGAaGTTGaCATTGAAGATATGAAGAAGAAGACCCTGCAGTTAGCCGTGGCCACTCACCA. The repair oligonucleotide was synthesized by BiOligo Biotechnology Co., Ltd., and the sgRNAs were provided by GenScript. Cas9 mRNA, sgRNAs, and the repair oligonucleotide were injected into mouse embryos as described previously.43 The genotyping of DOCK8A1949D knock-in mice was performed by sequencing a 346-bp DNA fragment flanking exon 45 using the following pair of PCR primers: forward 5′-CCATCTCTGTGTGACCTGTTCA-3′ and reverse 5′-AACAAACAAGCCCTAACCAAGC-3′.
4.3 Cells preparation
Mouse spleen, thymus, pLn, and mLn were collected and grounded. Cells were suspended in an HBSS solution containing 2% serum and then filtered with a nylon membrane after lysed red blood cells with Red Cell Lysis Buffer to obtain mononuclear cells. Reagents details are presented in Table S1.
4.4 Purification and activation of CD4+ T cell
Purified CD4+ T cells were isolated by murine CD4+ T cell Isolation Kit. For the activation of CD4+ T cells, sorted cells were cultured in 10 µg/mL plate-bound anti-CD3 plus anti-CD28 for 5 h at 37°C as described previously.44 Reagents details are presented in Table S1.
4.5 Flow cytometry
For cell surface staining, mononuclear cells (1 × 106) from the thymus, spleen, pLn, and mLn were stained with antibodies on ice for 30 min after incubation with the Fc blocker. For cell intracellular staining, cells (2 × 106) were fixed, permeabilized, and subsequently cultured with antibodies on ice for 30 min. For cytokine analysis, mononuclear cells (2 × 106) were cultured with PMA (50 ng/mL), GolgiStop (1:1000), and lonomycin (1 µM) at 37°C for 5 h. Then cells were collected and stained with antibodies on ice for 30 min. Finally, cells were measured using Attune NxT (Thermo Fisher, AFC2) and analyzed by FlowJo 10 software (TreeStar). Antibodies’ details are presented in Table S1.
4.6 Confocal
Purified CD4+ T cells (1.5 × 106) were cultured with 10 µg/mL plate-bound anti-CD3 plus anti-CD28 for 5 h at 37°C. To prepare ROS and PK Mito analysis, the cells were cultured with anti-CD4 and DHE or PK Mito for 15 min at 37°C. Then, the cells were analyzed by a confocal microscope (Nikon). The MFI was analyzed using NIS-elements AR 5.01. Over 50 individual cells were analyzed for confocal analysis. Reagents details are presented in Table S1.
4.7 Western blotting
Sorted and stimulated CD4+ T cells (2 × 106) were lysed using RIPA buffer containing cocktail, NaF (1 M), and Na3VO3 (100 Mm). Then, cell lysates were examined by SDS-PAGE, and immunoblotting was performed using antibodies (Bio-Rad). Antibodies details are presented in Table S1.
4.8 Seahorse analysis
Purified CD4+ T cells were prestimulated in 24-well plates with 10 µg/mL anti-CD3 plus anti-CD28 for 24 h at 37°C, and then seeded the cells into culture plates at 37°C for 1 h. For ECAR analysis, cells were stimulated with 2 uM Oligomycin, 10 mM Glucose, and 5 mM 2-DG. For OCR analysis, cells were stimulated with 1 µM FCCP, 1.5 µM oligomycin, 1 µM antimycin A, and 500 nM rotenone as described previously.45 Finally, cells were examined by the Seahorse XFe24 Cell Metabolism Analyser (Agilent). Reagents details are presented in Table S1.
4.9 ELISA
Levels of IgE in serum were measured using a mouse IgE ELISA Kit. Reagents details are presented in Table S1.
4.10 LCMV infection
Mice were injected intraperitoneally with PBS containing 5 × 105 PFU of LCMV-Armstrong 53b (200 µL). One week later, the mice were executed, and T cells from the spleen were examined. The level of IgE in serum was tested by ELISA (INFINITE 200 PRO, TECAN).
4.11 BM chimeras
BM cells were collected from WT (CD45.1 recipients), DOCK8 mutation (CD45.2, donor), and WT (CD45.2, donor) mice as described previously.45 After 8 weeks postinjection, spleen, thymus, and pLn cells were collected and isolated for staining by flow cytometry.
4.12 Transcriptome sequencing
For RNA-seq libraries, VAHTS Universal V8 RNA-seq Library Prep Kit for Illumina was constructed and then sequenced on the BGISEQ platform. Then, low-quality reads were removed by the BGISEQ platform and aligned reads to the mouse reference genome.
4.13 RT-PCR
In brief, total RNA was isolated from CD4+ cells (1 × 106) using the trizol reagent and detected using real-time reverse transcription PCR assay as described previously.46 The primer sequences refer to Table S2.
4.14 Statistics analysis
Data were presented as mean ± SEM and statistical analyses were presented using Prism 8.0 (GraphPad). When comparing two groups, an unpaired two-tailed Student's t-test was used. Asterisks indicate significant difference: *p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
AUTHOR CONTRIBUTIONS
Panpan Jiang and Siyu Zhao wrote the paper and performed the experiments and the statistical analysis. Xiaoyu Li, Shiyan Hu, Shuhan Chen, Lichen Zhang, Liaoxun Lu, and Yanmei Huang performed the flow cytometry, confocal experiments, and western blotting. Guofeng Fang performed RNA-seq analysis. Yinming Liang, Lu Yang, Heather Miller, Fei Guan, and Jiahui Lei revised the manuscript. Chaohong Liu designed the research. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We are grateful to the Medical Subcenter of HUST Analytical & Testing Center for data collection. This study was funded by International Cooperation in Science and Technology Innovation between Governments (2021YFE0108200), the National Natural Science Foundation of China (NSFC32311530061, 31970839, and 32100709) and the Fundamental Research Funds for the Central Universities.
CONFLICT OF INTEREST STATEMENT
Heather Miller is an employee of Cytek Biosciences but has no potential relevant financial or non-financial interests to disclose. The other authors declare no conflicts of interest.
ETHICS STATEMENT
All animal experiments were approved by the Animal Experiment Ethics Committee of Tongji Medical College (approval number, 3954).
Open Research
DATA AVAILABILITY STATEMENT
The raw data of RNA-bulk sequencing (No. CNP0006002) have been deposited in China National GeneBank DataBase (CNGBdb).




