Limited diagnostic possibilities for bloodstream infections with broad-range methods: A promising PCR/electrospray ionization-mass spectrometry platform is no longer available
Graphical Abstract
By using the polymerase chain reaction coupled with electrospray ionization-mass spectrometry (PCR/ESIMS) technology, we detected pathogenic agents in 29.4% (47 of 160) more samples than by using 16S rRNA PCR and/or blood culture. Thus, the PCR/ESI-MS represented an opportunity to make the bloodstream infection diagnostics highly sensitive and accurate.
Abstract
Fast and accurate detection of causative agents of bloodstream infections remains a challenge of today's microbiology. We compared the performance of cutting-edge technology based on polymerase chain reaction coupled with electrospray ionization-mass spectrometry (PCR/ESI-MS) with that of conventional broad-range 16S rRNA PCR and blood culture to address the current diagnostic possibilities for bloodstream infections. Of 160 blood samples tested, PCR/ESI-MS revealed clinically meaningful microbiological agents in 47 samples that were missed by conventional diagnostic approaches (29.4% of all analyzed samples). Notably, PCR/ESI-MS shortened the time to positivity of the blood culture-positive samples by an average of 34 hr. PCR/ESI-MS technology substantially improved current diagnostic tools and represented an opportunity to make bloodstream infections diagnostics sensitive, accurate, and timely with a broad spectrum of microorganisms covered.
The use of molecular methods in medical microbiology improves the sensitivity of examinations, shortens the turnaround time (TAT), and allows the detection of uncultivable microorganisms (Opota, Jaton, & Greub, 2015). However, their application in the diagnostics of bloodstream infections (BSI) is troublesome due to an extremely low bacterial load and the presence of an excessive amount of human DNA in whole blood (Opota et al., 2015). Therefore, classical blood culture (BC) remains the cornerstone of BSI diagnostics; however, its TAT often exceeds 24 hr, and the sensitivity can be as low as 50% (Opota et al., 2015; Peker, Couto, Sinha, & Rossen, 2018). Thus, reliable and robust BSI diagnostics are an unmet need of today's medical microbiology.
In this respect, a commercial platform IRIDICA (Abbott Molecular, Des Plaines, IL, USA) that combines a set of PCRs including broad-range PCRs with electrospray ionization-mass spectrometry (hereafter, PCR/ESI-MS) entered the European market in 2014, based on a very favorable outcome of a multicentre clinical trial (Vincent et al., 2015). The method is capable of detecting over 800 bacterial and Candida species associated with BSI and shortening the TAT by up to 6 hr (Bacconi et al., 2014). The width of detection, unprecedented for a commercial test, matches the conventional broad-range PCR strategy (Tkadlec et al., 2019) and goes far beyond the possibilities of any pathogen-specific PCR.
We aimed to evaluate the utility of the PCR/ESI-MS system in a clinical setting by comparing its real-life performance with conventional culture-dependent (BC) and culture-independent (16S ribosomal RNA PCR) broad-range tests.
Within 12 months, we examined 166 blood samples from 137 patients (median age: 64 years; range 22–94 years; 72% males) who had been hospitalized with suspected BSI at intensive care units of Motol University Hospital in Prague.
A BC was performed by using BACTEC™ FX (Becton Dickinson) with one pair of aerobic and anaerobic BC bottles collected. These BC bottles were incubated for 5 days before being concluded as negative. Additional (mycotic) BC bottles were collected if patients were suspected of a mycotic infection, and these bottles were incubated for up to 14 days before being concluded as negative. Positive BC bottles were streaked out on solid media, and upon overnight culture, the microorganisms were identified by using a MALDI-TOF mass spectrometer Biotyper v 3.1 (Bruker Daltonics). Two additional EDTA tubes were collected at the time of the BC blood draw. One tube was subjected to panbacterial/panfungal 16S/18S PCR assay (hereafter 16S-PCR) using UMD-SelectNA™ kit with Add-On10 extension (Molzym) to process up to 10 ml of whole blood as previously described (Tkadlec et al., 2019). The other tube with 5 ml whole blood was stored frozen at −20°C for up to 3 months until being processed with the PCR/ESI-MS system. The samples were run with the BAC BSI assay according to the manufacturer's instructions (Abbott Molecular).
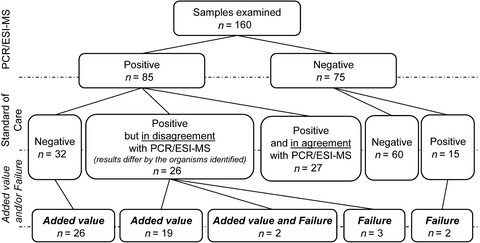
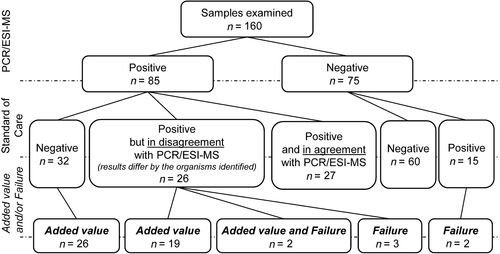
We assessed every PCR/ESI-MS result against the BC and 16S-PCR results, which are collectively referred to as standard-of-care (SoC) tests. When a discrepancy between PCR/ESI-MS and SoC occurred, the patient's medical records were carefully reviewed to determine whether the unique positivity of PCR/ESI-MS meant a false negativity of the SoC tests and vice versa. Detection of typical contaminating microbiota by any of the tested methods was not regarded as a relevant finding unless it was supported by microbiological investigation of other materials from the same patient. The unique positivity obtained by PCR/ESI-MS was considered of added value (Tkadlec et al., 2019) if a detected organism was found in another sample, collected close to the collection date of the sample for PCR/ESI-MS, and/or the detected microorganism was known to be associated with the BSI. Conversely, to the added value, failures referred to the results in which the PCR/ESI-MS missed relevant organism(s) that were detected by the SoC tests.
We obtained valid results from both PCR/ESI-MS and SoC methods from 160 samples (Figure 1). More than half of the samples were either complete negative or double positive with agreement in terms of recovering the same microorganism(s). PCR/ESI-MS as the only method detected the positivity or the presence of additional agent(s) in 58 samples. Out of them, 47 findings (i.e., 29.4% of all analyzed samples) were assessed to be of added value. Notably, 26 of the 47 samples were completely negative by the SoC tests, while 21 were positive by the SoC tests (5 with both BC and 16S-PCR, 5 with BC only, and 11 with 16S-PCR only); however, one or more clinically relevant microorganism(s) were left undetected (Appendix in Table A1). The failure of PCR/ESI-MS was seen in seven cases.
Time to culture positivity ranged from 4 to 116 hr for positive aerobic and anaerobic bottles and up to 271 hr for positive mycotic bottles. Time to culture positivity exceeded 24 hr in 16 of 27 BC samples, which had concordant results with the PCR/ESI-MS. On average, the PCR/ESI-MS result was delivered faster than that of BC by 34 hr if hands-on and instrument run times were taken into consideration.
Reliable and timely diagnostics of BSI-causing agents are critical for correct patient management. BC is the gold standard for BSI diagnostics, but due to its limitations, alternative detection systems are being sought. We tested the performance of PCR/ESI-MS as a novel BSI diagnostic method and found that: (a) The method was able to detect clinically relevant causes of BSI in 30% of samples (that would be determined as negative by the SoC methods); and (b) PCR/ESI-MS exhibited a considerably shorter time to positivity when compared to that of BC.
However, it is important to note that the majority of our patients were on antibiotic therapy at the time of sample collection and that the higher PCR/ESI-MS positivity rate could be also attributed to the detection of free circulating DNA, not necessarily of viable microbial cells. However, positivity of PCR/ESI-MS was already associated with increased mortality in patients with suspected sepsis, indicating the ability of the technology to correctly identify critically ill patients (O’Dwyer et al., 2017).
Despite these highly encouraging PCR/ESI-MS results (Karrasch et al., 2018; Makristathis et al., 2018; Tassinari et al., 2018; Vincent et al., 2015), the technology was unexpectedly suspended in 2017 (Özenci, Patel, Ullberg, & Stralin, 2018). Thus, 16S-PCR currently remains the only molecular genetic test with a panbacterial spectrum of detection that is applicable to blood. However, we previously demonstrated its low added value of 6.5% and high failure rate of 7.1% when blood samples from adults in intensive care units were checked in parallel with BC (Tkadlec et al., 2019). We believe that the discontinuation in the PCR/ESI-MS technology development is a loss and an unfortunate step back in the quest for ideal BSI diagnostics, and we strongly encourage the diagnostic industry to develop methods and equipment that can provide similar advantages to the clinical service of PCR/ESI-MS.
ACKNOWLEDGMENT
The authors are grateful to Dr. Veronica Barrioluengo Fernandez and Dr. Jessica Schilde for their technical assistance.
This work was supported by the Ministry of Health of the Czech Republic (grant no. 15-28157A) awarded to P.D.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTION
Jan Tkadlec: Conceptualization-Supporting, Data curation-Equal, Formal analysis-Equal, Investigation-Lead, Project administration-Lead, Writing-original draft-Equal; Eliska Bebrova: Data curation-Supporting, Formal analysis-Supporting, Supervision-Supporting, Validation-Supporting; Jan Berousek: Methodology-Supporting, Resources-Supporting, Validation-Supporting, Writing-original draft-Supporting; Tomas Vymazal: Formal analysis-Supporting, Investigation-Supporting, Methodology-Supporting, Project administration-Supporting, Resources-Supporting, Supervision-Supporting, Writing-original draft-Supporting; Jaroslava Adamkova: Methodology-Supporting, Project administration-Supporting, Validation-Supporting; Vendula Martinkova: Formal analysis-Supporting, Methodology-Supporting, Project administration-Supporting, Validation-Supporting; Claus Moser: Methodology-Supporting, Resources-Supporting, Writing-original draft-Supporting; Dragos Florea: Methodology-Supporting, Resources-Supporting, Supervision-Supporting, Writing-original draft-Supporting; Pavel Drevinek: Conceptualization-Lead, Data curation-Equal, Formal analysis-Equal, Funding acquisition-Lead, Investigation-Supporting, Methodology-Lead, Project administration-Supporting, Resources-Lead, Supervision-Lead, Validation-Lead, Writing-original draft-Equal.
ETHICS STATEMENT
Study protocol had been reviewed and approved by the institutional Ethics Committee of the Motol University Hospital, Prague, Czech Republic (date of the application for approval: 17 July 2014). All subjects or their legal representatives provided written informed consent.
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality reasons but are available from the corresponding author upon reasonable request.
APPENDIX A
| No. | PCR-ESI/MS | 16S-PCR | BC |
|---|---|---|---|
| Added value | |||
| 1 | Escherichia coli | Negative | Negative |
| 2 | Enterobacter cloacae complex | Negative | Negative |
| 3 | Escherichia coli, Streptococcus pseudopneumoniae, Corynebacterium sp. | CoNS | Negative |
| 4 | Klebsiella pneumoniae | Negative | Negative |
| 5 | Leclercia adecarboxylata, Klebsiella pneumoniae, Enterobacter cloacae complex | Enterobacter sp., Streptococcus oralis group | Enterobacter cloacae |
| 6 | Enterobacter cloacae complex, Gemella haemolysans, Streptococcus sp., Candida glabrata/ C. albicans | Bacillus sp. | Negative |
| 7a | Klebsiella pneumoniae | Negative | Negative |
| 8a | Enterococcus faecium | Negative | Negative |
| 9 | Enterococcus faecalis , Candida albicans | Negative | Candida albicans |
| 10 | Enterobacter cloacae complex, Escherichia coli | Negative | Negative |
| 11 | Fusobacterium necrophorum | Negative | Negative |
| 12 | Klebsiella variicola | Negative | Negative |
| 13b | Enterococcus faecium | pos. (with no ID retrieved) | Negative |
| 14b | Klebsiella oxytoca , Staphylococcus haemolyticus | CoNS, Propionibacterium acnes | Staphylococcus haemolyticus |
| 15 | Odoribacter splanchnicus, Fusobacterium necrophorum, Escherichia coli, Enterobacter cloacae complex | Negative | Negative |
| 16 | Salmonella bongori | Negative | Negative |
| 17 | Klebsiella pneumoniae, Escherichia coli, Morganella morganii | Negative | Negative |
| 18 | Klebsiella pneumoniae | Staphylococcus epidermidis | Negative |
| 19 | Klebsiella variicola , Streptococcus pyogenes | Streptococcus pyogenes | Negative |
| 20c | Serratia marcescens | Negative | Negative |
| 21c | Serratia marcescens | Negative | Staphylococcus hominis |
| 22c | Serratia marcescens | Negative | Negative |
| 23 | Burkholderia cepacia complex, Enterobacter cloacae complex | Negative | Negative |
| 24 | Enterococcus faecium, Enterobacter cloaceae complex | CoNS | Negative |
| 25 | Enterobacter cloacae complex, Escherichia coli | Bacillus sp. | Negative |
| 26 | Bacteroides uniformis , Shigella boydii | Negative | Negative |
| 27 | Escherichia coli, Nocardia sp., Candida tropicalis | Negative | Nocardia sp. |
| 28 | Escherichia coli, Klebsiella pneumoniae, Bacteroides fragilis | Negative | Negative |
| 29 | Klebsiella pneumoniae | Negative | Negative |
| 30 | Pseudomonas aeruginosa | Negative | Negative |
| 31 | Escherichia coli, Klebsiella pneumoniae | Klebsiella pneumoniae | Negative |
| 32 | Escherichia coli | CoNS | Negative |
| 33 | Streptococcus pyogenes | negative | Staphylococcus epidermidis |
| 34d | Klebsiella sp. , Propionibacterium acnes | negative | negative |
| 35d | Klebsiella pneumoniae | negative | negative |
| 36 | Clostridium perfringens, Escherichia coli | Lactobacillus sp. | negative |
| 37 | Leclercia adecarboxylata, Klebsiella oxytoca, Enterobacter cloacae complex | Bacillus sp., Massilia sp. | negative |
| 38 | Candida parapsilosis , Proteus mirabilis | Negative | Proteus mirabilis |
| 39 | Klebsiella pneumoniae | Negative | negative |
| 40 | Streptococcus pneumoniae | Negative | negative |
| 41 | Klebsiella pneumoniae | Negative | negative |
| 42 | Streptococcus agalactiae, Staphylococcus aureus | Staphylococcus aureus | Staphylococcus aureus, Staphylococcus epidermidis |
| 43e | Pseudomonas aeruginosa | Negative | Negative |
| 44e | Pseudomonas aeruginosa | Negative | Negative |
| 45 | Escherichia coli | Negative | Negative |
| Failure | |||
| 46 | Clostridium sticklandii | Streptococcus pyogenes, Fusobacterium nucleatum, Gemmela sp., Parvimonas micra, Peptostreptococcus sp. | Streptococcus pyogenes |
| 47 | Escherichia coli | Escherichia coli | Klebsiella pneumoniae , Escherichia coli |
| 48b | x | Haemophilus parainfluenzae | Negative |
| 49 | Fusobacterium nucleatum | Parvimonas micra , Fusobacterium nucleatum | Negative |
| 50 | x | Negative | Burkholderia cepacia complex |
| Added value and Failure | |||
| 51 | Streptococcus pneumoniae, Group G Streptococcus, Pseudomonas aeruginosa, Propionibacterium acnes | Enterococcus sp., CoNS | Enterococcus faecalis , Pseudomonas aeruginosa |
| 52 | Enterococcus faecium , Klebsiella pneumoniae | Klebsiella pneumoniae | Escherichia coli |
Note
- Microorganisms in bold were detected by one diagnostic approach only (either by PCR/ESI-MS or by SoC) and assessed to be of added value or relevant. CoNS: coagulase negative Staphylococci.
- Samples collected from the same patient are marked with the same index. The dates of their blood collections were as follows:
- a 27 July 2016 (id 7); 23 August 2016 (id 8).
- b 23 March 2016 (id 48); 6 April 2016 (id 13); 1 June 2016 (id 14).
- c 27 August 2016 (id 20); 3 September 2016 (id 21); 4 September 2016 (id 22).
- d 16 April 2016 (id 34); 17 April 2016 (id 35).
- e 10 April 2016 (id 43); 11 April 2016 (id 44).