Enhancing CO2 Transport Across the PEG/PPG-Based Crosslinked Rubbery Polymer Membranes with a Sterically Bulky Carbazole-Based ROMP Comonomer
Abstract
A series of poly(ethylene glycol)-block-poly(propylene glycol) (PEG/PPG)- and 5,6-di(9H-carbazol-9-yl)isoindoline-1,3-dione (2CZPImide)-based crosslinked rubbery polymer membranes, denoted as PEG/PPG-2CZPImide (x:y), are prepared from the norbornene-functionalized PEG/PPG oligomer (NB-PEG/PPG-NB) and 2-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-5,6-di(9H-carbazol-9-yl)isoindoline-1,3-dione (2CZPImide-NB) via ring-opening metathesis polymerization (ROMP). The molar ratio (x:y) of the NB-PEG/PPG-NB (x) to 2CZPImide-NB (y) monomers is varied from 10:1 to 6:1. X-ray diffraction (XRD), scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS), and pure gas permeability studies reveal that the comonomer 2CZPImide-NB successfully increases the d-spacing among the crystalline PEG/PPG segments, hence enhancing the diffusivity of gases through the membranes. The synthesized membranes exhibit good CO2 separation performance, with CO2 permeabilities ranging from 311.1 to 418.1 Barrer and CO2/N2 and CO2/CH4 selectivities of 39.4–52.0 and 13.4–16.0, respectively, approaching the 2008 Robeson upper bound. Moreover, PEG/PPG-2CZPImide (6:1), displaying optimal CO2 permeability and CO2/N2 and CO2/CH4 selectivities, shows long-term stability against physical aging and plasticization resistance up to 20 days and 10 atm, respectively.
1 Introduction
Significant attention has been directed toward the conceptualization, advancement, and utilization of membrane processes for gas separation. This can mainly be attributed to the inherent advantages of membranes, such as their relative simplicity, low costs,[1] good processability,[2-5] eco-friendliness, and easy scale-up, compared to other gas separation technologies.[6, 7] However, the prevailing limitation of membrane-based gas separation is the well-known “trade-off” between permeability and selectivity for a specific gas,[8] indicated in the Robeson plot. Nevertheless, this limitation has been overcome via the careful selection of membrane materials; this can be achieved by, for example, fine-tuning the polymer structure, incorporating porous materials, using molecules that can interact with a specific gas, etc.
In the case of polymer membrane-based CO2 separation, in particular, several advanced materials with specifically designed polymeric structures,[9] metal–organic frameworks,[10, 11] covalent organic frameworks,[12] ionic liquids,[13] and organic–inorganic hybrid structures[14-16] have been employed to boost CO2 solubility and/or CO2 diffusivity across the membrane. Such materials result in membranes with high CO2 permeability and/or selectivity that surpass Robeson's upper bound.
Poly(ethylene glycol) (PEG) and its derivatives, possessing ethylene oxide units, are another class of polymers that have been widely used for CO2 separation due to their high CO2-philicity.[17-19] The polar ether groups of PEG exhibit a strong quadruple–quadruple interaction with CO2 and, hence, have high CO2 solubility. This then contributes to enhanced selectivity toward CO2 over other gases. The highly crystalline nature of the ethylene oxide segments, particularly in high-molecular-weight PEG, however, can lead to a reduction in CO2 permeability.[20, 21]
On the other hand, the low-molecular-weight poly(ethylene glycol)-block-poly(propylene glycol) copolymer (PEG/PPG), possessing ─CH3 side chains in the PPG segment, can impede the dense chain packing of the PEG segment, reducing the crystallinity of PEG/PPG and, thereby, expanding its utility in gas separation.[22] Nevertheless, the copolymer still exhibits insufficient mechanical properties to form a self-standing membrane for use in gas separation applications.
Meanwhile, crosslinking has significantly impacted membrane-based gas separation and led to the production of polymer membranes with desirable properties.[23] Crosslinking polymer chains enhances the mechanical, thermal, and physicochemical properties of the corresponding polymer membranes, which then become suitable for gas separation applications, even under harsh conditions (e.g., at elevated temperatures or pressures).[24] In addition, crosslinking provides resistance against plasticization and aging, which are some of the main drawbacks of highly permeable polymer membranes with large free volumes and, hence, high diffusivity.[25, 26]
By crosslinking PEG/PPG, the structural freedom of the PEG segment is suppressed, thereby reducing its crystallinity and allowing for a high level of amorphous PEG that can easily transport gas.[27] Crosslinking can also compensate for the poor mechanical properties of low-molecular-weight PEG/PPG.[28]
Our group has recently developed novel crosslinked polymer membranes by polymerizing two rubbery comonomers: PEG/PPG, serving as a highly CO2-soluble unit, and polydimethylsiloxane (PDMS), a highly CO2-permeable unit due to its high gas diffusivity. Both PEG/PPG and PDMS were functionalized with bisnorbornene at the terminus to enable ring-opening metathesis polymerization (ROMP) for in-situ polymerization and crosslinking. The resultant crosslinked PEG/PPG–PDMS-based polymer membranes exhibited favorable thermomechanical properties applicable to gas separation; moderate permeability, up to 561 Barrer; and significantly high CO2 selectivity, up to 59.[28] Additional research was conducted to further improve the CO2 diffusivity – and, hence, the CO2 permeability – by introducing an additional bulky adamantane group into the crosslinked polymer structure; the resultant PEG/PPG–PDMS–adamantane membranes displayed further increases in CO2 permeability while maintaining high CO2 selectivity.[29]
Meanwhile, carbazole-based organic polymer membranes have recently been used for CO2 separation due to unique properties such as structural rigidity, leading to porosity, a high specific surface area, and a high gas uptake capacity.[30, 31] Additionally, the nitrogen atom in the carbazole moiety can increase the affinity of the polymer membranes for CO2, hence increasing their selectivity toward CO2 compared to N2 or CH4.[32] In particular, biscarbazole-substituted phthalimide (2CZPImide)-based polymer membranes, with two bulky carbazole moieties located at ortho positions and twisted ≈60° relative to the imide plane in the phthalimide, have exhibited ultra-micropores able to selectively absorb CO2 gas molecules over N2.[33]
Based on these previous findings, we herein developed crosslinked PEG/PPG and biscarbazole-based polymer membranes for CO2 separation. Combining the bulky and twisted structure of the biscarbazole unit with the highly CO2-soluble PEG/PPG unit, these crosslinked bimolecular polymer membranes are expected to provide high CO2 permeability and selectivity, even in the absence of PDMS, a highly permeable elastic polymer. The two monomers, PEG-PPG-substituted bis-norbornene (NB-PEG/PPG-NB) and bicarbazole-substituted norbornene (2CZPImide-NB), were polymerized and crosslinked in situ via ROMP to produce the PEG/PPG–bicarbazole-based membranes, 1. Unlike our previous studies, the bulky bicarbazole group was incorporated into mono-norbornene, not bis-norbornene, to reduce the crosslinking density of the resultant polymer membranes and, consequently, enhance the diffusivity of CO2 molecules. Only the PEG-PPG-substituted bis-norbornene (NB-PEG/PPG-NB) was, therefore, designed to form a crosslinked structure.
The ratio between the two comonomers was controlled by varying the feed ratio of 2CZPImide-NB to NB-PEG/PPG-NB. The physicochemical and morphological properties of the corresponding polymer membranes, together with their gas separation characteristics, were then thoroughly investigated.
2 Results and Discussion
2.1 Preparation of the Crosslinked Copolymer Membranes (PEG/PPG:2CZPImide) (x:y) 1
An in-situ membrane casting followed by a polymerization and crosslinking technique was employed to synthesis all the crosslinked copolymer membranes 1 with varying compositions of NB-PEG/PPG-NB 2 and NB-2CZPImide 3 monomers (Scheme 1).
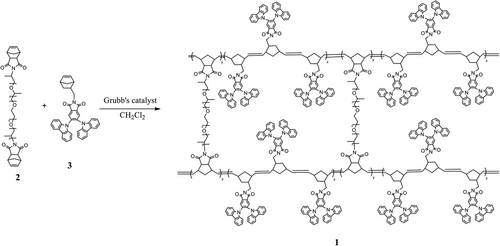
The required monomers 2 and 3 were synthesized as follows. The PEG/PPG-functionalized dinorbornene (NB-PEG/PPG-NB, 2) was prepared by the reaction of α,ω-diaminofunctionalilzed polypropyleneglycol-b-polyethyleneglycol-b-polypropyleneglycol monomer (NH2-PEG/PPG-NH2) with norbornene dicarboxylic anhydride in acetic acid as a catalyst (Scheme S1, Supporting Information).
The NH2-PEG/PPG-NH2 monomer with a molecular weight of 1900 g mol−1 was selected. The membranes prepared from lower molecular weight PEG/PPG would give poor CO2 solubility, whereas membranes with poor mechanical properties would be expected from membranes from higher molecular weight PEG/PPG.[28]
For the synthesis of norbornene-functionalized 2CZPImide monomer 3, sequential reactions were performed (Scheme S2, Supporting Information) by reacting carbazole with 4,5-dichlorophthalonitrile to produce 4,5-di(9H-carbazole-9-yl)phthalonitrile (2CzPN) a, which then undergoes hydrolysis to produce the corresponding phthalic acid (2CZPA) b, which in turn was converted to the corresponding anhydride 2CZPAnhydride c on heating with acetic anhydride. Finally, the anhydride c reacts with 5-norbornene-2-methyl amine to produce the desired NB-2CZPImide 3 with exo- and endoisomer forms.
The synthesis of each compound was confirmed by comparative spectroscopic methods using 1H NMR and ATR-FTIR spectra. Figure S1 (Supporting Information) compares the NMR spectra of NB, NH2-PEG/PPG-NH2, and NB-PEG/PPG-NB monomers. All the peaks corresponding to the NB monomers were shielded, and the peaks corresponding to the NH2-PEG/PPG-NH2 were deshielded in the NMR spectrum of NB-PEG/PPG-NB. Furthermore, individual changes to peaks suggested the formation of an imide bond [CH─NC(CO)2─] coinciding with the loss of amine bonds.
Figure S2 (Supporting Information) compares the FTIR spectra of NB, NH2-PEG/PPG-NH2, and NB-PEG/PPG-NB monomers. The anhydride functional group of NB transformed to imide C═O asymmetric and imide C═O symmetric stretching in the product NB-PEG/PPG-NB, indicating the conversion of the amide into an imide functionality. Moreover, a new peak in NB-PEG/PPG-NB further supports the formation of an amide bond in the structure.
The conversion from 2CZPN to 2CZPA was confirmed in 1H NMR spectroscopy by the shielding of aromatic proton Ha of 2CZPN (Figure S3a, Supporting Information) in 2CZPA (Figure S3b, Supporting Information). In the ATR-FTIR spectra (Figure S4, Supporting Information), the ─CN functionality in 2CZPN disappeared during conversion into 2CZPA. Additionally, a broad peak in the OH stretching region and two characteristic peaks of symmetrical and asymmetrical stretching of ─C═O bonds appeared in the FTIR spectrum of 2CZPA, confirming the presence of two adjacent -COOH functional groups in 2CZPA.
In a similar way, the conversion from 2CZPA to 2CZPAnhydride was confirmed by a deshielding of aromatic proton Ha of 2CZPA (Figure S3b, Supporting Information) in 2CZPAnhydride (Figure S3c, Supporting Information). In the ATR-FTIR spectrum of 2CZPAnhydride (Figure S4, Supporting Information), the broad peak in the region 2502 –3361 cm−1 disappeared. Moreover, during the conversion from 2CZPA to 2CZPAnhydride, the symmetric and asymmetric ─C═O stretching vibrations shifted, indicating the conversion of the ─COOH functionality to anhydride functionality.
Figure S5a,b (Supporting Information) presents the 1H NMR of 5-norbornene-2-methyl amine and NB-2CZPImide, respectively. These data indicated the conversion of the ─NH2 functional group in 5-norbornene-2-methyl amine to the imide group in NB-2CZPImide.
In the ATR-FTIR spectra of the norbornene-functionalized monomer NB-2CZPImide (Figure S4, Supporting Information), peaks corresponding to the symmetric and asymmetric stretching vibrations of C═O bonds in the imide functionality, the C(sp3) ─N stretching vibration of the imide functional group, C(sp2)-N vibrations of the carbazole moiety, and various aliphatic and aromatic C─H bonds were observed.[28, 34] This indicates the successful synthesis of NB-2CZPImide.
An in-situ membrane casting followed by polymerization and crosslinking was carried out to get the crosslinked membrane 1 from the designed monomers 2 and 3 (Scheme 1). Monomers were subjected to a controlled ROMP polymerization technique using 2nd generation Grubb's catalyst to obtain a sufficiently viscous casting solution. A slow evaporation of the casting solution over polypropylene petridish turned out into self-standing, homogeneous, and flexible membranes of variable thickness (75–100 µm).
The resulting membranes had different molar ratios of the constituent monomers of NB-PEG/PPG-NB and NB-2CZPImide and were denoted as PEG/PPG-2CZPImide (x:y), where the numerical values of x and y represent the molar ratio of NB-PEG/PPG-NB and NB-2CZPImide monomers in the feed. Figure 1 presents photographs of these membranes.

All the crosslinked PEG/PPG-2CZPImide (10:1), PEG/PPG-2CZPImide (8:1) and PEG/PPG-2CZPImide (6:1) membranes prepared were insoluble in all the commonly used solvents tested, including dichloromethane, tetrahydrofuran, chloroform, dimethylsulphoxide, dimethylformamide, dimethylacetamide, etc, suggesting that they were crosslinked.
The structure of the crosslinked PEG/PPG-2CZPImide (10:1), PEG/PPG-2CZPImide (8:1), and PEG/PPG-2CZPImide (6:1) membranes were confirmed by the comparative spectroscopic methods using FTIR. Figure S6 (Supporting Information) represents the IR spectra of monomers (norbornene-functionalized PEG/PPG and norbornene-functionalized 2CZPImide) and all the synthesized crosslinked polymers. With the increase of mol% of 2CZPImide in NB-PEG/PPG-NB, in the final crosslinked polymer, the peaks at 754 and 724 cm−1 corresponding to the aromatic C─H bending vibration of the carbazole moiety increased in intensity while the intensity of all the other peaks corresponds for NB-PEG/PPG-NB monomer moiety remained identical. The peaks at 788 and 722 cm−1 correspond to the 1,2-disubstituted C─H bond (cis) bending vibration, and the peak at 642 cm−1 corresponds to the 1,2-disubstituted C═C bond (cis) bending in the norbornene moiety of NB-PEG/NB monomer. These peaks disappeared in the final polymers, which provides evidence for the successful polymerization of the monomers during the ROMP polymerization reaction.
The gel fraction, an indirect index of the crosslinking density for the crosslinked polymers, was measured. It was found that all three PEG/PPG-2CZPImide (10:1), PEG/PPG-2CZPImide (8:1), and PEG/PPG-2CZPImide (6:1) membranes with different 2CZPImide contents showed a very high gel fraction over 97%, strongly indicating a high degree of crosslinking (Table 1).
| Membrane Code | Feed ratio | Density [gm mL−1] | Gel fraction | Tg [°C] | Tm [°C] | Tmax [°C] | ΔHm Jg−1 | Jg−1 | XC [%] | 2θ [°] | d-spacing [Å] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEG/PPG-2CZPImide (6:1) | 6:1 | 1.13389 | 97.45 | −50.96 | 25.29 | 413.25 | 56.9221 | 166.4 | 34.21 | 20.209 | 4.39 |
| PEG/PPG-2CZPImide (8:1) | 8:1 | 1.14222 | 97.81 | −40.67 | 26.80 | 413.85 | 41.9690 | 25.22 | 20.279 | 4.37 | |
| PEG/PPG-2CZPImide (10:1) | 10:1 | 1.14273 | 98.01 | −47.35 | 17.95 | 405.46 | 51.4177 | 30.90 | 20.334 | 4.36 |
Next, the density of the crosslinked polymers was measured, and it was found that the density decreased with the increase of 2CZPImide contents, suggesting that the introduction of the bulky 2CZPIimide has increased the free volume of the crosslinked membranes (Table 1).
Unlike NB-PEG/PPG-NB, the NB-2CZPImide only one norbornene unit, hence cannot form a crosslinked structure but is rather randomly distributed in the crosslinked PEG/PPG unit. This creates an incomplete crosslinked polymer network, with randomly distributed pores throughout the polymer matrix. A reduced density for the resultant crosslinked membrane is hence expected, which in turn helps gas molecules move throughout the polymer membrane more easily.
2.2 Thermal Properties of the Crosslinked PEG/PPG-2CZPImide (x:y) 1 Membrane
A thermogravimetric analysis (TGA) was carried out to investigate the thermal properties of the three crosslinked PEG/PPG-2CZPImide (x:y) membranes having different 2CZPImide contents.
The TGA and differential scanning calorimetry (DSC) curves of the crosslinked polymers and their respective monomers are represented in Figure 2 respectively. The TGA curves show two major weight losses (Figure 2a). The first weight loss below 100 °C corresponds to the loss or evaporation of water that is absorbed in the membrane as a consequence of the presence of polar and hydrophilic ─CH2─O─ unit. In the second step, the maximum weight loss (Tmax) corresponds to the scission of the backbone chain of the crosslinked polymer observed in between the temperature range 405.46–413.85 °C with an onset decomposition temperature ranging between 381.0–393.22 °C. These results demonstrated that our newly synthesized membranes have high thermal stabilities.
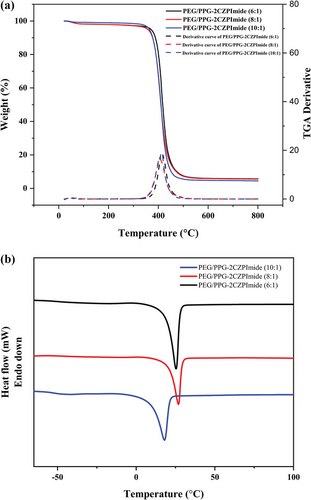
At high temperatures beyond 450 °C, all the bonds among the constituent elements in the membrane were broken, and residue formed. The amount of residue at the end of the measurement at 800 °C was shown to increase gradually with the increase in the 2CZPImide monomer content in the final feed during membrane synthesis.
The three crosslinked polymer membranes were further analyzed for their glass transition temperature (Tg) and melting temperature (Tm) using DSC within a temperature range from −80 to 150 °C (Figure 2b). DSC measurement revealed a single glass transition temperature (Tg) with a single melting temperature (Tm) for each polymer membrane. The Tg was found to be in the range of −50.96–−47.35 °C with the Tm value from 17.95–25.29 °C (Table 1). Having a Tg much lower than room temperature indicates that all membranes still maintain their rubbery properties even after the cross-linked network structure is formed through ROMP polymerization. It was also found that the increment of 2CZPImide (decrement of molar ratio of NB-PEG/PPG-NB to NB-2CZPImide) content from 10:1 to 8:1 in the crosslinked polymers leads to increase both the Tg and Tm from −47.35 to −40.67 °C and from 17.95 to 26.80 °C, respectively. This is ascribed to the fact that the sterically bulky 2CZPImide unit may partially hinder the free rotation of the PEG/PPG chains, thereby orienting the crosslinked polymers into a somewhat regular geometric structure, which in turn results in an increase in stiffness among the polymer chains as well as increment in both the glass transition temperature and the melting temperature of the polymer membranes. A further increase of 2CZPImide from 8:1 to 6:1 relative to NB-PEG-NB, however, lowered both the glass transition temperature and melting temperature. The 2CZPImide is a bulky molecule that does not contribute to cross-linking. When introduced in excess of a certain amount, it is thought to form voids between the network structures of the PEG/PPG chains, allowing the PEG/PPG chains to have more mobility. This is reflected in the decrease in both the glass transition temperature and melting temperature of the 6:1 membrane compared to the 8:1 membrane.
The latent heat of melting (∆Hm) for each crosslinked copolymer was estimated by calculating the area under the melting peak obtained from the second heating curves in DSC analysis. The percentages of crystallinity (Xc) of all the crosslinked copolymer membranes were calculated (S2) and were found in between 25.22% and 34.21% (Table 1). Around 65.79% to 74.78% of the crosslinked polymers were composed of the amorphous polymer moiety.
2.3 Morphological Analysis by XRD and SEM
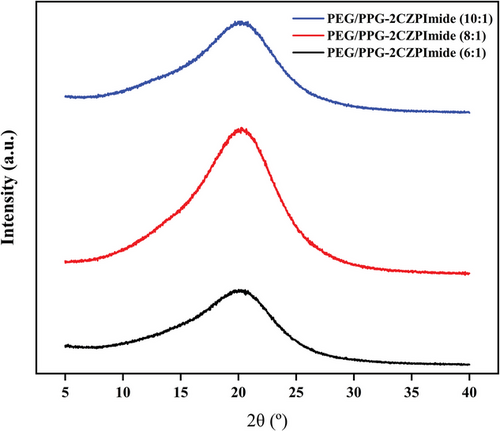
Wide-angle X-ray diffraction (WAXS) measurement was performed on three crosslinked membranes to obtain information on how the spacing between polymer chain segments changes at a microscopic level with compositional changes between NB-PEG/PPG-NB and NB-2CZPImide. The interchain distance between polymer chain segments plays an important role in gas transport. That is, membranes with large d-spacing values allow facile diffusion of gas molecules through the membrane, resulting in higher gas permeation flux.[35, 36]
The XRD curves of the three membranes are represented in Figure 3. A broad peak was observed centered on d-spacing values ranging from 4.36 to 4.39 Å, indicating that all three crosslinked polymers were amorphous. The peaks originate from the crystalline PEG units of NB-PEG/PPG-NB monomers.[28, 37] It was also found that an increase in 2CZPImide content with respect to PEG/PPG resulted in the increase of d-spacing value from 4.36 Å (for 10:1) to 4.39 Å (for 6:1). It is believed that the randomly distributed bulky NB-2CZPimide moiety cannot contribute to the crosslinking and hence act as spacer groups between the PEG/PPG chain segments, hence increasing the d-spacings. As a result, enhanced gas permeability is hence expected for the membrane with higher NB-2CZPimide contents (from 10:1 to 6:1).
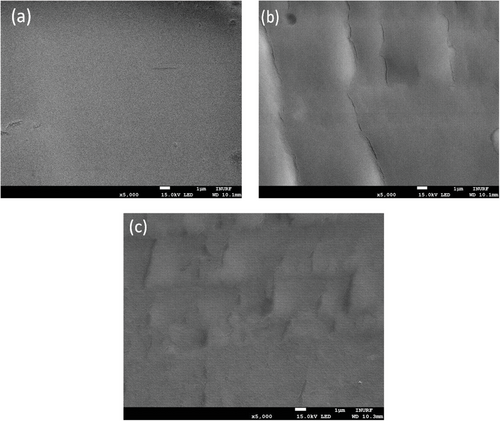
In addition, the cross-sectional SEM images of all the cross-linked membranes, viz, PEG/PPG-2CZPImide (10:1), PEG/PPG-2CZPImide (8:1), and PEG/PPG-2CZPImide (6:1) were analyzed (Figure 4). Dense morphologies were observed for all three membranes. Nevertheless, there are some changes in the morphologies of the crosslinked membranes with the increase of the 2CZPImide content. For PEG/PPG-2CZPImide (10:1), a smooth surface having no phase separation was observed, indicating that the poly(2CZPImide) segments mixed well with the poly(PEG/PPG) segments throughout the crosslinked network. However, some clefts and irregularly shaped dark spots appeared in the SEM images of PEG/PPG-2CZPImide (8:1) and PEG/PPG-2CZPImide (6:1) with higher 2CZPImide contents. These irregularly shaped black dots are presumed to be a collection of C-rich 2CzPImide moieties that act as spacer groups in the crosslinked PEG/PPG segments and create pores.[38] This space generated between the PEG/PPG segments by increasing 2CZPImide loading is believed to increase gas diffusivity through the membrane (this will be discussed later).
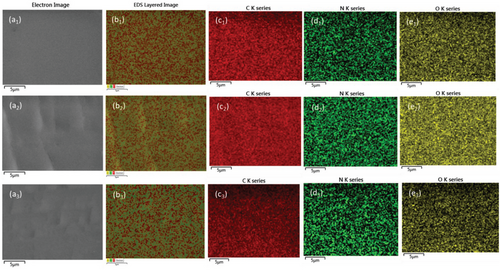
Energy dispersive spectroscopy (EDS) mapping was further performed to investigate the distribution of elements (C, N, and O) and their relative content (at.%) (Figure 5; Table S1, Supporting Information). The results showed that all C, N, and O elements are evenly distributed in all three crosslinked polymers. Since the NB-PEG/PPG-NB monomer has a ─CH2─O─ repeating unit in its backbone, as a result, it contains a high oxygen-to-carbon ratio. On the other hand, the monomer 2CZPImide is rich in carbon as compared to oxygen and contains less oxygen-to-carbon ratio as compared to NB-PEG/PPG-NB monomer. The amount of oxygen and the oxygen/carbon ratio decreases as we move from PEG/PPG-2CZPImide (10:1) to PEG/PPG-2CZPImide (6:1). This phenomenon also supports the quantitative increment of 2CZPImide monomer in the polymer matrix as we go from PEG/PPG-2CZPImide (10:1) to PEG/PPG-2CZPImide (6:1).
2.4 Gas Separation Performance of the Cross-Linked Copolymer Membranes
The single gas permeability of the three crosslinked membranes, with different NB-2CZPImide compositions (denoted as PEG/PPG-2CZPImide), for N2, CH4, and CO2 was measured at 1 atm and 30 °C under a constant volume and variable pressure, as outlined in Tables 2 and 3.
| Membrane code | Permeability, Pa) [Barrer] | Selectivity, α | Reference | |||
|---|---|---|---|---|---|---|
| CO2 | N2 | CH4 | CO2/N2 | CO2/CH4 | ||
| PEG/PPG-2CZPImide (10:1) | 311.10 | 5.98 | 19.44 | 52.02 | 16.00 | This work |
| PEG/PPG-2CZPImide (8:1) | 356.73 | 8.87 | 24.24 | 40.21 | 14.70 | This work |
| PEG/PPG-2CZPImide (6:1) | 418.16 | 10.60 | 31.08 | 39.44 | 13.45 | This work |
| Crosslinked PEG/PPG | 301.23 | 5.09 | 16.85 | 59.2 | 17.52 | [28] |
- a) P in Barrer, where 1 Barrer = 10–10 cm3 (STP)·cm/cm2·s·cmHg.
| Membrane code | Diffusivity, Da) | Diffusivity selectivity | Solubility, Sb) | Solubility selectivity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | N2 | CH4 | CO2/N2 | CO2/CH4 | CO2 | N2 | CH4 | CO2/N2 | CO2/CH4 | |
| PEG/PPG-2CZPImide (10:1) | 57.6 | 10.3 | 16.2 | 5.59 | 1.99 | 5.40 | 0.58 | 1.20 | 9.31 | 4.50 |
| PEG/PPG-2CZPImide (8:1) | 69.27 | 20.4 | 24.8 | 3.15 | 1.99 | 5.13 | 0.52 | 0.98 | 9.86 | 5.23 |
| PEG/PPG-2CZPImide (6:1) | 82.2 | 30.71 | 33.2 | 2.68 | 2.48 | 5.08 | 0.33 | 0.94 | 15.40 | 5.40 |
- a) Diffusivity in 10–8 cm2 s−1;
- b) Solubility in 10–2 cm3 (STP)·cm/cm3·cmHg.
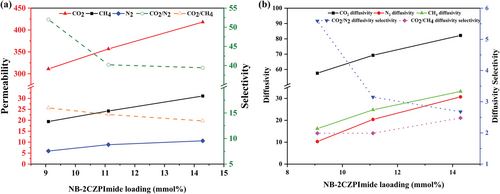
All crosslinked membranes show a significantly higher permeability for CO2 than N2 and CH4. Notably, the permeability of CO2 is 40–52-fold higher than that of N2 and almost 14–16-fold higher than that of CH4, suggesting high CO2/N2 and CO2/CH4 selectivities, as shown in Figure 6.
In addition, the permeabilities of all the crosslinked PEG/PPG-2CZPImide membranes decrease in the order CO2 > CH4 > N2, following the condensability of the gases – CO2 (195 K) > CH4 (149 K) > N2 (71 K) – rather than their molecular sizes – CH4 (3.8 Å) > N2 (3.64 Å) > CO2 (3.3 Å). This pattern is consistent with behavior commonly observed in rubber polymer membranes, corresponding to a solubility-selective mechanism for gas permeation.[39] In rubbery polymer membranes, the critical temperature of each gas is the main factor affecting the gas permeability trend. Specifically, gases with higher critical temperatures, such as CO2 (304.2 K), exhibit greater solubility coefficients and condensabilities than nonpolar gases, such as CH4 and N2. This confirms that the predominant factor contributing to high CO2 selectivity in our PEG/PPG-2CZPImide membranes is their solubility selectivity.
Furthermore, the relationship between the gas permeability of the three membranes and the NB-2CZPImide content is linear, indicating a distinct boost in gas permeability as the NB-2CZPImide content increases. This phenomenon may be attributed to the enhanced diffusivity of the crosslinked polymer membranes arising from an increased free volume (Figure 6b). A surge in the free volume is a direct outcome of the enhanced chain mobility resulting from the incorporation of more NB-2CZPImide units; this, consequently, facilitates the diffusion of gas molecules.
The addition of NB-2CZPImide to the NB-PEG/PPG-NB monomer during the synthesis of the PEG/PPG-2CZPImide membranes results in an increase in the permeability of all gases. A consistent rise in permeability with a simultaneous drop in selectivity compared to the pure NB-PEG/PPG-NB polymer membrane can be observed in the case of the PEG/PPG-2CZPImide (10:1) membrane. For example, compared to the pure NB-PEG/PPG-NB membrane, the CO2 permeability increases by ≈10 Barrer in the case of PEG/PPG-2CZPImide (10:1) that contains 9.09 mol% of 2CZPImide moiety in polymer matrix, whereas the CO2/N2 and CO2/CH4 selectivities drop to 52.02 and 16.00, respectively. Further increases in the 2CZPImide content to 11.11 and 14.28 mol% respectively increase the CO2 permeability to 356.73 Barrer for PEG/PPG-2CZPImide (8:1) and 418.16 Barrer for PEG/PPG-2CZPImide (6:1).
The analysis of the diffusivity and selectivity of the gases reveals that the CO2 diffusivity increases significantly – by ≈30% – in the PEG/PPG-2CZPImide (6:1) film compared to the PEG/PPG-2CZPImide (10:1) film. This increase in diffusivity corresponds to a similar increase in the CO2 permeability rate (Tables 2 and 3). This indicates that the 2CZPImide moiety can enhance the diffusivity of CO2 as it forms a non-crosslinked structure that leads to voids between the PEG/PPG chain segments, thereby facilitating gas diffusion. Moreover, the presence of two adjacent sterically bulky carbazole moieties in the benzene ring of the 2CZPImide monomer hinders the successful chain packing of the PEG/PPG polymer chain segments, which, in turn, enhances the diffusivity of passing gas molecules. This is consistent with the SEM-EDS (conformation of increment of 2CZPImide moiety in the matrix) and XRD results (conformation of increment in d-spacing). Furthermore, with the increase in the NB-2CZPImide content, the CO2/N2 and CO2/CH4 selectivities decrease, which is believed to be largely influenced by diffusivity.
The diffusivity generally depends on the kinetic diameter of the gas. As the flexibility of the polymer matrix increases, the diffusivities of gases with a higher kinetic diameter – namely, N2 and CH4 exhibit a more pronounced increase compared to the case for gases with a lower kinetic diameter . A larger NB-2CZPImide percentage in the crosslinked polymer matrix results in more flexible polymer chains, which, in turn, leads to a noticeable rise in the diffusivity of N2 and CH4 compared to CO2. As a result, the permeabilities of N2 and CH4 are more pronounced compared to that of CO2 with the increase in the 2CZPImide content. Hence, the CO2/N2 and CO2/CH4 selectivities drop accordingly from PEG/PPG-2CZPImide (10:1) to PEG/PPG-2CZPImide (6:1).
The solubility of all the gases decreases as the 2CZPImide content increases. Furthermore, the nonpolar gases N2 and CH4 lead to a more pronounced reduction in solubility compared to the polar CO2 molecule (Table 3). Thus, the CO2/N2 and CO2/CH4 solubility selectivities increase with increasing 2CZPImide content. This is because the 2CZPImide moiety, composed of only one polar imide functional group and two aromatic carbazole moieties, exhibits a weaker interaction with the nonpolar N2 and CH4; hence, their solubilities decrease to a greater extent with the increase in 2CZPImide. The N heteroatom in the carbazole moiety of 2CZPImide can interact with the polar CO2. Moreover, the π-electrons of the aromatic moiety in 2CZPImide can interact with CO2, thereby further enhancing its solubility.[40, 41] Nevertheless, since PEG/PPG has a more polar epoxy unit compared to 2CZPImide, the increment in solubility is more pronounced in the case of PEG/PPG compared to 2CZPImide. Overall, the increase in the 2CZPImide content in the polymer matrix leads to a marginal decrease in the solubility of CO2 gas.
2.5 Permeability Versus Selectivity
To assess the gas separation performance of the crosslinked membranes, the gas separation values were plotted together with the Robeson upper bound-2008 and recent upper bound-2019 (Figure 7). Previously reported permeability and selectivity data for PEO/PEG-based membranes,[42-45] PEG-containing crosslinked membranes,[25, 46-48] PEG-conjugated polymer membranes[49, 50] and PEG-containing ROMP membranes[28, 29, 51] are also displayed in the plots for comparison.
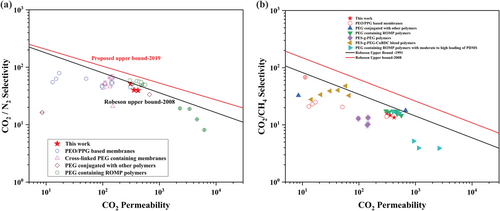
The membranes synthesized herein display a high separation performance with enhanced CO2 permeabilities and high CO2/N2 selectivities that approach the Robeson upper bound 2008.
Although the CO2 permeability of the PEG/PPG-2CZPImide (10:1) membrane is the lowest among the three crosslinked membranes, it still lies on the Robeson upper bound-2008 line due to its comparatively high CO2/N2 selectivity. The remaining two crosslinked membranes – PEG/PPG-2CZPImide (8:1) and PEG/PPG-2CZPImide (6:1) – lie just below the Robeson upper bound-2008. Additionally, all the membranes lie below the Robeson upper bound-1991 line for CO2/CH4 separation. This is because our crosslinked membranes are rubbery in nature, which increases the solubility of CH4 compared to N2 and reduces the CO2/CH4 selectivity. Nevertheless, our membranes still perform better than most PEO- or PEG-containing membranes and those conjugated with other PEG-containing polymers.
Although the crosslinked membranes herein do not contain a highly permeable PDMS counterpart, we achieved CO2 permeabilities similar to those of the PEG/PPG-PDMS- and PEG/PPG-PDMS-adamantane-containing membranes previously synthesized by our group.[28, 29]
2.6 Anti-Aging and Anti-Plasticization Performance
PEG/PPG-2CZPImide (6:1), with the highest CO2 permeability and moderate selectivity among the three crosslinked membranes, was used to test the long-term stability over 10 days. The CO2 and N2 permeability and perm-selectivity were determined by measuring the permeability of each gas at 2 atm and 30 °C during this time. In evaluating the long-term stability, the PEG/PPG-2CZPImide (6:1) membrane was first aged for 10 days after casting, and the CO2 and N2 permeability and perm-selectivity were measured (as mentioned above) over another 10 days; the normalized CO2 permeability along with the CO2/N2 selectivity were then compared (Figure 8a; Table S2, Supporting Information).
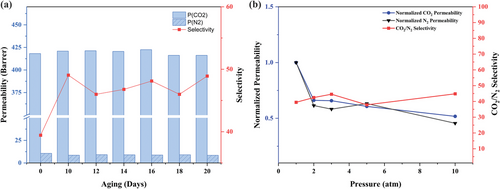
The results reveal that, except for a slight increase at the start of the test, PEG/PPG-2CZPImide (6:1) shows almost the same CO2 permeability throughout the period of analysis. The initial increase in CO2 permeability is due to the CO2-induced swelling of chain segments within the membrane. Nevertheless, due to its crosslinked structure, this swelling is not severe; the membrane thus regains its initial CO2 permeability after 16 days of aging, indicating its excellent anti-aging performance.
In addition, the CO2/N2 selectivity fluctuates within the range of 45.99–49.06 in the aging test due to gradual changes in the CO2 and N2 permeability. These are assumed to arise from changes in the free volume fraction of the crosslinked network due to conformational changes of both the PEG/PPG chain segments and 2CZPImide moiety.
Next, the anti-plasticization of the PEG/PPG-2CZPImide (6:1) membrane was analyzed by measuring the N2 and CO2 gas permeabilities over the pressure range of 1–10 atm. The normalized permeabilities and selectivities of CO2 and N2 over this range were then determined (Figure 8b).
The results indicate that the CO2 and N2 permeabilities decrease slightly with increasing pressure. This occurs due to the pressure-induced densification of the membrane, which reduces the free volume of the polymer matrix. Because N2 has a higher kinetic diameter than CO2, the densification of the polymer chain segments at high pressure (up to 3 atm) leads to a greater reduction in the permeability of N2 compared to CO2. Thus, the CO2/N2 selectivity is increased up to 3 atm. At 5 atm, a small drop in CO2/N2 selectivity was observed, ascribed to the pressure-driven increase of N2 permeability compared to CO2. However, at 10 atm pressure, the CO2/N2 selectivity again increases to 44.82. The stability in the CO2/N2 selectivity over the entire pressure range is ascribed to the rigid and interlocked structure of the polymer matrix in PEG/PPG-2CZPImide (6:1). From the above results, it is concluded that the PEG/PPG-2CZPImide (6:1) membrane exhibited an anti-plasticization behavior under the test condition of 10 atm pressure.
3 Conclusion
A novel sterically bulky norbornene-functionalized NB-2CZPImide monomer was synthesized, characterized, and combined with a dinorbornene-functionalized NB-PEG/PPG-NB monomer via in situ ROMP and membrane casting to form crosslinked PEG/PPG-2CZPImide membranes. The structures of all the synthesized monomers were characterized by 1H NMR and FTIR spectroscopic analysis. The structure and elemental compositions of all the crosslinked membranes and their thermal properties and morphologies were investigated by comparative FTIR spectroscopy, TGA, DSC, XRD, and SEM-EDS analysis. From the thermal analysis, it was evident that all the synthesized crosslinked membranes have good thermal stability. Notably, gradually increasing the amount of 2CZPImide moiety (as evident from the SEM-EDS analysis) in the crosslinked membranes enhanced the d-spacing of adjacent PEG/PPG chain segments in the membranes (as seen using XRD), which in turn augmented the CO2 transport across the membrane.
The synthesized membranes exhibit exceptional CO2 separation capabilities, showcasing CO2 permeabilities ranging from 311.10 to 418.16 Barrer, along with CO2/N2 and CO2/CH4 selectivities from 39.44 to 52.02 and 13.45 to 16.00, respectively, which approach the Robeson upper bound-2008. Notably, the PEG/PPG-2CZPImide (6:1) membrane exhibits the highest CO2 permeability (418.16 Barrer) and acceptable CO2/N2 and CO2/CH4 selectivities of 39.44 and 13.45, respectively, compared to the remaining membranes. Furthermore, it exhibits excellent long-term stability against physical aging (up to 20 days) and displays anti-plasticization behavior at a pressure of 10 atm.
4 Experimental Section
Synthesis of Norbornene-Functionalized Precursor Monomer and Oligomer—Synthesis of 4,5-Di(9H-carbazol-9-yl)phthalonitrile a
The required 4,5-Bis(9H-carbazol-9- yl)phthalonitrile a was synthesized by following a procedure reported by Shereen A. Majeed et al. and detailed in the Supporting Information.[34]
Synthesis of Norbornene-Functionalized Precursor Monomer and Oligomer—Synthesis of 4,5-Bis(9H-carbazol-9- yl)phthalic Acid (2CzPA) b
The required amount of 4,5-Di(9H-carbazol-9-yl)phthalonitrile a (5.0280 gm, 10.92 mmol) and KOH (12.25 gm, 218.41 mmol) was added to EtOH/H2O (1:1, v/v, 120 mL) solution kept in a 100 mL round-bottomed flasks and fitted with a reflux condenser. After completion of the reaction that had been stirred at 120 °C for 48 h, the reaction mixture was immediately diluted with excess ethanol and then left for cooling to room temperature. The solution was filtered and then weakly acidified (pH–4-5) with 1M hydrochloric acid. The resulting precipitate was filtered and washed with water several times until its pH reached ≈7. The yellow precipitate was collected by filtration and then dried overnight in an 80 °C oven.
Yield 4.72 gm (87%); 1 H NMR (400 MHz, DMSO-d6): δ, ppm: 8.14 ppm (s, 2H), 7.94–7.95 (d, 4H), 7.27–7.29 (d, 4H), 7.11 (m, 8H); ATR IR (cm−1): 3361–2502 (b, ─OH stretching), 1694 (symmetrical stretching of ─C═O bonds in two adjacent ─COOH groups) and 1722 (asymmetrical stretching of ─C═O bonds in two adjacent ─COOH groups).
Synthesis of Norbornene-Functionalized Precursor Monomer and Oligomer—Synthesis of 2-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)−5,6-di(9H-carbazol-9-yl)isoindoline-1,3-dione (2CZPI-NB) 3
2CZPA (2.96 gm, 5.94 mmol) and 50 mL of acetic anhydride were taken in a 50 mL round-bottomed flask fitted with a reflux condenser and heated at 140 °C for 4 h in a nitrogen atmosphere. After completion of the reaction, the anhydride of the corresponding 4,5-Bis(9H-carbazol-9- yl)phthalic acid c was formed. The product was recovered from the reaction mixture by evaporating the remaining acetic anhydride. The anhydride c was dried at 80 °C under reduced pressure for 24 h and was used for the next reaction without any purification.
2.18 g (4.53 mmol) of the obtained solid anhydride c and 0.84 gm (6.80 mmol) of 5-Norbornene-2-methyl amine were charged into a 50 mL round-bottomed flask containing 25 mL of acetic acid. The reaction mixture was refluxed at 130 °C for 48 h and then the acetic acid was evaporated to obtain a greenish-yellow crude product mixture. Finally, the desired norbornene-functionalized 2CZPI-NB 3 was purified from the crude product mixture through silica gel column chromatography using methylene chloride/hexane (1/4 to 1/1, v/v) solvent mixture as an eluent.
Yield 2.25 gm (84.79%), m.p; 268.2 °C; 1 H NMR (400 MHz, CDCl3): δ, ppm: 8.37 ppm (s, 2H; H9), 7.79–7.81 (d, 4H; H10), 7.03–7.17 (m, H11, H12, H13;); 6.31 (s, 1H, H5), 6.25 (s, 1H, H6), 3.78–3.91(m, 1 H, H8(Endo)) 3.49–3.61 (m, 1H, H8 (Exo)), 2.87-2.89 (m, H7α), 2.67 (m, H7β), 1.96–2.01 (m, H4(Exo)), 1.50–1.62 (m, H4(Exo)), 1.38–1.39 (m, H2(Endo)), 1.28–1.32 (m, H2 (Exo)) 0.82–0.93 (m, H3 (Endo)), 0.76–0.79 (d. m, H3 (Exo)); ATR-IR (cm−1): 1767 (imide C═O asymmetric stretching), 1700 (imide C═O symmetric stretching), 1356 (imide C─N stretching).
Synthesis of Norbornene-Functionalized Precursor Monomer and Oligomer—Norbornene-Functionalized PEG/PPG Oligomer 2
The required NB-PPG/PEG-NB was synthesized by following a procedure reported by Iqubal et al.[28] For the synthesis of NB-PPG/PEG-NB 2, 1.79 g of NB (10.53 mmol), 10.0 g of O,Oʹ-bis(2- aminopropyl) polypropylene glycol-block-polyethylene glycol-block-polypropylene glycol (NH2-PEG/PPG-NH2) (Mn = 1900) (5.26 mmol) and 25 mL of acetic acid were charged into a 100 mL round-bottomed flask fitted with a reflux condenser and the mixture was heated at 120 °C for 12 h. After cooling the mixture to room temperature, the acetic acid was evaporated. The viscous solution thus obtained was washed with diethyl ether after which a cloudy solution was obtained. The solution was refrigerated to produce a white precipitate of the product, which was separated by filtration. This process was repeated several times to remove unreacted reaction residue. Finally, the residue was dried at a 40 °C oven for 24 h to obtain a white wax-like solid product. (White solid, yield 8.77 gm; 75.5%); δH (400 MHz, CDCl3): δ, ppm: 6.27 (4H, s, CH═CH), 4.42–4.32 (2H, br. signal, CH─N(CO)2, Hb), 4.06–3.96 (4H, br signal, OCH2CH), 3.83–3.26 (186 H, br signal CH2─O─CH2 and CH─O), 3.24–3.20 (4H, br signal, CH), 2.66–2.57 (4H, br signal, CH), 1.50–1.44 (4H, br signal, CH2),1.30–1.28 (6H, br signal, C─CH3) and 1.17–1.01 (12H, br signal, C─CH3); ATR-IR (cm−1): 2880 (C─H stretching), 1768 (imide C═O asymmetric stretching), 1696 (imide C═O symmetric stretching), 1464 (CH2 deformation), 1340 (CH2 deformation), 1100 (C─O─C stretching)
Synthesis of Norbornene-Functionalized Precursor Monomer and Oligomer—Synthesis of In Situ Crosslinked Copolymer Membrane, PEG/PPG:2CZPImide (x:y) 1
The crosslinked copolymer membranes, PEG/PPG:2CZPImide (x:y) 1 having varying molar ratio of NB-PEG/PPG-NB monomer to NB-2CZPI monomer, were prepared by following the same experimental procedure as follows: The reaction solvent DCM was prepared by distillation under N2 atmosphere followed by drying over CaH2 overnight. The dissolved gas in the DCM was removed by taking it into a Schlenk flask and applying five freeze-pump-thaw cycles. The monomers, DCM, and catalyst were then taken in a N2-filled glove box. Stoichiometric amounts of monomer 2 and 3 were taken in a glass vial inside a nitrogen-filled glove box and dissolved into the degassed DCM by stirring for 1 h. For example, to maintain the feed ratio of 10:1 in between NB-PEG/PPG-NB and NB-2CZPI in the final crosslinked copolymer PEG/PPG-2CZPImide (x:y), 600 mg of NB-PEG/PPG-NB (0.27 mmol) and 16.02 mg of NB-2CZPI (0.027 mmol) were dissolved into 5.5 mL of DCM. Next, 2 mg of Grubb's catalyst was dissolved in 1 mL of dry DCM in a separate vial. After adding Grubb's catalyst solution to the monomer solution, the mixture was stirred constantly for ≈8 min. Subsequently, the mixture was transferred onto a polypropylene Petri dish, which was wrapped with aluminum foil, leaving a very small opening to regulate the gradual solvent evaporation process in the N2 environment inside the glove box. The sample was left to dry overnight at ambient temperature to produce the membrane. After that, a few drops of EVE were spread over the membrane to quench the polymerization reaction, and the membrane was dried in a 40 °C oven under vacuum. The greenish-yellow colored self-standing and transparent membranes (Figure 6) thus obtained were peeled from the Petri dish, washed very carefully with dichloromethane two times, and finally dried in a 40 °C oven for 48 h. The same approach has been implemented for making other membranes.
ATR-IR (cm−1) for (PEG/PPG-2CZPImide) (10:1): 2863 (C─H asymmetric and symmetric stretching), 1769 (imide C═O asymmetric stretching), 1698 (imide C═O symmetric stretching), 1453 (CH2 bending), 1351–1370 (imide C─N stretching), 1248 (CH2─O─CH2 asymmetric stretching), 1095 (CH2─O─CH2 symmetric stretching), 945 (CH2─O─CH2 symmetric stretching).
Synthesis of Norbornene-Functionalized Precursor Monomer and Oligomer—Characterization and Measurements
All characterization and measurements are detailed in the Supporting Information.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) (NRF-2022R1A2C2002746). Part of this work was also supported by the Core Research Institute (CRI) Program, the Basic Science Research Program through the National Research Foundation of Korea (NRF), Ministry of Education (NRF-2017R1A6A1A06015181).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




