Exosome nanovesicles: biomarkers and new strategies for treatment of human diseases
Abstract
Exosomes are nanoscale vesicles of cellular origin. One of the main characteristics of exosomes is their ability to carry a wide range of biomolecules from their parental cells, which are important mediators of intercellular communication and play an important role in physiological and pathological processes. Exosomes have the advantages of biocompatibility, low immunogenicity, and wide biodistribution. As researchers’ understanding of exosomes has increased, various strategies have been proposed for their use in diagnosing and treating diseases. Here, we provide an overview of the biogenesis and composition of exosomes, describe the relationship between exosomes and disease progression, and focus on the use of exosomes as biomarkers for early screening, disease monitoring, and guiding therapy in refractory diseases such as tumors and neurodegenerative diseases. We also summarize the current applications of exosomes, especially engineered exosomes, for efficient drug delivery, targeted therapies, gene therapies, and immune vaccines. Finally, the current challenges and potential research directions for the clinical application of exosomes are also discussed. In conclusion, exosomes, as an emerging molecule that can be used in the diagnosis and treatment of diseases, combined with multidisciplinary innovative solutions, will play an important role in clinical applications.
1 INTRODUCTION
Extracellular vehicles (EVs) are lipid bilayer vesicles released from the cell and have been recognized as crucial entities that play an important role in intercellular, intertissue, and interorgan communicatio.1-3 Exosomes are tiny vesicles 30−150 nm4, 5 in diameter, a subclass of EVs, which can be secreted by a variety of cells,6, 4, 7-10 and are enriched in specific biologically active molecules such as proteins, lipids, nucleic acids, depending on the source.11-13 Since the role of exosomes in intercellular communication was first discovered by Sebastian Amigorena's research group in 1998, it has been recognized as a new mode of intercellular communication as research has progressed. Exosomes can directly activate intracellular pathways in target cells by binding to specific receptor ligands,14 and can also stimulate target cells through membrane fusion or through endocytosis/phagocytosis to deliver a large amount of information,15 such as proteins, lipids, and nucleic acids, to the receptor cells, completing the signaling in the receptor cells,16, 17 thus participating in the regulation of physiological and pathological processes such as immune response, antigen presentation, and tumor invasion.18
Exosomes can be isolated from a variety of body fluids, including bronchoalveolar fluid,19 cerebrospinal fluid,20 blood,21 urine,22, 23 saliva,21, 24 breast milk,21 semen,25 amniotic fluid,26 synovial fluid,13 as well as fluid from diseased lesions including ascites and pleural fluid,27 and can be subjected to multicomponent analyses.28 Excitingly, exosomes are mediators of intercellular communication, and their secretion and content change as the disease progresses.29 Most notably, they can circulate steadily in body fluids and can be used for dynamic tracking.30 These properties determine the use of exosomes for liquid biopsies, which have been demonstrated in a wide range of diseases. Especially in tumor research, exosomes can be used as specific cancer biomarkers and diagnostic biomarkers for tumor diagnosis, treatment, efficacy assessment, and so on.31-35 Similarly, studies have proved that exosomes play an important role as biomarkers in neurodegenerative diseases,36-38 cardiovascular,39-41 hepatic,42, 43 infectious diseases,44-46 and autoimmune disease.47-50 It is currently recognized as the most promising biomarker in the field of liquid biopsy.51-53
Despite the advances in contemporary medicine, there are still serious challenges in the diagnosis and treatment of many diseases. It is well known that early diagnosis of diseases before they reach the clinical stage is very important for effective prevention, treatment, and significant improvement of prognosis.54 However, there are many limitations to early diagnosis, such as invasiveness, low sensitivity, high cost, and low acceptance.55-58 In the treatment of some intractable diseases (tumors, neuropsychiatric disorders, autoimmune diseases, and infectious diseases), despite the addition of many new drugs, the therapeutic effect has been seriously affected by the problems of blood–brain barrier (BBB) obstruction, drug tolerance, and poor drug targeting.59
Exosomes, as excellent key mediators of cell communication, can not only be used as biomarkers for early diagnosis and monitoring of diseases, but also play an active role in drug delivery, targeted therapy, and immune regulation60 due to their biocompatibility and protective bilayer lipid structure to protect genetic cargo from degradation, coupled with reduced immunogenicity. Because their small size and membrane composition allow them to cross the BBB,61 they also shed new light on neurological and psychiatric disorders.62, 63
In this review, our main aim is to explore the potential of exosomes in disease management. We introduces the key advances of exosomes in liquid biopsy, drug delivery, targeted therapy, immune regulation, and gene therapy. Revealed the achievements of extracellular vesicles in clinical research, pointed out the challenges faced in clinical translation, and finally explored the impact of extracellular vesicles in precision medicine and personalized therapy.
2 EXOSOME BIOGENESIS AND COMPOSITION
2.1 Biogenesis
Exosome formation begins with endocytosis of the cytoplasmic membrane in three main stages.64, 65 (1) Early endosomes are formed by the inward folding of the cell membrane. They are essentially lipid vesicles. (2) Multivesicular bodies (MVB) are formed. Early sorting endosomes progress to late sorting endosomes, which are membrane invaginated to form smaller nano-vesicles (30–150 nm)66 called intraluminal vesicles (ILVs), which restrict membrane endocytosis to form MVBs containing multiple ILVs. (3) The MVB fuses with the plasma membrane and releases ILVs with specific cargoes called exosomes64 (Figure 1). MVB formation is essential for exosome biogenesis and can occur through two pathways, the endosomal sorting complex required for transport (ESCRT)-dependent pathway and the ESCRT-nondependent pathway.67 ESCRT is involved in the development of the MVB and ILV, which contains four protein complexes, ESCRT-0, -I, -II, and -III and vacuolar protein sorting related protein 4.67 The nonclassical pathway includes specific lipid molecule-driven mechanisms68 and tetraspanin-mediated drive mechanism.69, 70 Specific lipid molecules that are involved in the regulation of MVB formation include mainly ceramide, phosphatidic acid, and sphingosine-1-phosphate,68, 71 which participate in inward budding and lead to ILV formation through lipid raft-mediated and amino acid-dependent pathways.72, 73 It has been found that the tetraspanin involved in secretion regulation (CD63, CD9, CD82, CD81, etc.) can polymerize with each other to dynamically recruit multiple transmembrane protein molecules or signaling molecules on the cell surface or organelle membranes to form specific multimeric complexes.74-76 This “tetraspanin web” can effectively promote the sorting and enrichment of other cargo proteins on endosomal membranes and the formation of ILVs.77 The fact that these two pathways for the biogenesis of exosomes are not entirely separate,78 they seem to work synergistically, and the diversity of exocytosis depends on the different machinery, as well as the cell types involved.77
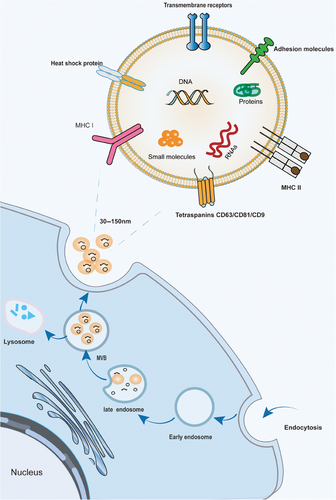
2.2 Composition
Exosomes induce intracellular signaling by delivering their contents to receptor cells via either receptor–ligand binding or plasma membrane fusion. The composition of exosomes is therefore an important factor in determining the effectiveness of their action. Lipids are one of the most essential components of exosomes, as the lipid bilayer composition determines their potential to be internalized by recipient cells and utilized for therapeutic development.79 Several lipid molecules in exosomes, including sphingolipids, cholesterol, and phosphatidylserine, are located in the membrane.80 Sphingolipids and cholesterol play a major role in the stability of the exosome phospholipid bilayer,81, 82 whereas phosphatidylserine promotes the fusion of exosomes with target cell membranes and acts as a signaling molecule in signal transduction.83
Based on the proteomic analysis, more than 4400 relevant proteins have been selectively enriched in specific exosomes,84 which are classified into five main groups,85 including (1) MVB manufacturing proteins (Alix, Tsg, tetraspanins, clathrinid) and transport proteins(annexins, flotillin, Rab); (2) heat shock proteins (HSP; HSP70, HS9083); (3) signaling molecules (HIF-1α, PI3K, ADP-ribosylation factor 1 (ARF1), β-catenin); (4) cytoskeletal proteins (actin, tubulin, cofilin, profiling, myosin, vimentin, fibronectin, meosin, keratins, talin); (5) various enzymes (GAPDH, ATPase, pgk1). Some of the aforementioned proteins participate in the normal physiological activities of exosomes, whereas other proteins mediate interactions between exosomes and recipient cells.
In addition, EVs also contain large amounts of nucleic acids (e.g., mRNAs, micro RNAs, noncoding RNAs, piRNA, and DNA).64 These functional RNAs can affect the transcriptome of recipient cells,86, 87 with miRNAs being the most extensively studied. They can inhibit the expression or translation of target genes after transcription, disrupt the stability of mRNA and inhibit its translation, regulate the expression of target genes88 in different types of cells, and participate in important biological processes such as cell proliferation, differentiation, apoptosis, and metabolism.89 Kahlert et al.90 identified large fragments (>10 kb) of genomic double-stranded DNA containing all chromosomes found in exosomes and can be used to detect mutated DNA in serum, which can be used for cancer prediction, treatment, and therapy resistance.
The composition of exosomes depends to some extent on source cell type and can also be affected by different cell conditions or treatments.91 In addition, the exosomes released by a cell line may be highly heterogeneous, which determines the functional properties of the exosomes.92 This ability of exosomes to deliver their contents to receptor cells produces a bi-directional effect, which can be involved in the diagnosis and treatment of disease, and is also closely related to certain diseases' development and progression.
2.3 The role of exosomal cargo in disease
Exosomes act as important messengers for the exchange of substances between cells,93, 94 facilitating the transfer of important information between cells and regulating cellular functions through the transmission of contents.95 Detection of specific molecules carried by exosomes provides a new way to understand the mechanism of disease occurrence and development. In recent years the contents of exosomes are associated with the development of a variety of diseases, especially in the field of cancer,78 which has received sustained attention.
2.3.1 Cancer
Exosomes regulate tumor and host cell functions by docking with receptor cells and delivering the proteins, nucleic acids, lipids, and other contents they carry. Exosomes derived from tumor cells can participate in the bioregulation, proangiogenesis, matrix remodeling and immune evasion of tumor cells (Figure 2). All these mechanisms suggest that exosomes are closely related to tumors and help promote tumor cell development and metastasis.
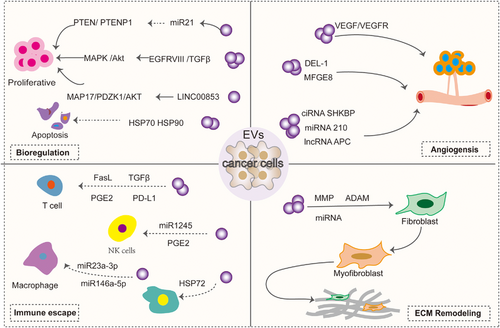
Biomodulation of tumor cells
High epidermal growth factor receptor (EGFR) expression is strongly associated with neurogliomas. EVs derived from brain tumors have been identified to contain both EGFR and EGFRvIII (constitutively active mutant receptors). In U373 glioma cells EGFRvIII can be transferred via EV to distant cells lacking EGFRvIII expression, while activating downstream signaling pathways in these cells and cell transformation is induced.96, 97 In addition, ligands such as epidermal growth factor(EGF), transforming growth factor alpha(TGFα), and two-regulator proteins have been found in the EV of breast and colorectal cancer cell lines. These EV-containing ligands can stimulate recipient cells and induce proliferation.98 Similarly, HCC cells produce the exosome miR-21, which regulates the expression of the tumor suppressor genes PTEN and PTENp1 through a variety of mechanisms, thereby affecting the proliferation of HCC cells.99 HCC cell-derived exosomes are also enriched in Golgi membrane protein 1, which activates the GSK-3β/MMPs signaling axis in the recipient cells and accelerates cell proliferation and migration.100 Survivin in tumor exosomes, as well as the heat shock proteins HSP70 and HSP90, have also been shown to inhibit apoptosis and increase cell proliferation and invasion, stimulate the tumor microenvironment, and promote the growth and spread of primary tumors.98, 101 Recently, LINC00853 in gastric cancer tissues was found to promote cell proliferation, invasion, and migration by regulating the MAP17/PDZK1/AKT pathway.98 MiR-92a, a cancer-associated fibroblasts(CAF)-derived exosome, acted as a facilitator of breast cancer cell migration and invasion by reducing G3BP2.102
Inducing angiogenesis
Angiogenesis is the formation of new blood vessels from preexisting ones.103 Abnormal angiogenesis is a feature of several pathologies including cancer. Tumor EVs promote angiogenesis by promoting vascular endothelial generation response. It has been found that tumor-derived EVs contain a variety of proteins associated with angiogenesis. For example, EV derived from A431 squamous carcinoma cells can transfer oncogenic EGFR to endothelial cells, activate the MAPK and AKT pathways, as well as increase the expression of endogenous vascular endothelial growth factor (VEGF) and induce angiogenesis.104 Glioblastoma-derived exosomes containing proangiogenic proteins, angiopoietins, VEGF, IL-6, and IL-8 stimulate increases in endothelial tube length and angiogenic proteins and stimulate in vitro angiogenesis in the brain microvascular endothelial tubule formation assay.105 In particular, EVs released in hypoxic environments increase the activation of ERK1/2 MAPK, PI3K/AKT, and FAK pathways in recipient endothelial cells, leading to more endothelial cell sprouting. Breast cancer tumor-derived EV promotes VEGF receptors and tumor angiogenesis via VEGF90K.106 Hegmans et al.107 analyzed EV secreted by several mesothelioma cells by MALDI-TOF MS and identified DEL-1 in EV, which is thought to play a role in angiogenesis.108 MFGE8, a major component of EV from immature dendritic cells and tumor cells, promotes VEGF-induced cell survival and thus induces angiogenesis.109, 110 Similarly, proteins associated with angiogenesis have been found in exosomes derived from hepatocellular carcinoma, ovarian carcinoma, and nasopharyngeal carcinoma.
The study also highlights that noncoding RNAs in exosomes play a key role in tumor angiogenesis. Circ-RNAs (circ-SHKBP,111 circRNA-100338112) in tumor exosomes can act as ceRNAs to inhibit miR-877-3p, thereby increasing VEGFA expression,111 and can also regulate angiogenesis by interacting with the RNA-binding protein NOVA2.112 LncRNAs(lncRNA-H19,113 lncRNA-APC,114 lncRNA-MALAT1,115 lncRNA-TUG,116 lncRNA-p21,117 lncRNA-GAS5,118 lncRNA-AHIF,119, 120 lncRNA-HOTAIR,121 lncRNA-CCAT2,122 lncRNA-POU3F3123) promote the expression of key proangiogenic genes and proteins by acting as ceRNAs or binding to mRNAs. A variety of EV miRNAs are involved in tumor angiogenesis by regulating endothelial cell activity (e.g., miR-23a,124 miR-25-3p,125 miR-26a,126 miR-182-5p,127 miR-221,128 miR21,129 miR-210,130 miR-9131). MiRNAs also can promote angiogenesis by inhibiting FOXO1 expression.132
Immune escape
Immune escape is one of the major hallmarks of cancer. During tumorigenesis and progression, EV suppresses immunity through a variety of mechanisms, a process mainly mediated by exosomes released from tumors.133 Tumor-derived EVs can carry immunosuppressive cargoes that prevent immune cells from attacking circulating tumor cells. Among these, protein programmed death ligand 1 (PD-L1) is the predominant cargo. For example, binds to T cells and induces immunosuppression134; inhibits the activation of NK and CD8+ cells through miR1245, which downregulates the expression of the NKG2D receptor135, 136; and it can also stimulate prostaglandin E2 secretion by increasing the myeloid-derived suppressor cell (MDSC),137 which inhibits NK cells as well as CD4 and CD8 lymphocytes.138 HMGB1 in tumor-derived exosomes also promotes immune evasion in HCC by promoting the expression of TIM-1 and regulating the growth of B cells.139 Tumor exosomes contain nucleic acids that are also involved in immune evasion. For example, the thyroid-derived exosome miR-519e-5p can aid tumor immune escape from distant organs by inhibiting the Notch signaling pathway.140 Exosomal circCCAR1 released by HCC cells contributes to immunosuppression by facilitating CD8 + T-cell dysfunction in HCC.141 Exosomal lncRNA-TUC339, circTMEM181, miR-23a-3p, and miR-146a-5p, components of the tumor-derived exosome, promote M2 macrophage polarization, thereby impeding the efficiency of CD8+Tcells.142 In addition, some EVs produced by cells with immunosuppressive properties are involved in immune evasion, such as Hsp72, the content of MDSC-derived EV, activates signaling and transcriptional activator 3 (Stat3), which stimulates MDSC immunosuppressive function.143, 144
Extracellular matrix remodeling
Abnormal deposition or loss of extracellular matrix (ECM) components may bring about many changes in the microenvironment that are associated with disease progression. Tumor-derived EVs enriched with proteases, including a variety of MMP (MMP14, MMP9, MMP13, MMP1, and MMP3), as well as the ADAM family of disintegrins, can regulate the ECM, thereby favoring tumor progression, as described in detail by Nawaz et al.145 Tumor exosomes can also remodel ECM by recruiting and reprogramming normal fibroblasts into cancer-associated fibroblasts (CAF). It has been shown that TGF-β expressed on the surface of cancer-derived EVs triggers the TGF-β/SMAD3 signaling pathway in recipient fibroblasts, which induces the expression of a myofibroblast-like phenotype (α-SMA, FGF2)146; EV also generates CAFs through a nonclassical fibronectin-dependent pathway.147 Furthermore, tumor exosomes contain miRNAs that reprogram recipient cells, which produce CAF.148
2.3.2 Neurological diseases
Research has shown that EVs transfer proteins and genomic materials between neurons, which is crucial for the progression of neurodegenerative diseases. These vesicles contribute to the propagation of these neurotoxic substances, leading to synaptic dysfunction and widespread neuronal damage.60 EV derived from neurons contains amyloid-β peptides and tau proteins.149 During the development of Alzheimer's disease (AD), pathological tau protein is distributed in exosomes released by microglia.150 Insoluble aggregates of tau proteins can induce ROS production in a calcium-dependent manner through activation of NADPH oxidase, leading to neuronal damage. Exosomes carrying α-synuclein disseminate and accumulate between neurons, leading to degeneration and death of dopamine neurons.151 Studies have also highlighted that exosomal miRNAs are closely associated with the development of neurodegenerative diseases.152 MiRNAs play a key role in the upregulation of PD-associated proteins such as α-synuclein, tau proteins, DJ-1, and so on.153 EV is also able to translocate miR-21-5P from the neuron to the microglial cell, leading to microglial M1 polarization and excessive release of proinflammatory cytokines, thereby aggravate neurological damage.154
2.3.3 Immune system diseases
EV contents also play a pathogenic role in autoimmune diseases. EV carries the peptide-major histocompatibility complex (MHC), which presents antigens to T cells and participates in the formation of immune complexes,155 inducing an inflammatory response by stimulating the production of tumor necrosis factor (TNF) by macrophages.148 LncRNAs in exosomes that mediate NF-κB and Wnt/β-catenin signaling pathways are involved in the pathogenesis of rheumatoid arthritis (RA).156 Additionally, cytokines carried by EVs, such as interleukin-6 (IL-6) and TNF-α, contribute to the inflammatory environment.157 Exosomal contents miRNAs are also involved in disease pathogenesis by regulating the secretion of key pathogenic cytokines158 and the direction of immune cell differentiation.159
Similarly, it has been proven that the proteins and nucleic acids carried by EVs play a pathogenic role in infectious diseases, cardiovascular diseases,160 blood diseases, and more.60
3 EXOSOME BIOMARKERS
Exosomes from different cell types have different signaling molecules because of their unique contents. With a full understanding of the relationship between exosomal contents and disease occurrence, exosomes are considered potential therapeutic targets and biomarkers.161 Moreover, it stably exists in various body fluids and has the advantages of high sensitivity, low invasiveness, and easy access to materials compared with other tests,17 which can help in the early diagnosis of diseases, detection of diseases, and evaluation of therapeutic effects (Table 1).32, 162
| Diseases | Biomarker | Appliance | References |
|---|---|---|---|
| Cancer | |||
| Pancreatic | miR-1293 | Prognostic biomarker | 163 |
| Liver | miR-638 | Prognostic biomarker | 164 |
| Ovarian | miRNA-205 | Diagnostic biomarker | 165 |
| miR-1290 | Diagnostic biomarker | 166 | |
| ZNF587B | Diagnostic biomarker | 167 | |
| Intestinal tract | miR-3127-5p | Diagnostic biomarker | 168 |
| miR-1470 | Diagnostic biomarker | 169 | |
| Lung | circ_0061407 circ_0008103 | Diagnostic biomarker | 170 |
| Cholangiocarcinoma | circ-0000367 | Diagnostic and monitoring biomarker | 171 |
| circ-0021647 | |||
| circ-0000288 | |||
| Glioblastoma | EGFR EGFRvIII | Diagnostic | 172 |
| miR-151-3p | Diagnostic | 173 | |
| Breast | miR-21 | Diagnostic | 174 |
| miR-27a | |||
| miR-375 | |||
| Immune disease | |||
| IgA nephropathy | miR-451a | Prognosis biomarker | 175 |
| Rheumatoid arthritis | lncRNA:ENST00000433825.1 | Prognosis biomarker | 176 |
| miR-885-5p | Diagnostic | 47 | |
| miR-6894-3p | |||
| miR-1268a | |||
| Idiopathic pulmonary fibrosis | hsa_circ_0044226、 | Diagnostic | 177 |
| hsa_circ_0004099 | |||
| hsa_circ_0008898 | |||
| SLE | miR-451a | Diagnostic | 178 |
| Cardiovascular | |||
| Acute myocardial infarction | circ_0001558 | Diagnostic | 179 |
| PTEN | Diagnostic | 180 | |
| miR-126 | |||
| miR-21 | |||
| Coronary heart disease | circ_0001360 | Diagnostic | 181 |
| circ_0000038 | |||
| Neurodegenerative | |||
| AD | miRNA-125b | Diagnostic | 182 |
| miRNA-451a | |||
| miR-135a | Diagnostic | 183 | |
| PD | miR-331-5p | Diagnostic | 184 |
| miR-505 | |||
| miR-153 | Diagnostic | 185 | |
| miR-223 | |||
| Communicable disease | |||
| Tuberculosis | miR-484 | Diagnostic | 46 |
| miR-96 | |||
| miR-425 | |||
| miR-let7e-5p | Treatment response monitoring | 186 | |
| M. tuberculosis-derived RNA | |||
| HIV | miR-378 | Treatment response | 187 |
| miR-630 | |||
| miR-378 | |||
| miR-630 | |||
- Abbreviations: AD, Alzheimer's disease; EGFR, epidermal growth factor receptor; PD, Parkinson's disease; PTEN, phosphatase and tensin homolog deleted on chromosome ten; SLE, systemic lupus erythematosus..
3.1 Cancer
As is well known, early diagnosis of tumors is particularly important in cancer treatment, closely related to the success rate and prognosis of patients.188 Exosomes contain a large amount of information about cancer cells,189 which is closely related to cell proliferation, angiogenesis, metastasis, and immune response regulation processes. They can be used as biomarkers for liquid biopsy to reflect in vivo tumor activity. At present, research has confirmed that exosomes have become an important tool for early cancer screening and detection.190 The most extensively studied area is miRNA, and Jafari et al.191 provided a detailed review of the application of extracellular vesicle miRNA as a biomarker in early cancer diagnosis. zNF587B plays a crucial role in early ovarian cancer detection.167
In addition, exosomes circulate in body fluids and can provide a wealth of information about the status of the cancer,192 offering valuable insights at all stages of disease progression. A meta-analysis shows that up- and downregulated circulating exomiRs may serve as a valid indicator of inferior survival outcomes in patients with gastrointestinal malignancies.193 In fact, serum-specific exomiRs have also been associated with the prognosis of several cancers. Circulating miR-122 expression is upregulated in BC, non-small cell lung cancer (NSCLC), associated with distant metastasis and reduced OS and PFS, which are considered prognostic factors.194 Low levels of miR320 were associated with advanced stage, LNM, and poorer OS in BC patients,195 as well as serum exosomal miR-940 levels reflecting the presence of lymph node metastases and EGFR type 2 status, which may be a biomarker for metastasis in breast cancer.196 Elevated ExomiR-301a was associated with the recurrence of gliomas.197 Recently, it has also been found that the exosome miR-5100 in hypoxic head and neck squamous cell carcinoma (HNSCC) promotes CAF activation by coordinating the QKI/AKT/STAT3 axis, which further facilitates the metastasis of HNSCC. miR-5100 has been associated with the malignant progression of HNSCC and may be a potential biomarker and therapeutic target of HNSCC.198
Other nucleic acids and proteins of exosome contents may serve as biomarkers for cancer surveillance. Serum exosomes circ_0061407 and circ_0008103 may be involved in NSCLC progression by interacting with microRNAs and proteins, and may serve as developmental and potential diagnostic biomarkers for NSCLC.170 Plasma exosome-derived Cx43 has been shown to be a possible prospective prognostic indicator of 5-year OS and 5-year DFS in melanoma patients.199 Meanwhile, cancer cell-derived exosomes have also demonstrated a promising role in monitoring resistance to chemotherapy and targeted drugs.200, 201
In summary, the use of exosomal cargoes as biomarkers can track early changes in cancer, progression, and drug efficacy over time.
3.2 Neurodegenerative diseases
The ability of exosomes to easily cross the BBB makes them very excellent biomarkers for determining CNS disease and response to therapy. Differential expression of specific proteins, miRNAs, and cirRNAs contained in exosomes offers potential for diagnosis and treatment of neurodegenerative diseases.202 Studies have demonstrated the consistency of blood and cerebrospinal fluid exosomes as biomarkers to answer questions about brain-related diseases,203, 204 for early detection of disease and monitoring of disease progression in a noninvasive manner.
Pathologic changes in neurodegenerative diseases begin 10−20 years before clinical manifestations,205 and it is particularly important to find early diagnostic biomarkers with high specificity. Exosomes, as traceable molecules, are now hot biomarkers for early detection and monitoring of progression. A case–control study confirms that levels of P-S96-tau, P-T181-tau, and Aβ1-42 in neurogenic blood exosomal extracts predict the onset of AD up to 10 years prior to the clinical onset of the disease.206 This was followed by studies confirming that blood neuroendocrine synaptic proteins, such as GAP43, SNAP25, neurogranin, and synaptotagmin 1, can be used as reliable biomarkers to predict AD 5−7 years before cognitive decline.207 In the Parkinson's study, investigators found that pathologically soluble a-syn detected in plasma-derived neuronal extracellular vesicles distinguished patients with Parkinson's disease from healthy controls.208 AD L1EV-Synaptic nucleoprotein measurements in combination with specific precursor markers (such as olfactory or cognitive impairment, rapid eye movement sleep behavior disorder, or glucosinolates genetic status), can be used to help stratify individuals at high risk for Parkinson's disease and related Lewy body disorders.209
Exosomes also play an important role in monitoring disease progression and determining severity. The SNAP25 (synaptosome-associated protein of 25 kDa) and synaptophysin-I binding protein in neuron-derived exosomes can predict the development of AD.210 Complement proteins (C1q, C3b, and C3d) and complement regulatory proteins (CD59, CD46, decay-accelerating factor, and complement receptor type 1) in astrocyte-derived exosomes (ADEs) can be further elevated or decreased as the disease progresses, and therefore, measurement of the levels of complement proteins in the exosomes can predict AD progression.210 In addition, reduced levels of specific excitatory synaptic proteins contained in neuron-derived exosomes in plasma may indicate a correlation between the degree of cognitive loss and the severity of AD progression.211 The ratio of α-syn oligomers to α-syn in plasma exosomes of PD patients correlates with the severity of PD,212 and the mitochondrial respiration-related markers they contain may be potential biomarkers for assessing the effect of restoration of mitochondrial function in PD recovery.213
3.3 Cardiovascular disease
Exosome-borne elements also show great clinical potential in the diagnosis and prognostic monitoring of cardiovascular diseases.214 Exosomes containing noncoding RNAs (miRNAs,215-217 lncRNAs,218 cirRNAs181, 179) have been suggested to function as coronary artery disease (CAD) biomarkers and to be part of their specific therapeutic strategies, especially miRNAs. Levels of miR-942-5p, miR-149-5p, and miR-32-5p in blood exosomes correlate with atherosclerosis formation and may serve as a novel diagnostic and prognostic indicator of the disease.219 MiRNAs are also highly specific for cardiac injury,220 with higher sensitivity and specificity than the detection of troponin T. They can be used as indicators for the early diagnosis of myocardial infarction, and these elevated miRNAs (miR-192, miR-194, miR-34a) may be predictors of the development of heart failure (HF) after acute myocardial infarction.221 Many miRNAs are involved in the diagnosis/prognosis of HF222 and have the ability to differentiate aspects of CAD patients with HF from those without HF.223 To further facilitate detection, the investigators also monitored circulating stained EVs by specifically staining atherosclerotic plaque (AS) foam cell-derived exosomes directly in the circulation by fluorescence spectrometry or zymography, enabling early detection and real-time differentiation of lesion vulnerability during AS progression, thereby facilitating effective CVD management.224
Recent studies report plasma exosomes to identify three early warning biomarkers of OST4, PKIG, and RPL23 associated with the genetic characterization of cardiac exosomes and used to create noninvasive and direct columnar graphic models to accurately predict the risk of CAD deterioration.225 Li et al.226 also found that increased expression of plasma exosome-derived cysteine-rich protein 61 (Cyr61) was associated with the acute coronary syndrome (ACS) and could be used in the diagnosis and prognosis of ACS. In addition, observation of urinary UMOD levels can be used as an early diagnostic biomarker for myocardial infarction.227 Of course, exosomes have been shown to be excellent in predicting early recurrence of atrial fibrillation,228 detection of cardiotoxicity of anticancer drugs,229 and diagnosis of aortic dissection.41
3.4 Infectious diseases
In the early stage of HIV infection, EVs can carry HIV viral RNA and proteins,230 and their content can change with HIV infection and treatment,231 so analyzing the size, number and molecular content of EVs can help in early diagnosis and observation of disease progression and treatment efficacy.232, 233 MiRNA-21 is a promising and valuable prognostic biomarker in elite controller patients infected with HIV-1 virus.234 Neuron-derived EV isolated from plasma can be an excellent source of liquid biopsy biomarkers for identifying neurocognitive deficits in HIV patients.235, 204 HMGB1, NF-L, and A proteins in exosomes are potential HIV-1 prognostic biomarkers,236 alpha-2-macroglobulin, properdin, and hemopexin may serve as potential markers of substance abuse in HIV-infected individuals who are regular smokers and alcohol drinkers.237 In addition, urinary EV collected from HIV-positive individuals may provide a good source of HIV biomarkers, including p24.238
In viral hepatitis, exosomes released from infected hepatocytes contain diagnostically significant amounts of viral RNA, core proteins, envelope proteins, and DNA.236 Researchers have shown that exosomes can store HBV DNA and exosome-based diagnostics exosomes may help predict the likelihood of HBV relapse.239 When a patient's serum HBV-DNA is negative, exosomal HBV-DNA accurately reflects the level of HBV replication in the body and allows for tracking of treatment.240 Characterization of Mycobacterium exocytosis RNA from infected macrophages reveals unique mRNA fingerprints and preferential exocytosis markers for host-mRNA–miRNA and TB RNA in Mycobacterium tuberculosis infection.241 Distinctive expression patterns of exosomal miRNAs (miR-148a-3p, miR-150-5p, and miR-451a) in pleural effusions from benign wounds and tuberculosis could serve as potential biomarkers for tuberculosis pathology.242 In addition, by analyzing the expression patterns of various genes, the investigators clarified the role of exosomal RNA biomarkers in the differential diagnosis of latent TB infection and active TB disease.243
The study of COVID-19 also showed that exosomes in the blood can serve as potential biomarkers for predicting infection outcomes. Exosome surface CD142 is a platelet tissue factor (an extrinsic pathway for activation of the coagulation cascade) that has been associated with morbidity and mortality in COVID-19 and is considered to be the most unique exosome surface protein for determining prognosis in COVID-19.244, 245 Measuring the surface-specific C-reactive protein (CRP), alpha 1-acidic glycoproteins (A1AG1 and A1AG2), and CXCL7 protein levels of exosomes can also help identify critically ill COVID-19 patients.246
3.5 Autoimmune diseases
Exosomes containing immune-related substances have also made a mark as biomarkers for autoimmune diseases. Circulating exosomes purified from the serum of patients with systemic lupus erythematosus (SLE) are immunologically active, and their relative levels correlate with disease activity in SLE patients, with dysregulation of exosomal miRNAs being the most studied.247 The circulating exosomes miR-21 and miR-155 are upregulated in SLE and are potential biomarkers for the diagnosis of SLE and LN.248 Circulating exosome miR-451a levels were significantly lower in the group without renal damage SLE than in the group with renal damage, and can be used to assess SLE activity and detect the risk of renal injury.178
Researchers note that exosomal miRNAs in SLE patients are predominantly enriched in urine.249 Urinary exosome miR-146a negatively correlates with traditional nephritis biomarkers (C3, C4) and proteinuria and is a predictor of SLE disease activity.250 In patients with LN, miR-29c levels in urinary exosomes are highly sensitive and specific for early assessment of renal fibrosis and prediction of LN severity.251 MiR-26a levels in urinary exosomes from LN patients compared with healthy controls that correlated positively with urinary protein levels, suggesting its potential use as a predictive biomarker of podocyte injury.252 Urinary exosomal miRNAs can also predict the response to treatment in LN. Urinary exosomal miR-31, miR-107, and miR-135b-5p levels were elevated in the post-treatment response group, suggesting that they can be used as early biomarkers of LN prognosis.253
In recent years, various extracellular vesicle-related biomarkers have been developed for RA. Among circulating exosomes, miR-335-5p, miR-486-5p, SRSF4,254 miR-25-3p,255, 256 and DPYSL3 and PSME1 proteins257 can be used for early diagnosis. Exosomal lncRNAs have also been extensively studied in RA due to their advantages in regulating gene expression. Research has shown that lncRNAs in RA plasma exosomes have characteristic expression profiles and potential for diagnostic biomarkers and therapeutic targets.258 The high expression level of HOTAIR in the blood monocyte exosomes of RA patients suggests that it may be a potential biomarker for the diagnosis of RA.259 NEAT1 and PCGEM1 have the potential to help clinicians better diagnose RA.156 In terms of disease monitoring, exosomal miR-548a-3p is negatively correlated with key laboratory indicators related to RA disease activity, which may be a predictive factor for RA disease activity.260 After tofacitinib administration, RA-HCV patients had a significant increase in has-mir-155-5p and has-mir-122-5p compared with patients without HCV, which may be potential biomarkers of treatment efficacy in HCV RA patients.261 Recently, in the analysis of exosomes derived from synovial fluid in RA patients, multiple differentially expressed lncRNAs were discovered. Among them, ENST000000433825.1 is highly uniquely expressed in RA and significantly positively correlated with CRP, which may provide diagnostic and therapeutic biomarkers for RA.176
EV levels and content also showed significant differences in patients with multiple sclerosis (MS),262-266 allowing for monitoring of disease activity and prediction of disease progression.264 The neurologic and immunologic subclasses of EV contain AMBP, FIBB, GELS, IGHG4, and MBP, which provide information about the pathology of MS, reflect disease activity, and can differentiate between patients' disease phenotypes.267 Analysis of serum-derived EV microRNAs also demonstrated that a variety of microRNAs were associated with MS recurrence and activity,264, 268, 269 and can be used to support personalized treatment decisions. Exosomes are also used in the diagnosis and monitoring of immune disorders such as multifocal leukoencephalopathy (PML),270 Sjögren's syndrome,271 autoimmune skin diseases,272 IgA neuropathies,273 adult myasthenia gravis,274 and inflammatory bowel disease.275, 276
In addition to the above diseases, exosomes also show the potential as noninvasive biomarkers in the diagnosis, prediction, and treatment of preeclampsia,277 corneal diseases,278 and transplant rejection.279 In summary, exosomes play an important role as biomarkers in the diagnosis, progression, and even efficacy monitoring of various diseases, which can help clinicians detect diseases earlier, evaluate efficacy, and provide a basis for individualized treatment of diseases.
4 EXOSOME THERAPY STRATEGIES
Exosomes are nanoscale vesicular structures containing a variety of specific contents with the ability to target specific tissues, low immunogenicity, easy transport of hydrophilic or hydrophobic biomolecules, and the ability to cross biological barriers and penetrate tissue structures. This advantage allows exosomes to play multiple roles in disease treatment (Figure 3).
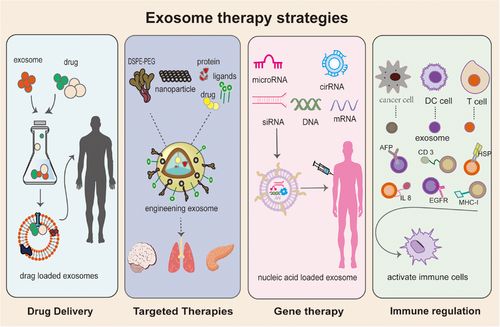
4.1 Drug delivery
For many years, research on drug presentation through nanoparticles (NPs) such as liposomes, micellar NPs, and superparamagnetic iron oxide NPs (SPION) has been going on, and some results have been achieved.280, 281 However, there are side effects such as early elimination, biological incompatibility, long-term toxicity, and so on.282 Exosomes, as high-quality delivery materials, have obvious advantages in terms of drug delivery capacity and toxicity.283 Nowadays, a large number of experiments have confirmed that exosomes can improve the precision of drug delivery and are efficient drug carriers without toxic side effects.
Back in 2015 researchers’ paclitaxel and adriamycin loaded into brain endothelial cell-derived exosomes, and exosomal delivery of anticancer drugs in a zebrafish brain cancer model significantly reduced the fluorescence intensity and tumor growth markers of xenografted cancer cells, confirming the hypothesis that exosomes can deliver anticancer drugs to the BBB for the treatment of brain cancer.284 Qu et al.285 subsequently succeeded in delivering dopamine to the brain via exosomes in the blood, and in a mouse model of PD, exosomes loaded with dopamine showed better therapeutic efficacy and lower systemic toxicity than free dopamine after intravenous administration. Since then, several studies of exosomes as small molecule drug carriers loaded with chemotherapeutic agents have been reported, such as exosomal delivery of the chemotherapeutic agents adriamycin,286 paclitaxel,287, 288 DDP,289 and gemcitabine,290 which have been shown to enhance the cytotoxicity of the drugs on cancer cells and accelerate tumor regression in a variety of tumors. Exosomes loaded with curcumin291 and rosmarinic acid292 were also developed to enhance anti-inflammatory activity. Loading brain-derived neurotrophic factor (BDNF) in neural stem cell exosomes reduces the volume of ischemic cerebral infarction and improves neurological function.293 In addition to intravenous administration, in recent years EV has been found to help overcome the limitations of oral administration.294 For example, exosomes derived from grapefruit, loaded with methotrexate, are used to treat diseases associated with intestinal inflammation.295 Encapsulation of anthocyanidins into milk exosomes results in more potent antitumor effects without toxic side effects, providing an effective, cost-efficient, and safe alternative to oral anthocyanidins administration.296 Hesperidin (HES), a flavonoid present in various citrus fruits, has excellent anticancer activity. Exo-HES was formulated by loading it onto exosomes and showed better anticancer activity and oral bioavailability in a mouse model of melanoma, which could improve the therapeutic efficacy of HES in melanoma treatment.297
Of course, exosomes can also serve as delivery vehicles for therapeutic macromolecules. For example, binding trastuzumab (T-DM1) to exosomes derived from HER2-positive cancer cells may be carried by exosomes to other cancer cells, resulting in reduced viability of the recipient cells.298 Happily, exosomes can also enable gene therapy through nucleic acid presentation and genetically engineered specific target presentation. We will describe this in detail below.
4.2 Targeted therapy for EV
Multiple studies have shown that extracellular vesicles have natural targeting capabilities based on donor cells.299 Currently, techniques for improving specific targeting of extracellular vesicles can be divided into direct modification (direct loading of therapeutic cargoes such as genetic elements, biomolecules, and drugs into exosomes) and indirect modification (modification using physical and genetic techniques).300, 301 Decorated exosomes can enhance drug targeting, increase drug accumulation, and reduce off-targeting, thereby improving efficacy and reducing side effects302 (Table 2).
| Therapeutic cargo | Targeting ligand | Target cells | Function | References |
|---|---|---|---|---|
| siRNA targeting STAT3 | Angiopep-2 | Glioma (U251) | Enhanced drug BBB penetration and targeting ability | 303 |
| miR-588 | C(RGDyk) |
MB-231 HEK-293 |
Reshape immunosuppressive TME to enhance the antitumor effect | 304 |
| PTX | EGFR-targeted peptides |
H358 HCC827 |
Improving efficacy in EGFR-positive tumors | 305 |
| PTX | AA | Murine lung cancer (3LL-M27) | Improved normalization of blood vessels to make cancer sensitive to chemotherapy | 306 |
| Doxorubicin | iRGD | Breast cancer cells | Highly effective targeted DOX | 307 |
| Indocyanine green | Folic acid | Breast cancer cells | Increasing the efficacy of ultrasonodynamic therapy for breast cancer | 308 |
| siRNA targeting PD-L1 | RGD peptide | Glioma cell | strategy significantly improves GBM targeting efficiency as well as CD8 cytotoxic T-cell activity | 309 |
| 5-Fluorouracil anti-miRNA-21 | zHER affibody | Colorectal cancer (HCT-116) | Targeted delivery of 5-fu for increased efficacy | 310 |
| KGN | E7 peptide | SF-MSCs | Cartilage regeneration | 311 |
| Curcumin | C(RGDyk) | Cerebral vascular endothelial cells | Reduce inflammation and inhibit apoptosis in ischemic stroke | 312 |
| Curcumin-SPION | Neuropillin-1-targeted peptide | Glioma (U251) | Simultaneous diagnosis and treatment of glioma | 313 |
- Abbreviations: AA, aminoethylanisamide; KGN, Kartogenin; PTX, paclitaxel; STAT3, signal transducer and activator of transcription 3; SF-MSCs, synovial fluid-derived mesenchymal stem cells.
Direct exosome engineering allows the loading of various types of ligands onto exosome membranes through coupling and hydrophobic insertion.314 DSPE–PEG is a widely used module for anchoring targeting molecules to the exosome surface in lipid insertion.315 Exosomes modified with aminoethyl anis amide-PEG (AA-PEG) through DSPE–PEG insertion has been shown to target sigma receptors overexpressing lung cancers and can accumulate in large numbers in cancer cells, resulting in better therapeutic efficacy.306 Application of DSPE–PEG-modified angiopep-2-EX to enhance BBB penetration and targeted drug delivery for the treatment of glioblastoma.303 In the study of triple-negative breast cancer (TNBC), this technology was also used to modify the c(RGDyK) peptide on the surface of EV to deliver miR-588, which can anchor CCL5 affected by the vicious circle of immune escape to reshape immunosuppressive TME to enhance the antitumor effect.304 The c(RGDyK) peptide also modifies EVs by chemical linkage. Engineered RGDyK-conjugated exosomes (cRGD-Exo) modify curcumin-loaded MSC-EVs, which can be targeted to diseased regions of the ischemic brain, leading to a strong inhibition of inflammatory responses and apoptosis in diseased regions.312 The same research team also confirmed that the engineered exosomes loaded with cholesterol-modified miR-210 (RGD exo: miR-210) can increase the aggregation of miR-210 in brain lesions, which is beneficial for the treatment of cerebral ischemia.316 Qi et al.317 used a receptor–ligand binding method to affix superparamagnetic magnetite colloidal nanocrystal clusters to the surface of exosomes for targeted drug delivery to tumors. Both in vivo and in vitro experiments showed that exosome-based drug carriers displayed excellent in vivo targeting ability and cancer inhibition.317 Vandergriff et al.318 coupled cardiac homing peptide to exosome membranes via hydrophobic interaction. Engineered exosomes target the infarcted heart, reducing fibrosis and scar size and increasing cell proliferation and angiogenesis.318 Through direct modification, researchers have also created many exosomes with specific targeting properties, such as EV coupling with EGFR-targeted peptides or anti-EGFR nano-antibodies to promote their accumulation in EGFR-positive cancer cells, which can enable PTX delivery to significantly enhance drug efficacy in EGFR-positive lung cancer.319 Jayasinghe et al.305 demonstrated that exosomes modified by enzyme engineering have a strong antigen-specific targeting ability to efficiently and specifically target cells and deliver therapeutic drugs. Further in vivo experiments demonstrated that these engineered EVs were able to improve the biodistribution of encapsulated and conjugated drugs and significantly slow down cancer progression, both through enzymatic ligation of peptide drugs and exogenous loading of RNA.305
To achieve better targeting, indirect modification can also be achieved by genetically modifying cells that produce exosomes. This technology has been extensively studied in the treatment of tumors. The genetic modification of exosome-producing cells is achieved by transfecting genes expressing targeted molecules (e.g., peptides, receptors, and antibodies), which fuse with extracellular vesicle membrane components (such as the C1C2 domain of ubiquitin, Lamp2b, and milk adhesive protein).320 Currently, LAMP-2B is the most widely used exosome surface protein modification. Research has shown that the N-terminus of LAMP-2B is displayed on the surface of exosomes, which can attach targeted sequences and is the most widely used surface protein modification of exosomes.321 LAMP-2B can be fused to specific binding peptides for tissue cell-specific targeting. Using this technique, it was first observed that neuron-specific RVG peptides fused to Lamp2b could specifically deliver siRNAs and induce knockdown of brain signaling specificity. However, EV targeting was affected by the degradation of the targeting peptide.321 Subsequently, in neuroblastoma studies, exosome-targeted delivery was enhanced by the addition of a glycosylated glycan of the Lamp2b protein to prevent fusion protein degradation.322 In another experiment, the αv integrin-specific (iRGD)peptide fused to exosome surface Lamp2b exhibits efficient targeting and delivery of DOX in αv integrin-positive breast cancer.323 Similarly, RGD peptide and Lamp2b fusion-modified exosome can target delivery of miR-484, reprogramming the tumor vascular system and promoting chemosensitivity.324 The NSCLC homing peptide Tlyp-1 coupled with Lamp2b selectively delivered siRNA to human NSCLC cells, thereby knocking down target genes and reducing the stemness of cancer stem cells.325
LAMP-2B can also be genetically fused with targeting proteins or antibody fragments to display antibodies on exosomes.320 For example, the affinity molecule zHER, which targets HER2 binding, fuses with green fluorescent protein to the N-terminus of Lamp2 on the surface of exosomes. zHER exosomes exhibit higher affinity and selectivity toward HCT-116 colon cancer cells, allowing for more accurate delivery of 5-FU and miR-21i, thereby enabling the treatment of colorectal cancer.326 Chronic myeloid leukemia (CML) mother cells overexpress IL-3 receptors (IL3-R) on the cell surface, and fusion of IL-3 receptors with EV Lamp2b can enhance EV accumulation in tumors. IL3-EVs carrying imatinib and BCR-ABL siRNA can target CML cells, effectively killing CML cells.327 In addition, the transmembrane proteins platelet-derived growth factor receptor,328 the four-transmembrane protein superfamily CD63/CD9/CD81329 and C1C2330 in exosomes have also been used for targeted modifications. For example, Apo-A1 is coupled with CD63 to functionally deliver miRNA-26a to SR-B1 expressing HepG2 cells by utilizing purified exosomes from ApoA-1 donor cells, thereby inhibiting their proliferation and migration.331 Exosomes can also be targeted by loading proteins. Hong et al.332 loaded PH20 hyaluronidase into exosomes inhibited tumor growth, increased T-cell infiltration of tumors, and inhibited tumor growth better when coadministered with a chemotherapeutic agent (doxorubicin).
It can be seen that engineered EVs have achieved promising results in targeted drug delivery to specific cells and organs, as well as improving therapeutic efficacy.
4.3 Gene therapy
In recent years, exosomes as nonviral vectors have been at the forefront of gene therapy, particularly for applications in refractory diseases and regenerative medicines.333, 334
Exosomal nucleic acid delivery is one of the major highlights of gene therapy.335 For instance, HeLa cell-derived exosomes deliver siRNAs for RAD51 and RAD52, which activate apoptosis in recipient cancer cells.336 Delivery of siRNA-containing nanovesicles to Yes-associated protein 1 (YAP) directs hepatocyte differentiation to normal hepatocyte-like cells by activating downstream transcription factors, leading to tumor regression.337 Further studies have shown that siRNA-loaded milk-derived EV is a safe and achievable vehicle for oral nucleic acid therapies (e.g., RNA) in the management of intestinal diseases.338 Interestingly, it was found that exosome-mediated siRNA successfully knocks out the therapeutic target BACE1 for AD through the BBB delivery through the BBB.321 Recently, it was found that exosomes of engineered chondrocytes, CAP-Exo, which targets delivery of siRNA for MMP13, stimulated cartilage regeneration and attenuated cartilage degeneration.339 In the study of delivering miRNAs, researchers developed exosomes delivering miR-204-5p and found that they significantly inhibited the proliferation of tumor cells and increased sensitivity to chemotherapeutic drugs.340 Exosomal delivery of miR146a and miR-200b ameliorates colitis341 and inhibits intestinal fibrosis.342 The genetically engineered exosomes are also highly efficient in delivering mRNA and have shown therapeutic effects in combination with other anticancer drugs.343 For example, exosomes loaded with peroxidase mRNA cross the BBB and improve the prognosis of Parkinson's disease in a mouse model of the disease.344 Researchers also loaded mRNA onto a charge-reversed cationic exosome designed for the treatment of osteoarthritis, which overcame joint clearance and rapidly penetrated cartilage to promote cartilage regeneration.345 In another study, encapsulation of ECM α1 type I collagen (COL1A1) mRNA into EV effectively reduced collagen loss due to photoaging in a mouse model.346 Using a similar approach, exosomally encapsulated low-density lipoprotein receptor mRNA can be used to treat familial hypercholesterolemia in mice.347
EVs can also be delivery vectors for CRISPR/Cas9 and participate in gene therapy. Xu et al.348 used chimeric antigen receptor-modified EVs as a biologically safe delivery platform for the CRISPR/Cas9 system and showed that the CRISPR/Cas9 system was able to efficiently release anti-MYC oncogenes in vivo and in vitro. Chen et al.349 constructed artificially designed extracellular vesicles for delivery of CRISPR/Cas9 targeting miR-29b (EVs-Cas9-29b) could protect against muscle atrophy induced by dexamethasone (Dex), angiotensin II, and TNF-α.
4.4 Modulating immunity
Exosomes can express a variety of immunomodulatory proteins on their surface, thus increasing their affinity and binding affinity to target receptors or ligands on immune cells and diseased cells, which can promote the formation of immune synapses and thus enhance the activation of the immune system.350 Studies have demonstrated that exosomes derived from tumor cells, CD4 T cells, DC cells, NK cells, macrophages (M1), and CAR-T cells can directly or indirectly improve the immune response.351-353 The exosome derivatives produced after modification are more capable of enhancing immunomodulation and are widely used in tumor immunotherapy and cancer vaccine research. For example, IL-18 and IL-2 genetically modified TEX stimulate antitumor immune responses.354, 355 Antitumor immune responses are also induced by surface anchoring of superantigenic staphylococcal enterotoxin A.356
The efficacy of tumor immunotherapy depends largely on the sustained activation and expansion of tumor-specific T cells, especially tumor-infiltrating cytotoxic T lymphocytes (CTLs).357 The ability of exosomes to activate T cells is enhanced by the addition of adjuvants.358 HSP are highly enriched in TEX and have potent adjuvant capabilities. HSP treatment increasing the immunostimulatory activities of TEXs has been demonstrated in A20 lymphoma/leukemia cells.359 Epigenetic drug MS-275 upregulates the expression of Hsp70, MHC-I, and MICB in exosomes, which significantly enhances the cytotoxicity of NK cells.360 Expi293F cell-derived exosomes were loaded with monoclonal antibodies against human T cell CD3 and EGFR, as well as immune checkpoint modulators, PD-1 and OX40 ligand (OX40L). The resulting genetically engineered multifunctional immunomodulatory exosome (GEMINI-Exos) not only redirects and activates T cells to kill EGFR-positive TNBC cells but also induces potent anticancer immunity.350 Exosomes can also enhance immunity by regulating immunosuppression. Surface modification of bone marrow mesenchymal stem cell exosomes with oxaliplatin predrugs followed by delivery of galactaglutinin-9 siRNA to PDAC tissues inhibits macrophage polarization, CTL recruitment, and downregulation of Tregs to stimulate antitumor immunity in the tumor.361
In the development of cancer vaccines, DC-derived EVs (DEVs) are the most studied because of their ability to interact with CD4 and CD8+ T cells as well as NK cells to elicit an effective immune response.362 A personalized cancer vaccine based on recombinant adenovirus-infected DC membranes genetically engineered to significantly improve antigen delivery to lymphoid organs and generate broad-spectrum T-cell responses.363 Exosomes prepared from ovalbumin (OVA)-pulsed, activated DC were modified with anti-CTLA-4 antibody (EXO–OVA–mAb), which increased the migration of CD4 and CD8 T cells to the tumor site and increased the ratio of CTLs/Tregs in the microenvironment of the B16 melanoma tumor model.364 Munich et al.365 used mouse BMDC DEVs loaded with TNF, FasL, and TRAIL and observed that these vesicles were able to directly kill B16 tumor cells by promoting apoptosis and also induced NK cell activation and production of IFNγ.
In addition, the researchers have developed FAP genetically engineered tumor cell-derived exosome-like nanovesicles (eNVs-FAP). As a tumor vaccine, eNVs-FAP, which suppresses tumor growth by inducing a strong and specific CTL immune response directed against tumor cells and FAPCAF, as well as reprogramming immunosuppressive TMEs in models of colon, melanoma, lung, and breast cancer.366 In another, a prophylactic vaccine against ESC-exo/granulocyte-macrophage colony-stimulating factor was developed, which inhibited the progression of metastatic lung tumors in mice.367
In conclusion, the exosome derivatives produced after processing are superior to native exosomes in modulating immunity and can be used both as novel and effective cancer vaccines and in immunotherapy.
5 CLINICAL APPLICATIONS AND CHALLENGES
Exosome-based therapeutic strategies, many preclinical studies have demonstrated the broad significance of exosomes in the diagnosis, monitoring, prognostic assessment, vaccine development and treatment of diseases, and have led to the entry of exosomes into clinical studies for the diagnosis and treatment of diseases.
5.1 Clinical use as biomarkers in disease diagnosis and prognosis
Exosomes are now being translated to the clinic as diagnostic markers for cancer. Yoshioka et al.368 developed “ExoScreen,” an analytical technique for early detection of colorectal cancer, based on the fact that from raw blood samples from patients, EVs are trapped by CD147 (colorectal cancer-specific antibody) and CD9 (exosomal membrane marker), and detected by photosensitizing beads. McKiernan et al.369 developed the “ExoDx Prostate (Intelliscore) EPI test” for the detection of prostate cancer, which is approved for clinical use by the Food and Drug Administration. This is based on the isolation of exosomal mRNA and the detection of PCA3 and ERG genes in a urine sample from the patient. On top of that, the results of several clinical trials using EV as a biomarker for diagnosis and disease surveillance have been published. For example, In a single-center observational cohort study (NCT04928534), serum exosomes CHL1, KIF2A, miR-1183, and miR-297 were found to be potential biomarkers of CTE in patients with repetitive mild traumatic brain injury.370 A previous prospective, multicenter clinical utility study of the ExoDx Prostate test as a predictor of prognosis in high-grade prostate cancer (NCT03235687) showed that the ExoDx Prostate (EPI) test had a significant impact on prostate biopsy decisions.371 The team recently reported interim results with 2.5 years of follow-up, suggesting that prebiopsy EPI testing also leads to better outcomes when followed for 2.5 years after the initial biopsy decision-making process.372 Another prospective study confirmed the efficacy of EV derived from adipocytes as an immediate biomarker for evaluating treatment response to nonalcoholic fatty liver disease.373 Mengfan's team demonstrated374 that complement proteins in plasma ADEs are associated with cognitive impairment in patients with obstructive sleep apnea (OSA) and can be used as a marker of cognitive impairment in OSA patients (ChiCTR1900021544). Another recent study on early diagnosis in patients with AD found that MiR-125b-1-3p and miR-451a have sufficient specificity and sensitivity for AD and are potential diagnostic biomarkers.182
Although EV has yielded good results in biomarkers, it also has some problems. As with conventional tumor markers, exosome markers can have false positive and false negative results. The contents secreted by normal cells may mask tumor secretion, and the same ones that are highly expressed in tumors may be positive in certain inflammatory conditions.375, 376 For example, miR-21 is significantly expressed in glioblastoma but is also positively expressed in patients infected with Apache encephalitis virus infection.377 Coupled with the fact that the accuracy of the assay is correlated with the number of exosomes, the method of sampling and isolation of exosomes also puts limitations on clinical translation. To improve diagnostic accuracy, it is suggested that combining assays with multiple markers may provide a more precise diagnosis.378, 379
5.2 Therapy and efficacy of exosomes in clinical studies
Most of the ongoing clinical studies of exosomes for therapeutic use are in the early stages (phases I and II), with only a few in Phase III, covering a wide range of different disease indications, including cancer, cardiovascular disease, infectious diseases, and others (Table 3). According to a search on clinicaltrials.gov, 26 registered trials of exosome therapy have been completed, most of which unfortunately have not yet published their final results. The following is a description of the test for the published data.
| Application | Clinical setting | Stage | Start year | Source of exosome | Sponsor | Clinical trial number |
|---|---|---|---|---|---|---|
| Biomarker | Ocular muscle myasthenia gravis | NA | 2023 | Serum | First Affiliated Hospital of Jinan University | NCT05888558 |
| Immunotherapy in renal cell carcinoma | NA | 2023 | Blood and urine | Zhejiang Cancer Hospital | NCT05705583 | |
| Locally advanced breast cancer | NA | 2021 | Serum | Samsung Medical Center | NCT05955521 | |
| Gastrointestinal cancers | NA | 2024 | Blood | Beijing Friendship Hospital | NCT06278064 | |
| Intrahepatic cholangiocarcinoma | NA | 2023 | Blood | City of Hope Medical Center | NCT06381648 | |
| Drug delivery | Inflammatory responses (several diseases) | 1 | 2023 | Engineered exosome | ILIAS Biologics Inc. | NCT05843799 |
| Inflammatory bowel disease | NA | 2018 | Ginger exosomes | University of Louisville | NCT04879810 | |
| Homozygous familial hypercholesterolemia | 1 | 2021 | Plasma | Tang-Du Hospital | NCT05043181 | |
| Therapy | Diabetic foot ulcers | 2 | 2024 | Purified Exosome Product | Rion Inc. | NCT06319287 |
| Acute ischemic stroke | 1 | 2024 | Pluripotent stem cell | Xuanwu Hospital, Beijing | NCT06138210 | |
| Decompensated liver cirrhosis | 2 | 2023 | Mesenchymal Stem Cells | Research Institute for Gastroenterology and Liver Diseases | NCT05871463 | |
| Refractory focal epilepsy | 1 | 2023 | Engineered exosome | Peking Union Medical College Hospital | NCT05886205 | |
COVID-19 Acute respiratory distress syndrome |
1 2 |
2023 | Mesenchymal Stem Cell | AVEM HealthCare | NCT04798716 | |
| Metastatic pancreas cancer | 1 | 2021 | Engineered exosome | M.D. Anderson Cancer Center | NCT03608631 | |
| Degenerative meniscal injury | 2 | 2022 | Mesenchymal Stem Cell | Eskisehir Osmangazi University | NCT05261360 | |
| Acute respiratory distress syndrome | 3 | 2022 | Bone marrow mesenchymal stem cell | Direct Biologics, LLC | NCT05354141 | |
| Retinitis pigmentosa | 2 3 |
2022 | Mesenchymal stem cells | TC Erciyes University | NCT05413148 |
- Data sources—clinical registration website.
Results of two phase I studies of DC-derived exosomes for use as vaccines were published as early as 2005, two trials of DC-derived exosomes (Dex) loaded with MAGE tumor antigens and MAGE 3 peptides in patients with unresectable lung cancers380 and melanoma,381 although one trial did not elicit an immune response, both trials confirmed the feasibility and safety of exosome administration. Also, in clinical trials of Dex modulation of immunity, investigators in two phase I trials in end-stage cancers observed that the first generation of Dex (without IFN-γ) could exert an NK cell effect. Since then with preclinical studies confirming that adjuvants (TLR4L or interferon (IFN) γ) enhance the stimulatory function of Dex on T-cells using the treatment,382 the team went on to produce a second generation of Dex (IFN-γ-Dex), optimized for treatments aimed at boosting the immune response of both NK and T-cells. This phase II clinical trial demonstrated that IFN-γ-DexDex, loaded with MHC class I and class II-restricted cancer antigens, enhanced antitumor immunity in patients with advanced NSCLC.383
In a study of malignant pleural effusions, there was a significant reduction in tumor cells and reversal of TRC resistance after 7 consecutive days of intrapleural injections of exosomes loaded with DDP, compared with patients who received only DDP.384 The same results were subsequently obtained in another study of loaded MTX exosomes for the treatment of malignant pleural effusions.385 Based on the above, the investigators designed another randomized controlled clinical trial with a slightly larger sample to evaluate the safety and efficacy of intrapleural administration of MV–MTX in combination with pemetrexed and DDP chemotherapy for the treatment of malignant pleural effusion in advanced nonsquamous NSCLC. The results of the study showed that the amount of pleural effusion was significantly reduced in the MV–MTX group compared with the control group. At 1 year after treatment, the survival rate was 77.5% in the MV–MTX group and 59.0% in the placebo group, demonstrating that EVs can be used as an effective delivery vehicle for chemotherapeutic agents for cancer treatment.386
Due to the prevalence of COVID-19 in previous years, researchers have also done a lot of research on introducing EVs for the treatment of COVID-19. In a nonrandomized open-label cohort study evaluating exosomes from allogeneic bone marrow mesenchymal stem cells (EXOFlo) for the treatment of severe COVID-19, EXOFlo significantly improved symptoms, reduced mortality, and no adverse effects were observed within 72 h of treatment.387 Immediately following this, an RCT of ExoFlo infusion for the treatment of COVID-19 respiratory failure yielded the same results and demonstrated the efficacy of a 15 mL dose of ExoFlo in COVID-19-induced asthma.388 Subsequently, researchers explored the safety and efficacy of aerosolized inhaled human adipose mesenchymal stem cell exosomes (haMSC-Exos) in COVID-19 patients. In this Phase IIa single-arm, open-label, interventional trial. After aerosol inhalation of haMSC-Exos, all patients experienced varying degrees of regression of lung lesions. All COVID-19 patients tolerated haMSC-Exos aerosol therapy well.389
Exosomes have also yielded good results in studies of other diseases. Exosomes from mesenchymal stromal cells (MSC-exo) administered as eye drops significantly alleviated dry eye associated with GVHD, in a prospective clinical trial.390 The first phase I/II clinical trial of three-arm drug intervention has also released partial results regarding the safety and efficacy of allogeneic human adipose MSCs Exos (ahaMSCs Exos) in mild to moderate AD patients. In the medium dose group, the Alzheimer's Disease Assessment Scale Cognitive Scale (ADAS cog) score decreased by 2.33 at week 12.391 There have also been two randomized trials showing that adipose tissue stems cell-derived exosomes (ASCEs) combined with microneedling/fractional CO2 lasers can have a synergistic effect on facial ageing392 and will have a significant improvement on atrophic acne scars.393
Building on the results of preclinical studies, scientists are currently conducting extensive clinical studies, which are briefly described below. In a study examining the ability of plant exosomes to deliver curcumin to normal and malignant colon tissues (NCT01294072), curcumin was loaded into plant exosomes and acted as a drug delivery system in normal and colon cancer tissues. Prior preclinical studies found that plant exosomes release exosomal particles that bind tightly to a variety of hydrophobic drugs, including curcumin, and are taken up by intestinal cells and intestinal immune cells.394 Chimeric exosomes iExosomes effectively used in preclinical studies were included in the clinical trial (NCT03608631). siRNA Kras G12D was loaded into exosomes derived from mesenchymal stromal cells and used as an anticancer drug for the treatment of patients with metastatic pancreatic cancer in this ongoing trial. Preclinical findings suggest that exosomes released from the placenta carry specific cargoes that lead to preeclampsia,395, 396 and by isolating these exosomes from maternal blood and placental tissues of patients diagnosed with preeclampsia and investigating their biochemical, cellular, and molecular mechanisms in animal models, the researchers hope to elucidate the key role that exosomal cargoes play in the development of preeclampsia and cardiovascular remodeling (NCT04154332). Several previous Phase I∖II studies have confirmed the utility of ExoFlo in respiratory distress syndrome. To further investigate the safety and efficacy of ExoFlo in the treatment of hospitalized patients with moderate to severe acute respiratory distress syndrome (ARDS), the investigators designed a Phase III, multicenter, randomized, double-blind, placebo-controlled trial (NCT05354141).
These clinical studies demonstrate the great possibilities of exosomes in medicine and provide an important basis for clinical translation.
5.3 Regulation and challenges of exosome therapy
The authoritative report from BCC Research in 2024 shows that the global market for exosome diagnostic, therapeutic, and research tools is expected to grow from $227.5 million in 2023 to $1.3 billion in 2028. Undoubtedly, exosomes have sparked a wave of biomedical research.
However, with the increase in research, the regulatory issues of exosome therapy have not been addressed. Current considerations include the following: (1) Standardization of preparation and storage. The preparation of exosomes varies from enterprise to enterprise in terms of purification, quantification storage methods, and so on, and there is a lack of mature industry standards. (2) Scale-up preparation. Current cell culture and exosome purification methods limit the implementation of large-scale production of exosomes.397 Although methods such as ultrafiltration, size exclusion chromatography, and aqueous two-phase systems that are compatible with large-scale production have been developed, there are no comparisons between them, and there is a lack of recommendations for regulated scale-up preparation methods. (3) Standardized control of exosome quality. According to the different states of different parental cells, exosomes are highly heterogeneous in carrying nucleic acids and proteins. Standardized quality control is the guarantee for the clinical application of exosomes. (4) Regulatory gaps. Currently, there is no standardized set of violations for using extracellular vesicles to serve supervision.
Of course, exosomes also face various challenges from preclinical to market. Due to the small size and complex composition of EVs, detecting and evaluating changes in the quality of electric vehicle products is challenging. The production of EVs, especially the selection and modification of donor cells to produce exosomes, is hampered by low yields, high prices, and difficulties in industrial production. How to produce exosomes quickly, economically, and in large quantities is a major challenge for clinical translation. Exosomes have become a key element in the field of therapeutic delivery, but the large number of natural components carried by exosomes themselves makes cargo loading very complex and restricted, and it has been a challenge to develop exosome engineering techniques for efficient and reproducible loading of therapeutics into EVs. Exosomes are highly heterogeneous and separating homogeneous exosomes from heterogeneous exosomal motifs is technically challenging. Quantifying the exact amount of EV per unit therapeutically remains difficult, and finding a strategy that allows precise control of exosome content is also challenging for scientists.
For these challenges, in the future, interdisciplinary teams from science, chemistry, engineering, and medicine will collaborate in what is expected to be a transformation of exosomes from the laboratory to clinical practice.
5.4 Prospects and potential R&D directions
With the development of biotechnology and an in-depth understanding of exosome functions, research on exosome technology has made great progress. The emerging magnetic NP capture technology has effectively improved the purity and efficiency of exosome isolation. Microfluidic microarray technology enables richer and more precise biological information to be obtained from individual exosomes. In addition, the development of Surface Enhanced Raman Spectroscopy sensors, immunomagnetic bead detection technology, and fluorescence technology has improved detection sensitivity and specificity.398 We have reason to believe that exosomes will revolutionize the medical field and promote the advancement of human health.
Exosomes will play an increasingly important role in the future of medical technology. It not only improves the precision and efficiency of drug delivery but also aids in the early detection and treatment of disease. With the deepening of scientists' understanding of exosomes, exosomes can also develop many potential research directions. First, current exosome research mainly focuses on plant and animal sources, and little is involved in microbe-derived exosomes. Can these bacterial and viral-derived exosomes, which carry information from their parents and are modified, play a beneficial role in infectious diseases, tumors, and other intractable diseases? Second, exosomes are mainly administered intravenously, but can also be sprayed and given orally. With the development of biomaterials, it will be possible to develop customized drug delivery methods for specific diseases by wrapping exosomes with special materials. Furthermore, exosomes have achieved some results in vaccine research. In the treatment of tumors, can synergistic effects be achieved by the combined application of exosome vaccines with radiotherapy and immunotherapy? Finally, Exosomal contents are complex, and the effects of many RNAs and DNAs on the body through cellular communication are not yet fully understood. Deepening research in this area may open up new horizons in the treatment of exosomes.
6 SUMMARY
Exosomes, as nanoscale vesicles, are one of the most effective means of intercellular communication, which regulates the function of receptor cells. Through extensive research, researchers have found that exosomes play an important role in disease treatment. Exosomes can carry information from parental cells and exist in various body fluids, making them the most promising biomarkers for liquid biopsy. It can be used for early diagnosis of diseases, and more importantly, it has been found to serve as an indicator for monitoring drug efficacy, disease progression, and to detect the sensitivity of diseases to certain drugs. More recently, this technology set has been combined with machine learning and artificial intelligence (AI), which has had a significant impact on the fast-reading screening of diseases, especially the diagnosis of cancer.399 Since exosomes have a natural advantage over other NPs, they are also a favorable contender for drug delivery. EVs can accurately transport a variety of molecules, proteins, and even nucleic acids to targeted cells. Most encouragingly, EVs show a broad biodistribution that crosses the BBB and holds promise for the treatment of CNS diseases. The exosomes secreted by some stem cells and immune cells have an indispensable role in tissue repair and immune response. In recent years exosomes have gained attention in tissue regeneration, tumor immunotherapy, and immune vaccine development, and some results have been achieved. Especially with genetically engineered exosomes, researchers have seen encouraging results in drug delivery, targeted therapy, and immune regulation. Next the combined application of exosomes and 3D printing has also brought new hope in tissue repair.
With the continuous progress of medical science and technology, the diagnosis and treatment of diseases have entered the era of precision medicine. Exosomes, as the most emerging molecules, will have a great role in promoting individualized and precise treatment. Exosomes as molecular markers can achieve precise detection of disease-causing genes, and through the mediation of targeting and gene therapy, precise treatment of diseases can be achieved, and the development of nano-vaccines opens up a new way of personalized immune regulation. In the future, the close integration of exosomes with emerging fields such as AI and bioengineering, will help to create diagnostic models for identifying specific diseases and new personalized treatment options. Exosome research and applications will revolutionize the field of medicine.
AUTHOR CONTRIBUTIONS
Writing—ideas, original drafts, graphic preparation, and literature searches: Chuan Xu. Writing—original draft: Chaoyang Jiang. Writing—original draft and funding acquisition: Zhihui Li. Writing—original draft: Hui Gao. Writing—original draft: Jing Xian. Writing—literature search: Wenyan Guo. Writing—literature search: Dan He. Writing—Review: Xingchen Peng. Review: Daijun Zhou. Writing—review and editing: Dong Li. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We appreciate Adobe Illustrator for supporting the materials in graphics in this article. This study was funded by the Talent Incubation Programme of the General Hospital of the Western Theatre Command of the People's Liberation Army (PLA) (2021-XZYG-C49).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




