Recent advances in smart-responsive hydrogels for tissue repairing
Abstract
The rapid development of biomedical materials and tissue engineering technology has played an increasingly important role in the process of tissue repair in recent years. Smart-responsive hydrogels are three-dimensional network structures formed by cross-linking of hydrophilic polymers. In addition to having conventional hydrogels that approximate the natural extracellular matrix structure and serve as delivery vehicles for functional molecules (drugs and proteins). More importantly, smart-responsive hydrogels can achieve relevant changes in material morphology or properties under the conditions of changes in physical, chemical, and biological factors, thereby achieving controlled functional molecules release. It is more urgent to design and build smart-responsive hydrogels to achieve precise tissue repair with the introduction of the concept of precision medicine and drug delivery. In this review, we highlight different types of smart-responsive hydrogels and their mechanisms of response to different stimuli and discuss their potential for application in different types of tissue repair, such as chronic wound repair, damaged heart tissue repair, brain nerve tissue repair, and other fields. Finally, we present the prospects of smart-responsive hydrogels in tissue repair. In general, the current progress in the application of smart-responsive hydrogels in tissue repair lays the foundation for future applications in other diseases.
1 INTRODUCTION
After local tissues and cells are damaged or died to varying degrees under the action of some external or internal pathogenic factors, the body repairs the damaged tissue through the regeneration of adjacent healthy tissues or cells, thereby restoring the integrity of the tissue, the process is called tissue repair.1-3 The ideal tissue repair is that the tissue defect is completely repaired by cells of the original nature and the original structure and function can be restored.4, 5 However, some injured tissues cannot be repaired by the original cells due to the different inherent proliferative abilities of various tissue cells in the human body.6, 7 The internal environment of the body is stable, but the shape and function of the newly grown tissue cannot be completely restored, which will bring aesthetic distress to the patient and even bring about psychological obstacles and life changes.8 In general, the tissue repair process in the body can be divided into three stages: (1) local inflammatory response stage; (2) cell proliferation, differentiation, and matrix deposition stage between cells; (3) tissue repair and shaping stage.9 During these stages, macrophages, endothelial cells, fibroblasts, and various mediators are involved.10, 11
When people's limbs, hearts, bones, brains, and spines are severely traumatized by external forces or internal factors, the injured tissues will try to repair themselves, but the results are often unsatisfactory. Thus, they need to accept external treatment interventions.12 The rapid development of biomedical materials and regenerative medicine has brought new hope for tissue repair.13, 14 At present, tissue repair, and regenerative medicine are constantly improving, based on traditional treatment techniques and are benefiting countless patients. Among all biomaterials, hydrogels have become the focus of contemporary biomedical materials research because of their natural advantages similar to human tissue properties.15, 16 Hydrogel is a three-dimensional network structure with a water content of more than 90%.17 When the hydrogel is implanted into the damaged tissue sites, the superior mechanical properties of the hydrogel are widely used for structural support.18, 19 The porous structure of the hydrogel itself is not only conducive to the migration and proliferation of surrounding tissue cells but also can be used as a drug delivery carrier to achieve in situ delivery of functional molecules such as drugs and proteins in damaged tissue sites.20-22 In addition, the biggest advantage of hydrogels with injectable and self-healing properties is that they can treat some diseases in a minimally invasive form, such as heart damage, cartilage repair, stroke, and so forth.23, 24 And the self-healing properties of hydrogels can achieve sufficient and uniform filling in irregular defect sites, thereby effectively reducing intraoperative pain, postoperative infection risk, and patient operating costs, and has become one of the main clinical forms of materials.25
People use the mechanical support and delivery carrier characteristics of hydrogels to fully stimulate and improve the self-repairing ability of the human body by endowing the hydrogel materials with different biological structures and functions.22 Hydrogels have been widely used in different tissue repairs, such as chronic wound skin tissue,26 damaged heart tissue,18 brain nerve tissue,27 bone tissue,28, 29 and corneal tissue.30 With the deepening of research, it has been found that functional molecules such as drugs or proteins encapsulated in traditional hydrogels have the disadvantage of uncontrollable release at the site of tissue damage. It will lead to problems such as the low therapeutic efficiency of functional molecules such as drugs or proteins.31 The emergence of smart-responsive hydrogels effectively solves the problem. Compared with conventional (no-stimuli-responsive) hydrogels, smart-responsive hydrogels can achieve the effect of the controllable on-demand release of functional molecules at the site of tissue damage.32, 33 That is more drugs or proteins are released when the body needs more and when the body needs less, fewer drugs or proteins are released.34 In recent years, compared with conventional hydrogels, smart responsive hydrogels with various response mechanisms have attracted extensive attention in the field of tissue repair (Figure 1).35
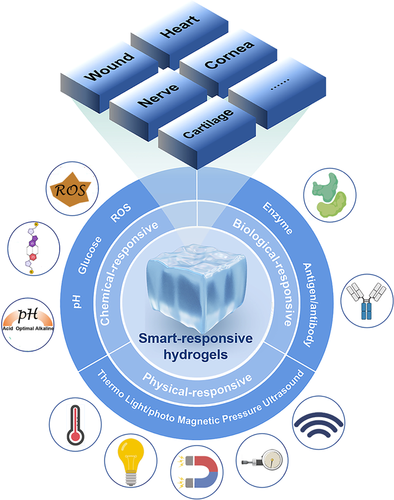
The review summarizes the current progress and advantages of smart responsive hydrogels in the field of tissue repair. We will focus on the classification of smart-responsive hydrogels and the current application of smart-responsive hydrogels in chronic wound skin tissue, damaged heart tissue, brain tissue, and other tissues. The current challenges and future development directions of smart-responsive hydrogels are also discussed to guide the application of smart responsive hydrogels in tissue repair. It will help to further provide new solutions for the treatment of clinical diseases through an in-depth understanding of smart-responsive hydrogels in tissue repair.
2 CHALLENGES TO USING CONVENTIONAL HYDROGELS IN TISSUE REPAIRING APPLICATIONS
Over the past few decades, a variety of hydrogels have been designed for tissue repair.36, 37 Due to the good swelling properties of hydrogels, they can be used as a swelling material to fill space in the treatment of diseases such as tissue defects. Hydrogels can be used as wound dressings to repair wounds.26 In addition, hydrogels have properties similar to biological glues, so they can also be used as adhesives for soft tissues.38 Because of its hydrophilic three-dimensional network structure, hydrogels can provide an environment for cell proliferation and adhesion and can be used as a transfection carrier for tissue cells. Due to the good permeability of hydrogels to small molecule solutes, they can be used as carriers for functional molecules (drugs or proteins), so that drugs or proteins can be delivered to local or specific tissues.21
However, hydrogels still face many problems in the application process, such as the contradiction between the mechanical strength and injectability of hydrogels. Hydrogels with high mechanical strength have poor injectability or cannot be injected, while injectable hydrogels tend to have poor mechanical strength and cannot provide effective mechanical support.39, 40 At the same time, the hydrogel also has the problem that the crosslinking density is not uniform, which leads to the uncontrollable overall performance of the hydrogel. The addition of chemical cross-linking agents inevitably brings the risk of the biotoxicity of hydrogels and the poor water solubility of some chemical cross-linking agents also seriously limits the clinical application of hydrogels.41 On the contrary, the biosafety and in vivo biodegradability of hydrogels have been puzzling people.42 The hydrogel applied to the human body must have good biological safety and cannot cause an acute inflammatory response in the body. And the hydrogel can be continuously degraded accompanied by continuous tissue repair. The degraded products are nontoxic to the body and can be quickly absorbed and metabolized by the body.42 More importantly, when conventional hydrogels are used as transport carriers for drugs or proteins, the release of drugs or proteins is an uncontrollable release process.20 The uncontrolled release will reduce the therapeutic effect of the disease and increase the toxic side effects of the drug. The above limitations limit further research and translation of conventional hydrogels. In recent years, it has been found that environmentally responsive hydrogels have many advantages while maintaining the inherent characteristics of conventional hydrogels, which greatly expands the applicability of hydrogels.33, 43
3 SMART-RESPONSIVE HYDROGELS
3.1 Advantages of smart-responsive hydrogels
The smart-responsive hydrogels can sense the small changes in the surrounding microenvironment (such as temperature, light, pressure, electric field, magnetic field, pH, ions, enzymes, redox, etc.) and enable precise and controllable release of drugs at disease sites by breaking and degrading of chemical bonds.44 Compared with conventional hydrogels, smart-responsive hydrogels have the following advantages: fixed-point/fixed-timing/fixed-quantitative; precise drug delivery and precise treatment; improving drug bioavailability; enhancing treatment effect of drugs; reducing the frequency of administration; reducing side effects of drugs; improving safety and reliability of drugs.45 The smart-responsive hydrogels greatly improve the diagnosis and treatment experience and have great prospects for clinical transformation. Hydrogels with environmentally responsive functions have received extensive attention. It has been widely used in the fields of regenerative medicine and tissue repair.46
3.2 Classifications of smart-responsive hydrogels
Smart-responsive hydrogels are mainly divided into the following three categories according to the type of response: physical-responsive hydrogels, chemical-responsive hydrogels, and biological-responsive hydrogels.47 Physical-responsive hydrogels are mainly based on smart materials that can respond to changes in external environments such as light, temperature, electric field, ultrasound, pressure, and magnetic fields. Chemical-responsive hydrogels are mainly divided into pH-responsive hydrogels, reduction–oxidation hydrogels, and glucose-responsive hydrogels. Biological-responsive hydrogels are the hydrogels that can simulate the molecular recognition phenomenon in the body's life activities and recognize specific biomolecules. Biological-responsive hydrogels possess the feedback and balance functions of biological systems. A wide variety of smart-responsive hydrogels have been developed for different biomedical applications (Table 1).
| Type of smart-responsive hydrogel | Stimuli | Smart-responsive hydrogel system | Biomedical applications | References |
|---|---|---|---|---|
| Physical-responsive hydrogels | Thermo | K9-C peptide-based thermosensitive hydrogel | Tissue damage in diabetic patients | [48] |
| Poly-n-isopropyl acrylamide (PNIPAM) hydrogel | Prevent tendon adhesion | [49] | ||
| Poly(N-isopropylacrylamide) hybrid hydrogel dressing | Wound repairing | [50] | ||
| Light/photo | A dodecyl-modified and Schiff base-linked chitosan hydrogel (a photothermal agent) | Wound repairing | [51] | |
| Calcium phosphate nanoparticle-coordinated poly (dimethylaminoethyl methacrylate-co-2-hydroxyethyl methacrylate) hydrogel | Bone repairing | [52] | ||
| Gelatin derivatives functioned with dibenzylcyclooctyne and azidated azobenzene | Tissue repairing | [53] | ||
| Magnetic | Magnetic nanoparticles in scaffolds | Bone repairing | [54] | |
| Magnetic nanoparticles in alginate hydrogels | Parkinson's disease | [55] | ||
| Pressure | Sodium carboxymethylcellulose microsheets and polyacrylamide network | Strain sensor | [56] | |
| Stretchable elastomer and microgel depots containing drug-loaded nanoparticles | Blood sugar monitoring | [96] | ||
| Microscale aggregates of nanoparticles | Stroke, atherosclerosis | [57] | ||
| Ultrasound | PEG-grafted gelatin | Stroke and acute myocardial infarction | [58] | |
| High-performance hydrogel nanogenerators | Electrical stimulation of nerve systems | [59] | ||
| Chemical-responsive hydrogels | pH | Acryloyl-6-aminocaproic acid and AA-g-N-hydroxysuccinimide | Wound repairing | [60] |
| pH-responsive FER-8 peptide hydrogel | Tumor | [61] | ||
| Chitosan quaternary ammonium salt and aldehyde groups in oxidized dextran-dopamine | Wound repairing | [62] | ||
| Glucose | A phenylboronic acid/galactosyl-based glucose-responsive insulin delivery system | Treatment of diabetes | [63] | |
| Dynamic boronic esters bonds between phenylboronic acid-grafted γ-Polyglutamic acid and konjac glucomannan | Diabetic nephropathy | [64] | ||
| Glucose oxidase, phenylboronic acid, and glucose-binding molecules | Treatment of diabetes | [65] | ||
| ROS | Silver nanoparticle-contained polyacrylic acid and Fe2+/Fe3+-contained polyglutamic acid | Wound repairing | [66] | |
| A thioketal-containing and ROS-scavenging hydrogel | Repair of spinal cord injury | [67] | ||
| A thioketal-containing and ROS-scavenging hydrogel | Wound repairing | [68] | ||
| Biological-responsive hydrogels | Enzyme | Injectable matrix metalloproteinase enzyme responsive hydrogel that loaded TMZ and O6-benzylamine | Brain tumor | [69] |
| Enzyme-sensitivity nanogel was constructed through an oxidation reaction initiate the chemical cross-link of the functional groups on the periphery of PAMAM dendrimers | Tumor | [70] | ||
| Crosslinking allyl glycidyl ether modified carboxymethyl chitosan with MMP-2 substrate peptide CPLGLAGC | Peritendinous anti-adhesion | [71] | ||
| Antigen/antibody | Hybridization between one designed DNA strand containing the OTA aptamer and two complementary DNA strands grafting on linear polyacrylamide chains | Detection of the toxin Ochratoxin A | [72] | |
| N-isopropylacrylamide and N,N′-methylenebis (acrylamide) using redox initiators | Immunoassay | [73] |
- Abbreviations: MMP-2, matrix metalloproteinase-2; OTA, ochratoxin A; ROS, reactive oxygen species; TMZ, temozolomide.
3.2.1 Physical-responsive hydrogels
Thermoresponsive hydrogels
Thermoresponsive hydrogels are hydrogels formed through hydrophobic interactions. The reason for the sol–gel phase transition of hydrogels is that thermoresponsive hydrogels are composed of amphiphilic polymers, which have a hydrophilic part and a hydrophobic part.74 Most thermoresponsive hydrogels can form hydrogels at higher temperatures and can return to the liquid state at lower temperatures within a certain range. The phase transition process does not require the help of any other factors and is considered to be a benign phase transition process.75 Due to the simple preparation process and the lack of toxic chemical cross-linking agents, thermoresponsive hydrogels exhibit good biocompatibility. Meanwhile, thermoresponsive hydrogels also have the greatest advantage of being able to undergo phase transitions at physiological temperatures.76 Specifically, it can form a hydrogel at the temperature of the human body and is in a liquid state at a temperature lower than the human body. This is convenient for injection and can form a hydrogel in situ in the diseased part of the human body, which has a very wide range of applications in the field of biomedicine.77
Diabetes mellitus is a chronic disease of metabolic disorder characterized by hyperglycemia. In diabetic patients, the initial vascular damage and dysfunction lead to macrovascular and microvascular complications. To address the problem, Lee et al.48 developed a K9-C peptide-based thermosensitive hydrogel. The experimental results demonstrated that the k9-c-peptide hydrogel provided prolonged preventive effects on oxidative stress, inflammatory response, and cell apoptosis in the animal model. These results suggested that the K9-C peptide hydrogel was suitable for long-term administration in people with diabetic vascular dysfunction. The thermoresponsive hydrogel can be used as a barrier material against tendon adhesion, preventing the formation of tissue adhesion between the damaged tendon and its surrounding tissue. Chou et al.49 reported that a thermoresponsive hydrogel based on poly-n-isopropyl acrylamide can effectively prevent postoperative peritendon adhesions.
Conventional wound closure and dressing are two crucial, time-consuming but isolated principles in wound care. Yu et al.50 developed a series of adhesive thermal-contracting hydrogels integrating suitable mechanical properties via a dual physical crosslinking method of host–guest interaction and hydrogen bonding. The multifunctional hydrogels enabled the most rapid closure of incisions by the strong tissue adhesion and thermal contraction of PNIPAm polymer.
Light-/photoresponsive hydrogels
Light has been regarded as a promising trigger in recent years due to its advantages of noninvasiveness, high spatiotemporal resolution, and low pollution.78 Commonly used light sources include near-infrared, visible light, and ultraviolet. Light-responsive hydrogels have received extensive attention because they can convert optical signals into multiple functions.79 Light-responsive hydrogels change their properties mainly in three ways.80 The first is that the hydrogels undergo a phase transition-triggered response after absorbing photons of certain energy by grafting photosensitive groups, which is the most common photoresponse mechanism. The commonly used photoactive groups include azobenzene, diaryne, spiropyrans, photoreversible dimerization groups (e.g., coumarin, anthracene, and pyrimidine derivatives), and nitrobenzyl derivatives.81 The second light-responsive mechanism is that the hydrogels contain photoactive molecules, leading to reactions with the network structure of the hydrogels or swelling due to the changes in osmotic pressure.82 Third, hydrogels containing photosensitive compounds can change their properties of hydrogels in response to changes in the environment by absorbing photon energy.83 Yang et al.51 prepared a novel near-infrared light-responsive hydrogel for antibacterial and wound repair. The multifunctional dressing has good injectable, adaptive, and tissue adhesion. When illuminated by near-infrared light, the photothermal agent generates a large amount of heat and triggers the on-demand release of antibiotics at the wound site to kill bacteria. Therapies that break the imbalance of osteoblasts and osteoclasts are effective ways to promote bone regeneration and are key to the treatment of patients with osteoporosis. Here, Kuang et al.52 developed multifunctional in-situ generations of calcium phosphate nanoparticles-coordinated poly (dimethylaminoethyl methacrylate-co-2-hydroxyethyl methacrylate) ester hydrogel, the multifunctional hydrogel-loaded parathyroid hormone can be released in response to near-infrared light stimulation to promote bone regeneration. The experimental results exhibited that the hydrogel could simultaneously enhance the activity of osteoblasts and osteoclasts and successfully repair the calvarial defect.
Magnetic responsive hydrogels
Magnetic responsive hydrogels were designed and prepared by adding magnetic nanomaterials into the hydrogel, which have great potential in tissue repair due to their fast magnetic responses, precise spatiotemporal control, and noninvasive remote actuation.84 Magnetic nanomaterials generally refer to iron oxides (Fe3O4, γ-Fe2O3), transition metal ferrites (CoFe2O4, MnFe2O4, etc.), and transition metal alloys (FePt).85, 86 Among these magnetic nanomaterials, Fe3O4 nanoparticles were widely used due to their good biocompatibility, strong magnetization ability, relatively easy preparation, and functionalization.87 Under the effect of an external magnetic field, the prepared responsive hydrogels can be treated with various response modes such as motion, deformation, and heat generation without being limited by the depth of tissue penetration.88 Magnetic responsive hydrogels are composite materials whose properties depend on a variety of factors. Among them, the network structure of the hydrogels, the type, content, size, and distribution of the magnetic nanomaterials have a great influence on the physicochemical properties of the magnetic responsive hydrogel.89 Therefore, selecting suitable hydrogel components and magnetic nanomaterials is the key to designing magnetic responsive hydrogels with specific properties. Xu and Gu54 explored the synergistic effect of magnetic responsive scaffolds on in situ bone regeneration under the action of an external magnetic field. Kondaveeti et al.55 prepared magnetic responsive hydrogels with in situ magnetic nanoparticles, alginate, and xanthan gum. The results showed that the magnetic alginate-xanthan gum hydrogels have high charge density, molecular weight, and superior mechanical properties. The magnetic alginate-xanthan gum hydrogel could promote cell proliferation and specific differentiation under the stimulation of the external magnetic field, which provides a rational design scheme for the drug delivery of magnetic responsive hydrogels.
Pressure-responsive hydrogels
Pressure-responsive hydrogels could undergo structural phase transitions with changes in external pressure.46 They could sense the pressure exerted on them and can exhibit different response behaviors under different pressures.90 At present, pressure-responsive hydrogels have been widely used in biomedical fields.91, 92 The pressure-responsive hydrogels are manifested in hyperelastic, cellular, and nanofibrous hydrogels, such as those produced by composites of alginate and flexible SiO2 nanofibers, in the presence of large amounts of water. Although these hydrogels have uniformly distributed network structures, they tend to lose mechanical properties in high water–water environments.93-95 Zhu et al.56 prepared a semi-interpenetrating network hydrogel sensor via monomer polymerization process as a novel stretchable piezoresistive strain sensor. The hydrogel strain sensor with a high gauge factor showed excellent mechanical properties and recoverability, which was capable of monitoring very weak vibrations and human motions. Mechanical force-based stimulus provided a simple and easily accessible manner for controlled drug delivery. Di et al.96 described a wearable and tensile strain-triggered drug delivery device consisting of stretchable elastomer- and microgel-loading drug-loaded nanoparticles. The release of drug from the microdepot was promoted when a tensile strain was applied, this was due to the enlarged surface area for diffusion and Poisson's ratio-induced compression on the microdepots. Korin et al.57 developed a drug delivery system that could be activated to release drugs preferentially under abnormally high shear stress at sites of vascular stenosis. This design strategy was primarily based on the use of microscale aggregates of shear-sensitive nanoparticles that disintegrate into individual nanoscale components in regions of unusually high fluid shear stress that adhere to the surface of narrow blood vessels. The approach provided a potential new therapeutic approach for the treatment of hemodynamic diseases caused by pulmonary embolism, stroke, and atherosclerosis.57
Ultrasound-responsive hydrogels
As a new type of smart hydrogels, ultrasound-responsive hydrogels show increasingly powerful functions in biomedical applications due to their noninvasive, controllable, and safe properties.97, 98 Ultrasound-responsive hydrogels have been used for drug delivery, biomedical imaging, smart materials, and shape memory.58, 99 Zhou et al.97 summarized the research progress of ultrasound-responsive hydrogels in the biomedical field in detail in recent years and discussed the challenges and prospects they faced. Chen et al.59 prepared a battery-free vagal nerve stimulator based on programmable ultrasonic remote actuation and implantable high-performance hydrogel and successfully applied it in the anti-inflammatory treatment of sepsis. Using endotoxin-induced systemic inflammation as a model, ultrasound-responsive constant pressure pulse stimulation of vagal nerve stimulators significantly inhibited proinflammatory cytokines. The work provided a new strategy for developing implantable soft nanogenerators for radio stimulation of the nervous system.
3.2.2 Chemical-responsive hydrogels
pH-responsive-responsive hydrogels
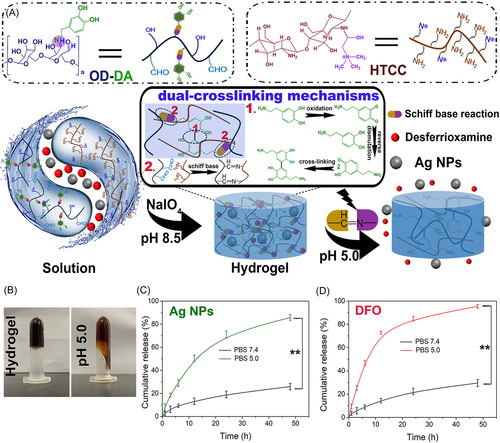
pH-responsive hydrogels can exhibit changes in physical and chemical properties within a specific pH range.100 The acidic groups in the polymer chain are deprotonated at a high pH value, while the basic groups are protonated at a low pH value. And the association and dissociation of various ions with the polymer chain lead to changes in the hydrogel structure.101 Because some chronic diseases such as tumors and wounds often exhibit a weakly acidic environment, in recent years, various pH-responsive hydrogels have been developed for the treatment of tumors and chronic wounds.60, 61 Based on the weakly acidic microenvironment of chronically infected wounds, Hu et al.62 prepared a pH-responsive injectable multifunctional hydrogel. At chronic wound sites, pH-responsive hydrogels rapidly released silver nanoparticles (Ag NPs) to effectively kill bacteria, while the drug deferoxamine (DFO) accelerated the remodeling of new blood vessels, thereby accelerating the repair of chronic skin wounds (Figure 2).
Glucose-responsive hydrogels
Glucose-responsive hydrogels can respond to different concentrations of glucose.63, 64 Glucose-responsive hydrogels are particularly suitable for the fabrication of blood glucose sensors and self-regulating drug delivery systems.65 At present, they are mainly divided into three categories, which are based on glucose oxidase (GOx), concanavalin A (Con A), and phenylboronic acid (PBA). Among them, GOx is an enzyme that catalyzes the oxidation of glucose, but its by-product H2O2 causes inflammation. Con A is a lectin with four glucose binding sites, however, it is somewhat cytotoxic. PBA is a synthetic molecule with high stability. Compared with GOx and Con A, PBA is more convenient and economical to use.102, 103 There has been no effective clinical treatment for diabetic chronic wounds at present. Yang et al.104 designed a glucose-responsive multifunctional hydrogel, which could modify the wound microbiome of hyperglycemia at chronic diabetic wound sites. On the contrary, it accelerated the release of zinc ions and DFO, showing synergistic antibacterial and proangiogenic functions. This innovative design strategy may be great potential for application in chronic wound therapy. Furthermore, Xu et al.105 designed a novel glucose-responsive hydrogel by modifying phenylboronic acid (PBA) onto a hyaluronic acid chain and then combining it with polyethylene glycol diacrylate. The multifunctional hydrogel could realize the controllable release of myricetin with antioxidant activity under glucose conditions and effectively scavenge reactive oxygen species.
ROS-responsive hydrogels
Reactive oxygen species (ROS) play an important role in regulating various life activities of the body; however, in areas of tissue damage, the increased concentration of ROS may further disrupt intracellular homeostasis, leading to a series of diseases.106 Therefore, using cellular products as triggers to target and modulate the structure and activity of polymers is a promising therapeutic approach. ROS-responsive hydrogels could change the structure in the state of highly ROS to control the release of drugs and reduce toxic side effects on normal cells.66 Currently, more and more data showed that many pathological conditions are associated with elevated levels of ROS. Therefore, ROS-responsive hydrogels have become an increasing research focus.107 Studies have shown that spinal cord injury is a pathological process associated with complex inflammation, such as excessive ROS leading to apoptosis of nerve cells and glial cells. Li et al.67 prepared a ROS-scavenging hydrogel containing thioketones to encapsulate bone marrow mesenchymal stem cells. By reducing ROS-mediated oxidative damage and downregulating the expression of inflammatory cytokines, the ROS-scavenging hydrogel improved the nerve regeneration of spinal cord tissue. ROS produced by wounds or bacterial infections is a key factor hindering wound healing. Using polyvinyl alcohol (PVA) as raw material, Zhao et al.68 prepared a hydrogel that can effectively scavenge ROS by cross-linking with a ROS-responsive linker. The hydrogel promoted wound healing by reducing ROS levels and upregulating M2 phenotype macrophages. Li et al.108 reported the design of a multifunctional ROS-responsive hydrogel coating tightly adhered to the Tβ4-loaded titanium implants to promote vascular reconstruction and bone formation by immunomodulation. Zheng et al.109 designed a ROS-responsive PAMB-G-TK/4-arm-PEG-SG hydrogel for localized drug-loaded liposome delivery to consume the overproduced pathological ROS for improved cardiomyocytes activity and enhanced myocardial infarction treatment.
3.2.3 Biological-responsive hydrogels
Enzyme-responsive hydrogels
Enzyme-responsive hydrogels are a new class of smart hydrogel materials. Under the selective catalysis of related enzymes, the structure of hydrogels will change.110, 111 In recent years, the use of enzymes as stimuli to trigger the response of hydrogels has been used in the field of biomedical engineering. Gliomas are the most aggressive primary malignant brain tumors. Temozolomide (TMZ) is a clinical drug for the treatment of glioma after surgery, but its therapeutic effect is not good. Zhao et al.69 prepared a matrix metalloproteinase (MMP) enzyme-responsive injectable drug-loaded hydrogel for the removal of residual drug-resistant gliomas after surgery. Drugs were released under the action of high concentrations of MMP enzymes after glioma surgery, significantly improving the efficiency of TMZ in inhibiting glioma growth. Wang et al.70 used the highly expressed elastase in tumor tissue as a trigger to prepare enzyme-responsive nanoaggregates by covalently modifying RGDC, RAADyC, and PEG chains on the outside of G4 PAMAM dendrimers. And enzyme-responsive nanogels were prepared by chemical cross-linking of peripheral functional groups initiated by an oxidation reaction. In the presence of elastase, the nanogels were broken down resulting in sustained drug release. The dendrimer-based nanogels may be a potential drug delivery nanocarrier for tumor therapy. Cai et al.71 crosslinked the allyl glycidyl ether grafted carboxymethyl chitosan with the substrate peptide CPLGLAGC to prepare the MMP-2 degradable hydrogels. The siRNA complex loaded with TGF-β1 was then used to inhibit the proliferation of fibroblasts. In the microenvironment of MMP-2 overexpression in vivo, TGF-β1 siRNA was released from hydrogels for peritendinous anti-adhesion.
Antigen/antibody-responsive hydrogels
Antigen/antibody-responsive hydrogels mainly rely on the specific binding reaction between the antigen and the antibody.112 Antigen/antibody-responsive hydrogels can be prepared by incorporating antigens into hydrogels or by chemically conjugating antigens or copolymerizing their binding fragments with hydrogels.113 Yang et al. designed and synthesized an aptamer-crosslinked hydrogel for the visual and quantitative detection of ochratoxin A (OTA). Hydrogels were formed by hybridization of DNA strands containing OTA aptamers with polyacrylamide grafted complementary DNA strands. Combining responsive hydrogels with a portable enrichment technique provided a new way of detecting the toxin OTA.72 Choi et al.114 proposed a label-free specific detection technology for immunoglobulin G antibodies, which is simple in-process and low in cost. The three-dimensional hydrogel structure showed the high immobilization ability of biomolecules and the advantages of high signal-to-noise ratio and sensitivity, which could interact with target ligands with the greatest probability. In addition, antigen-responsive hydrogels can also be used as antigen-sensing devices115 or as drug delivery vehicles.73
4 SMART-RESPONSIVE HYDROGELS EMPLOYED FOR DIFFERENT TISSUE REPAIRING APPLICATIONS
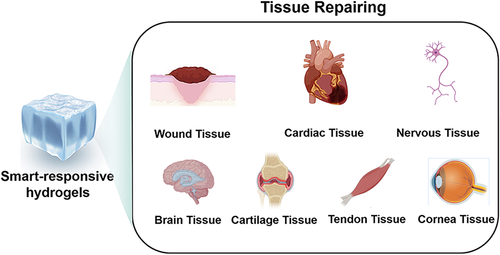
In recent years, the application of smart-responsive hydrogels in the field of tissue repair has attracted extensive attention. It has been widely used in wound repair, damaged myocardial tissue repair, nerve tissue repair, brain tissue repair, cartilage tissue repair, tendon tissue repair, and corneal tissue repair (Figure 3).
4.1 Application of smart-responsive hydrogels in chronic wound tissue repair
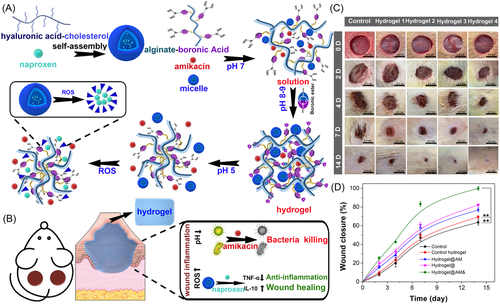
Smart-responsive hydrogels have very broad prospects in the fields of biomedical engineering and tissue repair. The complex process of chronic wound repair and four sequential yet intersecting stages lead to potentially different requirements for active substances during different stages of healing.116 Over the past few decades, gauze, films, foams, sprays, and hydrogels have been used to treat chronic wounds. These dressings are easy to absorb blood and exudate secreted by the wound and have certain antibacterial and anti-inflammatory effects.117 However, traditional products cannot meet the needs of different wound healing stages, resulting in a chronic wound healing rate of less than 30% and a high recurrence rate.118 Smart-responsive hydrogels respond rapidly to changes in pH, temperature, enzymes, or external light at the wound by utilizing smart materials. They can allow precise control of drug release in chronic wounds and the ability to adapt to the wound changes in the microenvironment and adjust the drug release curve in time to meet the needs of antibacterial, anti-inflammatory, pro-angiogenesis, and other therapeutic substances in different wound healing stages.119 Therefore, the development of smart-responsive hydrogels and their application in chronic wound repair has been a hot topic in recent years. Next, the review will focus on the application of smart-responsive hydrogels developed by our research group in the treatment of chronic wounds. In our previous work, Hu et al.120 successfully designed and prepared a smart injectable hydrogel with an inflammatory response function for the treatment of chronically infected wounds. Experimental results in vitro exhibited that the inflammatory response hydrogel had good biocompatibility and rheological properties and the results in vivo showed that it has a good effect on promoting wound healing (Figure 4). In another research work, an inflammatory response drug-loaded hydrogel with programmed hemostatic, antibacterial and anti-inflammatory properties was successfully prepared to reduce wound bleeding and bacterial resistance caused by antibiotic use. The results in vitro and in vivo showed the hydrogel has good hemostatic, bactericidal, and anti-inflammatory properties, and finally accelerated the repair of chronic diabetic wounds.121 An important reason for preventing the repair of chronic wounds in diabetes is that the formation of new blood vessels is difficult. Therefore, Wu et al.122 prepared double cross-linking spatiotemporal delivery of hydrogels using caffeic acid-modified ε-polylysine and 3-aminophenyl boronic acid-modified oxidized dextran. The rapid response release of the anti-inflammatory drug diclofenac sodium and the sustained release of mangiferin (promoting angiogenesis) enable the hydrogel to have the functions of anti-inflammatory first and then promoting vascular reconstruction, thereby accelerating the repair of chronic diabetic wounds. Inspired by the excellent effect of the recombinant humanized collagen type III (rhCol III) in promoting endothelial cell proliferation, Wang et al. designed a programmed delivery multifunctional hydrogel loaded with silver nanoparticles and rhCol III, and explored its effect on wound repair of chronic infection. The results of in vitro and in vivo showed that the silver ions released rapidly in the early stage can effectively remove bacteria and the sustained release of rhCol III in the later stage could effectively promote the migration of endothelial cells and the reconstruction of new blood vessels, thereby accelerating the repair of chronically infected wounds in diabetes.123-125
4.2 Application of smart-responsive hydrogels in the repair of damaged myocardial tissue
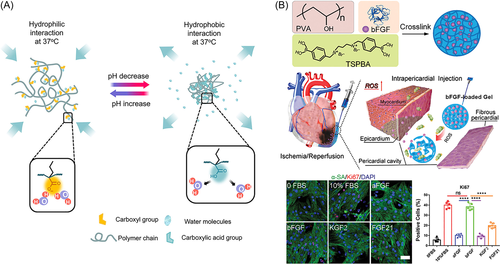
Cardiovascular disease is the leading cause of human death worldwide. Persistent ischemia-induced damage to part of the myocardium caused by coronary occlusion is a major factor in exacerbating myocardial infarction (MI) and leading to death in patients with heart failure (HF).126 Limited treatment options and poor prognosis result in high mortality and readmission rates with HF, with a 5-year survival rate of only 50%.127 According to statistics, the number of people with HF worldwide has risen to 64.3 million in 2017.128 After MI, a large number of cardiomyocytes die and cannot re-enter the cell cycle and proliferate, which makes the self-repair of the damaged heart extremely difficult and limits the development of treatment strategies for HF. At present, the main methods of clinical treatment of HF include drugs, medical device implantation, and heart transplantation. Among them, drug and medical device implantation can only relieve the clinical symptoms of patients to a certain extent, but cannot inhibit the pathological remodeling process after MI. Heart transplantation is the only life-saving straw for patients with end-stage HF. However, due to the current scarcity of cardiac donors and immune complications, these patients eventually face death.129 In recent years, the exploration of novel and promising HF treatment strategies has become a research hotspot. Injectable hydrogel as a minimally invasive technique overcomes the clinical and surgical limitations of traditional stent implantation. It can not only provide continuous mechanical support for the weakened ventricular wall but also serve as a delivery vehicle to locally deliver various drugs. Injectable hydrogel is widely used in the repair and treatment of damaged hearts and the safety and effectiveness of this technology in improving cardiac function after MI have been confirmed through a large number of experimental models and clinical trial studies.18, 130 However, due to the single structure and function of existing hydrogel, they cannot effectively cope with the complex microenvironment of the MI area and cannot meet the different needs of multistage repair of damaged myocardium, which often leads to poor therapeutic effect.131 In addition, the mechanism of hydrogels in various stages of damaged myocardial repair remains to be further studied, which has crucial practical significance and clinical application value for the development of injectable hydrogels for the treatment of MI.132 The repair of damaged myocardial tissue is a chronic, spontaneous, and multistage progressive process that can be divided into three distinct but overlapping stages: inflammatory, proliferative, and remodeling.133, 134 Li et al.135 developed a pH-responsive and temperature-responsive hydrogel. At 37°C, when the pH of the solution was adjusted to 8.0, the hydrogel could be injected through the catheter and formed a solid hydrogel in an acidic (pH 6.5) environment similar to infarcted heart tissue. In addition to cardiac applications, hydrogels can also be used to deliver drugs or cells into other inflamed soft tissues, which typically have a pH of 6–7 (Figure 5A). Blood reperfusion of ischemic tissue leads to excessive production of ROS. Li et al.136 synthesized ROS-sensitive polyvinyl alcohol (PVA) hydrogel for delivering basic fibroblast growth factor (bFGF) for the treatment of MI. bFGF released in response to ROS can protect cardiac function, reduce cardiac fibrosis, and enhance angiogenesis (Figure 5B).136 Under pathophysiological conditions after MI, persistent overexpression of MMPs leads to adaptive changes in tissue structure and function. Wei et al.137 used matrix metalloproteinase-sensitive peptide (MMP-SP) to construct a matrix metalloproteinase-responsive hydrogel to restore cardiac function after MI by stabilizing hypoxia-inducible factor-1α (HIF-1α).
4.3 Application of smart-responsive hydrogels in nervous tissue repair
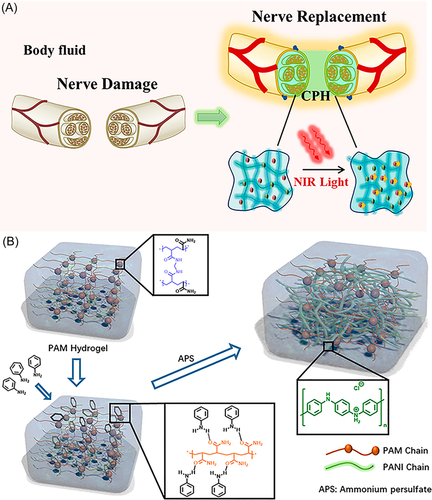
Brain-related diseases mainly include traumatic brain injury and stroke and stroke is further divided into ischemic stroke and hemorrhagic stroke.138 Traumatic brain injury is a neurological injury and a leading cause of death and disability worldwide. There is often damage to brain structure and function caused by external forces, which in turn affects the cognitive behavior of the body.139 The incidence of ischemic stroke is slightly higher than that of hemorrhagic stroke, accounting for about 60%–70% of the total population.140 There is currently no effective clinical treatment for traumatic brain injury and stroke. The repair and rehabilitation of brain tissue damage is a long-standing and challenging problem. The presence of the blood–brain barrier results in poor permeability of therapeutic molecules such as drugs or proteins, ultimately resulting in limited therapeutic efficacy.141, 142 To address the limitations of current drug delivery, hydrogels hold superior promise for in situ treatment of brain tissue-damaging diseases.143 Considering the microenvironmental characteristics of brain tissue injury sites (such as acidic pH, platelet activation, ROS concentration, and enzyme concentration), some smart-responsive hydrogel-based strategies have been used for brain injury tissue repair.143, 144 In addition to stimuli from internal factors, external stimuli such as magnetic fields, electric fields, ultrasound, and light are also possible.129 Dong et al.145 developed a photoresponsive conducting polymer hydrogel. Under the irradiation of near-infrared light, the electrical conductivity of the conductive hydrogel enhanced and facilitated the conduction of bioelectrical signals. Furthermore, when the conductive hydrogel was mechanically elongated, it still exhibited high conduction durability which could accommodate the strain of neural tissue during exercise (Figure 6). Ghuman et al.146 used an acellular extracellular matrix (ECM) to prepare thermosensitive hydrogels, which could form hydrogels in situ at the stroke site. Different concentrations of ECM (0, 1, 2, 3, 4, and 8 mg/ml) were injected 14 days after stroke, the experimental results showed that 8 mg/ml ECM had the effect of significantly promoting endogenous repair and could be used for stroke treatment.
4.4 Application of smart-responsive hydrogels in other tissue repairs
With the deepening of related research, various hydrogels have been continuously developed for the repair of cartilage, tendon, cornea, meniscus and other tissues.147-149 Thermosensitive hydrogels have become a research hotspot for cartilage tissue engineering scaffolds due to their unique physical properties similar to natural ECM.150 Zhou et al.151 prepared a TGF-β1-loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) thermosensitive hydrogel. The results exhibited that the hydrogel had good heat sensitivity, injectability, and biodegradability. And the results indicated that thermosensitive hydrogel was capable of cartilage repair in vivo. Corneal inflammation and damage are major causes of corneal scarring and loss of transparency. Therefore, Lin et al.152 synthesized thermosensitive hydrogels with different ratios of hyaluronic acid and Pluronic F-127, which are liquid at low temperatures and hydrogel formation at human body temperature. The results showed that the hydrogels containing platelet-rich plasma exhibited higher cell viability and cell migration, reduced apoptosis, and rebuilt the corneal endothelium. Silva et al.153 reported a platelet lysate-loaded photocrosslinked magnetic responsive hydrogel made of chondroitin methacrylate sulfate. Iron-based magnetic nanoparticles were added to the hydrogel to impart the magnetic responsiveness of the hydrogel. The biological responsiveness of different types of cells suggested that magnetic systems are best suited as cell carriers. In addition, the related data results suggest that the magnetic responsive hydrogel may produce gradient organization. The site of intervertebral disc degeneration has a local inflammatory response. In response to this feature, Wang et al.154 designed an injectable inflammatory-responsive hydrogel for the responsive delivery of anti-inflammatory drugs curcumin and cholesterol-modified miRNA-21 inhibitors (Antagomir-21) for the treatment of intervertebral disc degeneration. The related data results demonstrated that the inflammatory-responsive hydrogels had good biocompatibility and the potential to promote intervertebral disc regeneration.154
5 CONCLUSION AND FUTURE PERCEPTIONS
In the past few decades, a lot of research on biomedical materials for tissue repair has been carried out and significant progress has been made. Among them, hydrogels have been widely used in the field of tissue repair. With the deepening of research, it has been found that the complexity of the microenvironment of tissue disease sites makes conventional hydrogels often have poor therapeutic effects. Therefore, hydrogels that can respond to physical, chemical, and biological factors have received extensive attention. In addition to the advantages of conventional hydrogels, smart-responsive hydrogels can also release functional molecules in an on-demand and controllable manner, which greatly improves the therapeutic effect of diseases. The review details the classification of smart-responsive hydrogels in recent years and their applications in different types of tissue repair. In the future, developing smart-responsive hydrogel platforms targeting tissue disease microenvironments is a potential area of research. In addition, it is also a good option to develop a programmatic delivery hydrogel platform that can respond to both the external environment and the disease microenvironment. Although significant progress and diversification have been made in the preparation of smart-responsive hydrogels, their biodegradability, responsiveness, microstructure, inflammation, and immune response are still important challenges in the preparation process. They will severely limit the application field expansion and clinical medical translation of smart-responsive hydrogels. Therefore, more attention should be paid to the preparation of smart-responsive hydrogels in the future, including their minimal immune response and optimal biosafety. Different solvents used in the preparation of smart-responsive hydrogels will also pose additional toxicity risks. Therefore, the use of natural polymers and cross-linking agents with good water solubility is also an important topic for further research in the future.
When smart-responsive hydrogels are used as delivery vehicles, the regulation of the release rate of therapeutic molecules such as drugs and proteins in smart-responsive hydrogels is crucial. Therefore, future research should emphasize the design and preparation of responsive hydrogels with precise responsive release properties. In general, although the synthesis of smart-responsive hydrogels, the regulation of physicochemical properties, and the regulation of the release rate of drugs and protein molecules are still issues to be further studied. However, when scientists overcome the above problems one by one in the foreseeable future, the structure and function of smart-responsive hydrogels will be continuously optimized. And tailored smart-hydrogel-based tissue repair strategies can be designed for different types of diseases. We believe that smart-responsive hydrogels can be applied in a wider range of fields including but not limited to tissue repair in the future. It is foreseeable that with the emergence of personalized precision medical technology, smart-responsive hydrogels will also receive more application prospects and attention in clinical practice.
AUTHOR CONTRIBUTIONS
Cheng Hu: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Project administration (lead); Resources (lead); Software (lead); Supervision (lead); Validation (lead); Visualization (lead); Writing – original draft (lead); Writing – review & editing (lead). Li Yang: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Software (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing – original draft (supporting); Writing – review & editing (supporting). Yunbing Wang: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Software (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing – original draft (supporting); Writing – review & editing (lead). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Juan Cao for helping them to smooth the manuscript. Figure 1 and Figure 3 were drawn with BioRender software, biorender.com. This study was supported by the funding listed as follows: The Fundamental Research Funds for the Central Universities (No. YJ2021115).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




