Low-dose ionizing radiation-induced RET/PTC1 rearrangement via the non-homologous end joining pathway to drive thyroid cancer
Yuhao Liu, Jiaojiao Zhu, and Shenghui Zhou contributed equally to this work.
Abstract
Thyroid cancer incidence increases worldwide annually, primarily due to factors such as ionizing radiation (IR), iodine intake, and genetics. Papillary carcinoma of the thyroid (PTC) accounts for about 80% of thyroid cancer cases. RET/PTC1 (coiled-coil domain containing 6 [CCDC6]-rearranged during transfection) rearrangement is a distinctive feature in over 70% of thyroid cancers who exposed to low doses of IR in Chernobyl and Hiroshima‒Nagasaki atomic bombings. This study aims to elucidate mechanism between RET/PTC1 rearrangement and IR in PTC. N-thy-ori-3-1 cells were subjected to varying doses of IR (2/1/0.5/0.2/0.1/0.05 Gy) of IR at different days, and result showed low-dose IR-induced RET/PTC1 rearrangement in a dose-dependent manner. RET/PTC1 has been observed to promote PTC both in vivo and in vitro. To delineate the role of different DNA repair pathways, SCR7, RI-1, and Olaparib were employed to inhibit non-homologous end joining (NHEJ), homologous recombination (HR), and microhomology-mediated end joining (MMEJ), respectively. Notably, inhibiting NHEJ enhanced HR repair efficiency and reduced IR-induced RET/PTC1 rearrangement. Conversely, inhibiting HR increased NHEJ repair efficiency and subsequent RET/PTC1 rearrangement. The MMEJ did not show a markable role in this progress. Additionally, inhibiting DNA-dependent protein kinase catalytic subunit (DNA-PKcs) decreased the efficiency of NHEJ and thus reduced IR-induced RET/PTC1 rearrangement. To conclude, the data suggest that NHEJ, rather than HR or MMEJ, is the critical cause of IR-induced RET/PTC1 rearrangement. Targeting DNA-PKcs to inhibit the NHEJ has emerged as a promising therapeutic strategy for addressing IR-induced RET/PTC1 rearrangement in PTC.
1 INTRODUCTION
Thyroid cancer is the most common endocrine cancer, and its incidence increases worldwide every year. The causes of thyroid cancer include iodine intake, genetics, and ionizing radiation (IR).1-3 Papillary carcinoma of the thyroid (PTC) accounts for about 80% of thyroid cancer cases. Numerous studies have demonstrated that the thyroid is sensitive to IR3; especially, exposure to IR during childhood or adolescence increases the risk of PTC.4, 5 Besides, among the survivors of Chernobyl and Hiroshima–Nagasaki atomic bombings, a significant increase in thyroid cancer incidence in adulthood was found after exposure to low doses of IR during adolescence and over 70% of thyroid cancers were accompanied by a distinctive feature: RET/PTC1 rearrangements.6-8 RET/PTC rearrangement is a prevalent genetic alteration observed in PTC and results in the oncogenic rearrangement of the RET gene. It is estimated that this fusion gene accounts for approximately 20%−40% of adult sporadic PTC cases.9-11 RET/PTC1 is a fusion protein formed by the fusion of the tyrosine kinase domain of the RET proto-oncogene, which is situated on human chromosome 10, with the H4 gene (also known as CCDC6), located at 10q21 on the same chromosome. Chromosomal rearrangement always results from DNA recombination, repair, and so on.12, 13 RET/PTC rearrangements were observed in immortalized human thyroid cells (HTori-3) after gamma radiation exposure.14 The detailed mechanism between RET/PTC1 rearrangement and DNA damage repair has not been reported.
Generally, rearrangement arises from the DNA damage repair after a double-strand break (DSB). DNA DSB always resulted in reactive oxygen species from IR, oxidative metabolism, and chemical agents. The mechanisms of chromosomal rearrangement, including canonical non-homologous end joining (NHEJ) and microhomology-mediated end joining (MMEJ), have been well discussed.15-19 NHEJ has been identified that can form different rearrangements, such as translocations, inversions, and deletions.12, 16, 20 Whether NHEJ could participate in the RET/PTC rearrangement is not very clear. DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is a serine/threonine protein kinase that is a critical regulator of NHEJ, which is involved in DNA damage repair. It has been widely discussed in DNA damage repair,21 tumors, and natural immunity.22, 23 The RET chromosomal rearrangement induced by low dose of IR has not been reported to be associated with DNA-PKcs.
This study aimed to investigate the function and mechanism of low-dose IR-induced RET/PTC1 rearrangement in PTC. We performed nested PCR on normal human thyroid follicular epithelial cell line (N-thy-ori-3-1) treated with different low dose of IR to determine the RET/PTC1 rearrangement corresponding to low dose of IR and performed in vitro and in vivo functional validation to identify the function of RET/PTC1 rearrangement to PTC. Mechanistically, we investigated the key role of NHEJ in RET/PTC1 rearrangement and the specific regulatory relationships between NHEJ and DNA-PKcs in normal human thyroid follicular epithelial cells exposed to low-dose IR.
2 RESULTS
2.1 IR induces RET/PTC1 rearrangement in thyroid follicular epithelial cells in a dose-dependent manner
It could be detected when RET/PTC1 rearrangement occurs in one cell and is mixed in 106 cells (Figure S2A,B). RET/PTC1 rearrangement was not observed in either 0.2 or 0.5 Gy after 3 days of exposure to IR (Figure S2C). Three RET/PTC1 rearrangement-positive wells were detected in the 0.2 Gy group, six RET/PTC1 rearrangement-positive wells were detected in the 0.5 Gy group at the sixth day (Figure S2C), and 6 days was determined as the collection time in the following experiments.
To further identify the association between the dose of IR and the number of RET/PTC1 rearrangement-positive wells in normal human thyroid epithelial cells, N-thy-ori-3-1 cells were exposed to five doses (2/1/0.5/0.2/0.1/0.05 Gy), and detected the RET/PTC1 rearrangement at sixth day after IR (Figure 1A and Table 1). The result showed that the RET/PTC1 rearrangement increased after IR in a dose-dependent manner (Figure 1B,C). Therefore, IR-induced RET/PTC1 rearrangement might be a potential mechanism of IR-induced PTC.
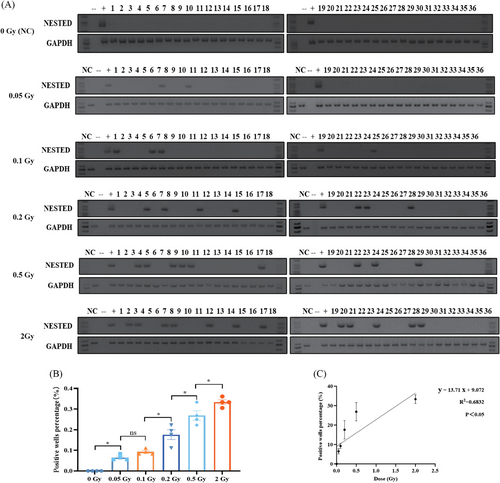
| Treatments | Wells tested | Wells RET/PTC1 positives | RET/PTC1 (%) | |
|---|---|---|---|---|
| Experiment 1 | Control | 36 | 0 | 0 |
| 0.05 Gy | 36 | 3 | 8.3 | |
| 0.1 Gy | 36 | 4 | 11.1 | |
| 0.2 Gy | 36 | 7 | 19.4 | |
| 0.5 Gy | 36 | 9 | 25 | |
| 2 Gy | 36 | 12 | 33.3 | |
| Experiment 2 | Control | 36 | 0 | 0 |
| 0.05 Gy | 36 | 3 | 8.3 | |
| 0.1 Gy | 36 | 3 | 8.3 | |
| 0.2 Gy | 36 | 4 | 11.1 | |
| 0.5 Gy | 36 | 8 | 22.2 | |
| 2 Gy | 36 | 11 | 30.6 | |
| Experiment 3 | Control | 36 | 0 | 0 |
| 0.05 Gy | 36 | 2 | 5.6 | |
| 0.1 Gy | 36 | 3 | 8.3 | |
| 0.2 Gy | 36 | 8 | 22 | |
| 0.5 Gy | 36 | 9 | 25 | |
| 2 Gy | 36 | 13 | 36.1 |
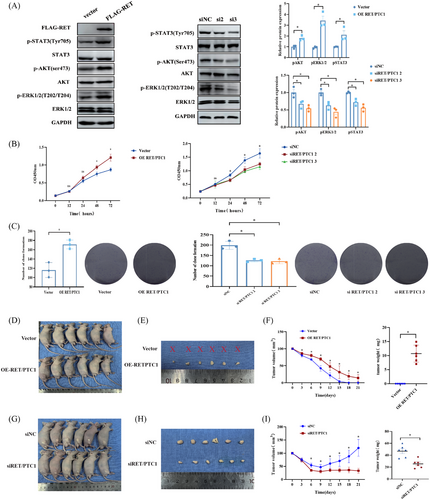
2.2 RET/PTC1 rearrangement promotes PTC tumorigenesis and progression in vitro and in vivo
We further assessed function of RET/PTC1 rearrangement in PTC and discovered that over-expression of RET/PTC1 in the N-thy-ori-3-1 cells could activate AKT, ERK, and STAT3, all of which have been shown to be oncogenesis key signals that can be activated by RET/PTC1 (Figures 2A and S3A). In contrast, the inhibition of RET/PTC1 in the TPC-1 cells could inactivate these oncogenesis signals (Figure 2A and S3B). In vitro, over-expression of RET/PTC1 in N-thy-ori 3-1 cells markedly increased the cell proliferation, as determined via cell counting assays (CCK-8) and plate colony formation assays (Figure 2B,C). However, the CCK-8 was used to identify that RET/PTC1 deletion could reduce the cell proliferation (Figure 2B). Concordantly, RET/PTC1 depletion also decreased the number of cell colonies, as determined by plate colony formation assays (Figure 2C). Furthermore, we verified the oncogenicity of RET/PTC1 in vivo. We observed that over-expression of RET/PTC1 in N-thy-ori 3-1 cells leads to a significant increase in the growth of subcutaneous xenograft tumors in nude mice (Figure 2D), including the sizes and weights of tumors (Figure 2E,F). In addition, knockdown of RET/PTC1 in TPC-1 cells leads to a significant reduction in the growth of subcutaneous xenograft tumors in nude mice (Figure 2H,I). These results indicated that RET/PTC1 drives the formation and progression of PTC in vitro and in vivo.
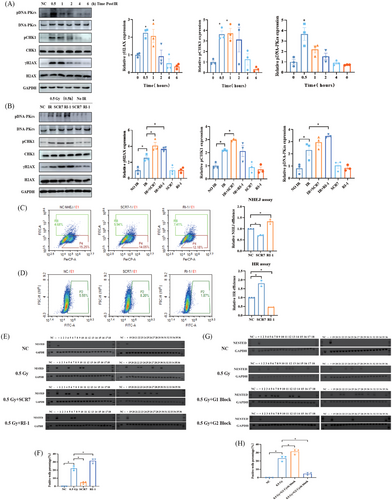
2.3 NHEJ repair contributes to RET/PTC1 rearrangement induced by IR in thyroid follicular epithelial cells
To determine the underlying mechanism of RET/PTC1 rearrangement, we found that γ-H2AX and pCHK1 were highly expressed at 0.5 h exposed with 0.5 Gy IR, indicating that DNA damage reached its maximum after 0.5 h of IR (Figure 3A). The NHEJ repair pathway inhibitor SCR7 and the homologous recombination (HR) repair pathway inhibitor RI-1 were employed to target DNA ligase IV and Rad51, respectively, which are key proteins in both pathways. A more increase in γ-H2AX expression was detected after 0.5 Gy of IR combining use of SCR7 and RI-1 than IR, and pCHK1 showed a same increase after the use of NHEJ inhibitors (Figure 3B). NHEJ and HR repair efficiency experiments showed that RI-1 decreased the HR repair efficiency and increased the NHEJ repair efficiency (Figure 3C). Conversely, SCR7 decreased the NHEJ repair efficiency and increased the HR repair efficiency (Figure 3D). These findings indicate that NHEJ inhibition promoted HR repair and that HR increased that's of NHEJ (Figure 3C,D). Next, we investigated whether the DNA damage repair efficiency can regulate the rate of RET/PTC1 arrangement. The results showed that the number of RET/PTC1 rearrangement-positive wells significantly increased after HR inhibition and decreased after NHEJ inhibition (Figure 3E,F and Table 2). In addition to canonical NHEJ and HR, the MMEJ is also an important DNA damage repair mechanism. To ascertain the role of MMEJ on RET/PTC1 rearrangement induced by IR, the MMEJ inhibitor Olaparib was employed to treat N-thy-ori-3-1 cells. Compared with NC group, the NHEJ and HR repair efficiency showed no significant alterations after treated with Olaparib (Figure S4A). Furthermore, the positive rate of IR-induced RET/PTC1 rearrangement remained unmodified upon Olaparib treatment (Figure S4B,C). Additionally, the combining use of SCR7 and RI-1 could also reduce the formation of RET/PTC1 induced by IR, and this reduction extend is consistent with the use of SCR7 (Figure S4B,C and Table S1). These findings indicted that the NHEJ not HR or MMEJ is the cause of IR-induced RET/PTC1 rearrangement.
| Treatments | Wells tested | Wells RET/PTC1 positives | RET/PTC1 (%) | |
|---|---|---|---|---|
| Experiment 1 | Control | 36 | 0 | 0 |
| 0. 5 Gy | 36 | 7 | 19.4 | |
| 0.5 Gy + SCR7 | 36 | 1 | 2.8 | |
| 0.5 Gy + RI-1 | 36 | 10 | 27.8 | |
| Experiment 2 | Control | 36 | 0 | 0 |
| 0.05 Gy | 36 | 9 | 25 | |
| 0.5 Gy + SCR7 | 36 | 2 | 5.6 | |
| 0.5 Gy + RI-1 | 36 | 12 | 33.3 | |
| Experiment 3 | Control | 36 | 0 | 0 |
| 0.05 Gy | 36 | 9 | 25 | |
| 0.5 Gy + SCR7 | 36 | 2 | 5.6 | |
| 0.5 Gy + RI-1 | 36 | 12 | 33.3 |
To further elucidate the involvement of NHEJ in RET/PTC1 rearrangement induced by IR. Studies have shown that the cell cycle phase is associated with the selective of DNA damage repair mechanisms: cells in the G1 phase predominantly utilize the NHEJ pathway, whereas cells in the G2 phase are more inclined to employ the HR pathway.24, 25 Therefore, the N-thy-ori-3-1 cells were treated with thymidine to achieve cell synchronization, and the G1 and G2 phase cells were separately exposed to IR (Figure S5A–C). Results indicated a significant increase in RET/PTC1 rearrangement in G1 phase cells compared to the control IR group (Figure 3G,H). In contrast, a notable decrease in RET/PTC1 rearrangement was observed in G2 phase cells relative to the IR group (Figure 3G,H and Table 3). These findings indicate that the induction of RET/PTC1 rearrangement by IR is specific to the G1 phase, emphasizing the role of NHEJ in this process.
| Treatments | Wells tested | Wells RET/PTC1 positives | RET/PTC1 (%) | |
|---|---|---|---|---|
| Experiment 1 | Control | 36 | 0 | 0 |
| 0.5 Gy | 36 | 9 | 25 | |
| 0.5 Gy + G1 block | 36 | 15 | 41.7 | |
| 0.5 Gy + G2 block | 36 | 3 | 8.3 | |
| Experiment 2 | Control | 36 | 0 | 0 |
| 0. 5 Gy | 36 | 9 | 25 | |
| 0.5 Gy + G1 block | 36 | 12 | 33.3 | |
| 0.5 Gy + G2 block | 36 | 1 | 2.8 | |
| Experiment 3 | Control | 36 | 0 | 0 |
| 0. 5 Gy | 36 | 10 | 27.8 | |
| 0.5 Gy + G1 block | 36 | 15 | 41.7 | |
| 0.5 Gy + G2 block | 36 | 1 | 2.8 |
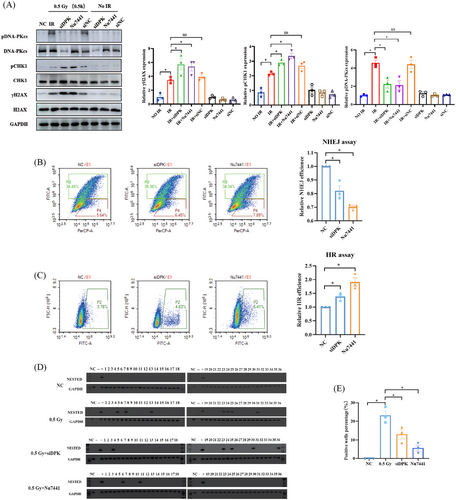
2.4 DNA-PKcs-mediated NHEJ contributes to IR-induced RET/PTC1 rearrangement in thyroid follicular epithelial cells
In view of the pivotal role of DNA-PKcs in response to IR and NHEJ, we found that the pDNA-PKcs was highly expressed at 0.5 Gy after 0.5 h (Figure 3A). Inhibition of DNA-PKcs with siRNA-DNA-PKcs (siDPK) and kinase inhibitor Nu7441 could significantly promoted the IR-induced pCHK1 expression increase (Figure 4A) and decrease the NHEJ repair efficiency (Figure 4A–C). To investigate the role of DNA-PKcs in IR-induced RET/PTC1 rearrangement generation, N-thy-ori-3-1 cells were subjected to simultaneous DNA-PKcs inhibition and 0.5 Gy IR treatment using siDPK or Nu7441. The results showed a significant decrease in the number of RET/PTC1 rearrangement-positive wells following DNA-PKcs inhibition (Figure 4D,E and Table 4), suggesting that DNA-PKcs-mediated NHEJ was critical for the occurrence of RET/PTC1 rearrangements induced by IR. Taken together, these results demonstrated that DNA-PKcs-mediated NHEJ plays a crucial role in the occurrence of IR-induced RET/PTC1 rearrangement.
| Treatments | Wells tested | Wells RET/PTC1 positives | RET/PTC1 (%) | |
|---|---|---|---|---|
| Experiment 1 | Control | 36 | 0 | 0 |
| 0.5 Gy | 36 | 7 | 19.4 | |
| 0.5 Gy + siDPK | 36 | 5 | 13.9 | |
| 0.5 Gy + Nu7441 | 36 | 2 | 5.6 | |
| Experiment 2 | Control | 36 | 0 | 0 |
| 0.05 Gy | 36 | 8 | 22.2 | |
| 0.5 Gy + siDPK | 36 | 3 | 8.3 | |
| 0.5 Gy + Nu7441 | 36 | 3 | 8.3 | |
| Experiment 3 | Control | 36 | 0 | 0 |
| 0.05 Gy | 36 | 10 | 27.8 | |
| 0.5 Gy + siDPK | 36 | 6 | 16.7 | |
| 0.5 Gy + Nu7441 | 36 | 1 | 2.8 |
3 DISCUSSION
The escalating levels of global environmental pollutants, such as metal ions,26 air pollution,27 and endocrine-disrupting chemicals,28 have been associated with a rise in thyroid cancer incidence both directly and indirectly. Thyroid cancer is a well-known risk associated with exposure to IR,14 in addition to abnormal iodine consumption29 and family history.30 It has been observed that workers in the nuclear industry as well as doctors practicing nuclear medicine have also been exposed to such radiation following events such as Chernobyl and Fukushima.31 After the Chernobyl incident, there has been a significant surge in thyroid cancer occurrences, particularly PTC, which exhibited chromosomal rearrangements.31, 32 Conventional chromosomal rearrangements and the subsequent cellular consequences have been extensively recorded. However, the alarming rise in thyroid cancer incidence without a corresponding increase in mortality, may be attributed to the overdiagnosis of thyroid nodules detected by ultrasound.33-35 This issue of overdiagnosis has been observed in various geographical regions, including areas outside the contaminated zones following the Chernobyl accident, where there has been a heightened awareness and increased use of diagnostic imaging.
There are two types of chromosomal rearrangements in thyroid follicular epithelial cells caused by IR: RET/PTC1 and RET/PTC3.14 Previous reports indicate that RET/PTC1 occurs more frequently after exposing with IR.36 Nonetheless, the precise origins of thyroid cancer rearrangements remain unknown, with only the triggering conditions being outlined rather than the mechanism.37-39 Our findings revealed that even at low dose of IR (0.05 Gy) could induce RET/PTC1 rearrangement in normal thyroid follicular epithelial cells. And there is a dose-dependent manner of IR induced RET/PTC1 rearrangement, which is consistent with previous study.14 Notably, previous studies have shown that RET/PTC1 rearrangement can be detected at 914 or 1440 days after IR, whereas our study found that they can be detected at sixth day. This difference in detection time may be due to the limitation of the number of RET/PTC1 rearrangement-positive cells. RET/PTC1 rearrangement has been confirmed in many PTC cases and thought to be a tumor driver gene.41-43 In this study, overexpression of RET/PTC1 induced the N-thy-ori-3-1 cells to have clonal and tumorigenic ability. We confirmed the carcinogenesis of RET/PTC1 rearrangement in PTC through both in vivo and in vitro experiments.
The RET/PTC1 rearrangement is a fusion gene resulting from RET and CCDC6. The fusion may occur due to a chromosome break caused by IR which ultimately leads to the formation of RET/PTC1.44-46 The potential regulatory mechanism responsible for this fusion gene in IR-induced PTC has yet to be determined. In general, the fusion genes always form from two genes whose proximity is located at chromosome through DNA damage repair following a DNA DSB. DNA DSBs can be caused by both extrinsic and intrinsic factors. Extrinsic factors include sources such as IR and reactive oxygen species. Intrinsic factors include examples such as DNA replication errors and V(D)J recombination.21, 47-49 NHEJ is the main pathway for repairing of two-ended DSBs and has been identified that can form different rearrangements such as translocations, inversions, and deletions.50, 51 In addition to the well-established DNA damage repair pathways, such as HR and NHEJ, there are also non-canonical end joining pathways, such as MMEJ, which might be a potentially regulator of chromosomal rearrangements.21 To date, no studies have been found that the association between RET/PTC1 and NHEJ after IR. Here, we employed three specific inhibitors: SCR7 to inhibit NHEJ, RI-1 to obstruct HR, and Olaparib to inhibit MMEJ. We observed that the number of RET/PTC1 rearrangement decreased when NHEJ was inhibited by SCR7 after exposure to 0.5 Gy IR, while it increased with RI-1. No significant alteration in RET/PTC1 rearrangements was detected with Olaparib after 0.5 Gy IR exposure. Thus, our results suggested that not HR or MMEJ but NHEJ plays a crucial role in the formation of RTE/PTC1 rearrangements induced by low dose of IR.
DNA-PK is recognized as a critical protein kinase in the NHEJ pathway, primarily due to its essential role in the assembly of the holoenzyme complex. This assembly is vital for recruiting NHEJ-related ligases and repair proteins, such as DNA ligase IV, XRCC4, XRCC4-like factor, and Artemis.52, 53 It is generally believed that heterodimers composed of Ku70 and Ku80 first recognize DSBs, subsequently recruiting and activating DNA-PKcs.54-57 DNA-PKcs, a serine/threonine kinase, has been demonstrated to phosphorylate various proteins in vitro, such as p53, transcription factors, RNA polymerases, and Ku70/Ku80.22, 58-60 Despite DNA-PKcs's crucial role in NHEJ, its involvement in IR-induced chromosome rearrangement in thyroid follicular epithelial cells remains unclear.22, 61 Our findings revealed that both knockdown of DNA-PKcs and inhibition of its phosphorylation reduced in NHEJ efficiency, thereby reducing the incidence of RET/PTC1 rearrangements in thyroid follicular epithelial cells exposed to 0.5 Gy IR. In this study, we have only been briefly examined the extensive capabilities of DNA-PKcs in relation to its function in the NHEJ repair efficiency domain. DNA-PKcs, within the human body, generates its own protective effects against IR and oxidative stress. For instance, it facilitates the production of superoxide dismutase, enabling organs to endure higher doses of IR compared to isolated cell lines, even when both experience equivalent levels of damage from exposure.23, 62-65 Consequently, exposure doses on cell lines and experiments can only serve as a cautionary measure for individuals at risk of IR exposure. Our current study has identified that DNA-PKcs-mediated alterations in NHEJ plays a crucial role in the formation of RET/PTC1 rearrangements in thyroid follicular epithelial cells following low-dose IR exposure.
In summary, we identify that the response of thyroid follicular epithelial cells to low dose of IR can undergo RET/PTC1 rearrangement. Our data emphasize the importance of NHEJ between RET/PTC1 rearrangement and IR, particularly highlighting the involvement of DNA-PKcs in facilitating RET/PTC1 rearrangement. Consequently, targeting DNA-PKcs to inhibit the NHEJ pathway has emerged as a promising therapeutic strategy for addressing IR-induced RET/PTC1 rearrangement in PTC. Despite this, there are three aspects of IR-induced RET/PTC1 in PTC that warrant further study. First, it is crucial to develop specific protective agents against thyroid exposure to low dose IR, particularly from the perspective of NHEJ. Second, although the RET and CCDC6 genes are located on the same chromosome, they are positioned far apart, which allows for the formation of various RET/PTC rearrangements uniquely in thyroid cells.44, 46 This raises the question of whether the chromosomal structure of thyroid cells differs fundamentally from that of other cell types, thereby facilitating RET/PTC rearrangement. Third, both high and low doses of IR can lead to RET/PTC1 rearrangement, suggesting potentially distinct underlying mechanisms that need thorough investigation. Research into these areas will enhance our understanding of the molecular processes involved and may lead to better prevention and treatment strategies for PTC.
4 MATERIALS AND METHODS
4.1 Cell culture
The human normal thyroid epithelial cell line (N-thy-ori-3-1) and thyroid papillary carcinoma cell line (TPC-1: CVCL_6298), purchased from the Pricella Life Science & Technology, were grown in Roswell Park Memorial Institute 1640 (RPMI 1640 Sigma, Inc.), supplemented with 1% antibiotics/antimycotics (Invitrogen) and 10% fetal bovine serum (FBS) (ExCell Bio). The HLF cell line (Human Lung Fibroblasts) purchased from Pricella Life Science & Technology, was cultured in Dulbecco's Modified Eagle Medium (DMEM, Sigma, Inc.) supplemented with 10% FBS (ExCell Bio) and 1% antibiotics/antimycotics (Invitrogen).
4.2 Cell irradiation
Trypsin digestion of well-grown N-thy-ori-3-1 cells was carried out and counted to ensure that the number of cells per well in a six-well plate was 2 × 104 cells. After that, the cells were passaged into six-well plates for 16 h and then exposed with a single dose of IR from a γ 60Co source at a dose rate of 60 cGy/min. Cobalt-60 (60Co), an artificial radionuclide that emits high-energy electrons, is a commonly acceptable source of gamma-ray radiation. Cells were cultured in a humidified thermostatic incubator at 37°C containing 5% CO2, according to the experimental design, following grown for 3−6 days and harvested.
4.3 Gradient dilution experiment
To assess the sensitivity of the nested PCR for the detection of RET/PTC1, and to determine the number of cells to be inoculated and the limits of the assay for each subsequent independent experiment. A gradient dilution experiment was performed by mixing 106, 105, 104,103, 102, 101, and 100 TPC-1 cells with 106 thyroid follicular epithelial cells to test the accuracy of the experiment. The sensitivity of the assay was determined to be one positive cell mixed in 107 during this study. To ensure the accuracy of the experiments, 106 cells were used, and irradiation was performed after inoculating them in six-well plates.
4.4 Detection of RET/PTC1 rearrangements
Total RNA was isolated by TRIzol from cells (Ambion, Thermo Fisher Scientific) and eluted in 20 µL of RNase/DNase-free buffer (Biomed, RA114-02). A Nanodrop 2000c spectrophotometer (Thermo Fisher Scientific) was employed to determine RNA concentration and quality. RNA reverse transcription was performed with Rever Tra Ace qPCR RT Master Mix and gDNA Removal Kit (Large Edition). The reverse transcription system was carried by Applied Biosystems PCR (Ambion, Thermo Fisher Scientific). The first and the nested PCRs were carried out with 35 cycles in Applied Biosystems PCR (Applied Biosystems). The first-round PCR products were pooled, diluted 1:5, and then, 1 µL was used for one nested PCR. The PCR amplification products were analyzed with 2%−3% agarose (Beyotime, ST004L) gel electrophoresis and ethidium bromide staining (Thermo Fisher Scientific, 15585011). The specificity of the amplification product with predicted size was confirmed by Sanger sequencing. TPC-1 cells were used as a positive control and HLF cells were used as a negative control.40, 66 The primers used are listed in Table S2.40, 67
4.5 Antibodies and chemicals
Anti-GAPDH (Santa Cruz, sc-32233), anti-H2AX (Santa Cruz, sc-517336), anti-γH2AX (Santa Cruz, sc-517348), anti-CHK1 (Santa Cruz, sc-84084), anti-pCHK1 Ser345 (CST, 2348S), anti-DNA-PKcs (Invitrogen; Thermo Fisher Scientific, Inc.; MA5-13238), anti-pDNA-PKcs S2056 (Abcam; cat. no. ab18192), anti-STAT3 (CST, 9139S), anti-p-STAT3-Tyr705 (CST, 9145S), anti-AKT (CST, 9272S), anti-pAKT-Ser473 (CST, 4060S), anti-ERK1/2 (CST, 9102S), and anti-p-ERK1/2-Thr202/Tyr204 (CST, 9102S). All antibodies were diluted at 1:1000. The chemical Rad51 inhibitor RI-1 (cat. no. S8077), DNA ligase IV inhibitor SCR7 (cat. no. S7742), DNA-PK inhibitor Nu7441 (cat. no. S2638), Parp1 inhibitor Olaparib (cat. no. S1060), and thymidine (cat. no. S4803) were purchased from Selleck Chemicals.
4.6 Western blotting procedures
At 4°C, cells were washed with phosphate buffered saline(PBS), collected into EP tubes by centrifuging at 3000 rpm for 3 min, lysed on ice for 30 min with RIPA (Beyotime, P0013B), and centrifuged at 12,000 rpm for 10 min. The protein concentration was measured by Nanodrop 2000c spectrophotometer and 40 µg was loaded per lane on 6%−12% SDS–PAGE gels according to molecular weight of proteins. Proteins were transferred to nitrocellulose membranes and blocked with 5% bovine serum albumin(BSA) for 1 h at room temperature. Membranes were incubated overnight at 4°C with primary antibodies. After washing, membranes were incubated with secondary antibodies (1:4000) at room temperature for 1 h. After washing, SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific) was uniformly added to the membrane. The bands were visualized using an Image Quant LAS 500 and the Image Quant LAS 500 1.1.0 software (GE Healthcare Life Sciences).
4.7 Transfection
The siRNA and plasmid used in the present study were purchased from Gene Pharma (Gene Pharma); the sequences were as follows: siRET/PTC1.1—5′-AACTATCAAACGTGTCC-3′; siRET/PTC1.2—5′-AACATCTCGGGACAAGC-3′; siRET/PTC1.3—5′-TATCTTCAGCACCTTGTTC-3′; siDPK1—5′-GGGCGCTAATCGTACTGAA-3′; and siDPK2—5′-GGAAGAAGGUGAAGGAGAATT-3′. All transfections were conducted using lipofectamine 2000 (Invitrogen, 11668019) according to the manufacturer's instructions.
4.8 NHEJ assay
NHEJ-GFP plasmid was digested with HindIII enzyme overnight at 37°C and recovered using AxyPrep DNA Gel Extraction Kit (Axygen; Corning, Inc.) according to the manufacturer's instructions. N-thy-ori-3-1 cells (3 × 105) were pretreated with SCR7 (50 µM) or RI-1 (30 µM) for 1 h. The cells were transfected with 0.25 µg of pCherry and 1 µg of the digested NHEJ-GFP plasmid and mixed with 2.5 µL of Lipofectamine 2000. Following 6 h, the culture medium of the transfected cells was replaced with medium containing SCR7 (50 µM) or RI-1 (30 µM) and further cultured for 2 days. The detailed procedure has been described.68 The cells were trypsinized (0.25%) and resuspended in PBS. The cellular fluorescence was measured by flow cytometry analysis (NovoCyte; ACEA Biosciences, Inc.) and the NovoExpress 1.3.0 software (ACEA Biosciences, Inc.).
4.9 HR assay
N-thy-ori-3-1 cells (3 × 105) were pretreated with SCR7 (50 µM) or RI-1 (30 µM) for 1 h. Then, they were transfected with a single copy of a DR-GFP, I-SceI expression plasmid and with a pCherry plasmid used as a transfection efficiency control. Following 6 h, the culture medium of the transfected cells was replaced with medium containing SCR7 (50 µM) or RI-1 (30 µM), after which the cells were further cultured for 2 days and subjected to flow cytometry analysis (NovoCyte; ACEA Biosciences, Inc.) and the NovoExpress 1.3.0 software (ACEA Biosciences, Inc.), and then, the GFP-positive cell population was measured. The mean values were obtained from three independent experiments. Little variation was observed among the three independent experiments.68
4.10 Real-time PCR assay
Total cellular RNA was extracted by TRIzol and the reverse transcription procedure was carried out according to the product instructions. The primers were designed and synthesized by Tingke Biotech. β-Actin was used for normalization as a control.
4.11 Animal experiments
BALB/c-nu mice (4 weeks old) were purchased from SPF (Beijing) Biotechnology Co., Ltd., and experiment was conducted after 1 week of stabilization. TPC-1 (siRET/PTC1) and N-thy-ori-3-1 (OE RET/PTC1) cells were digested down separately with trypsin, washed twice with PBS, and 0.2 mL cell suspension was taken at a concentration of 3 × 107/mL and injected into the right subcutaneous side of nude mice. The animals were housed in ventilated microisolators with free access to sterile food and water. Twenty-one days after injection, BALB/c-nu mice were sacrificed, and tumors were observed, collected, weighted, and measured.
4.12 Cell proliferation assay
For CCK-8 assay, cells were diluted with complete medium to a concentration of 15,000/mL and added to 96-well plates for culture at 100 µL per well. Absorbance values were measured at 12/24/48/72 h after cell inoculation. For the colony formation assay, cells were diluted with complete medium to a concentration of 1000/mL and added to six-well plates for culture. After 10 days of culture, the cell colonies were fixed with 4% paraformaldehyde (Beyotime, P0099) and stained with Giemsa (Beyotime, C0133).
4.13 Cell cycle synchronization using thymidine double block
The cells were initially cultured to approximately 50%‒60% confluence in complete growth medium. The first block was achieved by incubating cells with 2 mM thymidine for 16 h. Following this, cells were released from the block by washing three times with PBS and incubating them in fresh thymidine-free medium for 8−10 h to allow cell cycle progression. A second thymidine block was then applied by re-treating the cells with 2 mM thymidine for an additional 16 h. After the second block, cells were washed thoroughly with PBS to remove residual thymidine and incubated in fresh medium. Synchronization efficiency was assessed by flow cytometry.
4.14 Statistical analysis
Statistical analyses were conducted with GraphPad Prism (version 8.0) software. The data were derived from at least three independent experiments and are presented as mean ± SEM. p < 0.05 was considered statistically significant.
AUTHOR CONTRIBUTIONS
Yuhao Liu and Jiaojiao Zhu conducted the experiments, performed data analysis, and drafted the manuscript. Shenghui Zhou conducted the experiments. Yifan Hou, Ziyan Yan, Xingkun Ao, Ping Wang, and Lin Zhou provided experimental support. Huixi Chen, Xinxin Liang, Hua Guan, Shanshan Gao, and Dafei Xie provided experimental reagents and materials. Yongqing Gu and Ping-Kun Zhou supervised the project, and designed, edited, and led out the experiments of this study. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank Ma Teng laboratory for kindly providing NHEJ-GFP plasmid and DR-GFP plasmid. This research was funded by grants from the National Natural Science Foundation of China (82073488, 32171238, 82273568, 82230108, 81773359, and 31470827).
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
All the animal experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals and were performed according to the guidelines for the Laboratory Animal Guideline of Welfare and Ethics of China. All experimental procedures were approved by the Animal Care and Use Committee at the Military Academy of Medical Sciences. (IACUC-DWZX-2024-531).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




