Dysregulation of Astrocytic ATP/Adenosine Release in the Hippocampus Cause Cognitive and Affective Disorders: Molecular Mechanisms, Diagnosis, and Therapy
Funding: The authors were supported by the grants from NSFC-RSF (82261138557), NSFC (82274668; 82230127), the Sichuan Science and Technology Program (2021YFH0096; 2022YFH0006), and the Sichuan Provincial Administration of Traditional Chinese Medicine (2023zd024). Support by the São Paulo Research Foundation (FAPESP; 2018/07366-4, 2022/10950-5) and the National Council for Scientific and Technological Development (CNPq; 406396/2021-3).
ABSTRACT
The gliotransmitter adenosine 5'-triphosphate (ATP) and its enzymatic degradation product adenosine play a major role in orchestrating in the hippocampus cognitive and affective functions via P2 purinoceptors (P2X, P2Y) and P1 adenosine receptors (A1, A2A). Although numerous reviews exist on purinoceptors that modulate these functions, there is an apparent gap relating to the involvement of astrocyte-derived extracellular ATP. Our review focuses on the following issues: An impeded release of ATP from hippocampal astrocytes through vesicular mechanisms or connexin hemichannels and pannexin channels interferes with spatial working memory in rodents. The pharmacological blockade of P2Y1 receptors (P2Y1Rs) reverses the deficits in learning/memory performance in mouse models of familial Alzheimer's disease (AD). Similarly, in mouse models of major depressive disorder (MDD), based on acute or chronic stress-induced development of depressive-like behavior, a reduced exocytotic/channel-mediated ATP release from hippocampal astrocytes results in the deterioration of these behavioral responses. However, on the opposite, the increased stimulation of the microglial/astrocytic P2X7R-channel by ATP causes neuroinflammation and in consequence depressive-like behavior. In conclusion, there is strong evidence for the assumption that gliotransmitter ATP is intimately involved in the pathophysiology of cognitive and affective neuron/astrocyte-based human illnesses opening new diagnostic and therapeutic vistas for AD and MDD.
1 Introduction
The major non-neuronal cell population of the CNS consists of glial cells such as astrocytes, oligodendrocytes, and microglia. These cell types interact with neurons to shape various functions of the brain and spinal cord. In short, astrocytes appear to be important players in serving ionic and neurotransmitter homeostasis with special emphasis on the clearance of extracellular potassium and glutamate [1, 2]. In addition, after the release of glutamate/GABA from neurons and subsequent uptake into astrocytes, these cells synthesize and release glutamine, which is taken up into neurons and is used as a neurotransmitter precursor for the production of glutamate and GABA [3]. Astrocytes enwrapping synapses also control the extracellular space volume and hence the extracellular concentrations and diffusion of neuroactive substances [4]. Besides these homeostatic functions, astrocytes are endowed with a range of neurotransmitter receptors, whose activation may result in elaborate [Ca2+]i transients to induce the release of gliotransmitters (ATP, glutamate, D-serine) stimulating their receptors at neuronal terminals/cell bodies and establishing thereby a modulation of synaptic functions [5, 6]. The functional entity of pre- and postsynaptic neurons as well as the involved astrocytic processes are termed the “tripartite synapse” giving credit to the ability of astrocytes to modulate neuronal functions [7].
Eventually, astrocytes mediate neurovascular signaling to capillary pericytes in order to cause vasodilation via the arachidonic acid metabolites prostaglandin (PG) E2 and 20-hyroxyeicosatetraenoic acid [8]. In sleep-promoting neurons of the ventrolateral preoptic nucleus, PGD2Rs are expressed in astrocytes mediating increased adenosine release, which exerts on its behalf a control of local blood flow [9]. Adenosine appeared to be involved also in the blood oxygenation level-dependent functional magnetic resonance imaging signals of the rat somatosensory cortex during periods of enhanced neuronal activity [10]. It has been suggested that during exacerbated neuronal firing, astrocytes respond to neuronal ATP, which propagates astrocytic activation, stimulates release of vasoactive substances (e.g., adenosine), and thereby dilation of cerebral vasculature.
ATP is intimately involved in the pathophysiology of cognitive and affective neuron/astrocyte-based illnesses. In this respect, the subject of our overview is defined by the title, which points out a restriction to the emotional and cognitive functions of ATP released by astrocytes and also to similar functions of adenosine generated by the enzymatic degradation of astrocytic ATP. Release means in our understanding an active process, such as Ca2+-dependent exocytosis, passage through (hemi)channels after their opening, and operation of equilibrative nucleoside transporters (ENT), but by no-way passive outflow from damaged cells (see Section 2.1). Thus, we concentrate ourselves on astrocytic, but not neuronal release of ATP and adenosine, the latter of which is generated from this ATP via enzymatic decomposition or is alternatively driven out of cells by a concentration-dependent transporter. This limitation is necessary because otherwise the review would include a considerable amount of data about ATP/adenosine and their receptors published over many years, without giving consideration to these specific issues raised.
As indicated above, our aim was to summarize the reported effects of astrocyte-derived ATP (and its enzymatic degradation product adenosine) on cognitive and affective functions and their disturbances in the respective rodent models. It is expected that these considerations will have diagnostic and therapeutic consequences especially for Alzheimer's disease (AD) and major depressive disorder (MDD) in human medicine.
2 Astrocytic ATP and its Enzymatic Degradation Product Adenosine
2.1 Astrocyte-Derived Release of ATP and its Degradation to Adenosine
A manipulation that interferes with astrocyte vesicular release of gliotransmitters via overexpression of a dominant-negative domain of vesicular soluble N-ethylmaleimide-sensitive-factor attachment receptor (dnSNARE) has led to the realization of astrocytic involvement in processes that were traditionally considered strictly neuronal, including the sleep–wake cycle, long-term potentiation (LTP), and cognition [11]. Although the astrocyte specificity of this manipulation was questioned later, by demonstrating widespread expression of the dnSNARE transgene in cortical neurons, and the finding that the activity of cortical neurons is reversibly suppressed in dnSNARE mice [12], these animals are still widely used in order to demonstrate the release of gliotransmitters, such as ATP/adenosine from astrocytes (e.g., [13, 14].
Hence, ATP is besides its general signaling function in the CNS (e.g., outflow from endothelial cells on shear stress from brain capillaries; [15]), also a neurotransmitter, released from synaptic-type vesicles of nerve terminals [16], and a gliotransmitter, released via exocytotic, Ca2+- and SNARE complex-dependent mechanisms from astrocytic lysosomes [17, 18]. While in neurons, the immediate stimulus for exocytotic (vesicular) transmitter release is extracellular Ca2+ passing via voltage-sensitive Ca2+ channels the plasma membrane and thereby increasing [Ca2+]i in astrocytes, this increase is fueled by the activation of G proteins coupled to the inositol 1,4,5-triphosphate receptor subtype 2 (IP3R2) release channels, located at the endoplasmic reticulum and triggering [Ca2+]i increase [19]. Combined epifluorescence and total internal reflection fluorescence microscopy was used to monitor individual quinacrine-loaded, ATP-containing vesicles undergoing exocytosis in cultured astrocytes [20]. Two populations of ATP-containing vesicles with distinct (fast and slow) time-course of cargo release were identified. These ATP pools were thought to represent synaptic-type vesicles and secretory lysosomes in astrocytes [21]; the speed of transmitter release was proven to be at least two orders of magnitude slower than the kinetics of regulated exocytosis recorded in neurons [22]. A prerequisite for ATP release is the active accumulation of this nucleotide into the secretory vesicles/lysosomes by a vesicular nucleotide transporter (VNUT; [23]).
ATP may leave astrocytes also by nonexocytotic pathways, through connexin hemichannels (mostly connexin-43 [Cx43]; [24]) and pannexin 1 (Panx-1) channels [25]. Maxi-anion channels [26], volume-regulated anion channels [27], the Ca2+-dependent Cl− channel bestrophin [28], the calcium homeostasis modulator 1-3 (CALHM1-3; [29]), and the purinergic receptor P2X7R [30, 31] also participate in the accumulation of ATP outside of cells. Hence, neurotransmitters (e.g., noradrenaline) may stimulate their astrocytic receptors, resulting in the subsequent release of the gliotransmitter ATP, which in turn modulates synaptic functions [32, 33].
Extracellular adenosine in the CNS derives from the enzymatic degradation of neuronal and astrocytic ATP by ectonucleotide tri(di)phosphohydrolase (ENTPD; CD39) and the subsequent degradation of AMP by ecto-5’-nucleotidase (CD73) [34, 35]. Besides this “conventional” and Ca2+-dependent, although indirect generation of adenosine, the nucleoside may be also outpoured from astrocytes by the operation of ENT1 and 2. There is some evidence for the exocytotic, Ca2+-dependent release of adenosine itself from separate neuronal/astrocytic synaptic vesicles [36, 37], or the Ca2+-dependent operation of ENT-1 [37], generating increased adenosine concentrations in the extracellular space. These latter release mechanisms are, however, far from being generally accepted as sources of extracellular adenosine.
2.2 Astrocytic Purinoceptors
Extracellular ATP released by vesicular and nonvesicular means stimulates ionotropic P2XRs (assembled as trimeric homo- or heteromeric receptors from seven subunits, P2X1-7) and metabotropic P2YRs (eight subtypes, P2Y1, 2, 4, 6, 11, 12, 13, 14; [38, 39]), most of which are localized also at astrocytes [40, 41]. P2XRs open nonselective cationic channels, while P2YRs are coupled to Gq, Gs, or Gi/o proteins and their obligatory second messenger systems. Whereas P2XRs respond only to ATP [42, 43], P2YRs respond to ATP/ADP, UTP/UDP, or UDP sugars [44, 45]. Adenosine, the enzymatic degradation product of ATP, acts on G protein-coupled P2Rs (Gs, Gi/o), which either stimulate (A2A, A2B) or inhibit (A1, A3) adenylate cyclase [46, 47]. The two behaviorally relevant high-affinity receptors, A1 and A2A, however, exert their effects not exclusively via the inhibition and stimulation of adenylate cyclase production (see Section 4.7).
Behavioral effects of adenosine are mediated in the CNS both by A1 and A2ARs [48, 49]. Signaling through these receptors is terminated by rapid uptake through the function of ENT1-2 [50] and to a lesser extent by the conversion of adenosine to the only slightly active inosine in the extracellular space by the already mentioned 5’-nucleotidase [34, 35] (see in Section 4.6.1). Another possibility of enzymatic conversion of adenosine is that to AMP by adenosine kinase, which is however an intracellular enzyme, unable to bind extracellular adenosine to terminate its action [51]. Astrogliosis, via overexpression of adenosine kinase, induces a deficiency in the homeostatic tone of adenosine, which is a common hallmark of epilepsy, AD, Parkinson's disease (PD), and amyotrophic lateral sclerosis [52].
The ionotropic P2X7R is activated by concentrations of ATP in the pathologically high micromolar/millimolar range, in contrast to the other P2XR subtypes, which are activated already by lower micromolar ATP concentrations [53, 54]. Further, although P2X7Rs cause nonselective cationic (Na+, K+, Ca2+) currents, when in initial contact with ATP, longer-lasting or repetitive stimulation by ATP allows the passage of larger molecules (up to 900 Da) through the cell membrane [55]. These pores are believed to play a direct role in apoptosis/pyroptosis and inflammation [56, 57]. The P2X7R is widely expressed by cells of the innate and adaptive immune systems all over the animal kingdom including the human species [56, 57]. P2X7Rs are preferentially located on microglia, the resident macrophages of the brain, but they also occur at astrocytes [56] (Figure 1). P2X7Rs, in association with toll-like receptors (TLRs), may cause (neuro)inflammation mainly by releasing the cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) mediated by the nucleotide-binding, leucine-rich repeat, pyrin domain containing (NLRP3) inflammasome [56, 58].
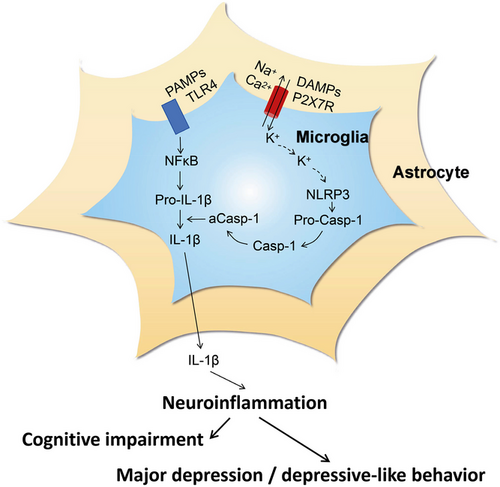
3 Regulation of Cognitive and Affective Functions by the Hippocampus
The hippocampus, as part of the limbic system, is involved in memory, learning (especially spatial learning [59, 60]), and emotion [61, 62]. On the one hand, the hippocampus transfers short-term memory to long-term storage; its functions are typically curtailed in illnesses, such as AD or posttraumatic stress disorder. There is broad consensus on the idea that hippocampal LTP and long-term depression (LTD) are forms of synaptic plasticity, which comprise at the cellular level the physiological correlates of associative learning [63]. On the other hand, emotional behavior is also regulated by the hippocampus, and therefore mood disorders, such as MDD, are thought to be due to disturbed network functions, governed by the hippocampus in association with numerous regions of the brain, for example, the medial or dorsolateral prefrontal cortex (PFC) [64, 65]. In this respect, it should be noted that the hippocampal–prefrontal pathway (a monosynaptic unilateral projection) is highly sensitive to stress, which is a major precipitating factor for the symptoms of depression [66]. The wide range of bioactive molecules released by reactive astrocytes disturbs CNS homeostasis and thereby causes cognitive impairment and major depression (Figure 2).
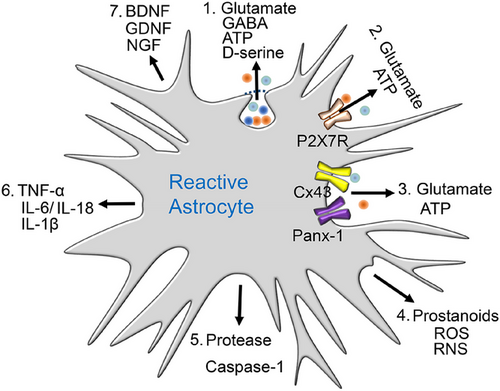
4 Purinergic Regulation of Cognition
4.1 Cognitive Disturbances and their Animal Models
Learning is a neural process that enables both humans and animals to adapt to their environment, based on previous experiences; memory is the neural process by which experience acquired during learning is stored and eventually accessed [68]. Memory systems can be classified to declarative and nondeclarative memory [69]. The former can be expressed in words, while the latter encompasses information related to motor skills. Another distinction of cognitive performances is made between long-term, short-term, and working memory, based on the duration of time after which previous experiences have to be recalled [70].
Cognitive disorders frequently accompany neurodegenerative disorders, such as AD, PD, stroke, and so on. They may be connected with altered ATP release from astrocytes or altered effects of ATP/ADP at astrocytic purinoceptors [71]. Astrocytes may modify neuronal activities in three different manners. First, astrocytes affect neurons according to their genetic identity—for example, dopamine D1 and D2R-containing medium spiny neurons (MSNs) are governed differentially [72]. Second, astrocytes exert neurotransmitter-specific effects on different neuronal types [73]; and third, they exhibit task-specific effects, for example, depending on the presence or absence of learning processes [74]. Hence, the gliotransmitter ATP and its astrocytic purinoceptors may interfere with the learning ability of rodents and possibly also humans through at least the three above-cited mechanisms.
Learning/memory disorders may be investigated in various rodent models. The simple and most often used ones are the following: Novel object recognition (recognition memory), T-maze or Y-maze (exploratory/spatial memory), Morris water maze (spatial and long-term reference memory), Barnes maze (spatial learning and memory), passive avoidance (emotional learning and long-term memory), and contextual fear conditioning (associative context-driven learning) [75-77]. Of course, all these test systems only incompletely model the complex cognitive disturbances of humans; nevertheless, they are of considerable help in collecting preclinical evidence and in allowing some extrapolation to patients in the clinical praxis.
4.2 Impeded Channel-Mediated ATP Release from Astrocytes in the Hippocampus is a Cause of Cognitive Impairments
Gap junctions of vertebrates comprise of two opposed connexin hemichannels that link the cytosol of adjacent cells, whereas in invertebrates, they are built up of innexins of related structure [78, 79]. Homologous to innexins are the vertebrate pannexins; however, these do not form gap junctions and exist only in form of channels. As already mentioned earlier, both unopposed connexins and pannexins are release pathways for ATP from astrocytes (Figure 3). Gap junction connections, which ensure the undisturbed network activity of astrocytic populations through the cell-to-cell diffusion of Ca2+, called Ca2+ waves, are also of high physiological significance. Ca2+ waves are due to the passive diffusion of Ca2+ through the gap junctions, but also to the release of ATP in one astrocyte and the stimulation in the next one of metabotropic P2Y1 and P2Y2Rs releasing Ca2+ from the endoplasmic reticulum [80].
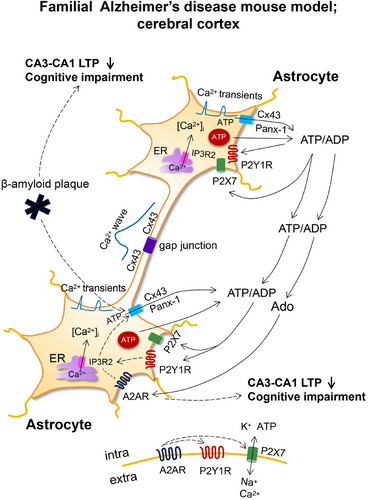
There are a number of publications that present evidence on the interrelationship between connexin hemichannel- and pannexin channel-mediated release of ATP from astrocytes, and learning/memory performance of rodents [81] (see below). In particular, Cx43 (a connexin with an approximate molecular weight of 43 kDa) is located on astrocytes in contrast to neurons and has been specifically implicated in activity-induced heterosynaptic metaplasticity [82].
The microinfusion of transactivator of transcription-linked Gap19 (TAT-Gap19) peptide (highly selective to Cx43) into the brain ventricle of mice did not affect the spatial working memory in a spontaneous alternation Y maze task, but impaired the spatial short-term memory in a delayed spontaneous alternation Y maze task [83]. Along this line of thinking, infrasonic noise impaired cognitive functions as measured in the Morris water maze test; this effect was closely related to the activation of Cx43 hemichannels in astroglia [84]. Blockade of spatial learning/memory by infrasonic noise depended on Cx43, as proven by restitution of cognitive functions after Cx43 blockade with the intrahippocampal injection of shRNA or TAT-Gap19 peptide targeting this connexin, or alternatively fluorocitrate, a general metabolic toxin of astrocytes.
When the learning/memory capabilities of Panx1−/− mice were compared with their Panx1+/+ counterparts, prominent differences were observed [85]. In the novel object recognition and cookie finding tests, the KO mice showed limited performance, confirming the participation of Panx-1 and the release of gliotransmitters in object recognition and spatial memory. In another study, experiments with a mouse genetically deficient in Panx-1 documented in the eight arm radial maze that long-term spatial reference memory, but not spatial working memory was deficient, in comparison with the achievement of the respective control mice [86].
The tight coupling of P2X7Rs with Panx-1 channels has been verified about two decades ago and it was even concluded that these two channel types act as associated proteins [87, 88]. Hence, Panx-1 has been assumed to be necessary for the processing of caspase-1 and the generation of mature IL-1β induced by P2X7R activation.
Although connexin hemichannels and pannexin channels are known to be expressed both in neurons and astrocytes, it is increasingly believed that P2X7Rs fail to be expressed at neurons; the effects observed appear to be indirect, and due to astrocytic/microglial receptor activation via the release of gliotransmitters/signaling molecules [31, 89-91].
4.3 Anxiety Disorders and Fear Memory
Contextual fear conditioning appears to depend on the hippocampus, basolateral amygdala, and ventromedial PFC [92, 93]. An abundance of data support the assumption that P2X7R antagonists [94] or the genetic deletion of this receptor type [95] interfered in mice with contextual fear recall [96]. When liquid chromatography and mass spectrometry were used to identify substrates of the protein degradation process in the amygdala of male rats following fear conditioning [97], proteins involved in the cytoskeleton, ATP synthesis, and cell signaling were significantly altered during contextual fear acquisition and extinction. Thus, it was suggested that these processes are regulated by P2X7Rs, which open astrocytic Cx43 channels.
In a series of experiments, rats were fear conditioned, using parings of neutral tone (conditional stimulus) with an aversive foot shock (unconditional stimulus; [98]). All animals exhibited equal levels of freezing, when tested 1 day later for their tone fear memories. Intraperitoneal (i.p.) application of the general gap junction blocker carbenoxolone or the selective Cx43 blocker mefloquine significantly reduced context fear. Microinfusion into the rat basolateral amygdala of TAT-Cx43L2, a peptide that selectively inhibits Cx43 hemichannel opening during memory consolidation, induced amnesia for auditory fear conditioning [99]. Learning capacity was recovered after coinfusion of TATCx43L2 with a mixture of possible gliotransmitters including ATP.
4.4 Involvement of P2X and P2Y Receptors in Cognitive Deterioration; AD
Dementia is the leading cause of disability in the elderly population and AD is the most prevalent of all dementias, leading to early deficits in episodic, short-term memory, followed by progressive impairment in declarative and nondeclarative memory [100, 101]. There is abundant experimental evidence that P2X7R activation and the consequent release of cytokines, chemokines, reactive oxygen/nitrogen species, and the passage through the P2X7R-channels themselves of the cytotoxic glutamate may cause neurodegeneration superimposed on the primary causes of the disease, which are thought to be the deposition of toxic extra- and intracellular protein aggregates (β-amyloid, hyperphosphorylated tau [79, 102-104]). We do not discuss these results in detail because there is no definite evidence for the exclusively astrocytic localization of the P2X7R [31, 89].
In vivo two-photon microscopy demonstrated in astrocytes of the somatosensory cortex the increase of spontaneous [Ca2+]i transients in a mouse model of familial AD (APPPS1 mice [105]; Figure 3). This was most pronounced in reactive astrocytes around β-amyloid plaques and consisted of single-cell [Ca2+]i transients and oscillations. Calcium responses were augmented by increasing ADP (an endogenous agonist of the P2Y1R) levels as consequence of blocking its degradation by apyrase and was reduced by blocking P2Y1Rs with MRS2179 or inhibiting the release of ATP/ADP through connexin hemichannels with carbenoxolone.
In a successor paper of this group of authors, the significance of their findings to the cognitive abilities of the APPPS1 mice was further extended [106]. They documented that the high-frequency stimulation of Schaffer collaterals induced LTP in the hippocampal CA1 neurons. The extent of LTP was higher in brain slices taken from wild-type than from the AD-model animals; however, the application of MRS2179 normalized this difference. Thus, the pathological increase of strength in the CA3–CA1 synapses caused by astrocytic hyperactivity could be normalized by P2Y1R inhibition. In accordance with this cellular model of hippocampal learning, chronic P2Y1R inhibition through application of MRS2179 via osmotic minipumps reversed spatial reference learning and memory deficits in APPPS1 mice. The learning/memory performance was tested in the Barnes maze and Morris water maze tests. Moreover, both elementary memory processes and spatial learning performance could be improved in a mouse model of genetic AD by rescuing normal astrocytic [Ca2+]i activity not only by the pharmacological blockade of P2Y1Rs but also by the deletion of metabotropic signaling downstream of P2Y1R activation. This latter effect was achieved by generating mice double transgenic for Appps1−/+ (AD model) and Ip3r2−/− (blockade of Ca2+ release from the endoplasmic reticulum).
As discussed above, astrocytic ATP release via Cx43 channels is a necessary prerequisite for cognitive performance. In apparent disagreement with these findings, in transgenic mice with overexpressed β-amyloid precursor protein (APP) and presenilin1 (PS1), an increase in astroglial connexin immunoreactivity around the β-amyloid plaques was documented [107]. Other studies proved the specific expression of astroglial Cx43 around the amyloid plaques of this rodent model of AD [108]. These findings perfectly correlate with the earlier detection of upregulated Cx43-immunoreactivity in cortical areas of postmortem brains of AD patients [109]. A possible explanation for this apparent controversy is that the increased density of Cx43 around the amyloid plaques is not a reason but a compensatory (maladaptive) consequence of the AD-induced functional limitation.
4.5 P2X and P2Y Receptor-Mediated Changes in Synaptic Plasticity Events
P2X and P2YRs have been shown to participate in synaptic plasticity events in the CNS, including cognition-relevant areas such as the hippocampus, and thereby were found to modulate learning and memory [33, 110-112] (Figure 4). It has been especially interesting to appreciate that downregulation of NMDAR-mediated signaling by the P2XR can have significant impact on LTP in the hippocampal CA1 area and the layer 2/3 pyramidal cells of the somatosensory cortex [113, 114].
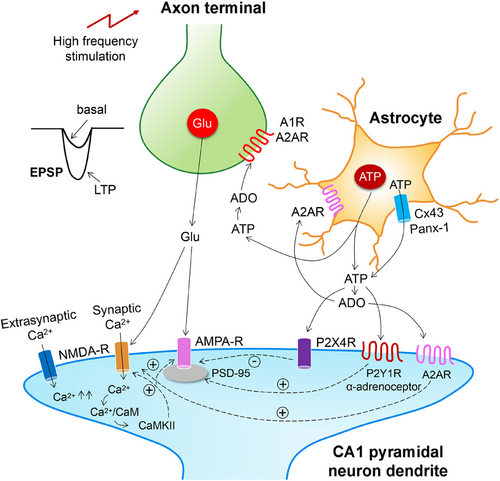
The combination of electrophysiological recording techniques and multiphoton fluorescent Ca2+ imaging in acutely isolated astrocytes from the somatosensory cortex showed that α1-adrenoceptor activation stimulates the vesicular release of ATP from astrocytes; this initiates a burst of P2XR-mediated currents in adjacent pyramidal neurons [14]. Such purinergic currents were depressed by intracellular perfusion of astrocytes with the exocytosis inhibitory tetanus toxin. Weak sub-threshold electrical stimulation can induce LTP, when astrocytes are additionally activated by the preferentially α1-agonistic noradrenaline. Treatment of the brain slices with tetanus toxin, or preparing the brain slices from dnSNARE mice with decreased ATP release from astrocytes abolished and depressed the induction of LTP, respectively. An alternative to α1-adrenoceptor-stimulation to increase intracellular [Ca2+]i in astrocytes was the application of ryanodine to induce the release of Ca2+ from its storage sites [120]. This procedure also enhanced LTP in the hippocampus and neocortex.
Heterosynaptic (h)LTD at untetanized synapses accompanying the induction of LTP, spatially sharpens the synaptic potentiation. It was found that hLTD in the hippocampal CA1 area is caused by stimulation-induced ATP release from astrocytes that suppresses transmitter release from untetanized synaptic terminals via activation of P2YRs [121]. P2YR activation was blocked by Reactive Blue-2, which at that time was thought to selectively inhibit P2YRs (this unfortunately did not prove to be true later) and more reliably by buffering astrocyte [Ca2+]i at a low level. The same methodological repertoire described above, allowed the group of Yuriy Pankratov to report that in the neocortex, ATP and glutamate are coreleased onto adjacent pyramidal neurons, and postsynaptic NMDARs are downregulated by simultaneously activated P2XRs [114]. Genetic deletion of postsynaptic density protein-95 (PSD-95) abrogated the P2XR-mediated downregulation of NMDARs. Pharmacological blockade of purinergic modulation of NMDARs by the P2X4R antagonist 5-BDBD in PSD-95-deleted mutant mice, dramatically decreased the threshold of LTP induction and increased the net magnitude of LTP. This is in perfect agreement with the reported finding that recombinant P2X4Rs interact with NMDARs when coexpressed in Xenopus laevis oocytes [122].
4.6 Changes in Extracellular Adenosine Levels are Causes of Cognitive Deterioration
4.6.1 Changes of Adenosine Levels Arising from the Operation of an ENT are Causes of Cognitive Deterioration
A subgroup of astrocytes in the dorso-medial striatum regulate the transition from habitual to goal-directed reward-seeking behavior [123]. Of the dopamine D1R-expressing direct pathway, and the dopamine D2 and the adenosine A2AR-coexpressing indirect pathway MSNs, the activation of designer receptors exclusively activated by designer drugs (DREADDs), fabricated in nearby astrocytes, reduced the frequency of sEPSCs in the indirect pathway MSNs, but not in the direct pathway MSNs. This chemogenetic stimulation of astrocytes abolished habitual reward-seeking behavior and promoted the transition to goal-directed reward seeking behavior. Mice genetically lacking ENT1 did not show such a transition, confirming that the endogenous adenosine level participates in this effect. These series of experiments agreed with the finding that L-α-aminoadipate (a selective metabolic toxin of astrocytes), both under ex vivo and in vivo conditions, depressed LTP magnitude in the CA1 area of the hippocampus and in addition impaired hippocampal-dependent memory (measured with the novel object recognition and object displacement tests) in mice [119].
The A2AR agonist CGS21680 facilitated adenosine uptake into hippocampal synaptosomes and also increased from these synaptosomes the depolarization-induced release of adenosine [124]. By contrast, either the blockade of the ENT by nitrobenzylthioinosine or inhibition of the A2AR by SCH58261 abrogated these effects. These results indicated that A2AR activation facilitated the activity of the nucleoside transporters and its blockade inhibited the outflow of adenosine from the synaptosomal preparations. By using a genetically encoded G protein-coupled receptor (GPCR)-activation-based adenosine fluorescent sensor (CRABAdo), it was discovered that neuronal activity releases adenosine from the somatodendritic compartments rather than from the nerve terminals of hippocampal neurons [125]. It was also shown that adenosine release depends on ENT and requires calcium influx through L-type Ca2+ channels. A similar ENT-2-dependent adenosine release was observed in the cerebral cortex after 40 Hz light flickering [126]. The resulting enhanced glymphatic flow was due to increased cerebrofluid adenosine levels activating astrocytic A2ARs, which themselves interacted with aquaporin-4 channels of the same astrocytes. Glymphatic flow is fundamental for the homeostasis of the brain milieu eliminating metabolic waste. Since both inhibition of ENT-2 and exclusion of A2ARs blocked the enhanced glymphatic flow, an astrocytic function appears to be involved, although the increased release of adenosine itself is due to a neuronal effect. In fact, cortical (glutamatergic and GABAergic) neurons, rather than astrocytes, were the cellular sources of adenosine outflow via the function of an ENT-2 as the molecular pathway [127]. The increased ENT-2-mediated release of adenosine enhanced non-rapid eye movement (non-REM) and REM sleep in mice, which by itself enhanced cognitive functions.
A small adenosine analogue J4 blocks ENT-1 and thereby prevented the decline in memory in the APP/PSI mouse model of AD [128]. Chronic treatment with J4 normalized LTP at CA3–CA1 synapses, as well as counteracted the aberrant expression of synaptic proteins, and the detrimental elevation in astrocytic A2AR expression in the hippocampus. Hence, the ENT1-mediated release of adenosine during AD activated A2ARs, causing neurodegenerative damage to the hippocampus and cortex. Similarly, treatment with the ENT1 inhibitor J4 exerted beneficial effects in a mouse model of tauopathy [129]. Thus, energy dysfunction (including mitochondrial impairment) was improved, pathological astrocytic activation was prevented, and synaptic functions/memory processes were ameliorated.
Neuroinflammation is involved in cognitive deficits and neurodegenerative illnesses. Application of lipopolysaccharide (LPS) to ENT-2 KO mice and their wild-type controls showed an attenuation of the LPS effects in the KO mice [130]. Thus, ENT-2 plays an important role in regulating inflammation-associated cognitive decline and neural damage.
ENT-1 and ENT-2 were found both on neuronal and astrocytic plasma membranes [131] just as A2ARs. Therefore, the functioning of, for example, an A2AR-modulated ENT-1 effect is by no way a proof for the astrocytic regulation of adenosine release into the extracellular space. However, the opposite notion does not hold true either. In the present section, some but not all data suggest astrocytic involvement in the regulatory functions of ENT1/2, but in many cases, this affirmation is doubtful or even can be excluded with high certainty.
4.6.2 Changes of Adenosine Levels Arising from the Degradation of Astrocytic ATP are Causes of Cognitive Deterioration
Further evidence also convincingly proved that in a β-amyloid (Aβ1–42)-based mouse model of early AD, an increased synaptic release of ATP coupled to an increased density and activity of CD73 is of causative significance for the cognitive deterioration [132]. CD73 was shown according to expectations to facilitate the formation of adenosine, which on its behalf selectively activated A2ARs. The enhanced CD73 activity was critically required for Aβ1–42 to impair synaptic plasticity (CA3–CA1–LTP in hippocampal slices) and memory, since the cognitive deficits were eliminated in A2AR KO mice as well as forebrain A2AR and CD73 KO mice. A2ARs were shown to control fear memory and the underlying processes of synaptic plasticity in the amygdala [133]. The repeated bilateral cerebroventricular injection of the CD73 inhibitor α,β-methylene ATP (α,β-meATP) imitated the effects of selective A2AR blockade by SCH58261 both on fear memory and amygdala-LTP, proving that the enzymatic degradation of released ATP to adenosine is the initiator of these effects. Similarly, the ATP-derived formation of extracellular adenosine bolstering A2AR activation was identified as a key pathway responsible for abnormal synaptic plasticity in circuits involved in the onset of PD motor symptoms [134].
Neuroligins, a family of cell adhesion molecules, are essential for synapse development and their dysfunction is linked to social memory disturbances (measured by three-chamber sociability and social novelty tests) via A2AR downregulation [135]. These data were generated in adult male mice with the astrocytic deletion of neuroligin-3 in the ventral hippocampus.
4.7 Involvement of A1 and A2A/A2B Receptors in Cognitive Deterioration
A1Rs are coupled via Gi,o proteins to adenylate cyclase and their activation decreases the cAMP concentration and thereby the tonic activity of protein kinase A (PKA) [133-136]. They depress neuronal excitability by a mixed pre- and postsynaptic effect; presynaptic A1Rs located at glutamatergic neuronal terminals decrease the release of the excitatory glutamate and postsynaptic A1Rs open inwardly rectifying potassium channels thereby causing hyperpolarization. A2ARs are coupled via Gs proteins to adenylate cyclase, and their activation increases cAMP concentration and thereby the stimulation of PKA in the striatum; presynaptic A2ARs located at glutamatergic nerve terminals increase the release of the excitotoxic glutamate and postsynaptically they lead to the opening of voltage-sensitive Ca2+ channels. Whereas A1Rs have a widespread distribution in the CNS, A2ARs are preferentially expressed in the striatum, although they can be found also in the hippocampus and cerebral cortex albeit at lower densities.
However, outside of the striatum, the behaviorally relevant signaling mechanisms of postsynaptic A2ARs (located at synapses between mossy fibers and CA3 pyramidal neurons) are independent from PKA stimulation. In the hippocampus, they spared mossy fiber stimulation-dependent AMPA–EPSCs, but potentiated NMDA–EPSCs involved in the induction of LTP in CA3 cells [136]. Thus, postsynaptic A2ARs might affect information processing in CA3 neuronal networks and possibly also memory performance.
The inhibition of gliotransmission in dnSNARE mice attenuated the accumulation of sleep pressure, assessed by measuring the slow wave activity of the EEG during non-REM sleep, and also prevented the cognitive deficits (measured by the novel object recognition test) associated with sleep loss [11, 137]. Since the sleep-suppressing effects of the A1R antagonist 8-cyclopentyl-1,3-dimethylxanthine (CPT) was prevented following gliotransmission inhibition, and because intracerebroventricular application of CPT to wild-type mice mimicked the transgenic phenotype, it was concluded that A1Rs were involved in the reported effects of sleep-deprivation.
The specific optogenetic stimulation of astrocytes in glial fibrillary acidic protein–channelrhodopsin 2 (ChR2)-enhanced yellow fluorescent protein rats disrupted memory consolidation of fear-related anxiety behavior [138]. Intracerebral blockade of A1Rs reversed the attenuation of fear memory, while intracerebral injection of an A1R agonist, 2-chloro-N6-cyclopentyladenosine (CCPA) mimicked the effect of astrocyte activation. Apparently, optogenetic stimulation caused adenosine release from astrocytes, which then activated A1Rs and interfered with the consolidation of anxiety behavior.
High frequency stimulation of the cortical inputs induced LTD, mediated by A1R activation at corticostriatal synapses of the direct pathway in the dorsolateral striatum [139]. It was found that cortical high frequency stimulation increased calcium levels in striatal astrocytes through activation of mGluR5-R signaling and that this astrocyte-mediated response is necessary for A1R-mediated LTD. In agreement with this finding when Gq receptors were chemogenetically activated, A1R-mediated synaptic depression evolved at cortico-dSPN synapses.
Caffeine is the most consumed psychostimulant in the world and is known to affect basic and fundamental neuronal processes such as sleep, arousal, cognition as well as learning and memory [140]. These effects are exclusively due to the blockade of A1 and A2ARs (and not of other actions, e.g., release of intracellular Ca2+, elicited by toxic concentrations of caffeine only), although the modulation of synaptic plasticity, and the beneficial effects of caffeine in the neurogenerative illnesses AD and PD are caused by A2AR inhibition [48, 49, 132, 141, 142].
A2ARs are potential candidates to modulate bidirectional communication between neurons and astrocytes, thus shaping synaptic plasticity, which underlies learning and memory [143]. The mixed A1/A2AR antagonist caffeine was found to decrease in hippocampal slices the CA3–CA1–LTP amplitude, an effect mimicked in A2AR KO mice or by pharmacological blockade of A2ARs by SCH58261 [141]. These findings suggest that endogenous adenosine acting at A2ARs potentiates LTP under control conditions, although there is no information with this experimental approach on the astrocytic or neuronal localization of A2ARs [141]. However, genetic silencing of A2ARs selectively in hippocampal astrocytes alters astrocytic morphology and leads to deficits of spatial reference memory (Y maze test), and compromises hippocampal synaptic plasticity, typified by a reduction of LTP magnitude and a shift of LTD towards LTP [144].
In another study, selective knockout of A2ARs in mouse astrocytes altered glutamate homeostasis by causing aberrant glutamate transporter-1 (GLT-1) activity [145]. The Na+-dependent GLT-1 controls the uptake of glutamate preferentially into astrocytes. The dysregulation of GLT-1 in consequence of the missing regulation by A2ARs led to a decrease of working memory. In good agreement with the regulation of astrocytic functions by A2ARs, the overexpression of GLT-1 promoted robust transcriptional changes, mostly affecting immune responses, angiogenesis and cell activation-related genes in a primary astrocytic culture [146]. Investigations of other authors also showed that altering astrocytic function with either glial toxins [119, 147], or different genetic [148, 149] or chemogenetic manipulations [74, 150] result in impaired spatial memory. Hence, both astrocytic and neuronal A2ARs may modulate hippocampal synaptic plasticity and memory, albeit with different consequences.
The dorsal and ventral hippocampal circuits both control mood via differential A1/A2AR-mediated modulatory mechanisms [151-153]. Nonetheless, these effects have not been reported to depend on the astrocytic release of adenosine.
Until now, we discussed the role of astrocytic A1/A2ARs in the regulation of cognitive performance because of their activation by low concentrations of adenosine; nonetheless, A2BRs, much less sensitive to adenosine, also appear to execute important functions [154]. Stimulation of A2BRs recruits a cAMP–PKA signaling pathway, resulting in rapid activation of astrocyte glucose metabolism and the release of lactate, which supplements the extracellular pool of readily available energy substrates. These data identify the A2BRs of astrocytes as sensors of neuronal activity, which results in the enhanced release of ATP/adenosine tuning brain metabolism.
4.8 Participation of A2A Receptors in AD
As already mentioned in Section 4.4, and further discussed in Section 4.6, AD is the most common form of dementia in the elderly, and is characterized by a deterioration of memory and other cognitive functions [155]. Aging, already independent of AD, differentially modulates A1 and A2AR-dependent synaptic plasticity in three age groups of rats [156]. The selective A2AR antagonist SCH58261 attenuated hippocampal CA3–CA1–LTP, with the largest effect in the aged in comparison with two younger groups of rats. By contrast, the selective A1R antagonist DPCPX increased the LTP magnitude in young adult rats, without an effect in the other age groups. In agreement with these findings, the A2AR–mRNA expression was the highest in the hippocampus of aged rats out of the three groups of animals. Similarly, a significant overexpression of A2ARs was reported in hippocampal neurons of aged humans, which was aggravated in AD patients [117]. Hence, the upregulation of A2ARs appeared to be sufficient to drive memory impairment observed in old and AD-afflicted humans.
Accordingly, it was found that the antagonism of A2ARs can recover memory deficits in animal models of AD. In rats, injected with Aβ1–42 via the intracerebroventricular route, both caffeine and the selective A2AR antagonist SCH58261 prevented synaptotoxicity and consequent cognitive impairment [155, 157, 158]. This was suggested to be associated with the increased levels of Gs-coupled A2ARs in astrocytes [150]. Conditional genetic removal of astrocytic A2ARs enhanced long-term memory in young and aging mice, while chemogenetic activation of astrocytic Gs-coupled signaling reduced long-term memory.
A number of astrocyte-dependent possible mechanisms were identified through which A2ARs might perform impairment of cognitive functions in AD. First, in astrocytic primary cultures prepared from rat cortices and exposed to Aβ1–42, ATP was released through Cx43 hemichannels, in a manner blocked by the A2AR antagonist SCH58261 and mimicked by an A2AR agonist CGS21680 [159]. Hence, Aβ1–42 triggered ATP release through Cx43 hemichannels, a process blocked by A2AR antagonists and mimicked by A2AR agonists. A2ARs directly regulated hemichannel activity and prevented Cx43 upregulation observed in Aβ1–42-exposed astrocytes. Second, it was confirmed that APP/PS1 AD-model mice display deficits in hippocampal-dependent memory (measured by the Morris water maze test), an accumulation of Aβ plaques and an increased astrocyte arbor complexity in the hippocampus [144]. In addition, enhanced activity of astrocytic Cx43 hemichannels was also observed in the hippocampus of these mice. The pharmacological blockade or genetic silencing (both global and astrocyte-specific) of A2ARs prevented Aβ1–42-induced hemichannel dysregulation in hippocampal slices. Third, in primary cultures of rat astrocytes exposed to Aβ1–42, ATP evoked Ca2+ responses had a lower amplitude but a longer duration than in Aβ1–42-untreated astrocytes and involved P2X7 and P2Y1R activities [160]. The A2AR antagonist SCH58261 regulated both P2×7 and P2Y1R-mediated [Ca2+]i responses in astrocytes, confirming that A2ARs controlled the P2X7 and P2Y1R-mediated [Ca2+]i dynamics, which are disrupted in conditions of early AD.
5 Purinergic Causes of Affective Diseases
5.1 Major Depressive and Bipolar Disorders and their Rodent Models
MDD is a mental illness with symptoms of extreme sadness, depressed mood, and loss of interest that persists for at least 2 weeks and interferes with the social functioning and working ability of the afflicted individuals [54, 102, 161]. Bipolar disorder is characterized by alternating depressive and manic episodes; the latter periods are defined by increased psychomotor activity and elevated self-esteem. Whereas the lifetime prevalence of MDD amounts to 15–18% in the general population [162, 163], that of bipolar disorder is only about 3% [164]. Both diseases are thought to be caused by the interaction of genetic, environmental, psychological and social factors. Especially long-lasting stress exposure is considered to be the main pathogenetic factor of MDD. Hence, routinely used rodent models of MDD are based on the delivery of acute (tail suspension test, TST; forced swim test, FST; foot shock combined with sucrose preference test, SPT) or chronic (chronic unpredictable mild stress, CUMS; chronic social defeat stress, CSDS) stressful stimuli [165, 166], although it is absolutely clear that acute in contrast to chronic stress does not lead to abnormal adaptive behaviors typical for MDD. The consequence of TST and FST is “behavioral despair,” manifesting itself in mice suspended by their tails or swimming in water, in prolongation of immobility due to giving up futile escape reactions. The classic rodent model of mania consists of the systemic application of amphetamine or ouabain and the measurement of increased locomotion [167].
5.2 Impeded Exocytotic ATP Release from Astrocytes in the Hippocampus and Medial (m)PFC are Causes of Depressive-Like Behavior
In the last couple of years, experimental work on rodent models has identified the gliotransmitter ATP as one of the primary executors for the development of depressive-like behavior [168-170]. Both decreased and increased ATP release is thought to be involved in the etiology of MDD; thus, the role of extracellular ATP by itself (or after degradation to adenosine) in the control of mood is multifaceted (see Sections 5.2–5.5). Furthermore, extracellular ATP concentrations in the brain of mice, that were susceptible to CSDS, were permanently low [11] (Figure 5). Blockade of vesicular ATP release from astrocytes either by knockout of IP3R2 or by generation of a transgenic dnSNARE mouse led to depressive-like reactions that could be rescued via the i.p. application of ATP. Moreover, the selective stimulation of ATP release from astrocytes of transgenic mice that expressed a Gq protein-coupled artificial receptor, induced antidepressant-like effects. Hypostimulation of P2X2Rs in the mPFC was supposed to be causally related to depressive-like behavior. In a follower paper of the same group of authors, the target of astrocyte-derived ATP was identified at P2X2Rs of mPFC pyramidal neurons [164]. Conditional knockout of P2X2Rs in these pyramidal neurons promoted resilience to chronic stress-induced depressive-like behaviors, and specific gain of P2X2Rs by their overexpression with adeno-associated virus (AAV)-P2X2 increased the vulnerability to such behaviors.
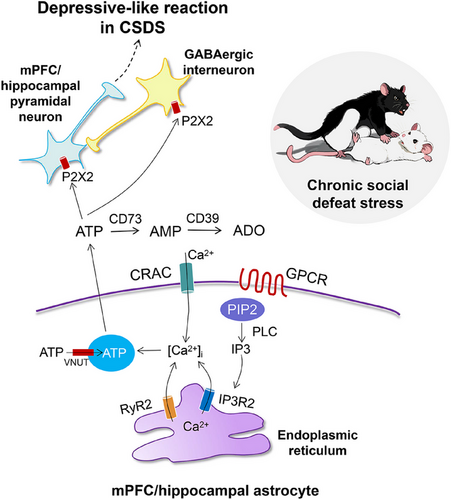
In perfect agreement with the above data, the blockade of the enzyme CD39 (ENTPD), which hydrolyses extracellular ATP, counteracted the degree of depressive-like behavior in the CSDS test [171]. Thus, increased ATP levels, in this case in the hippocampus by direct effect (but not by the indirect one through adenosine, see Section 5.5), beneficially modified the CSDS-induced depressive-like behavior of mice. Eventually, ex vivo slice electrophysiology documented that the observed extracellular ATP deficiency both in CSDS and IP3R2 KO mice reduced GABAergic inhibition and elevated excitability in lateral habenula-projecting, but not dorsal raphe-projecting mPFC neurons [172]. In contrast to previous results, it was found that GABAergic interneurons rather than pyramidal neurons were endowed with P2X2Rs and mediated ATPergic modulation of depressive-like behavior.
Classic antidepressive therapy with, for example, the selective 5-HT reuptake inhibitor fluoxetine might also act, at least partially, via increasing the pathologically low astrocytic ATP release [173]. ATP exocytosis relies on the unimpaired functioning of the VNUT. Fluoxetine-induced antidepressive-like behavior was decreased in mice, whose astrocyte-selective VNUT was genetically deleted, but was increased when astrocyte-selective VNUT was overexpressed. These findings suggest that in addition to neurons, fluoxetine acts on astrocytes and can mediate its therapeutic effect by increasing ATP gliotransmission. However, it has to be noted that of the routinely used classes of antidepressants, the tricyclic imipramine, but not the selective 5-HT reuptake inhibitor fluoxetine might exert an antidepressive-like effect by modulating astrogliogenesis in the dentate gyrus of rats exposed to CUMS [174].
Exocytotic gliotransmitter release may be due to Ca2+ influx triggered by ORAI1-3 channels. The calcium release-activated calcium channel protein 1 (CRAC1) is formed by the ORAI1-3 proteins and serves in non-neuronal cells store-operated Ca2+ entry [175]. The depletion of endoplasmic reticulum Ca2+ stores activates ORAI1-3 channels in the plasma membrane and thereby results in the replenishment of the intracellular Ca2+ stores. Knockout of this type of transmembrane channels has been reported to show amelioration of LPS-induced depression-like behaviors including learned helplessness and anhedonia [176] (for the participation of neuroinflammatory signaling in such behaviors see Section 5.4).
In clinical praxis, individuals with MDD frequently experience symptoms of anxiety or have comorbid anxiety disorders [177]. In this context it was interesting to learn that optogenetic hippocampal astrocyte activation elevating intracellular calcium, induced anxiolytic behavior in astrocyte-specific ChR2 transgenic mice [178]. ATP released from the activated hippocampal astrocytes increased excitatory synaptic transmission in dentate gyrus granule cells, which exerted anxiolytic effects.
5.3 Impeded Channel-Mediated ATP Release from Astrocytes in the Hippocampus and mPFC is a Cause of Depressive-Like Behavior
Ample evidence indicates that blockade of gap junctions and hemichannels induces depressive-like behavior in rodent models of MDD [179, 180]. For example, the expression of Cx30 and Cx43 was found to be significantly decreased in the mPFC and hippocampus of CSDS mice and was strongly associated with decreases in neuronal activity as measured by electrophysiological methods in slices prepared from these areas of the brain [181]. Moreover, overexpression of Cx30 and Cx43 in the mPFC and hippocampus increased neuronal activity and inhibited depressive-like behavior. The antimalarial drug mefloquine has frequent side-effects, such as depression and anxiety, and is known as an inhibitor of Panx-1 channels. In the CSDS mouse model of depressive-like behavior a decrease in the expression and function of Panx-1 channels has been observed in the mPFC of susceptible mice [182]. Pharmacological blockade of Panx-1 by carbenoxolone induced depressive-like behavior, which was prevented by preconditioning with ATP. Systemic and intra-mPFC injection of mefloquine inhibited the activity of Panx-1 and induced depressive-like and anxiety behaviors in mice.
As quite often, also in this case, there are two opposite results available in the literature. Dye uptake experiments in hippocampal slices revealed that acute restraint stress induces the opening of both Cx43 hemichannels and Panx-1 channels in astrocytes, which were further increased by chronic restraint stress [183]. Blockade of these channels reduced ATP and glutamate release from hippocampal slices of stressed mice. Why channel closure in one case and opening in the other induces depressive-like behavior is unclear, although it has to be mentioned that methodological differences (e.g., dye uptake versus electrophysiology) might be one of the explanatory factors.
In addition to connexin hemichannels and pannexin channels, other type of transmembrane channels may function in astrocytes as ATP secretory pathways. Such are the CALHM2 channels, which can directly mediate ATP release. Conventional knockout and conditional astrocyte-specific knockout of CALHM2 both led to significantly reduced ATP concentrations, loss of hippocampal spine number, and depression-like behaviors in mice [184]. All these reactions can be rescued by systemic ATP replenishment. It has been previously reported that inescapable foot shock caused an acute and persistent loss of spine synapses in all hippocampal subfields (CA1, CA3, dentate gyrus), which was associated with escape deficit in learned helplessness [185]. The analysis of single nucleotide polymorphism showed that the CALHM2 V136G small nucleotide polymorphism (SNP) is significantly associated with the ATP-release function of astrocytes and results in depressive-like behavior that is rescued by application of exogenous ATP [186].
5.4 Upregulation of P2X7 Receptor During Neuroinflammation is a Cause of Depressive- or Mania-Like Behavior
In extension of the previously reported data, we also refer herewith to results proving increased ATP release and the emergence of the P2X7R under neuro-inflammatory conditions, which is associated with stress-evoked depressive-like reactions.
Acute restraint stress rapidly increased the levels of extracellular ATP, inflammatory cytokines, and the active form of the NLRP3 inflammasome in the hippocampus of rats or mice [187]. Administration of P2X7R antagonists blocked the release of these cytokines and reversed also the anhedonic and anxiety behaviors caused by CUMS exposure. Moreover, deletion of the Nlrp3 gene, coding for the NLRP3 inflammasome rendered mice resistant to the development of depressive-like behaviors caused by CUMS. Similar effects were observed also by another group of authors, utilizing again chronic rodent models of MDD; they concluded that P2X7Rs located at hippocampal microglia rather than astrocytes mediate the depressive-like reactions [188]. Systemic injection of LPS initiated in mice an increase of the serum concentration of TNF-α, and increased the immobility time in TST and FST. Both the cytokine increase in the serum and the depressive-like responses were abrogated by the application of Brilliant Blue G, a selective P2X7R antagonist [189]. However, this experiment did not differentiate between microglia or astrocytes as sources of cytokine secretion.
P2X7Rs and the NLRP3 inflammatory pathway leading to neuroinflammation and in consequence major depression (depressive-like behavior) appears to be initiated not only by the activation of microglia, but most probably also by activated astrocytes [190, 191]. In fact, in a rodent model of MDD, in which chronic sleep deprivation induced depressive-like reactions, astrocytic, in contrast to microglial P2X7Rs turned out to be the major etiological factors [192]. Similarly, TST, FST, and SPT (after foot shock) determinations have shown in rodent models, the acquirement of P2×7R-medited behavioral despair and anhedonia reactions, respectively [161]. It was concluded by the use of drugs preferentially interfering with the function/metabolism/mitotic activity of microglia (minocycline), astrocytes (L-α-aminoadipate), and oligodendrocytes (cytosine-β-arabinoside), that pharmacological damage to microglia and astrocytes causes blockade of all types of acute depressive-like reactions, while injury to astrocytes inhibits only reactions induced by strong stressors, such as foot shock. When the expression profiles of mRNAs for Cx43, P2X7Rs, and 5’-nucleotidase were examined in cerebro-cortical and hippocampal astrocytes (identified by magnetic cell sorting), increased levels of these cellular constituents were noticed [193]. Thus, unequivocal evidence confirms the upregulation and behavioral involvement of P2X7Rs in rodents subsequent to acute or chronic stressful stimulation.
It is interesting to mention that brain-region specific alterations of epoxyeicosatrienoic acid (EET) signaling, which is an arachidonic acid metabolic pathway, was observed both in a mouse model of MDD (CSDS) and postmortem samples from patients with this disorder [194]. The enzymatic activity of soluble epoxide hydrolase (sHE), the key enzyme in EET signaling was selectively increased in the mPFC of susceptible mice after the application of CSDS, in an ATP release-dependent manner. Actually, sHE was primarily expressed in the lysosomes of astrocytes suggesting their involvement in vesicular ATP secretion. Accordingly; higher expression of sHE protein was found in the postmortem brain samples of patients with depression [195]. A study in women with gestational diabetes mellitus found association between depressive symptoms and several SNPs of epoxide hydrolase-2 encoding sHE [196].
Astrocytes highly express the VNUT, which takes up ATP into storage vesicles or secretory lysosomes of neurons/astrocytes [197]. Another ω-3-polyunsaturated fatty acid, eicosapentaenoic acid (EPA) has been shown to inhibit VNUT, thereby impairing vesicular ATP release from neurons, without affecting the vesicular release of other neurotransmitters. EPA potently attenuated neuropathic and inflammatory pain in wild-type mice but not in VNUT−/− mice [198]. In addition, ω-3-polyunsaturated fatty acids, especially EPA have been shown to have an overall beneficial effect on depression symptoms as reported for humans [199]. Hence, we tentatively suggest that the improvement by EET in CSDS-induced depressive-like behavior depends on the inhibition of the vesicular ATP release.
In contrast to MDD and depressive-like behavior, only few animal studies dealt with the participation of P2X7Rs in the rodent, amphetamine-model of mania, although they were without exception affirmative [200-203]. However, none of these studies indicated that astrocytic rather than neuronal P2X7Rs are the targets of endogenous ATP.
5.5 Changes in Adenosine Levels Arising from the Degradation of Astrocytic ATP are Causes of Depressive-Like Behavior
The enzymatic degradation product of ATP, adenosine has also been reported to be involved in the induction of depressive-like behavior. In agreement with the finding that purinergic signaling orchestrates neuron-glia communication [96, 204], modulation of adenosine synthesis, transport, catabolism, and receptors, all affected responses to acute or chronic stress in rodent models of MDD [205-208]. However, in these cases there was no indication for the astrocytic release of ATP identified as a source of adenosine or the astrocytic location of, for example, A1Rs in question [209-211]. By contrast, the systemic administration of the selective A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) counteracted the antidepressant activity of the NMDA-R antagonistic ketamine in the mouse FST by a purely neuronal mechanism [212].
Peripheral injection of LPS to mice evoked systemic inflammation, and rapidly increased the plasma levels of adenosine, triggering astrocyte reactivity via A1R activation [213]. The stimulation of A1Rs on their behalf increased the levels of inflammatory factors, thereby alleviating microglial reactions, causing among others depression-like behavior. A1R deficiency in astrocytes inhibited these effects, while chemogenetic stimulation of Gi protein signaling restored neuroinflammation and depressive-like behavior. Although the astrocytic source of ATP was not proven either, a particularly impactful study still deserves mentioning [160]. In chronically restraint stressed rats, an increased release of ATP, its extracellular catabolism through CD73 to adenosine, and the subsequent overactivation of A2ARs in the frontocortical and hippocampal regions of rats was demonstrated [160]. Synaptosomes prepared from the mentioned areas of restrain stressed rats exhibited increased ATP release on depolarization by elevated extracellular potassium concentration. The continuous intracerebroventricular delivery of the CD73 inhibitor α,βme-ATP during the restraint stress procedure attenuated mood and memory dysfunction and also prevented the otherwise decreased LTP in the prefrontocortical layer II/III-layer V, as well the hippocampal CA3–CA1 synapses. These findings are of great interest because of two reasons: (1) an increased rather than a decreased ATP release underlies the stress-induced depression-like reactions; and (2) the CD73-produced adenosine acting via A2ARs is the cause of the interference with normal mood and cognitive behavior (Figure 6; see also [208]).
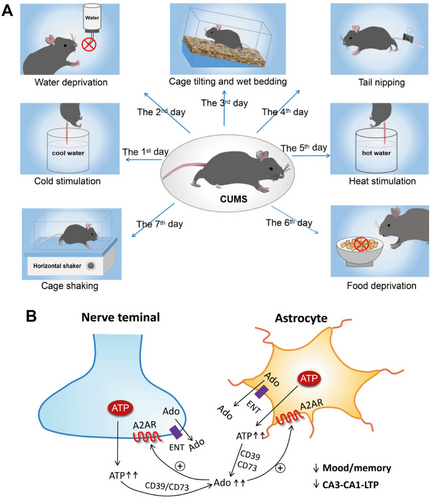
Stress-induced depressive-like reactions in mice TST and chronic restraint stress appear to be mediated by GABAergic A2AR-containing neurons localized in the lateral septum region of the brain, via projections sent to the dorsomedial hypothalamus and the lateral habenula [215]. However, A2ARs might be expressed both by astrocytes and microglia in the CA1 area of the ventral hippocampus participating in the anxiodepressive consequences of cheek pain. The astrocytic metabolic inhibitor fluorocitrate, the CD39 (ENTPD) inhibitor ARL 67156 and A2AR blockade attenuated the increases of extracellular ATP and adenosine, and the consequent pain-related anxiodepression [216].
6 P2X7 Receptor-Related Diagnostic and Therapeutic Maneuvers
6.1 Diagnostic Use of Blood Plasma or Serum Determination of P2X7 Receptors
A wealth of publications reports that P2X7Rs participate in various neurodegenerative diseases in general [217-219] and AD in particular [58, 79, 220]. All these articles unequivocally conclude that such CNS illnesses lead to increased levels of P2X7Rs accompanying neuroinflammation, inevitably superimposed on the core causative factor (usually the extracellular deposits of pathological protein aggregates).
It was shown as early as 2006 that stimulation of the P2X7R by its prototypic agonist dibenzoyl-ATP (Bz-ATP) in dendritic cells (DCs; professional antigen-presenting cells initiating the immune response) kept in culture led to fast microvesicle shedding from the DC plasma membrane [221]. These microvesicles contained P2X7Rs, major histocompatibility complex II, and CD39. Based on that, two parallel studies identified in blood plasma/serum of temporal lobe epileptic (TLE; [222]) or COVID-19 [223] patients, the increased level of soluble or shed (s) trimeric/monomeric P2X7Rs [224]. Soon afterward it was reported that AD patients also exhibit higher plasma concentrations of sP2X7R [225]. Hence, in CNS diseases (TLE, AD) and mixed central and peripheral illnesses (COVID-19) a uniform increase of the (neuro)inflammatory marker P2X7R was verified. Independent of these experiments, the blood levels of sP2X7Rs were shown to accord with the classic inflammatory marker C reactive protein [226]. Further, in this latter study the sP2X7R in the plasma was largely associated with microvesicles. Sustained activation of P2X7Rs apparently triggered the release of matrix metalloproteinase 2-dependent receptor cleavage [227]. Thus, the sP2X7R might be a full receptor protein, its single subunit, or only the ectodomain of the receptor.
6.2 Radioligand Targeting of P2X7Receptors
The in vivo imaging techniques positron emission tomography (PET) and single photon emission computed tomography (SPECT) are methods with high sensitivity for diagnosis and treatment-monitoring of P2X7R-related diseases [228, 229]. PET surmounts SPECT in its sensitivity, and has usually used to recognize a 18 kDa translocator protein (TSPO) as a marker for inflammatory changes of the microglial activation state [230]. However, TSPO had high inter-subject variability in binding affinity, and nonspecific binding in the human brain due to TSPO polymorphism. This has necessitated the need to look for an alternative neuroinflammatory marker, which was found in the P2X7R [231]. Tritium (3H), carbon (11C), fluorine (18F), and iodine (123J) were used for labelling radioligands targeting P2X7Rs [228]. These radioligands were allosteric rather than orthosteric antagonists, with only [11C]GSK1482160, [18F]JNJ-64413739, and [11C]SMW139 used in human studies [232-234]. The simultaneous application of shed P2X7 (sP2X7R) determination and the PET measurement of this receptor has a higher predictive value than that of only one of the methods.
6.3 P2X7 Receptors as Therapeutic Targets
We mentioned already previously that P2X7Rs are likely therapeutic targets for neurodegenerative diseases causing cognitive impairment and/or affective disturbances in animal models of these diseases [103, 235, 236]. P2X7R antagonists block the release of proinflammatory cytokines from macrophages and have been intensively investigated in peripheral autoimmune illnesses such as rheumatoid arthritis [237, 238] and Crohn's disease [239]. Nonetheless, these clinical studies were considered to be unsatisfactory, and were terminated [227, 240], although in moderate to severe Crohn's disease a favorable risk profile was observed [239]. Diverse pharmaceutical companies still continued their research activities for a couple of indications (rheumatoid arthritis, neuropathic pain, age-related retinal degeneration, diabetic retinopathy, glaucoma), but again without the expected success [224]. However, in a double-blind, placebo controlled, randomized clinical study, the blood–brain-permeable P2X7R antagonist, JNJ-54175446 albeit having no significant effects on mood perturbed by total sleep deprivation, reduced anhedonia caused by this manipulation [241].
Hence, the development of therapeutic agents to cure either central or peripheral inflammatory diseases, turned out to be of questionable value, although antagonists with high blood–brain barrier (BBB) permeability, and excellent peroral adsorption have been used for CNS therapeutic indications [242, 243]. Alternative approaches, with nanobodies (heavy chain antibodies) exhibiting high selectivity for P2X7Rs were not superior to the small molecular pharmacological antagonists, because in this case both the poor enteral adsorption and BBB permeability were serious hindrances [244, 245].
In spite of these mostly negative experiences with P2X7R antagonists, there is still room for further clinical studies, especially for the applicability in the case of CNS illnesses, such as cognitive and mood disorders, where experiments with rodents promise considerable beneficial effect. Unfortunately, AD and MDD investigations are most tedious, because the respective diseases are of chronic nature and a treatment has to be carried out over a long period of time.
Subgroups of patients, which are resistant to classic therapeutic approaches, might still respond to combinations of P2X7R antagonists with the presently known antidepressive or cognition enhancing drugs. Eventually, alternative therapeutic maneuvers in humans would be either the selective blockade of ATP release from microglia/astrocytes, or an interference with the multiple transduction mechanisms triggered by P2X7R activation. One of these latter possibilities would be the blockade of the inflammasome activation, which is an emerging field to combat neurodegenerative diseases. With respect to the blockade of the release of ATP and other gliotransmitters from astrocytes, metabolic toxins (L-α-aminoadipate, fluorocitric acid) were repeatedly used in animal experimentation, raising the hope that nontoxic substances with similar effects might also be developed for clinical praxis.
6.4 A2A Receptors as Therapeutic Targets
The A2AR appears to be involved in the pathophysiology of AD as already discussed in detail in Section 4.8. Approved drugs for AD primarily increase acetylcholine transmission and reduce glutamate excitotoxicity (donezepil, rivastigmine, galantamine, memantine [246, 247]). Moreover, recently a novel antibody decreasing extraneuronal Aβ protein accumulation in the brain, called aducanumab, has been approved by the US Food and Drug Administration (US FDA; [248]). However, with the first classes of drugs only a mild symptomatic treatment can be expected to occur, while aducanumab has only a marginal, although assumedly causative therapeutic effect. The chronic ingestion of caffeine, a most consumed preferential A2AR antagonist contained in coffee, results in decreased hippocampal tau hyperphosphorylation and neuroinflammation, as well as improvement of memory deficits [45, 249]. Accordingly, selective A2AR antagonists, such as istradefylline, applied over a short period (3 weeks) was found to restore memory performance in different rodent models of amyloid pathology [150, 250]. In consequence, there is sufficient experimental evidence to turn to the US FDA approved drug istradefylline (Nourianz) in clinical studies to investigate its possible anti-AD effect.
In fact, istradefylline has been introduced as an adjuvant therapy to L-DOPA in PD [251, 252]. Motor deficits characteristic of PD involve an overactivation of the striatopallidal afferent pathway of the dorsal striatum, due to the degeneration of dopaminergic projection neurons in the substantia nigra pars compacta [246]. A2AR antagonists inhibit striatal D2R binding, possibly through A2AR-D2R heteromers [253]. While PD is primarily characterized by motor symptoms, cognitive dysfunction also occurs both in the early and later stages of the disease process [254]. Recent studies demonstrated that the A2AR antagonists reversed working memory impairments in animal models of PD [255]. Unfortunately, the effect of istradefylline on cognition was hitherto not tested in PD patients; this however, should be made up leeway in the future.
7 Conclusions and Prospects
In the CNS, purinergic cotransmission may modulate the release/effect of the main transmitters (e.g., glutamate, GABA), but the primary modulatory function in this respect appears to be reserved for astrocyte-derived ATP and its enzymatic degradation product adenosine. Two important functions of the brain, cognition, and emotion are intimately regulated by the gliotransmitters ATP/adenosine. While cognitive and emotional disturbances are typical human disorders, experimental medicine makes use of various animal models, which, although incompletely, are able to model these diseases. In consequence, the aim of the present review was to elucidate the involvement of astrocyte-neuron interactions via the gliotransmitter ATP and its metabolite adenosine, in hippocampal and prefronto-cortical cognitive and affective processes.
Repetitive stimulation of the neuronal input of many synapses in the CNS results in LTP/LTD, regarded as cellular equivalent for learning and memory. ATP and adenosine can up- or downregulate both the changes in synaptic strength and the consequent learning performance in the respective animal (usually rodent) models. The hippocampus, which acts as a sieve and transformer for stimuli entering from the entorhinal cortex this tri-neuronal system (dentate gyrus, CA3, and CA1 pyramidal cells) and leaving it via axonal projections to various areas of the brain, is crucially involved in cognitive and emotional regulation. In the case of affective disorders (MDD, bipolar disorder), the eminent significance of a unidirectional projection connecting the hippocampus with the mPFC has to be taken into consideration. Difficulties encountered with the use of rodent models are still more prominent in relation to affective disorders, than in case of the cognitive ones. Most of these latter models are based on the application of acute stressful stimuli, although the human disorder MDD is caused by the interplay of chronic environmental/social stress and genetic factors. We pay particular attention to animal models because the present review reports data raised experimentally and only by extrapolation generates clinically relevant data.
After recognizing astrocytic modulation by the “purinome” (purinergic transmitters, their astrocytic release mechanisms, their receptors and synthesizing/decomposing enzymes) of cognitive processes, we discussed the dual hypothesis of depression-like behavior by increased or decreased levels of ATP in the hippocampus/mPFC. Both of these opposing changes might be of astrocytic origin, although the former is due to P2X7R-mediated modulation of pyramidal cell function, while the latter is due to activation of the apoptotic/necrotic/inflammatory P2X7R. The involvement of astrocyte-derived adenosine in cognitive deterioration or affective disorders has been hitherto proven only in a few cases, but this might be due to the limited interest directed to this specific question.
Animal experimentation has always the aim of clarifying some clinically important issues, and, therefore, the numerous caveats associated with the animal models are an apparent drawback. Thus, on the one hand, further clinical studies and work with postmortem human material is expected to generate relevant findings, and on the other hand, new techniques on the subcellular (transcriptomics, metabolomics), but also on the whole animal/human level (refined imaging methods) are expected to become particularly helpful. Eventually, a new and most promising field is the measurement of blood plasma levels of sP2X7Rs as a diagnostic tool to identify (neuro)inflammation.
Author Contributions
Peter Illes has written the first version of the manuscript. Patrizia Rubini, Henning Ulrich, Hai-Yan Yin, and Yong Tang improved and extended the paper. All authors consented with the final version.
Acknowledgments
Open access funding enabled and organized by Projekt DEAL.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
Author Peter Illes is an Editorial Board Member of MedComm. He was not involved in the journal's review of or decision related to this manuscript. All authors declared no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




