Chinese expert consensus on the diagnosis and treatment of coronary microvascular diseases (2023 Edition)
Abstract
Since the four working groups of the Chinese Society of Cardiology issued first expert consensus on coronary microvascular diseases (CMVD) in 2017, international consensus documents on CMVD have increased rapidly. Although some of these documents made preliminary recommendations for the diagnosis and treatment of CMVD, they did not provide classification of recommendations and levels of evidence. In order to summarize recent progress in the field of CMVD, standardize the methods and procedures of diagnosis and treatment, and identify the scientific questions for future research, the four working groups of the Chinese Society of Cardiology updated the 2017 version of the Chinese expert consensus on CMVD and adopted a series of measures to ensure the quality of this document. The current consensus has raised a new classification of CMVD, summarized new epidemiological findings for different types of CMVD, analyzed key pathological and molecular mechanisms, evaluated classical and novel diagnostic technologies, recommended diagnostic pathways and criteria, and therapeutic strategies and medications, for patients with CMVD. In view of the current progress and knowledge gaps of CMVD, future directions were proposed. It is hoped that this expert consensus will further expedite the research progress of CMVD in both basic and clinical scenarios.
1 INTRODUCTION
Since Likoff et al.1 first reported the clinical manifestations of a group of patients with a definite diagnosis of coronary heart disease (CHD) but a normal coronary angiogram in 1967, basic and clinical research on coronary microvascular diseases (CMVD) has continued for half a century. In 2013, the European Society of Cardiology (ESC) guidelines for the treatment of stable coronary artery disease (CAD) first recognized CMVD as a clinical type of CHD and made preliminary recommendations for the diagnosis and treatment of CMVD.2 In 2017, the Basic Research Group, Interventional Cardiology Group, Women's Heart Health Group, and Atherosclerosis and Coronary Heart Disease Group of the Chinese Society of Cardiology issued “Chinese expert consensus on the diagnosis and treatment of coronary microvascular diseases,” the first expert consensus on CMVD in the literature,3 which clarified a number of unclear issues and expedited the progress of basic and clinical research in the field of CMVD in China.
Since 2017, international consensus documents on CMVD have increased rapidly. In 2018, the Coronary Vasomotion Disorders International Study Group (COVADIS) recommended the diagnostic criteria for type 1 coronary artery microvascular dysfunction, namely primary CMVD.4 In 2019, the ESC published guidelines for the diagnosis and treatment of chronic coronary syndrome, in which microvascular angina (MVA) pectoris was classified as an important type of chronic coronary syndrome, and corresponding diagnostic and treatment strategies were recommended.5 In 2019, the American Heart Association (AHA) issued a scientific statement on the diagnosis and treatment of myocardial infarction (MI) with nonobstructive coronary arteries (MINOCA), stating that MVA pectoris, micro-vasospasm, and slow coronary flow are important causes of MINOCA.6 In 2020, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and ESC jointly issued a consensus document on ischemia with nonobstructive coronary arteries (INOCA), indicating that CMVD and epicardial coronary artery spasm are the main causes of INOCA.7 In 2020, ESC Working Group on Coronary Pathophysiology and Microcirculation published position paper on coronary microvascular dysfunction, dividing CMVD into six clinical types.8 These international documents focused on mainly research progress of CMVD, and although some of these documents made preliminary recommendations for the diagnosis and treatment of CMVD, they did not provide classification of recommendations and levels of evidence. Thus, the guidance value of these documents in clinical practice is limited.
In order to summarize recent progress in the field of CMVD, standardize the methods and procedures of diagnosis and treatment of CMVD, and identify the scientific questions for future research, the Basic Research Group, Atherosclerosis and Coronary Heart Disease Group, Interventional Cardiology Group, and Women's Heart Health Group of the Chinese Society of Cardiology held a meeting in November 2021, discussed current issues in the field of CMVD and decided to update the 2017 version of Chinese expert consensus on CMVD. The four working groups adopted the following measures to control the quality of this document: first, a consensus revision committee and a consensus drafting group were formed and their members were nominated by the four working groups; second, search keywords on CMVD in the literature were suggested by the drafting group and approved by the consensus revision committee; third, English and Chinese literatures published from 2016 to 2021 were extensively searched using approved key words and major search engines; fourth, poor quality, repetitive and small sample studies were deleted using the quality standard predefined by the consensus revision committee, and the remaining literatures were classified according to their research area; fifth, a consensus outline was suggested by the drafting group after a thorough investigation of classified literatures, and approved by the consensus revision committee; sixth, the consensus revision committee and drafting expert group held several meetings to discuss the manuscript, which were revised for several times and finally approved by the consensus revision committee and the Chinese Society of Cardiology.
The recommendations of diagnosis and treatment of CMVD in this consensus were based on ACC/AHA criteria as illustrated in Tables 1 and 2.9 The following aspects of CMVD were discussed in this consensus sequentially: (1) definition, clinical classification, and epidemiology of CMVD; (2) pathogenetic mechanism of CMVD; (3) diagnostic techniques for CMVD; (4) clinical manifestations and diagnostic criteria of CMVD; (5) treatment of CMVD; and (6) gaps of knowledge and future perspectives. The updated recommendations and opinions in the new consensus were given in Table 3 as compared to the Chinese expert consensus on the diagnosis and treatment of CMVD issued in 2017.
| Definition | |
|---|---|
| Class I | Treatments or procedures that have been proven and/or unanimously recognized to be beneficial, useful or effective, and recommended |
| Class II | Treatments or procedures for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy |
| Class IIa | The evidence/opinion tends to be useful and/or effective, and it is reasonable to apply these treatments or procedures |
| Class IIb | The relevant evidence/opinions have not been fully proven to be useful and/or valid and can be considered for application |
| Class III | Treatments or procedures that have been confirmed and/or unanimously recognized as useless and/or ineffective and may be harmful in some cases, and not recommended |
| Definition | |
|---|---|
| Level A | Evidence based on multiple randomized clinical trials or meta-analyses. |
| Level B | Evidence based on a single randomized clinical trial or multiple nonrandomized controlled trials |
| Level C | Consensus of expert opinion and/or evidence based on small-scale studies, retrospective studies, and registration studies |
| New concepts and recommendations in 2023 | ||
|---|---|---|
| Section 2.2 Clinical classifications | ||
| 1 | CMVD associated with myocardial ischemia | New |
| 2 | CMVD associated with myocardial infarction | |
| 3 | CMVD associated with coronary revascularization | |
| 4 | CMVD associated with non-atherosclerotic heart disease | |
| Section 3 Pathogenetic mechanism of CMVD | ||
| 1 | Regulatory mechanism of coronary blood flow | New |
| 2 | Structural abnormalities of the coronary microcirculation | Updated |
| 3 | Microvascular obstruction | New |
| 4 | Functional abnormalities of the coronary microcirculation | Updated |
| Section 4.1 Vasoactive drugs for evaluating coronary microvascular function | ||
| Regadenoson and nicorandil | New | |
| Section 4.2 Noninvasive techniques for evaluating coronary microvascular function | ||
| 1 | TTDE | Updated |
| 2 | MCE | New |
| 3 | SPECT | Updated |
| 4 | PET | Updated |
| 5 | CMR | Updated |
| 6 | CTP | New |
| Section 4.3 Invasive techniques for evaluating coronary microvascular function | ||
| 1 | CAG | Updated |
| 2 | Bolus and continuous thermodilution | Updated |
| 3 | Intracoronary Doppler flow velocity measurement | Updated |
| 4 | Diagnostic flow chart | New |
| Section 5 Clinical manifestations and diagnostic criteria of CMVD | ||
| Diagnostic criteria for different types of CMVD | New | |
| Section 6 Treatment of CMVD | ||
| 1 |
Treatment for CMVD associated with INOCA Risk factor management and lifestyle modifications Coronary and endothelial function test Stratified medical therapy |
Updated |
| 2 |
Treatment of CMVD associated with IOCA Lifestyle modifications Antiatherosclerosis therapy Stratified medical therapy Coronary revascularization |
Updated |
| 3 |
Treatment of CMVD associated with MINOCA Stratified medical therapy Secondary prevention |
Updated |
| 4 |
Treatment of CMVD associated with MIOCA Pharmacological treatment before PCI Pharmacological treatment during PCI Nonpharmacological treatment |
Updated |
| 5 |
Treatment of CMVD after successful intervention for AMI Pharmacological treatment Nonpharmacological treatment |
New |
| 6 |
Treatment of CMVD with non-atherosclerotic heart disease Treatment of CMVD with myocardial hypertrophy Treatment of CMVD without myocardial hypertrophy |
New |
- Abbreviations: AMI, acute myocardial infarction; CAG, coronary angiography; CTP, computed tomography perfusion; CMR, cardiovascular magnetic resonance; CMVD, coronary microvascular disease; INOCA, ischemia with nonobstructive coronary arteries; IOCA, ischemia with obstructive coronary arteries; MCE, myocardial contrast echocardiography; MINOCA, myocardial infarction with nonobstructive coronary arteries; MIOCA, myocardial infarction with obstructive coronary arteries; PCI, percutaneous coronary intervention; PET, positron emission tomography; SPECT, single-photon emission computed tomography; TTDE, transthoracic Doppler echocardiography.
2 DEFINITION, CLINICAL CLASSIFICATION, AND EPIDEMIOLOGY OF CMVD
2.1 Definition
CMVD is a clinical syndrome of acute and chronic myocardial ischemia caused by abnormalities in structure and function of coronary prearterioles, arterioles, and capillaries induced by atherosclerotic and non-atherosclerotic pathogenic factors.
2.2 Clinical classifications
This consensus divides CMVD into four major types and nine sub-types (Table 4).
| Classifications |
|---|
|
|
|
|
- Abbreviations: IOCA, ischemia with obstructive coronary arteries; CMVD, coronary microvascular disease; INOCA, ischemia with nonobstructive coronary arteries; MIOCA, myocardial infarction with obstructive coronary arteries; MINOCA, myocardial infarction with nonobstructive coronary arteries; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
2.3 Epidemiology
Most epidemiological studies of CMVD were performed in European and American populations, some of which included Asian populations as well. No studies have yet reported ethnic differences in the incidence of CMVD. The incidence of CMVD varies greatly in different studies, ranging from 10% to 80% due to the inconsistency of CMVD definition and diagnostic criteria. Likewise, owing to the differences in endpoints and follow-up duration, there were significant differences in mortality and the incidence of adverse cardiovascular events in CMVD patients in different studies.10 However, among INOCA patients, the incidence of CMVD in females is consistently higher than that in males.
2.3.1 CMVD associated with myocardial ischemia
Available studies showed that women had a higher incidence of CMVD associated with myocardial ischemia. In addition, CMVD increased the incidence of composite endpoints of cardiovascular events as an independent predictor of prognosis.
In a single center study, 329 consecutive patients with angina pectoris underwent coronary angiography (CAG) in whom coronary flow reserve (CFR) after stress testing were measured with myocardial perfusion positron emission tomography (PET) and followed for an average of 3.1 years. Major adverse cardiovascular events (MACE) included cardiovascular death and hospitalization due to heart failure or MI. The extent and severity of angiographically identified coronary disease were estimated using coronary artery disease prognostic index (CADPI) based on the number of stenotic vessels and the severity of coronary artery stenosis (50–100%), with three-vessel stenosis < 50% counted as zero. The results showed that the MACE rate at 1-year follow-up was higher in the CFR < 1.6 group with or without revascularization therapy, while the group with low CADPI and CFR≥1.6 had the highest rate without cardiovascular events. Multiple regression analysis showed that CFR was a more important predictor of the risk of MI and heart failure than CADPI with a hazard ratio (HR) of 2.02 and 1.17, respectively.11 The Women's Ischemia Syndrome Evaluation (WISE) study showed that patients with INOCA had a poor prognosis, and a decreased CFR was a strong independent predictor of MACE.12 The 2015−2018 international multicenter enrollment cohort study launched by the US COVADIS project team showed that in 686 patients (women 64%) with MVA pectoris excluding epicardial vessel stenosis > 50%, coronary microvascular spasm was the most common (42%), followed by decreased CFR (35%), increased microvascular resistance (14%), and slow coronary flow (6%). The incidence of composite endpoints, including cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalization for heart failure or angina, was 7.7% after 1 year of follow-up.13
In a retrospective study, the results of CAG performed in 1600 patients in 6 centers of China were analyzed, and the prevalence rate of INOCA was about 20%, and female was identified as a risk factor of INOCA.14 In a systematic review and meta-analysis that included 56 studies and 14,427 patients with INOCA, microvascular function was evaluated using invasive or noninvasive diagnostic methods, and the results showed that 41% of patients had coronary microvascular dysfunction, 40% had epicardial coronary spasm, and 24% had microvascular spasm. In addition, the incidence of coronary artery microvascular dysfunction in women was 1.45 times higher than that in men.15
2.3.2 CMVD associated with MI
CMVD-associated MI can be divided into two subtypes: CMVD associated with MIOCA and CMVD associated with MINOCA. The incidence of adverse cardiovascular events significantly increases when MIOCA coexists with CMVD. MINOCA is more common in women, and CMVD is an important pathogenic factor for MINOCA.
A systematic review and meta-analysis analyzed cardiovascular endpoints in 1094 patients (women 18.2%) with ST-segment elevation MI (STEMI) in six studies conducted between 2013 and 2020. The endpoints were all-cause death, nonfatal MI, and combined cardiovascular events with hospitalization for heart failure, and follow-up ranged from 6 months to 7 years. Using an index of microcirculatory resistance (IMR) > 40 or hyperemic microvascular resistance (HMR) > 3 mmHg/cm/s as an indicator for severe CMVD, the results showed that severe CMVD had a worse prognosis, with an HR of 3.42 compared with nonsevere CMVD.16 A meta-analysis of 46 studies showed an incidence of MINOCA of 6% (patients’ median age 55 years), which was more common in young women without hyperlipidemia, and 12-month all-cause mortality was lower in patients with MINOCA than MIOCA (4.7% vs. 6.7%).17 In the absence of culprit plaque rupture or erosion, microvascular dysfunction may have a critical role in MINOCA. Forty-four female patients with MINOCA underwent cardiovascular magnetic resonance (CMR), and late gadolinium enhancement imaging was observed in 59% of these patients, indicating myocardial ischemia caused by microvascular dysfunction.18 In addition, 96 patients with MINOCA underwent acetylcholine stress test, and one-third patients were found to have microvascular spasm.19
2.3.3 CMVD associated with coronary revascularization
CMVD associated with coronary revascularization is mainly manifested with no-reflow, which is more common and the prognosis is worse in women than in men. Long-term MACE increased significantly in patients with CMVD after elective percutaneous coronary intervention (PCI). Evidence of coronary microvascular dysfunction was also found in both short-term and long-term follow-up of patients undergoing coronary artery bypass grafting (CABG).
A 2010–2016 Italian-registration study included 2596 patients with STEMI (women 25.9%) who underwent primary PCI (PPCI). The primary endpoint was 30-day mortality. The results showed that women had a higher rate of primary endpoint than men (4.8% vs. 2.5%, odd ratio = 2.0). Multiple regression analysis showed that women were more likely to develop no-reflow (postprocedural thrombolysis in MI (TIMI) flow grade 0–2, HR = 1.68).20 An international, multicenter observational study recruited 572 patients with stable CAD who underwent coronary interventional therapy, and IMR ≥ 25 was defined as coronary microvascular dysfunction. After 4 years of follow-up, the cumulative MACE, including perioperative MI, recurrent MI, all-cause mortality and revascularization, was significantly higher in patients with a high IMR than in those with a low IMR (HR = 1.56, p = 0.001).21 In a retrospective study, 341 patients who had previously received CABG were followed for 638 days. After adjusting for known prognostic factors (regional ischemia, infarction), both stress myocardial blood flow (MBF) and myocardial perfusion reserve (MPR) independently predicted the composites of all-cause mortality and MACE. The adjusted HR for 1 mL/g/min of decrease in stress MBF was 2.56 (95% CI: 1.45–4.35) and for 1 unit of decrease in MPR was 1.61 (95% CI: 1.08–2.38).22 Spyrou et al.23 measured MBF at rest and during dipyridamole-induced hyperemia using PET in eight patients who underwent CABG, and found that coronary microvascular dysfunction was present at 1 and 6 months postoperatively, and the recovery was slow.
2.3.4 CMVD associated with non-atherosclerotic heart disease
CMVD has been reported in a variety of patients with non-atherosclerotic heart disease. CMVD with cardiac hypertrophy is common in hypertrophic cardiomyopathy (HCM),24 aortic stenosis (AS),25 cardiac amyloidosis,26 heart failure with preserved ejection fraction (HFpEF),27 and Anderson–Fabry disease. On the other hand, CMVD without myocardial hypertrophy is common in stress cardiomyopathy,28 diabetic cardiomyopathy,29 dilated cardiomyopathy,30 and autoimmune diseases.31, 32 However, most of these associations came from small sample studies.
3 PATHOGENETIC MECHANISM OF CMVD
3.1| Structural and physiological characteristics of the coronary artery
Coronary artery is a continuous vascular network consisting of four segments33: (1) epicardial coronary artery: the luminal diameter of the epicardial coronary arteries is 0.5–5 mm, which act as a capacitance vessel and offer little resistance to coronary blood flow (CBF); (2) prearteriole: the luminal diameter of the prearterioles is 100–500 μm, which are mainly responsible for regulating coronary artery perfusion pressure and exert a major effect on coronary blood flow resistance. The prearterioles can be further divided into two segments: the proximal prearterioles (150–500 μm) are more responsive to changes in flow, and the distal prearterioles (100–150 μm) are more sensitive to pressure variations; (3) arteriole: the luminal diameter of arterioles is <100 μm, which are sensitive to the change of myocardial metabolite concentration and play a major role in matching myocardial blood supply with oxygen consumption34; (4) capillary: myocardial capillaries are composed of monolayer endothelial cells, which provide 90% of the blood supply to the myocardium, and are mainly responsible for the exchange of myocardial oxygen, nutrients, and metabolites.35 Prearterioles, arterioles, and capillaries constitute coronary microcirculation. Pressure gradually decreases as blood flows from the aorta to the coronary capillaries: 10% pressure reduction occurs in epicardial coronary arteries, 30% in prearterioles, 40% in arterioles, and 20% in capillaries and venules. Therefore, the coronary microcirculation plays a crucial role in regulating coronary perfusion pressure and blood flow.36
3.1 Regulatory mechanism of CBF
3.1.1 Regulatory mechanism of coronary vasomotor
Vasomotor function in different coronary segments is regulated by different mechanism:
(1) Flow-mediated vasodilation: This mainly occurs at the epicardial coronary artery and prearteriole levels. When the shear stress of blood flow increases, endothelial cells release nitric oxide (NO), endothelium-dependent hyperpolarization factors (EDHFs) and prostacyclin to induce vasodilation.
(2) Coronary blood flow autoregulation: Under the condition of unchanged myocardial metabolism, the CBF remains consistent despite a wide variation of coronary perfusion pressure (60–100 mmHg). This phenomenon is called automatic regulation, and the mechanism may involve myogenic reaction at the distal prearterioles, that is, when the coronary perfusion pressure rises, the prearterioles contract and vice versa.
(3) Myocardial oxygen consumption: The maximum oxygen supply of coronary artery can be five times larger than that in the resting state due to a fully dilation of coronary resistance vessels to meet an increased demand of myocardial oxygen consumption, which has been termed “functional hyperemia.” A variety of mediators are involved in this mechanism, including neurotransmitters in circulation, NO, EDHFs, prostacyclin, and endothelin produced by vascular endothelial cells, histamine, kinin, and interleukin produced by vascular adventitial cells, and thromboxane A2 and serotonin produced by platelets.
(4) Myocardial metabolites: Hypoxia can induce adenosine production and stimulate the adenosine A2 receptor of smooth muscle cells to dilate the coronary artery. During myocardial ischemia or hypoxia, the accumulation of myocardial metabolites first dilates the coronary arterioles resulting in decreased coronary artery resistance and perfusion pressure in the prearterioles, which triggers a myogenic response of the coronary prearterioles, further dilates blood vessels, and increases the shear stress of coronary blood flow. The increased shear stress may trigger blood flow-mediated vascular dilation of the epicardial coronary arteries and larger prearterioles. This cascade regulatory mechanisms ensure that other mechanisms can compensate when one regulatory mechanism fails in pathological conditions, thus avoiding myocardial ischemia.37
3.1.2 CFR
CFR is defined as the ratio of CBF or MBF during maximum coronary dilation to the corresponding indicators in the resting state. It is an overall indicator of the reserve function of the entire coronary system. CFR is affected by four factors: resting CBF, the cross-sectional area of resistance vessels per unit volume of the myocardium, extravascular pressure, and coronary perfusion pressure. In clinical practice, factors affecting coronary or MBF at rest are as follows: age, sex, myocardial oxygen consumption (heart rate and blood pressure), medications, abnormal vascular endothelial function, and myocardial fibrosis. The following factors are known to affect coronary or MBF in the hyperemia state: age, inadequate coronary dilation, coronary perfusion pressure, caffeine, and its derivatives which may counteract the effects of adenosine or dipyridamole, microvascular anatomical remodeling, increased microvascular tension, increased extravascular tension, abnormal vascular endothelial function, and myocardial fibrosis.38
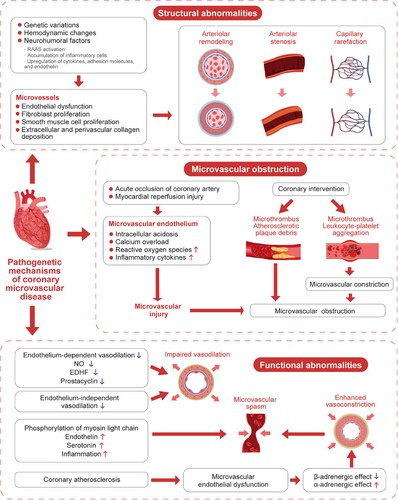
3.2 Structural abnormalities of the coronary microcirculation
Microvascular remodeling and stenosis are consistently documented in patients with HCM and hypertensive heart disease.39 The pathogenesis may involve genetic factors such as gene polymorphisms of the renin–angiotensin–aldosterone system (RAAS), peroxisome proliferator-activated receptors and endothelin, hemodynamic changes such as a high shear stress induced by blood pressure elevation, and neurohumoral mechanisms including RAAS activation in hypertension, high expression of cytokines, adhesion molecules, and endothelin, as well as inflammatory cell accumulation. All these mechanisms may result in endothelial dysfunction and proliferation of fibroblast and vascular smooth muscle cells, leading to varying degrees of intimal and medial thickening, perivascular fibrosis, intramural arteriolar remodeling and stenosis, and capillary rarefaction. Ultimately, these structural abnormalities cause increased coronary microvascular resistance and decreased CBF.40-42
3.3 Microvascular obstruction
Coronary microvascular obstruction (MVO) is commonly seen in emergency PCI and intervention of stenotic saphenous vein graft.43 The following mechanisms are involved: (1) acute coronary artery occlusion causes acute hypoxia of endothelial cells and reduced flow shear stress, leading to a series of biochemical and metabolic changes, such as increased anaerobic glycolysis, intracellular acidosis and calcium overload, and release of reactive oxygen species and inflammatory cytokines. These abrupt changes may lead to endothelial swelling and disruption, increase in vascular permeability, loss of vasodilator response, and constriction of smooth muscle cells, and finally cause injury and obstruction of the coronary microcirculation. In addition, myocardial reperfusion injury may further aggravate microvascular damage; (2) obstruction of distal coronary microvessels due to microthrombi and debris released from atherosclerotic plaques during various intervention procedures44; (3) microvascular compression due to myocardial cell apoptosis, myocardial edema and inflammation, which may lead to erythrocyte extravasation and intracardiac hemorrhage45; (4) coronary microvascular constriction caused by microthrombi or aggregated leukocyte-platelet.46
3.4 Functional abnormalities of the coronary microcirculation
3.4.1 Impaired endothelium-dependent vasodilation
Endothelium-dependent vasodilation dysfunction is more common in patients with risk factors for cardiovascular disease (such as diabetes, dyslipidemia, obesity, and smoking) or atherosclerosis. Prostaglandins, NO and EDHF synthesized and released by endothelial cells play a key role in regulating vascular tension, and endothelium-dependent coronary microcirculation dysfunction may be attributed to the decreased production or attenuated role of these vasodilators.47 EDHFs are more important than NO in the pathogenesis of CMVD due to their function of dilating coronary resistance vessels.47, 48
3.4.2 Impaired endothelium-independent vasodilation
In patients with diabetes, metabolic syndrome, dyslipidemia, hypertension, obesity, smoking, renal disease, and cardiomyopathy, the coronary artery dilation response to papaverine, adenosine, or dipyridamole is weakened, suggesting impaired endothelium-independent vasodilation.49 Consequently, these abnormalities may lead to decreased reactivity of coronary arteries to vasodilating substances, and declined CFR.
3.4.3 Microvascular spasm
Microvascular spasm is commonly seen in patients with angina or MINOCA.19, 50 Coronary microvascular spasm is primarily attributed to myosin light chain phosphorylation induced by Rho kinase, increased secretion of vasoconstrictive agonists (such as endothelin and serotonin), and increased vasoconstrictive reactivity of coronary microvessels due to inflammatory states.51-53
3.4.4 Dysfunction of cardiac sympathetic neurons
In the resting state, sympathetic nerves have a limited capacity to regulate coronary vasomotor function. During exercise, however, sympathetic nerves may release norepinephrine and regulate coronary tone. β-adrenergic stimulation activates β2-receptors in the coronary arteries and promotes coronary artery dilation to compensate for increased myocardial oxygen consumption. When endothelial dysfunction occurs due to coronary atherosclerosis, α1-adrenergic-mediated vasoconstriction is more prominent, resulting in decreased CBF and myocardial ischemia.54
It should be noted that microvascular dysfunction may be a systemic disease, and patients with CMVD may also have cerebral, retinal or renal small vessel disease.55 The pathogenetic mechanisms of CMVD are summarized in Figure 1.
4 DIAGNOSTIC TECHNIQUES FOR CMVD
4.1 Vasoactive drugs for evaluating coronary microvascular function
Coronary microvascular function is frequently assessed by evaluating the coronary microvascular response to vasodilators, because of the limitation of current imaging techniques in displaying morphological changes of coronary microvasculature. CFR is a commonly used index. Vasodilators include endothelium-independent vasodilators, which primarily act on vascular smooth muscle cells, and endothelium-dependent vasodilators, which primarily act on vascular endothelial cells.
(1) Adenosine: Adenosine is the most commonly used endothelium-independent vasodilator for assessing coronary microvascular function. Adenosine receptors are G protein-coupled glycoproteins classified as four types: A1, A2A, A2B, and A3. The effect of coronary dilation is related to A2 receptors, while the effect on ischemia–reperfusion injury is related to A2A receptors. Adenosine can be administered intravenously at a dose of 140 μg/(kg·min) or intracoronarily at a dose of 2–16 μg/(kg·min) with an injection duration of 1.5–6.0 min. The advantage of adenosine is a very short half-life of only 10 s. The common adverse effects include atrioventricular block, sinoatrial block, and bronchospasm. Once occurring, however, these side effects usually disappear quickly.56
(2) Dipyridamole: Dipyridamole acts by inhibiting adenosine degradation, with a pharmacological effect similar to adenosine. Dipyridamole is diluted with glucose into 5 or 10% solution and administrated intravenously at a dose of 0.56–0.84 mg/kg. After administration of adenosine or dipyridamole, a CFR value of <2.5 indicates abnormal coronary microvascular dilative function.57 In clinical practice, CFR < 2.0 is commonly regarded as a cutoff value for the diagnosis of microvascular dysfunction.58
(3) Acetylcholine: Acetylcholine is a commonly used vasodilator for evaluation of endothelium-dependent coronary microvascular function. It works by two different mechanisms: (a) stimulating endothelial cells to release NO, which results in vasodilation, and (b) binding to muscarinic acetylcholine receptors, which activates smooth muscle cells and results in vasoconstriction. When vascular endothelial function is normal, the vasodilative effect of acetylcholine predominates. However, in the setting of endothelial dysfunction, the vasoconstrictive effect becomes prominent, resulting in vasospasm. Acetylcholine diluted in solution is injected into coronary artery at an incremental dose.59 One minute after injection of acetylcholine, CAG is performed immediately, and the diameter of the coronary artery is measured by quantitative CAG to determine whether epicardial coronary spasm is present. Anginal symptoms and changes of electrocardiogram (ECG) are monitored simultaneously. If angina pectoris occurs or ischemic ST-T changes appear without detectable epicardial coronary artery spasm after injection, CMVD can be diagnosed. At the same time, nitroglycerin or nicorandil should be immediately injected into coronary artery to relieve coronary microvascular spasm.
(4) Regadenoson: Regadenoson is a selective agonist of adenosine A2A receptor. It dilates coronary arteries and increases coronary blood flow by selectively activating adenosine A2A receptors. The advantages of regadenoson lie in its selective dilatation of coronary arteries, rapid onset of pharmacological effect and a fixed dose recommended without the need for adjustment for patient's body weight. Regadenoson is administered at a bolus injection of 0.4 mg/5 mL in 10 s. Currently, regadenoson has been widely used as the drug of choice to evaluate coronary microcirculation function5 in a variety of pharmacological stress tests, including ECG, echocardiography, PET, CMR, and computed tomography (CT).60
(5) Nicorandil: Nicorandil is a nitrate and adenosine triphosphate (ATP)-sensitive potassium channel opener, which activates guanylate cyclase in cells and relax vascular smooth muscles, and dilates both epicardial coronary arteries and coronary microvessels without affecting myocardial contractility, blood pressure, myocardial oxygen consumption and heart rate. Its pharmacological effects and duration of maximum hyperemia are dose dependent. Nicorandil is injected into coronary artery at a bolus injection dose of 2–4 mg.61, 62 For fractional flow reserve (FFR) and IMR measurement, intracoronary injection of nicorandil at a dose of 3 mg is a suitable alternative to ATP. The advantages of nicorandil include direct intracoronary injection, maximum hyperemia achieved with short time, sufficient duration of hyperemia, few side effects which subside quickly after drug withdrawal. However, most studies examining microcirculation function before and after nicorandil injection were conducted in a single center, and the sample sizes were limited.
4.2 Noninvasive techniques for evaluating coronary microvascular function
4.2.1 Transthoracic Doppler echocardiography
Coronary blood flow of the distal left anterior descending artery (LAD) can be clearly visualized by transthoracic Doppler echocardiography (TTDE) in more than 90% of patients, and after intravenous injection of ultrasound contrast, the success rate of LAD flow imaging can be increased to 100%.63 The success rate for imaging the posterior descending artery ranges from 54% to 86%, and for imaging the left circumflex flow is lower. TTDE allows measurement of the maximal diastolic flow velocity in LAD at rest and during stress test with adenosine, dipyridamole, or regadenoson, and a ratio of stress to rest velocity can be calculated, which is called coronary flow velocity reserve (CFVR).64 In the absence of flow-limited stenosis of LAD, CFVR is a reliable measure of coronary microvascular function, and the cutoff of ≤2–2.5 indicates impaired coronary microvascular function.4
TTDE is a noninvasive, time-saving, feasible, repeatable, relatively inexpensive, and nonradiative technique. However, it requires extensive technical training and can only be used when the LAD flow is clearly visualized.65
4.2.2 Myocardial contrast echocardiography
Myocardial contrast echocardiography (MCE) is a noninvasive technique for evaluating coronary microvascular function based on myocardial backscatter signals detected by ultrasound technology after intravenous injection of microbubble contrast agent.66 CFR at the myocardial level can be derived by calculating the ratio of MBF during exercise or pharmacological stress to that at rest. MBF acquired by MCE correlated well with MBF by PET in clinical patients,67 and CFR measured by MCE during stress test has been used to assess the stenotic severity of epicardial coronary arteries and detect MVO after MI.68 However, there are few clinical studies of this technique to evaluate CMVD.
MCE is a noninvasive, feasible, and relatively inexpensive technique that can be performed at bedside and does not expose patients to radiation. However, image quality of MCE is operator dependent and affected by obesity, respiratory movement, and pulmonary disease. In addition, uneven distribution of ultrasound intensity in a two-dimensional sector may lead to attenuated myocardial contrast signals in the bilateral edges and far field, with a false positive finding of myocardial hypoperfusion.
4.2.3 Single-photon emission CT
This technique uses a tracer labeled with thallium-201 (201Ti) or technetium-99m (99mTc) to record myocardial radioactivity at rest and during stress test. Signs of segmental hypoperfusion, perfusion defect, or perfusion redistribution can be detected, which are indicative of CMVD in the absence of significant epicardial coronary artery stenosis. The newly developed three-dimensional single-photon emission CT (SPECT) technology can be used for quantitative assessment of MBF, but the accuracy is affected by respiration and heartbeat.69 SPECT/CT using a low-dose CT scan can overcome SPECT imaging attenuation, optimize the anatomical co-localization of the heart, and perform partial correction of volume effect. Thus, dynamic SPECT imaging technology combined with CT has become a new method for measuring MBF. A heart-specific SPECT using new semiconductor and cadmium zinc telluride crystal as a tracer has significantly improved spatial resolution and sensitivity of cardiac imaging, shortened scanning time, and reduced radiation dose.70
SPECT has a high diagnostic sensitivity and negative predictive value in evaluating coronary microvascular function, but it cannot measure CFR quantitatively using conventional techniques and is associated with radiation exposure and a low spatial resolution.
4.2.4 PET
This technique uses an intravenously injected radionuclide-labeled tracer, such as 15O, 13N, and 82Rb, to continuously monitor the radiation activity in the blood and myocardium. By recording the time-radioactivity curve reflecting the dynamic changes in radionuclide uptake of the left ventricular cavity and myocardium, MBF per gram of myocardium per minute can be calculated. When the myocardial load increases, the myocardial oxygen consumption increases, and the MBF will increase by three to four times. However, in the setting of coronary microcirculation dysfunction, MBF cannot meet the myocardial oxygen demand, leading to myocardial ischemia. The ratio of MBF measured after coronary vasodilator administration to MBF at rest is equivalent to CFR. Currently, MBF and CFR measured by PET are the gold standard for the noninvasive diagnosis of myocardial ischemia, and PET has a high reproducibility within a certain coronary blood flow range (0.5–6 mL/g/min).71, 72
Recent development of PET/CT technology has partially overcome the attenuation effect of PET through anatomical correction by CT.73 In addition, PET/magnetic resonance imaging (MRI) technology is expected to reduce PET attenuation through MRI correction, thereby improving its accuracy.74 Furthermore, the development of 3D-PET technology is expected to reduce radiation dose and increase the accuracy of PET scanning.75 Compared with SPECT, PET provides higher spatial resolution and better attenuation correction, resulting in enhanced image quality. In comparison with technetium-99m-labeled SPECT perfusion tracers, commonly used PET tracers have lower radiation exposure and better mobility in the myocardial microcirculation.76
The advantages of PET are an accurate quantification of MBF and CFR for both resting and hyperemic conditions, accurate evaluation of myocardial perfusion, and availability of multiple tracers. Its disadvantages are time-consuming, a high cost, a limited spatial resolution and radionuclide exposure.
4.2.5 CMR
CMR is a noninvasive imaging technique that can simultaneously assess cardiac anatomy, morphology, function and myocardial perfusion.68 The characteristic of CMR is to perform first-pass perfusion imaging of the myocardium based on the T1 relaxation properties of gadolinium as a contrast agent. In T1-weighted images, normally perfused myocardium shows a uniform increase in the first-pass signal intensity of the gadolinium. In the presence of microcirculation dysfunction, however, the signal intensity in the ischemic zone increases slowly relative to that at the adjacent normal myocardial segments, yielding a visible low signal area. MBF (mL/min/g) at the resting and hyperemic states can be measured from the intensity curves in myocardial area of interest.
A semi-quantitative MPR index (MPRI) can be obtained from routine CMR imaging, but a decreased MPRI may be caused by an increased resting myocardial perfusion or impaired microvascular function. The CMR myocardial perfusion measurement sequence corrected for respiratory motion, which is being tested in clinical trials, allows patients to breathe freely and quantifies MBF pixels during postprocessing.77 Fully quantitative MBF measured by CMR has been shown to correlate well with MBF determined by PET.78 Myocardial perfusion imaging by CMR during adenosine stress test is of diagnostic value, which has been used in clinical studies to assess coronary artery stenosis, detect CMVD and risk-stratify clinical patients.79, 80
There are a number of advantages of CMR including a high feasibility, high spatial resolution, absence of radiation exposure and signal attenuation, simultaneous detection of myocardial function, structural morphology, myocardial edema and myocardial perfusion, accurate differentiation of myocardial ischemia caused by coronary arterial stenosis or microvascular disfunction, as well as accurate evaluation of myocardial perfusion, coronary artery resistance and diastolic filling time. With these advantages, CMR has gradually become the gold standard for noninvasive evaluation of myocardial ischemia. The disadvantages of CMR are common subendocardial artifacts that can affect visual image analysis and MBF calculations,77 and adverse reactions caused by conventional gadolinium contrast in patients with renal insufficiency.
4.2.6 CT perfusion imaging
CT perfusion (CTP), which is based on computed tomographic angiography (CTA), has emerged as a novel noninvasive and “one-stop” solution for the comprehensive assessment of both anatomy and physiology of epicardial coronary arteries.81 The CTP protocol includes resting scanning (coronary CTA), and stress scanning (pharmacological stress). The former is used to reliably rule out any significant epicardial coronary stenosis, and the latter is used to assess microvascular function by qualitative or quantitative evaluation of MBF distribution based on the difference in CT values when blood flow goes through different myocardial segments. Stress scanning of CTP can be further divided into two modes82: first, static CTP, which acquires images of only one cardiac cycle when the contrast first passes through myocardium, and a visual qualitative assessment of myocardial perfusion is performed according to CT values in different myocardial segments; and second, dynamic CTP, which continuously acquires images when the contrast goes through myocardium to create a time-CT value curve and thereby calculate MBF and myocardial blood volume to achieve quantification of myocardial perfusion.83
Currently, CTP is the only noninvasive modality that allows simultaneous assessment of epicardial coronary arteries and coronary microcirculation. The accuracy of CTP to identify microcirculatory perfusion defects is comparable to that of SPECT.84 In addition, CTP has a low cost and is easy to accept by most patients. In patients in whom precise evaluation of coronary artery morphology is precluded due to coronary stenting, severe coronary calcification and imaging artifacts, CTP enables functional assessment of myocardial perfusion, thus breaking the diagnostic limitations of conventional coronary CTA.85 Furthermore, CTP offers several advantages over CT-FFR, which focuses only on a focal lesion. The disadvantages of CTP are an increased contrast dose and radiation exposure. The lack of a recognized cutoff value also limits its clinical use. The advantages, disadvantages, levels of evidence, and grades of recommendation of noninvasive techniques for assessing microvascular function are listed in Table 5.
| Technique | Method | Agent | Parameter | Diagnostic threshold | Advantages | Limitations | Clinical significance | Class of recommendation | Level of evidence | References |
|---|---|---|---|---|---|---|---|---|---|---|
| TTDE | Pulsed-wave Doppler on the proximal LAD artery |
Vasodilator: • Adenosine • Dipyridamole • Regadenoson |
CFVR | CFVR < 2 |
• Bedside • Safe • Readily available • Inexpensive • Radiation-free |
• Limited to the LAD region • Operator dependent • Technical pitfalls (poor acoustic window in obesity and lung diseases) • Obstructive CAD needs to be excluded • Very limited data with use in nonobstructive CAD |
Only applicable in patients without significant LAD obstructive stenosis | IIa | C | 64 |
| MCE | Backscatter signal of microbubbles from the microvasculature is detected using low-power harmonic ultrasound |
Vasodilator • Adenosine • Dipyridamole • Regadenoson Contrast agents: • Sulfur hexafluoride microbubbles for injection |
CFR | CFR < 2 |
• Readily available • Bedside • Radiation-free • Safe • Good correlation with MBF by PET |
• Operator dependent • Technical pitfalls (poor acoustic window in obesity and lung diseases) • Obstructive CAD needs to be excluded • Very limited data with use in nonobstructive CAD • Unavailability of validated commercial software for CFR quantification |
Allows evaluation of CFR in different myocardial segments or regions | I | A | 112, 113 |
| SPECT | Distribution of radionuclides indicates myocardial perfusion for both resting and stress conditions |
Vasodilator: • Adenosine • Dipyridamole • Regadenoson Tracers: • 201Ti • 99mTc |
MBF, CFR |
CFR < 2 |
• High sensitivity and specificity for detection of ischemia • Less expensive than PET |
• Limited ability for absolute quantification of MBF • Operator dependent • Limited spatial resolution • Radiation exposure |
• Distinguishes between CMVD and epicardial coronary lesions, based on tissue characterization • Allows evaluation of LV function |
IIa | B | 114 |
| PET | Radioactivity curves of the distribution of radionuclides are dynamically recorded |
Vasodilator: • Adenosine • Dipyridamole • Regadenoson Tracers: • 13N • 82Rb • 15O |
MBF, CFR |
CFR < 2 |
• Gold standard for noninvasive and quantitative assessment of coronary microvascular function • Reproducibility |
• High costs • Operator dependent • Limited availability • Limited spatial resolution • Radiation exposure • Limited diagnostic ability for microcirculation dysfunction with obstructive CAD |
• Comprehensive and accurate evaluation of MBF and CFR for both resting and stress conditions • Multiple available tracers |
I | A | 115-117 |
| CMR | Myocardial signal intensity is recorded during the first-pass contrast uptake for both resting and stress conditions |
Vasodilator: • Adenosine • Dipyridamole • Regadenoson Contrast agent: • Gadolinium-based |
MPR, MPRI, MBF, CFR |
MPRI < 2, MBF < 2.25 mL/g/min, CFR < 1.5–2 |
• Radiation-free • Excellent spatial resolution • Coronary territories can be evaluated simultaneously • Tissue characterization |
• High costs • Time-consuming • Poor patient compliance • Limited availability • Limited ability for absolute quantification of MBF • Contraindicated in patients with severe renal disease, claustrophobia, arrhythmias, and implanted devices |
• Applicable in patients with unobstructed coronary arteries and suspected primary CMVD • Distinguishes between CMVD and epicardial coronary lesions, based on tissue characterization |
I | A | 79, 118, 119 |
| CTP | Signal intensity of contrast medium is recorded at rest and stress conditions using dynamic first-pass effect |
Vasodilator: • Adenosine • Dipyridamole • Regadenoson Contrast agent: • Iodine-based |
MBF, MPR |
MPR < 2 |
• Combination of coronary anatomy and myocardial perfusion as “one-stop” solution • Simultaneous evaluation of all coronary territories • CCTA-derived FFR |
• Radiation exposure • Risk of kidney disease • Lacking standard cutoff for MBF • Still under investigation |
Comprehensive work-up of suspected CAD and/or CMVD | IIb | C | 83, 84 |
- Abbreviations: CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CFR, coronary flow reserve; CFVR, coronary flow velocity reserve; CMR, cardiovascular magnetic resonance; CMVD, coronary microvascular disease; CTP, computed tomographic perfusion; FFR, fractional flow reserve; LAD, left anterior descending branch; LV, left ventricle; MBF, myocardial blood flow; MCE, myocardial contrast echocardiography; MPR, myocardial perfusion reserve; MPRI, myocardial perfusion reserve index; PET, positron emission tomography; SPECT, single-photon emission computed tomography; TTDE, transthoracic Doppler echocardiography.
4.3 Invasive techniques for evaluating coronary microvascular function
4.3.1 Coronary angiography
Two methods are available to evaluate the epicardial coronary artery flow by CAG: (1) TIMI flow grading: TIMI (grades 0–3) is widely used to evaluate the patency of epicardial coronary artery, but is only a semi-quantitative parameter and cannot reflect coronary microvascular function86; (2) TIMI flow frame count (TFC): TFC is the number of frames from the beginning of coronary artery imaging to the standardized distal marker imaging, which overcomes the shortcomings of semi-quantitative nature of TIMI flow grading. However, it does not directly reflect coronary microvascular flow.87
“Slow coronary flow” is an angiographic phenomenon characterized by a delayed visualization of distal vessels of a nonobstructive coronary artery, which is considered a manifestation of CMVD. The definition for “slow coronary flow” varies between different studies, some using TIMI flow grades 1−2 while others using a modified TIMI flow count of >25 frames.4
Several indexes measured by CAG have been proposed to assess coronary microvascular function based on the speed of myocardial opacity after CAG, such as TIMI myocardial blush grade (TMBG), myocardial blush grade (MBG), and TIMI myocardial perfusion frame count (TMPFC). TMBG is classified into grades 0–3 according to the duration of ground glass appearance of the myocardium after CAG, which can be used as a semi-quantitative measure to reflect the patency of coronary microvessels.88 MBG is classified into grades 0–3 based on changes in myocardial contrast density after the contrast enters the myocardial tissue, which can be used as a semi-quantitative index to reflect the perfusion status of the coronary microcirculation.89 TMPFC is an index put forward by Chinese researchers to quantitatively evaluate myocardial reperfusion immediately after PCI by measuring the number of frames from the onset of myocardial blushing to contrast emptying from the myocardium.90 Studies have shown that an increased TMPFC predicted microvascular dysfunction defined by MRI gadolinium imaging after PCI.91
An advantage of selective coronary angiographic techniques is an immediate evaluation of coronary microvascular function after PCI, with a high technical feasibility and analytical simplicity. However, these indices are affected by coronary perfusion pressure and heart rate, and cannot reflect CFR.
In a proportion of patients with myocardial ischemia, coronary motor disorder at epicardial and/or microvascular levels may play a role, but a definitive diagnosis requires the use of coronary acetylcholine provocative test during CAG.92, 93 Accumulating clinical experience with this provocative test has proven its safety and usefulness in identifying patients at a high risk of future clinical events.94
4.3.2 Thermodilution technique
(1) Bolus thermodilution: The conventional thermodilution technique is the bolus thermodilution method. It is based on the principle of the Fick method, where cold saline at a given temperature and injection rate is bolus injected as a tracer through a catheter into the ostium of a coronary artery, and the blood temperature is measured at the distal coronary artery. The magnitude of blood temperature drop indicates the degree of tracer dilution, which is proportional to CBF, and thus CBF can be estimated. The time required for cold saline to leave the guide catheter to reach the guidewire sensor in distal coronary artery can be recorded from the temperature dilution curve, that is, the mean transient time (T). The T value is inversely proportional to CBF, and the ratio of T values recorded at baseline and maximal hyperemia is CFR (T baseline/T hyperemia).95
Index of microvascular resistance (IMR) has been recognized as a feasible index to evaluate the microvascular function distal to a stenotic lesion, which is defined as a ratio of distal coronary pressure to distal coronary flow, that is, the product of the distal coronary pressure (Pd) and mean transit time (T) of a saline bolus during maximal hyperemia. Pd and T are measured by a pressure guidewire equipped with a temperature sensor.96 IMR is independent of epicardial vascular function and able to specifically evaluate microvascular function with a good reproducibility.96-98
Of the bolus-thermodilution method, large intraobserver variability and overestimated CFR at higher values were observed due to manual rapid injection of saline. It is also susceptible to subjectivity with an alternative T value. Additionally, intravenous or intracoronary adenosine is associated with multiple side effects.
(2) Continuous thermodilution: Recently, a novel method based on continuous thermodilution has been validated,99, 100 which allows direct measurement of absolute coronary blood flow and resistance. A specialized monorail infusion catheter is inserted over the pressure wire with its tip being positioned in the proximal region of the coronary artery. Then, room-temperature saline is injected at a rate of 15−25 mL/min. After a steady-state maximum hyperemia is induced, the distal temperature can be recorded by the guidewire. By pulling back the temperature sensor to the opening of the infusion catheter, the infusion temperature can be determined. Absolute blood flow and vessel resistance are calculated automatically using these variables. It has been well verified that hyperemic flow measured by continuous thermodilution correlates well with the gold standard PET.101
Microvascular resistance reserve (MRR) is a novel metric specific for the microvasculature, defined as the ratio of true resting to HMR.102 It is calculated as follows: MRR = (Qmax/Qrest) × (Pa,rest/Pd,hyper), where Pa,rest represents aortic pressure at rest and Pd,hyper indicates distal coronary pressure measured at hyperemia, while Qrest and Qmax denote the actually measured resting and hyperemic blood flow. MRR can be easily measured invasively using intracoronary Doppler, continuous thermodilution, or bolus thermodilution. However, Doppler tracing was shown to be challenging in obtaining high quality signals, resulting in insufficient data in up to 30% of patients,103 whereas bolus thermodilution is susceptible to patient- and operator-dependent variability. The correlation between continuous thermodilution MRR and patient symptoms has been demonstrated, suggesting that it could be a superior technique to bolus thermodilution for reflecting the disease status of patients with CMVD.104
The optimal cutoff values of MRR remain to be determined. An exploratory analysis in patients with angina and nonobstructive CAD indicated that an MRR value of >2.7 ruled out CMVD with high certainty, whereas an MRR value < 2.1 highly suggested CMVD.105 The ILIAS registry study proposed MRR of 3 as the cutoff value to predict MACE and target vessel failure at 5-year follow-up in vessels with functionally significant epicardial disease.106 This unique metric seems useful in the occasion with adaptive coronary flow regulation such as AS.107 Measurement of MRR is accurate, reproducible, and safe, and is independent of epicardial coronary disease, hemodynamic variation, operator, autoregulation, epicardial resistance, and myocardial mass. With wider clinical applications, MRR will gain further clinical benefits.
4.3.3 Intracoronary Doppler flow velocity measurement
This technique uses Doppler flow velocity recorder connected with a Doppler flow wire, which is inserted into a distal coronary artery to record the flow spectrum. It is followed by intracoronary adenosine injection to measure the coronary flow velocity during maximal hyperemia.108 The CFR value is obtained by calculating the ratio of diastolic coronary flow velocity in the maximal hyperemia to that in the basal state. The major advantage of this technique is that it allows accurate measurement of flow velocity and CFR in each coronary artery. The disadvantage is that flow velocity is affected by the guidewire position in the lumen, coronary flow velocity profile, and luminal area changes following injection of vasodilators. In addition, a reduced CFR can be observed in patients with CMVD or severe epicardial coronary stenosis and thus, coronary microvascular function can only be evaluated in patients without obstructive epicardial coronary stenosis. The normal CFR value is >2.565, 109, 110 and CFR < 2.0 indicates the presence of coronary microvascular dysfunction.
HMR is a recently proposed index. This new technique uses an intracoronary guidewire equipped with the Doppler transducer and pressure sensor to measure the mean flow velocity and pressure in the cardiac cycle during maximal hyperemia in the distal end of a stenotic lesion (or distal coronary arteries in the absence of a stenotic lesion). The HMR value is calculated as follows: HMR = pressure/flow velocity.111 CFR < 2.5 and HMR > 1.7 mmHg/cm/s indicate coronary microvascular dysfunction. HMR is not affected by resting coronary blood flow, but the diagnostic cutoff value remains controversial.
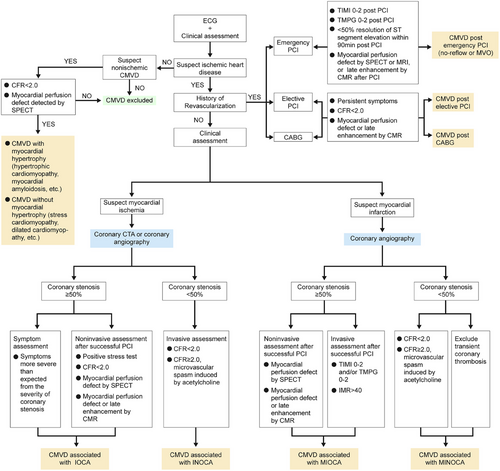
The advantages and disadvantages, level of evidence, and recommended grades of invasive techniques for evaluating coronary microvascular function are shown in Table 6. The clinical diagnostic flow chart of CMVD is shown in Figure 2.
| Technique | Method | Drug load | Parameter | Diagnostic thresholds | Advantages | Limitations | Clinical significance | Class of recommendation | Level of evidence | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Coronary angiography | Dynamic changes in the coronary artery filling with angiographic contrast using X-ray photography/TIMI frames | Iodine contrast media |
TIMI blood flow grade, TFC |
TIMI grading ≤ 2, TFC > 25 fps |
• Simple, feasible and inexpensive • Identification of epicardial coronary artery spasm and microvascular spasm after acetylcholine administration |
• Only semi-quantitative evaluation • Low sensitivity for CMVD diagnosis • Cannot reflect CFR Only used for resting state evaluation |
Is a semi-quantitative index to reflect the perfusion status of the coronary microcirculation | I | A | 86, 87, 89-91 |
| Intracoronary temperature-pressure measurement | Estimation of coronary blood flow using bolus injection to calculate mean transit time or using continuous thermal dilution technique |
Vasodilators: • Adenosine • Saline solution |
CFR, IMR, MRR |
CFR < 2–2.5, IMR > 25 U, MRR < 2.1 |
• CFR and IMR can be combined to assess both impaired dilation and enhanced contraction of microvessels • IMR specifically assesses microvascular function independent of resting-state hemodynamics • MRR is accurate, reproducible, safe and independent of the operator, the autoregulation, the epicardial resistance, and the myocardial mass |
• CFR does not distinguish between CMVD and epicardial coronary lesions • IMR cutoff value is still controversial • IMR correlates poorly with PET versus HMR • High intra- and inter-observer variability |
Can be used in a variety of clinical scenarios: post-PCI, post-STEMI, angina pectoris, myocardial ischemia with nonobstructive coronary arteries cardiomyopathy |
IIa | B | 97, 98, 100-102,105, 106, 108 |
| Intracoronary Doppler flow velocity measurement | Recording peak coronary flow velocity using Doppler technique | Vasodilator: Adenosine |
CFR, HMR |
CFR < 2.5, HMR > 1.7 mmHg/cm/s |
• CFR and HMR can be combined to assess both impaired dilation and enhanced contraction of microvessels • HMR independent of resting-state coronary blood flow • Simultaneous measurement of FFR is possible • Good correlation with clinical prognosis and measurements by noninvasive techniques • Good reproducibility |
• Complex technique • CFR unable to distinguish between CMVD and epicardial coronary lesions |
For assessment of microvascular function caused by suspected CMVD or post myocardial infarction | IIb | C | 97, 98, 109-111 |
| Coronary spasm induction test | Assessment of epicardial coronary and microvascular spasm using intracoronary infusion of vasoactive drugs |
Vasoactive drugs: • Acetylcholine • Ergonovine |
Angina pectoris symptoms, Electrocardiographic changes, Changes in the internal diameter of blood vessels |
• Epicardial coronary artery spasm: >90% reduction in the internal diameter of the vessel; • Microvascular spasm: Presence of angina and ECG ischemic changes without change in large vessel internal diameter |
• Simple, easy to perform, and inexpensive • Can distinguish between epicardial coronary spasm and coronary microvascular spasm |
• Additional contrast injection and radiation exposure • Inability to provide direct evidence of microcirculatory spasm • Risk of severe ventricular arrhythmias • Less clinical use |
For suspected coronary spasm angina | IIb | B | 65 |
- Abbreviations: CMVD, coronary microvascular disease; CFR, coronary flow reserve; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; IMR, index of microcirculatory resistance; MRR, microvascular resistance reserve; PCI, percutaneous coronary intervention; PET, positron emission tomography; STEMI, ST-segment elevation myocardial infarction; TFC, TIMI frame count; TIMI, thrombolysis in myocardial infarction.
5 CLINICAL MANIFESTATIONS AND DIAGNOSTIC CRITERIA OF CMVD
5.1 CMVD in patients with myocardial ischemia
5.1.1 CMVD associated with INOCA
This type of CMVD is also referred to as primary stable MVA,4 where patients exhibit symptoms of persistent myocardial ischemia with a low CFR or laboratory evidence of microvascular spasm without obstructive lesions of the epicardial coronary artery. The primary symptom is exertional chest pain, which is difficult to distinguish from chest pain in patients with severe coronary stenosis. The following characteristics suggest the possibility of CMVD: Women are common with the majority of cases occurring after menopause; Most patients experience labor-induced chest pain, and very few have chest pain during rest; A single episode of chest pain lasts for a relatively long time with more than 50% episodes lasting for more than 10 min, and the discomfort continues for several minutes after ceasing exercise; Nitroglycerin is ineffective against chest pain, or even make chest pain worse.
The following criteria are recommended for the diagnosis of CMVD associated with INOCA: (1) typical clinical symptoms; (2) at least one of the following objective evidence of myocardial ischemia: exertion-induced or spontaneous typical chest pain accompanied by ST-segment depression on ECG; reversible myocardial perfusion defect by SPECT; CFR reduction (<2.0) during stress by Doppler ultrasound or MPRI reduction (<2.0) by CMR; and metabolic evidence of myocardial ischemia by PET (3) CAG shows normal coronary artery, or irregular coronary walls or a luminal stenosis < 50%109; (4) If CMVD is highly suspected clinically but CFR is ≥2.0, intracoronary acetylcholine provocation test can be used under close supervision. The diagnosis of CMVD is confirmed if angina symptoms and ischemic ST-T changes on ECG appear without spasm in the epicardial coronary arteries; (5) noncardiac chest pain and other cardiac conditions, such as variant angina pectoris, cardiomyopathy, myocarditis, or valve heart disease should be ruled out.
5.1.2 CMVD associated with IOCA
When stable angina is caused by combined CMVD and epicardial coronary obstructive lesions, patients may experience a prolonged angina attack with a high variability in the threshold of physical activity triggering angina and an ineffective response of sublingual nitroglycerin. In addition, the severity of angina often exceeds that expected from the degree of coronary stenosis.
The following criteria are recommended for the diagnosis of CMVD associated with IOCA: (1) typical clinical symptoms; (2) a positive stress test early after a successful PCI; (3) CFR < 2.0, or a positive intracoronary acetylcholine provocation test that induces typical angina pectoris and ischemic ST-T changes without visible epicardial coronary artery spasm after coronary stenosis is relieved by PCI; (4) TIMI flow grade < 3 and/or TMPG < 3 in patients receiving elective PCI; (5) myocardial perfusion defect detected by SPECT or MRI, or late gadolinium enhancement displayed by MRI in patients receiving PCI before discharge. However, in post-PCI patients, whether PCI-related CMVD exists requires further clarification.
5.2 CMVD in patients with MI
5.2.1 CMVD associated with MINOCA
MINOCA is a syndrome with symptoms of non-STEMI and laboratory evidence of coronary microvascular dysfunction after excluding epicardial obstructive and spastic coronary lesions, transient coronary thrombosis, cardiomyopathy, and other cardiovascular diseases. The most frequent causes of MINOCA are plaque rupture or erosion, thromboembolism, hereditary thrombophilia, or MVO associated with microvascular spasm.6, 120 The clinical presentation is recurrent chest pain during rest or in the early morning, and chest pain induced by mild physical activity, which may last for 1–2 h, and nitroglycerin administration is ineffective. Ischemic and dynamic ST-T changes on ECG can be recorded during the onset of chest pain or on Holter monitoring. Patients with MINOCA have significantly higher MACE and lower quality of life, but the clinical diagnostic pathway is to be defined.
5.2.2 CMVD associated with MIOCA
This type of CMVD is frequently linked to MVO. The incidence of post-PCI left ventricular remodeling, heart failure, and mortality are higher in patients with STEMI and MVO.121, 122 One year follow-up after successful PCI found that one-third to one-half of patients experienced CMVD-related angina with left ventricular remodeling, cardiac dysfunction and cardiovascular events.123, 124 Endothelial dysfunction, oxidative stress, decreased NO generation, prior CMVD, and ischemia/reperfusion injury have been found to be contributing factors to CMVD in MVO. However, it is necessary to take into account of the possibility of emergency PCI-related CMVD.
5.3 CMVD associated with revascularization
5.3.1 CMVD associated with emergency PCI
The incidence of MVO is 5–50% in patients with STEMI receiving direct PCI. The following conditions indicate the existence of MVO: (1) TIMI blood flow grades 0–2 after PCI; (2) TMPG classes 0–2 after PCI; (3) ST-segment elevation on ECG resolves by <50% 90 min after PCI; (4) SPECT shows regional myocardial no-perfusion area before discharge, and MRI shows a first-pass perfusion defect, or late gadolinium enhancement. In addition, in patients receiving PCI due to acute coronary syndrome (ACS) or restenosis after great saphenous vein bypass grafting, atheromatous debris from the compressed plaque may go to the distal vessel and cause microembolization and small MI.
5.3.2 CMVD associated with elective PCI
About one-third of patients receiving elective PCI can develop CMVD, which is manifested by increased troponin levels after PCI, recurrent angina pectoris, and an increased risk of major cardiovascular events, death, MI, and repeated PCI. The mechanism involves the distal embolism of plaque materials during stent implantation, preexistence of CMVD before PCI, an increased α-adrenergic sympathetic nerve tension in coronary microvessels caused by balloon dilation, and the aggravation of the original endothelial dysfunction caused by stent-eluted drugs.125, 126 The diagnostic criteria of elective PCI-related CMVD include: (1) typical clinical manifestation; (2) CFR < 2.5 measured by intracoronary Doppler ultrasound post-PCI127; (3) IMR ≥ 25 measured immediately post-PCI.21
5.3.3 CMVD associated with CABG
If angina pectoris occurs repeatedly after CABG, the combined CMVD should be considered in most cases, and the mechanism involves structural and functional abnormalities of coronary microvessels.60, 126, 128 During CABG, many factors may affect the function of coronary microvessels, including cardiac arrest, extracorporeal circulation, myocardial ischemia, and inflammatory response. The effect of myocardial injury after CABG on the prognosis of patients is similar to that of PCI, indicating that regardless of the mechanism, prognosis ultimately depends on the extent of myocardial necrosis.128 Myocardial injury post-CABG may be related to CMVD-induced electrical instability or sustained myocardial ischemia. In addition, CMVD is also common in allograft coronary angiopathy in heart transplant recipients, which is independent from epicardial coronary angiopathy and associated with the risk of death.
5.4 CMVD associated with non-atherosclerotic heart disease
5.4.1 CMVD with myocardial hypertrophy
CMVD associated with HCM: Microvascular pathological features include arteriolar wall thickening, luminal narrowing, and capillary rarefaction. Many studies have shown that although CFR reduction is detected in non-hypertrophic myocardial areas, it is more significant in subendocardial and hypertrophic areas. Long-term CMVD may induce recurrent myocardial ischemia and myocardial cell death, leading to local myocardial fibrosis. In patients with HCM, CMR shows late gadolinium enhancement image. CMVD detected by PET is a reliable predictor of left ventricular remodeling, systolic dysfunction, clinical deterioration and death.42
CMVD associated with Anderson–Fabry disease: CMVD has become an important feature of Anderson–Fabry disease-related cardiomyopathy. A considerable number of patients with this disease experience angina pectoris without coronary artery stenosis. Recent studies have shown that mild coronary microvascular dysfunction is the phenotype characteristics before the occurrence of myocardial hypertrophy.
CMVD associated with cardiac amyloidosis: The pathogenesis includes arteriolar wall infiltration and thickening, luminal stenosis, microvascular dysfunction caused by autonomic nervous dysregulation and endothelial dysfunction, extramural compression by interstitial deposition of amyloid protein, and reduced microvascular perfusion pressure caused by elevated left ventricular filling pressure. In patients with systemic amyloidosis, angina pectoris with decreased CFR may occur before typical general manifestations.26
CMVD associated with AS: About 40% of patients with AS develop angina pectoris without epicardial CAD, which increases the risk of sudden death. These patients exhibit decreased MBF and CFR and weakened exercise tolerance. Reduced CFR is the only independent predictor of cardiovascular events in patients with AS.129 Thanscatheter and transthoracic aortic valve replacement can restore myocardial perfusion and contractility by reducing left ventricular wall stress, and improve coronary microvascular function.130
5.4.2 CMVD without myocardial hypertrophy
Stress cardiomyopathy (Takotsubo cardiomyopathy): A study has shown that CMVD may be involved in the pathogenesis of Takotsubo cardiomyopathy.131 Transthoracic Doppler ultrasound or PET showed decreased coronary microvascular blood flow and CFR during the acute phase of Takotsubo cardiomyopathy. However, CMVD was reversible in most patients. In another study, the perfusion defects observed in myocardial segments with weakened systolic function were improved after intracoronary injection of adenosine and completely resolved after 1 month of follow-up.132 The long-term prognosis of patients showing slow blood flow on CAG is poor.28
Dilated cardiomyopathy: Recent studies have found that myocardial ischemia caused by CMVD is an independent risk factor of the progression of dilated cardiomyopathy. Patients with dilated cardiomyopathy and moderate or severe left ventricular remodeling often have abnormal myocardial perfusion. In addition, the severity of CMVD is an independent predictor of the risk of death and aggravated heart failure in patients with dilated cardiomyopathy.133
HFpEF: HFpEF has been redefined as a systemic disease characterized by multiple organ inflammation and microvascular dysfunction. CMVD plays a key role in the pathogenesis and progression of HFpEF.134 In a small, prospective observational study, mean CFR in a group of patients with HFpEF was significantly reduced and mean IMR was substantially increased compared with the control group. In addition, more than one-third of these patients presented with evident CMVD, which was associated with death or hospitalization due to heart failure during follow-up.135, 136 In patients with HFpEF, coronary artery hemodynamics measured with Doppler guidewire demonstrated that CMVD was mainly caused by endothelium-dependent and endothelium-independent microvascular dysfunction.137 Female patients with CMVD often have an impaired left ventricular diastolic function, and CMVD increases the risk of HFpEF.138 However, the causal relationship between CMVD and HFpEF is still unclear.
Diabetic cardiomyopathy: Recent prospective studies suggested that diabetic cardiomyopathy may be a unique and high-risk clinical phenotype of DM-related HFpEF, which is characterized by increased serum levels of N-terminal pro-B-type natriuretic peptide, myocardial fibrosis, vascular endothelial dysfunction, and increased incidence and mortality of heart failure.139 The mechanism of microvascular disease in diabetic patients involves deposition of advanced glycation end products, vascular inflammation, reduced NO production, and endothelial cell apoptosis, leading to microvascular dysfunction and capillary rarefaction.
6 TREATMENT OF CMVD
To date, a number of small randomized clinical trials or nonrandomized observational studies have reported cardiovascular outcomes in patients with CMVD.140, 141 Large-sample randomized clinical trials with cardiovascular events as endpoints are underway. Different from the 2017 Chinese expert consensus, the current consensus recommended treatment strategies of CMVD based on evidence from the latest clinical outcome studies, and presented classes for recommendations and levels of evidence.
6.1 Treatment of CMVD associated with INOCA
6.1.1 Risk factor management
Atherosclerosis is the pathological basis for CMVD in most patients, and traditional risk factors of atherosclerosis such as smoking, hypertension, hyperlipidemia, and diabetes may promote development of CMVD. Thus, primary prevention of atherosclerosis for controlling risk factors may help alleviate CMVD and symptoms of angina.
(1) Hypertension: Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) are treatment of choice. Studies have shown that ACEI treatment improves angina symptoms and CFR in women with INOCA and hypertension, which is consistent with the overall WISE hypothesis.142 Another randomized clinical study found that enalapril increases CFR in patients with diabetes and hypertension.143 A meta-analysis showed that therapy with ACEIs and ARBs is associated with significantly improved CFR in patients with hypertension and CMVD.144
(2) Hyperlipidemia: Several small-sample studies have shown that statins significantly improve exercise tolerance, CFR, exercise-induced tissue hypoperfusion, and quality of life in patients with INOCA.144-147 The proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitors evolocumab and alirocumab significantly improve vascular endothelial function, inhibit inflammation and oxidative stress, and stabilize atherosclerotic plaques while reducing low-density lipoprotein cholesterol levels.5, 60, 148, 149 However, EVOCATION study found that evolocumab did not prevent microvascular dysfunction in patients undergoing PCI.150
(3) Diabetes: A study has shown that oral hypoglycemic drugs or insulin can improve coronary microvascular endothelial function.151 Metformin reduces body weight and insulin resistance, improves acetylcholine-mediated endothelium-dependent microvascular function, and alleviates ST-segment depression and angina symptoms.152, 153 Sodium-dependent glucose transporters 2 (SGLT2) inhibitors attenuate CMVD by improving vascular endothelial function and inhibiting smooth muscle cell proliferation, endothelial cell oxidative stress, and inflammatory responses.151, 154, 155
(4) Microembolism: Low-dose aspirin can reduce microembolism after PCI.156, 157 Tegregrel, a novel P2Y12 receptor inhibitor, can inhibit the degradation of adenosine and increase the level of adenosine, which may improve CMVD by dilating microvessels.142, 158
6.1.2 Lifestyle modifications
Lifestyle management in patients with CMVD is similar to that in those with atherosclerosis and includes healthy diet, smoking cessation, and weight control.141, 159 Individualized exercise programs and cardiac rehabilitation can improve angina symptoms, exercise tolerance, quality of life, and CFR in patients with CMVD.160-162 As coronary macrovascular and microvascular spasms are often induced by stress, especially in female patients, prevention from stress and psychological counseling may be necessary, and behavioral therapy may help relieve stress and reduce spastic angina.2, 163
6.1.3 Stratified treatment for CMVD
Based on the results of the CorMicA trial, coronary function test with invasive pressure guidewire, and endothelial function test with acetylcholine stress are recommended for patients with INOCA to differentiate MVA from vasospastic angina (VSA). The diagnostic criteria for MVA are FFR > 0.8, CFR < 2.0, IMR ≥ 25, and HMR ≥ 1.9 as measured by a coronary pressure guidewire, and occurrence of angina symptoms and ischemic ST-segment changes on ECG with epicardial coronary diameter constriction < 90% during acetylcholine testing. The diagnostic criteria for VSA are FFR > 0.8, CFR ≥ 2.0, IMR < 25, and HMR < 1.9 as measured by a coronary pressure guidewire, and occurrence of angina symptoms, and ischemic ST-segment changes on ECG with coronary diameter constriction ≥90% during acetylcholine testing. The diagnostic criteria for combined MVA and VSA are FFR > 0.8, CFR < 2.0, IMR ≥ 25, and HMR ≥ 1.9 as measured by a coronary pressure guidewire, and occurrence of angina symptoms, and ischemic ST-segment changes on ECG with coronary diameter constriction ≥90% during acetylcholine testing.140
Treatment of MVA. (1) β-Blockers: Several studies found that nebivolol, a highly selective inhibitor of β1-receptor, alleviates angina symptoms via increasing NO production and coronary artery dilation. Intracoronary injection of nebivolol increases CFR and reduces myocardial oxygen consumption. Carvedilol blocks both α- and β-receptors and has been proven beneficial for CMVD.164, 165
(2) Calcium channel blockers (CCBs): Mibefradil as both L-type and T-type CCB, reduces the frequency of angina in patients with coronary slow flow.166, 167 Amlodipine and benidipine as long-acting dihydropyridine agents relieve symptoms of MVA patients.168-170
(3) Nicorandil: Nicorandil is an ATP-sensitive potassium channel opener, which effectively dilates coronary microvessels and produces salutary effects on MVA. Available studies have shown that nicorandil improves microvascular function in patients with stable CHD and significantly lowers the incidence of adverse cardiovascular events in patients with MVA.171, 172
(4) Ranolazine: Ranolazine can inhibit Na+ inward flow, promote Ca+ outward flow to reduce intracellular calcium overload, and dilate coronary arteries to relieve MVA. An open-label, multicenter trial found that ranolazine significantly reduced angina symptoms and increased exercise tolerance in patients with MVA.173
(5) Trimetazidine: A study has shown that trimetazidine increases exercise tolerance and significantly reduces the duration of ST depression in patients with INOCA and CMVD.174
(6) ACEIs/ARBs: ACEIs/ARBs increase CFR by improving microvascular remodeling and dysfunction and decrease cardiac load. Studies have demonstrated that ACEIs/ARBs alleviate angina symptoms in patients with CMVD by increasing CFR, reducing cardiac load, and improving microvascular remodeling.175-177
(7) Ivabradine: Ivabradine is a specific inhibitor of cardiac pacing current, which acts as an anti-MVA agent by slowing heart rate and reducing myocardial oxygen consumption. Clinical studies demonstrated that ivabradine reduced MVA symptoms in patients with INOCA.178
(8) Endothelin receptor antagonists: Increased serum endothelin-1 (ET-1) levels in patients with MVA lowers the exercise threshold to trigger angina and reduces CFR in women. The CorMicA trial found that patients with INOCA showed enhanced response to ET-1. In a randomized controlled study, the endothelin receptor antagonist zibotentan improved vascular endothelial function in patients with CMVD.179
(9) Tricyclic antidepressants: In patients with refractory angina despite routine therapies, low-dose tricyclic antidepressants, such as amitriptyline, imipramine, or desmethylimipramine, might be considered as an alternative therapy.142, 180, 181
(10) Nonpharmacological treatment: In patients with refractory MVA and failed medical treatment, spinal cord electrical stimulation (SCS) or enhanced external counter pulsation (EECP) may be considered.163 Studies have shown that SCS reduces MVA frequency and duration, and improves Seattle Angina Questionnaire scores and quality of life in patients with CMVD.163, 182, 183 EECP relieves MVA by increasing retrograde aortic blood flow, prolonging diastolic period, and augmenting coronary perfusion, venous return flow, and cardiac output.184
Treatment of VSA. (1) CCBs: CCBs are the drug of choice for the treatment of VSA, and the dose of CCBs should be doubled in patients with severe coronary spasms. Combined administration of dihydropyridine and non-dihydropyridine CCBs, such as diltiazem, is necessary in special cases.185, 186 Benidipine, a controlled-release dihydropyridine CCB, reduces the symptoms and improves the prognosis of patients with VSA.168, 187
(2) Nitrates: Long-acting nitrates alone or in combination with CCBs can reduce the frequency of angina in patients with VSA.185, 188-190
(3) Nicorandil: Nicorandil improves VSA by opening ATP-sensitive potassium channels and dilating microvessels.7, 191, 192
(4) Rho kinase inhibitors: Fasudil, a Rho kinase inhibitor, inhibits acetylcholine-induced microvasospasm and alleviates VSA. In patients with VSA and elevated IMR, fasudil significantly decreases IMR.193, 194
(5) Phosphodiesterase (PDE) inhibitors: Cilostazol, a PDE3 inhibitor, inhibits adenosine degradation and exerts antiplatelet, anti-inflammatory, and vasodilatation effects. Cilostazol alleviates spontaneous or ergometrine-induced VSA and refractory angina unresponsive to calcium antagonists and nitrates.195 Sildenafil, a PDE5 inhibitor, dilates coronary arteries and improves endothelium-dependent vasodilation in patients with CHD. Moreover, sildenafil improves CFR and relieves angina symptoms in female patients with INOCA whose CFR is <2.5.196
(6) β-Blockers: In patients with VSA, β-blockers, especially nonselective β-blockers such as propranolol, should be avoided.197
Treatment of combined MVA and VSA. Statins improve vascular endothelial function and inhibit vascular inflammation.7 Recommendations for treatment of CMVD in INOCA are shown in Table 7.
| Treatment | Class of recommendation | Level of evidence | References |
|---|---|---|---|
| Risk factor management | |||
| Hypertension | |||
| ACEIs/ARBs | I | A | 142-144 |
| Hypercholesterolemia | |||
| Statins | I | A | 60, 144, 146, 147 |
| PCSK9 inhibitors | IIb | B | 60, 148 |
| Diabetes mellitus | |||
| Metformin | IIa | B | 152, 153 |
|
SGLT2 inhibitors dapagliflozin and empagliflozin |
IIa | B | 154, 155 |
| Thromboembolism | |||
| Low-dose aspirin | IIa | B | 156, 157 |
| Clopidogrel or ticagrelor in patients with aspirin intolerance | IIb | C | 158 |
| Lifestyle modifications | |||
| Smoking cessation, body weight control, exercise, cardiac rehabilitation, stress avoidance | I | A | 141, 159-163 |
| Stratified therapy for CMVD | |||
| Based on coronary function tests with an invasive pressure guidewire and endothelial function tests with acetylcholine injection to stratify diagnosis and treatment | IIa | B | 140, 145 |
| Treatment of MVA | |||
| β-Blockers such as nebivolol (not available in China) | I | A | 164, 165 |
| CCBs such as amlodipine or benidipine | I | B | 168-170 |
| Nicorandil | IIa | B | 171, 172 |
| Ranolazine | IIa | B | 173 |
| Trimetazidine | IIa | B | 174 |
| ACEIs /ARBs | IIa | B | 175-177 |
| Ivabradine | IIa | B | 178 |
| Endothelin receptor antagonists such as zibotentan (not available in China) | IIb | B | 179 |
| Imipramine (for patients less effective with conventional antianginal drugs or overreaction to cardiac pain) | IIb | B | 180, 181 |
| Spinal cord electrical stimulation or enhanced external counter pulsation (for patients with refractory MVA) | IIb | B | 163, 182-184 |
| Treatment of VSA | |||
| Amlodipine or diltiazem | IIa | B | 168, 185, 186 |
| Nitrates | IIa | B | 185, 188-190 |
| Nicorandil | IIa | C | 192 |
| Fasudil, cilostazol, sildenafil | IIb | B | 193-196 |
| Propranolol | III | C | 197 |
| Treatment of MVA combined with VSA | |||
| CCBs such as amlodipine, diltiazem, or verapamil | IIa | B | 168-170 |
| ACEIs /ARBs | IIa | B | 143, 144 |
| Nicorandil | IIa | B | 192 |
| Trimetazidine | IIa | B | 174 |
| Statins | IIa | C | 144, 146, 147 |
- Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCB, calcium channel blocker; CMVD, coronary microvascular disease; INOCA, ischemia with nonobstructive coronary arteries; MVA, microvascular angina; PSCK9, proprotein convertase subtilisin kexin type 9; SGLT2, sodium-dependent glucose transporters 2; VSA, vasospastic angina.
6.2 Treatment of CMVD associated with IOCA
6.2.1 Lifestyle modifications
Improvements in lifestyle, such as healthy diet, smoking cessation, physical exercise, cardiac rehabilitation, body weight control, stress avoidance, and psychological counseling, can improve the prognosis and CFR in patients with IOCA and CMVD.163, 198-200 Recommendations are shown in Table 8.
| Treatment | Class of recommendation | Level of evidence | References |
|---|---|---|---|
| Lifestyle modifications | |||
| Smoking cessation, body weight control, exercise, cardiac rehabilitation, stress avoidance | I | A | 163, 198-200 |
| Antiatherosclerosis therapy | |||
| Antiplatelet therapy | |||
| Low-dose aspirin | IIa | B | 157 |
| Clopidogrel or ticagrelor in patients with aspirin intolerance | IIb | C | 207 |
| Ticagrelor instead of clopidogrel in patients at high risk of ischemia | IIb | C | 158, 207 |
| Secondary prevention of coronary heart disease | |||
| Statins | I | A | 60, 144, 145, 201, 202 |
| ACEIs/ARBs | I | A | 143, 144, 203, 204 |
| β-Blockers | I | A | 205, 206 |
| Stratified therapy for CMVD | |||
| Based on coronary function tests with an invasive pressure guidewire and endothelial function tests with acetylcholine stress to stratify diagnosis and treatment | IIa | B | |
| Treatment of CMVD | Same as the treatment of CMVD in INOCA | Same as the treatment of CMVD in INOCA | Same as the treatment of CMVD in INOCA |
| Coronary revascularization | |||
| Revascularization can be performed when a major coronary artery stenosis is ≥90% or in case of extensive myocardial ischemia or FFR < 0.8 | I | A | 5, 208 |
- Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CMVD, coronary microvascular disease; INOCA, ischemia with nonobstructive coronary arteries; IOCA, ischemia with obstructive coronary arteries; MVA, microvascular angina; VSA, vasospastic angina.
6.2.2 Antiatherosclerosis therapy
Previous studies have shown that statins, ACEIs/ARBs, β-blockers, and aspirin/P2Y12 receptor inhibitors can improve the prognosis and CFR in IOCA patients with CMVD.5, 60, 142, 144, 145, 157, 201-207 The recommendations are shown in Table 8.
6.2.3 Stratified medical therapy for CMVD
Coronary function tests with an invasive pressure guidewire and endothelial function tests with acetylcholine stress are recommended to detect CFR and IMR, respectively, before and after revascularization and to stratify treatment for MVA and VSA. The treatment protocol is similar to the aforementioned treatment for patients with INOCA and CMVD5, 7, 140, 145 and shown in Table 8.
6.2.4 Coronary revascularization
Multiple coronary revascularization guidelines recommend direct intervention when a major coronary artery stenosis is ≥90%, or coronary stenosis is <90% in combination with evidence of extensive myocardial ischemia or FFR < 0.8. In patients with IOCA and CMVD, epicardial artery revascularization can improve angina symptoms and prognosis.5, 208 The recommendations are shown in Table 8.
6.3 Treatment of CMVD associated with MINOCA
6.3.1 Stratified medical therapy for CMVD
Classification, diagnosis, and treatment of CMVD according to the results of coronary artery function evaluation and acetylcholine-mediated endothelial function assessment are recommended. The recommended treatment is the same as that for INOCA-associated CMVD,6, 140, 145, 209 as shown in Table 9.
| Treatment | Class of recommendation | Level of evidence | References |
|---|---|---|---|
| Stratified medical therapy for CMVD | |||
| Based on coronary function tests with an invasive pressure guidewire and endothelial function tests with acetylcholine injection to stratify diagnosis and treatment | IIa | B | 140, 145 |
| Treatment of CMVD | Same as the treatment of CMVD in INOCA | Same as the treatment of CMVD in INOCA | Same as the treatment of CMVD in INOCA |
| Secondary prevention of CHD | |||
| Statins | I | A | 211-213 |
| ACEIs/ARBs | I | A | 211-213 |
| β-blockers | I | A | 211-213 |
| Aspirin combined with clopidogrel or ticagrelor | IIb | A | 211-213 |
- Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CHD, coronary heart disease; CMVD, coronary microvascular disease; INOCA, ischemia with nonobstructive coronary arteries; MINOCA, myocardial infarction with nonobstructive coronary arteries; MVA, microvascular angina; VSA, vasospastic angina.
6.3.2 Secondary prevention of MINOCA
Current observational or prospective studies suggest that secondary prevention of CHD improves long-term outcomes in patients with MINOCA. In the SWEDEHEART registry, 9136 patients with MINOCA received statins, ACEIs/ARBs, β-blockers, and dual-antiplatelet therapy, who were followed for an average of 4.1 years. The results showed that statins and β-blockers significantly reduced the incidence of major cardiovascular events (all-cause mortality, MI, ischemic stroke, and heart failure), while dual-antiplatelet therapy did not reduce major cardiovascular events.6, 210 Choo et al.211 followed 396 patients with MINOCA for 2 years and found that ACEIs/ARBs and statins significantly reduced all-cause mortality in patients with MINOCA. Paolisso et al.212 conducted a prospective study on 134 patients with MINOCA with an average follow-up of 20 months and found that ACEIs/ARBs significantly reduced the incidence of all-cause mortality and MACEs; however, dual-antiplatelet therapy, β-blockers, and statins did not produce significant benefits. Another retrospective study showed no benefits from aspirin therapy in patients with MINOCA due to coronary spasm.213 The ongoing PROMISE trail including 140 MINOCA patients aims to compare the precision medicine approach with standard medical treatment to improve the angina status and the rate of MACEs during 12-month follow-up.214 Therefore, statins, ACEIs/ARBs, and β-blockers may improve the long-term prognosis of patients with MINOCA, partly due to improved CMVD (Table 9).
6.4 Treatment of CMVD associated with MIOCA
Prevention of MVO and no-reflow in patients with STEMI receiving primary PCI is a key for the prevention and treatment of CMVD.215
6.4.1 Pharmacological treatment before PCI
(1) Dual-antiplatelet therapy: This is the cornerstone of antithrombotic therapy for STEMI. Ticagrelor combined with aspirin is recommended in patients with STEMI who have a low risk of bleeding, and clopidogrel can be used in patients who are intolerant to ticagrelor or have a high risk of bleeding.216-218
(2) Statins: MCE studies have shown that statin therapy before revascularization in patients with ACS significantly improves coronary microvascular perfusion.8, 219, 220
6.4.2 Pharmacological treatment during PCI
(1) Platelet glycoprotein IIb/IIIa receptor antagonists (tirofiban, abciximab, or eptifibatide; the latter two not commercially available in China): In patients receiving primary PCI with a high thrombus burden, intracoronary or intravenous administration of platelet glycoprotein IIb/IIIa receptor antagonists reduces MVO incidence and MI size, improves myocardial perfusion, and lowers the reinfarction rate and mortality.221
(2) Plasminogen activators: Low-dose streptokinase by intracoronary injection immediately after primary PCI improves myocardial reperfusion but not long-term left ventricular size or function.222 The T-TIME investigators found that in patients with STEMI, adjunctive low-dose intracoronary alteplase during primary PCI does not reduce MVO.223
(3) Adenosine: The REOPEN-AMI study showed that in patients with STEMI, intracoronary administration of adenosine after coronary thrombus aspiration improves MVO and left ventricular function and reduces rehospitalization.224
(4) Nicorandil: Studies have shown that intracoronary injection of nicorandil during PCI prevents or alleviates no-reflow and improves myocardial perfusion and clinical prognosis.225 The Effects of Nicorandil Administration on Infarct Size in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention (CHANGE) study showed that perioperative intravenous administration of nicorandil in patients with STEMI undergoing primary PCI reduces the occurrence of coronary no-reflow/slow flow and promotes ST-segment restoration.226
(5) Nitroprusside: Studies have demonstrated that intracoronary administration of nitroprusside improves no-reflow during primary PCI and increases myocardial perfusion.227, 228
(6) Verapamil and diltiazem: Both drugs reduce the occurrence of slow flow or no-reflow by dilating epicardial coronary arteries and coronary arterioles.229
6.4.3 Nonpharmacological treatment
(1) Coronary thrombus aspiration: Routine thrombus aspiration is not recommended. However, in cases with a high coronary thrombus burden, thrombus aspiration may be performed to reduce MVO and improve coronary microvascular function and myocardial reperfusion.230
(2) Proximal or distal protection devices: A randomized, prospective, multicenter clinical trial found that use of proximal and distal protection devices during saphenous vein graft intervention significantly reduces the incidence of perioperative MI.231
(3) Excimer laser coronary atherectomy (ELCA): ELCA ablation can be considered in patients with a high intracoronary thrombus burden or undergoing saphenous vein graft intervention to reduce the occurrence of no-reflow.232
(4) Deferred stenting: The DEFER-STEMI study showed that after thrombus aspiration or balloon dilation, if TIMI flow was restored to grade 3, coronary stenting could be deferred until 4−16 h later. Compared with direct PCI, deferred stenting had a lower incidence of no-reflow/slow reflow.233 The above treatment recommendations are listed in Table 10.
| Treatment | Class of recommendation | Level of evidence | References |
|---|---|---|---|
| Perioperative antiplatelet therapy | |||
| Aspirin | I | A | 216, 217 |
| Ticagrelor or clopidogrel | I | A | 216, 218 |
| Statins | I | A | 219, 220 |
| Platelet glycoprotein IIb/IIIa receptor antagonists | IIa | B | 221 |
| Plasminogen activators | IIb | B | 222 |
| Adenosine | IIa | B | 224 |
| Nicorandil | IIa | B | 225, 226 |
| Nitroprusside | IIa | C | 227, 228 |
| Verapamil or diltiazem | IIa | C | 229 |
| Thrombus aspiration | IIa | B | 230 |
| Excimer laser coronary atherectomy | IIa | B | 232 |
| Proximal or distal protection devices | IIb | B | 231 |
| Deferred stenting | IIb | B | 233 |
- Abbreviations: CMVD, coronary microvascular disease; MIOCA, myocardial infarction with obstructive coronary arteries.
6.5 Treatment of CMVD after successful intervention for acute MI
Successful opening of infarct-related artery in patients with STEMI is only the first step. The ultimate goal of treatment is to prevent CMVD, protect myocardial function, and improve patient prognosis.121, 234
6.5.1 Drugs
(1) β-Blockers: Studies have found that the third-generation β-blockers carvedilol and nebivolol improve microcirculatory perfusion and reduce infarct size in patients with STEMI. The Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) study showed that metoprolol significantly reduced myocardial infarct size at 6 months, improved LVEF, and significantly reduced the incidence of cardiovascular events at 2 years in patients with STEMI receiving intravenous metoprolol prior to PCI followed by oral metoprolol over 24 h. A CMR study revealed that metoprolol reduced MVO by 40% by a mechanism related to the inhibition of neutrophil activation.235, 236 In the EARLY-BAM study,237 patients with STEMI who received intravenous metoprolol before primary PCI did not demonstrate significant reduction in myocardial infarct size at 30 days in the treatment group. However, recent international guidelines still recommend intravenous β-blockers before PCI in the absence of heart failure and hypotension.215, 238
(2) Statins: The STATIN STEMI study found that high-dose atorvastatin loading before primary PCI in patients with STEMI reduced the incidence of MVO but not myocardial infarct size.239 A subgroup analysis of SECURE-PCI study showed that high-dose atorvastatin administered pre- or 24 h postprimary PCI reduced the 30-day cardiovascular event rate by 50%.240
(3) Adenosine: Studies have shown that continuous intravenous adenosine infusion during PCI significantly reduces the myocardial infarct size by 67% and improves coronary microcirculation in patients.241-243
(4) Nicorandil: Intracoronary injection of nicorandil during primary PCI improves myocardial perfusion, reduces myocardial infarct size, and ameliorates clinical prognosis.225 The CHANGE study showed that perioperative intravenous nicorandil administration for 24-h in patients with STEMI significantly increased LVEF after a 6-month follow-up.226
(5) Atrial natriuretic peptide (ANP): A study has shown that ANP administration reduces occurrence of MVO by inhibiting ET-1.244 The J-WIND study found that ANP agonists administered before PCI in patients with STEMI significantly reduced the myocardial infarct size.245
(6) Antiplatelet therapy: Pre-PCI administration of P2Y12 receptor inhibitors in patients with STEMI reduces MVO and infarct size and improves long-term prognosis. In addition to inhibition of platelet aggregation, ticagrelor dilates microvessels and improves CMVD through the adenosine pathway, similar to postischemic conditioning.158 The On-TIME-2 study found that routine high-dose tirofiban administration before PCI in patients with STEMI reduced microvascular injury, promoted ST-segment regression, and improved clinical prognosis.246 The INFUSE-AMI study showed that acute intracoronary administration of abciximab during PCI reduced the infarct size in patients with acute anterior MI.247
(7) Erythropoietin (EPO): In a Japanese study, intravenous EPO administration after PCI in patients with acute anterior MI reduced the incidence of transmural MI, significantly improved CFVR for 8 months, and reduced left atrial volume.248 However, more clinical studies are needed to confirm this therapeutic effect of EPO.
(8) Iron chelators: MVO and intramyocardial hemorrhage exacerbate iron deposition in the myocardial interstitium, which can induce an inflammatory response and exacerbate microvascular injury and left ventricular remodeling. Pre-PCI administration of iron chelators in patients with STEMI significantly reduces serum iron and oxidative stress levels.249
6.5.2 Nonpharmacological treatment
(1) Ischemic conditioning: Ischemic preconditioning refers to repeated coronary ballooning to block coronary arteries temporarily several times prior to coronary stenting to induce transient myocardial ischemia and protection against ischemia–reperfusion injury after PCI. Ischemic postconditioning refers to repeated coronary ballooning to block culprit coronary arteries temporarily several times after coronary stenting to induce transient myocardial ischemia and protection against ischemia–reperfusion injury after PCI. Studies have shown that ischemic postconditioning reduces MVO occurrence and promotes recovery of left ventricular function. Remote ischemic preconditioning and remote ischemic postconditioning involve the use of a cuff to inflate and deflate the upper limb several times before or after PCI in patients with STEMI, resulting in transient ischemia in the upper limb and local production of protective substances for myocardial ischemia, thereby promoting myocardial survival. The CONDI study, which included 166 patients with STEMI who received 5-min upper arm inflation and deflation four times in a prehospital ambulance, showed that remote ischemic preconditioning reduced the myocardial infarct size but did not improve CBF. At the end of the 4-year follow-up, all-cause mortality, cardiovascular events, and cerebrovascular events were significantly reduced in patients receiving remote ischemic preconditioning.250 White et al.251 found that remote ischemic preconditioning reduced myocardial infarct size and myocardial edema detected by CMR. However, the CONDI-2/ERIC-PPCI study found that remote ischemic conditioning offers no benefits on cardiovascular mortality and rehospitalization due to heart failure one year after primary PCI in patients with STEMI, and a CMR subgroup study showed that remote ischemic preconditioning did not reduce MI size or improved LVEF 6 months after PCI.252, 253
(2) Pressure-controlled intermittent coronary sinus occlusion (PICSO): PICSO improves coronary microcirculation and reduces the incidence of MVO during primary PCI by increasing the redistribution of MBF in ischemic regions, promoting the clearance of microvascular harmful substances, and inducing production of vascular growth factors.254The above treatment recommendations are summarized in Table 11.
| Treatment | Class of recommendation | Level of evidence | References |
|---|---|---|---|
| The ultimate goal of reperfusion therapy in patients with AMI is to attenuate CMVD and protect cardiac function | I | A | 121, 234 |
| β-Blockers | I | A | 235, 236, 238 |
| Statins | I | A | 239, 240 |
| Adenosine | IIa | B | 241-243 |
| Nicorandil | IIa | B | 225, 226 |
| Platelet glycoprotein IIb/IIIa receptor antagonists | IIa | B | 246, 247 |
| Ticagrelor | IIb | B | 158 |
| ANP | IIb | B | 245 |
| Erythropoietin | IIb | B | 248 |
| Iron chelators | IIb | C | 249 |
| Ischemic conditioning | IIb | B | 250-253 |
| PICSO | IIb | C | 254 |
- Abbreviations: AMI, acute myocardial infarction; ANP, atrial natriuretic peptide; CMVD, coronary microvascular disease; PCI, percutaneous coronary intervention; PICSO, pressure-controlled intermittent coronary sinus occlusion.
6.6 Treatment of CMVD associated with non-atherosclerotic heart disease
6.6.1 Treatment of CMVD with myocardial hypertrophy
(1) Treatment of primary disease: In patients with hypertensive left ventricular hypertrophy, ACEIs/ARBs or CCBs may improve ventricular remodeling and increase CFR.255-257 In patients with hypertrophic obstructive cardiomyopathy, septal resection or chemical ablation may reduce left ventricular outflow tract obstruction and increase CFR.258, 259 In patients with severe AS, aortic valve replacement may reduce transvalvular pressure differences and increase coronary perfusion pressure, thereby improving CFR.25, 260, 261
(2) Treatment of CMVD: A meta-analysis has shown that ACEIs and ARBs significantly improved the CFR in patients with hypertensive cardiac hypertrophy combined with CMVD.144 In patients with HCM, β-blockers and CCBs increased CFR by reducing left ventricular end-diastolic pressure and decreasing left ventricular outflow tract pressure differences.262-265 Another study showed that β-blockers significantly reduced the incidence of all-cause mortality, cardiovascular death, and sudden cardiac death in patients with mild-to-moderate asymptomatic AS.266
6.6.2 Treatment of CMVD without myocardial hypertrophy
ACEIs/ARBs are the first-line drugs for the treatment of dilated cardiomyopathy combined with heart failure. Enalapril and quinapril improve CFR in patients with dilated cardiomyopathy.142, 143, 267 Other studies suggested that early administration of carvedilol in patients with idiopathic dilated cardiomyopathy significantly increased CFR which was closely associated with improved long-term left ventricular function.268, 269 Nebivolol and esmolol increase CFR in patients with dilated cardiomyopathy.270, 271 Allopurinol significantly improves CFR and left ventricular systolic and diastolic functions while lowering serum uric acid levels in patients with dilated cardiomyopathy combined with hyperuricemia.272 Treatment recommendations are summarized in Table 12.
| Treatment | Class of recommendation | Level of evidence | References |
|---|---|---|---|
| CMVD with myocardial hypertrophy | |||
|
Hypertensive myocardial hypertrophy |
|||
| ACEIs/ARBs | I | A | 255-257 |
| CCBs | IIa | B | 255, 256 |
| Hypertrophic cardiomyopathy | |||
| Septal resection or chemical ablation | IIa | B | 258, 259 |
| Aortic stenosis | |||
| Aortic valve replacement | I | A | 25, 260, 261 |
| CMVD treatment | |||
| ACEIs/ARBs | I | A | 144, 255-257 |
| β-Blockers | I | A | 264, 266 |
| CCBs | IIb | B | 255, 265 |
| CMVD without myocardial hypertrophy | |||
| ACEIs/ARBs | I | A | 143, 267 |
| Beta-blockers | I | A | 268-271 |
| Allopurinol | IIb | B | 272 |
- Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CMVD, coronary microvascular disease; CCB, calcium channel blocker.
7 GAPS OF KNOWLEDGE AND FUTURE PERSPECTIVES
There exists significant gap of knowledge in the field of CMVD. First, current knowledge of pathogenic mechanism of CMVD was based on findings in animal models and vascular cells of aortic atherosclerosis, which is in many ways different from CMVD. Development of animal models with CMVD and culture of microvascular endothelial cells from these models are essential for further understanding of the pathogenesis of CMVD. Second, epidemiological data of CMVD in different populations and ethnics, and in patients with less common etiologies, such as those with cardiomyopathy, are lacking. Thus, further epidemiological studies in these populations are required. Third, standard techniques for detecting CMVD are mostly invasive and focusing on only LAD and its supplied territories. Thus, development of noninvasive imaging techniques to evaluate the structure and function of entire left ventricular microvasculature is highly warranted. Finally, at present, all published randomized clinical trials for assessing therapeutic effects of drugs in patients with CMVD used surrogate endpoint, mostly CFR, and clinical trials using hard endpoint, such as cardiovascular mortality, should be the future direction.
AUTHOR CONTRIBUTION
Wenqiang Chen, Mei Ni, He Huang, and Yundai Chen drafted the manuscript. Hongliang Cong, Xianghua Fu, Wei Gao, Yuejin Yang, Mengyue Yu, Xiantao Song, Meilin Liu, Zuyi Yuan, Bo Zhang, Zhaohui Wang, and Yan Wang participated in the writing and revision of the manuscript. Cheng Zhang and Yun Zhang designed the structure and contents of the consensus and corrected the manuscript. We confirmed that all authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by grants of the National Natural Science Foundation of China (No. 82241203, 81920108003, 82030051), State Key R&D Program, Chinese Ministry of Science and Technology (2021YFF0501403), Key R&D Program, Shandong Provincial Department of Science and Technology (2021SFGC0503, 2021ZLGX02, 2021ZDSYS05, 2020ZLYS05), Natural Science Foundation, Shandong Provincial Department of Science and Technology (ZR202102190727), and the Taishan Scholars Program, Shandong Provincial Department of Science and Technology (Zhang C).
CONFLICT OF INTEREST STATEMENT
All participants in the preparation of the manuscript declare that there is no conflict of interest relevant to this consensus.
ETHICS STATEMENT
Not relevant.
Open Research
DATA AVAILABILITY STATEMENT
All data associated with this consensus are within the paper itself.




