Thiol-X Chemistry: A Skeleton Key Unlocking Advanced Polymers in Additive Manufacturing
Abstract
Using additive manufacturing (AM) technologies for the fabrication of advanced polymeric objects is a logical progression to realize their potential within engineering applications that demand complex geometries. Thiol-X chemistry has presented itself as a diverse and invaluable toolkit to accomplish such, satisfying both the processing requirements and properties desired to fabricate a diverse range of advanced polymeric objects using a variety of AM technologies. The “click” nature of many thiol-X reactions, mechanisms of polymerization, functional group tolerance and orthogonality, and diverse and desirable properties achievable with thiol-X chemistry presents a synergistic opportunity that few other chemistries can as broadly facilitate. As a result, the use of thiol-X chemistry within AM has gained rapid traction in recent years and it is now the case that both thiol-X chemistry and additive manufacturing technologies are being viewed as two sides of the same coin, whereby the scope and limitations of the whole system are appreciated. This review highlights the advancements, status, scope, and opportunities of using thiol-X chemistry in AM to achieve a diverse range of advanced polymers by critically examining the structure-property-processing-performance relationships between different thiol-X chemistries and various AM technologies.
1 Introduction
The ongoing development of advanced polymeric materials has facilitated the progress of technologies within a myriad of engineering fields.[1-3] However, their successful implementation within engineering design often demands complex fabrication requirements. One of the challenges faced by emerging advanced polymers is that they are often unsuitable for drop-in use with well-established manufacturing technologies (e.g., injection molding, extrusion, thermoforming) or pose challenges for effective subtractive manufacturing.[4] Furthermore, these traditional fabrication technologies may also be limited in their achievable geometries and physical scale of fabrication. As a result, manufacturing complex geometries is either not possible or time-consuming and expensive, thus limiting their ability to adequately satisfy engineering design constraints. Additionally, conceptual phenomena such as multi-materials, those containing spatially varied polymer properties, are generally unfeasible using many of these traditional fabrication technologies.[5-7] It is thus the logical progression that additive manufacturing (AM) could provide an accessible and effective platform to facilitate complex object fabrication for these materials.[8] Several AM technologies are commonly used for polymeric materials, which can be broadly categorized into either a) melt-processing of thermoplastic polymers (e.g., fused filament fabrication (FFF) and selective laser melting (SLM)) or b) in situ polymerization of monomers such as stereolithography (SLA), digital light processing (DLP), masked stereolithography (mSLA), digital light synthesis (DLS, commercially known as continuous liquid interface polymerization (CLIP)), volumetric polymerization (VP), two-photon polymerization (TPP), direct laser write (DLW) printing, and UV assisted direct ink write (DIW) printing.[9, 10] The use of AM presents appealing opportunities for rapid fabrication of objects benefiting complex geometries, high-resolution fidelities down to micro- and nanoscale, and economic accessibility.[11, 12] However, in order to exploit these AM technologies, the chemistry underpinning the polymers needs to both satisfy the processing constraints imposed by AM, while still successfully imparting desirable properties within the polymer.[11] Within this context, the fact that AM requires spatially selective polymerization or material deposition for fabrication imposes appreciable constraints on the type of polymer that can be employed. It is thus the case that the conceptual marrying of previously siloed development between emerging polymers and AM technologies needs to be sought to fully exploit the potential of using advanced polymers in AM.[13]
In light of this, thiol-X chemistry has proved itself as a valuable toolkit of chemical reactions to satisfy these constraints. Thiol-X chemistry comprises of a family of chemical reactions between thiols and several other functional groups.[14] Many thiol-X reactions have been lauded as “click” type reactions after the landmark description by Sharpless et al.,[15] characterized by mild reaction conditions, high conversions, rapid kinetics, few byproducts, stereoselectivity, and solvent-free conditions. Furthermore, many thiol-X reactions display good functional group tolerance and can facilitate orthogonal reactions, thus enhancing its potential within molecular architectures containing multiple distinct chemistries and the development of multi-material polymers. Due to these oft-favorable properties of thiol-X reactions, their development has flourished, whereby the seminal reviews by Bowman, Hoyle, and co-workers[16-18] on thiol-ene polymers firmly embedded thiol-X chemistry within polymer science. While substantial efforts to incorporate thiol-X reactions as polymerization strategies have already been observed, it is only more recently that their potential within AM has begun being realized and gained traction. Nevertheless, within a relatively short time, a diverse assortment of thiol-X chemical reactions has already shown it as an effective platform for developing a broad array of different advanced polymers with potential in an almost endless list of important contemporary engineering applications. Furthermore, their suitability within several AM technologies makes these reactions synergistically favorable to facilitate both desirable properties and effective AM processing. Practically, many thiol-X reactions also benefit from “drop-in” capabilities within AM, mitigating bespoke AM technology development that inherently limits widespread adoption. Indeed, the vast majority of literature reviewed used unmodified commercially available 3D printers over a range of technologies, including most VAT photopolymerization AM, binder jet printing, DIW printing, and FFF printing.
The goal of this review is to highlight the instrumental role that thiol-X chemistry has contributed to the development of fabricating advanced polymers using AM, critically examine the complex structure-property-processing-performance relationships of thiol-X chemistry within the context of various AM technologies, and explore both the scope and future opportunities thiol-X chemistry may present within AM.
2 Overview of Thiol-X Chemistry
2.1 Properties and Chemistry of the Thiol Group
The thiol functional group is a sulfur analog of the hydroxyl group, although the S-H bond dissociation energy is relatively lower (typically ≈330–370 kJ mol−1), and thus the thiol group has a comparatively longer bond length.[19, 20] The relatively weak S-H bond makes the hydrogen on thiols susceptible to abstraction to form a thiyl radical, and thiols can also be deprotonated to form the thiolate conjugate base. Comparatively, the thiol group is more acidic than its hydroxyl analog, and the thiolate acts as a stronger nucleophile than alkoxides; conversely, the thiolate is also generally a weaker base than alkoxides.[21] Thiols and several thiol-X reaction products are amenable to further oxidation reactions under basic conditions, while thiols can also undergo an SN2 reaction with alkyl halides (Figure 1).[22] Remarkably, the thiol group and thioethers can also undergo conjugation with elemental gold.[23]
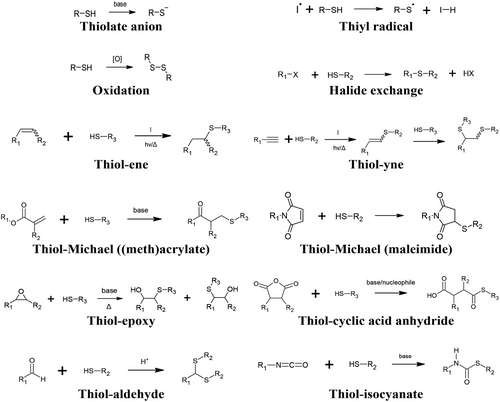
Thiols thus serve as a versatile functional group that can react with several other functional groups to form products useful within the context of polymer science (Figure 1). These include the reaction of thiols with carbon-carbon double bonds (ene) and triple bonds (yne), (meth)acrylates and maleimides, epoxies, cyclic acid anhydrides, aldehydes, and isocyanates. Further details of each reaction, its product, and properties are described in more detail within each subsection of the review. Most of the outlined chemical reactions are long known from classical organic chemistry and have merely been adopted into the field of polymer science in more recent years. In fact, thiol-X chemistry has been exploited by Nature for millions of years in macromolecules such as polypeptides,[24] with synthetic descriptions from 1905,[25] and yet the development of thiol-X chemistry has continued to generate attention in contemporary literature.[26-33]
In most instances, multifunctional compounds enable thiol-X chemistry to be exploited as appropriate monomers. However, the requirements for different thiol-X chemistries to form polymers vary depending on the specific AM technology. For melt processing AM (e.g., FFF), polymerization of thermoplastic polymers is performed prior to AM, making the polymerization mechanism and kinetics less important, although sufficient molecular weights need to be achieved. On the other hand, for vat photopolymerization AM and DIW printing, in situ polymerization is usually necessary for these technologies. In this case, the polymerization initiation mechanism and reaction kinetics become more important considerations. Within this context, the reaction kinetics of various thiol-X reactions has been well established, and generally present several opportunities for relatively rapid polymerizations.[34] Indeed, some thiol-X reactions are autocatalytic at room temperature and pressure, and thus while they are excellent as general “click” type reactions, they are not always immediately suitable as polymerization mechanisms in AM where spatiotemporally controlled polymerization by external stimulus is imperative. Similarly, in situ polymerization AM is also difficult to achieve when catalysis of the reaction cannot be spatiotemporally controlled. Nevertheless, in several cases, these reactions may be used in monomer/oligomer synthesis, and thus still hold relevance within the context of AM.[35] Furthermore, strategic photoactivation of catalysts for these reactions presents an avenue to access these reactions within AM. Alternatively, in situ mixing of monomers during AM is a logically viable strategy, although this approach comes with several processing considerations and typically entails bespoke AM technologies. Nevertheless, using different thiol-X chemistries either within the monomer structure or as a polymerization strategy, alongside the incorporation of other structural attributes and functional groups within the monomer, can be used to control and tailor desirable engineering properties. However, other factors such as system viscosity, polymerization stimulus, crystallinity effects, interlayer adhesion, polymerization shrinkage, and print resolution fidelity can impose additional constraints on the designing of polymers that can be successfully processed with AM.
2.2 Thiol Monomers
Several thiol monomers of differing molecular structure and functionality are commercially available (Figure 2) and regularly employed in AM. Many of these monomers are ethers and esters of 2-mercaptoethanol and 3-mercaptopropionate, respectively, which can make them hydrolytically labile.[36] On the other hand, the use of ester-free thiols has been demonstrated as an appropriate alternative for applications where hydrolytic stability is important.[36] For most thiol-X reactions, the thiol monomer is required to have a functionality of two or more in order to participate in polymerization, owing to a step-growth mechanism of polymerization. Alternatively, monofunctional thiols may be used to form pendant groups on the monomer/oligomer/polymer to impart desirable properties or functionality, although an alternative mechanism of polymerization is then required.[37]
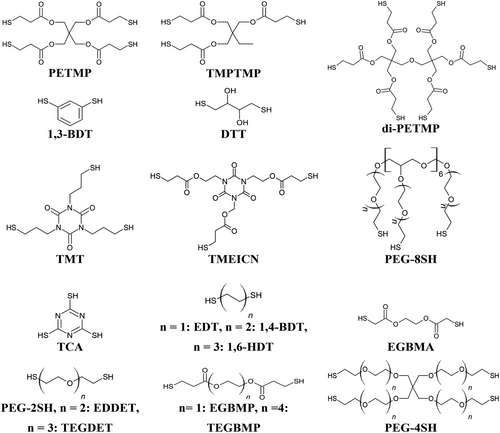
While a few naturally occurring thiol-bearing compounds can serve as suitable monomers (e.g., dithiothreitol, DTT, Figure 2), several synthetic strategies to impart thiol functional groups are also well known. These include the use of isothiouronium salts, alkyl halides, and alkali metal hydrogen sulfides, and the catalyzed reaction of hydrogen sulfide with a variety of functional groups.[38] Alternatively, the reaction of hydrogen sulfide with α,β-unsaturated carbonyls or alcohols is another efficient pathway for thiol preparation.[39, 40] On a laboratory scale, a number of other synthesis pathways present viable strategies to impart thiol functionality. For example, the thiol-ene coupling of thioacetone onto unsaturated compounds, followed by base hydrolysis of the thioacetate to yield free thiols has been established as a convenient synthetic pathway.[41] More recently, a similar synthetic strategy using thiourea has been described for the α,β-unsaturated carbonyl compound crotonic acid, yielding secondary thiols that could be coupled to polyols via simple Fisher esterification.[42] Aside from these examples, several other synthetic avenues have also been reported.[43-48] Although primary thiols are most commonly employed as monomers, secondary thiols are also known to serve as effective functional groups. A systematic investigation into the effect of primary, secondary, and tertiary thiols for DLP 3D printing was performed to ascertain the effect of thiol substitution on the reaction kinetics and resulting polymer properties. Interestingly, negligible differences in kinetics between primary and secondary thiols were observed, and secondary thiols provided improved mechanical properties and lower polymerization shrinkage in DLP-printed polymers.[49] On the other hand, it has been established that tertiary substituted thiols display polymerization rates almost 10-fold lower than primary thiols, yet still reach quantitative conversions similar to that of primary and secondary substituted thiols. Furthermore, this work established that the shelf-life of secondary thiol resins was around 100 times longer than for primary thiols.[50]
3 Thiol-Ene
3.1 Polymerization and Properties
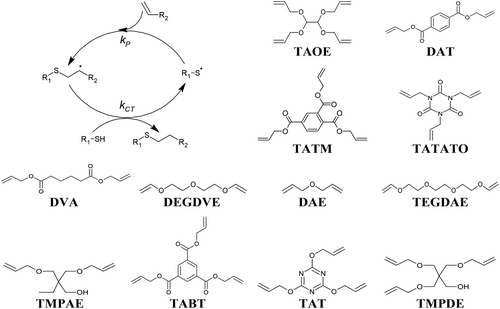
The thiol-ene reaction is well established, having first been described by Posner[25] in 1905 and further identified for photopolymerization by Braun and Murjahn[51] in 1926. The reaction between thiol and alkene (i.e., ene) functional groups results in an alkyl sulfide bond (also referred to as thioether) via light, radical generator, or heat-induced thiyl radical generation. Once the hydrogen has been abstracted from the thiol to form the thiyl radical, propagation occurs through anti-Markovnikov addition with an alkene to generate a carbon-centered radical. In turn, the carbon-centered radical can abstract a hydrogen from another thiol in a chain-transfer step, thus allowing multiple propagation steps for step-growth polymerization (Figure 3).[16] The thiol-ene reaction is thermodynamically favorable, whereby the enthalpy of reaction is ≈3× greater than that of typical free radical acrylate polymerization.[52] However, one of the challenges of the thiol-ene polymerization strategy is that, unlike free radical polymerization that generates a growing chain, the reaction generates a single covalent bond followed by chain transfer, thus lowering the maximum theoretical crosslinking density. Generally, electron-rich alkenes proceed almost exclusively via thiol-ene step-growth with virtually no homopolymerization about the carbon-centered radical.[53, 54] Kinetically, it is thus found that electron-rich alkenes are consumed faster than electron-poor enes.[55, 56] Comparatively, the chain transfer constant (Ctr, calculated as a ratio of thiyl radical propagation to chain transfer kinetic parameters) for norbornenes is 1, vinyl ethers 0.83, while methacrylates and acrylates are as low as 0.26 and 0.08, respectively.[57, 58] Herein, thiol-ene chemistry for polymerization refers to the case whereby the step-growth reaction predominates, and the competing reactivity ratio for chain-growth homopolymerization is negligible. Within this context, where the purely step-growth thiol-ene polymerization is expected, the thiol-ene reaction is characterized by longer induction periods before gel formation and vitrification owing to slower molecular weight increase of the step-growth mechanism, and thus facilitates an overall high degree of polymerization and ideal network structure formation, with reduced polymer shrinkage stresses.[59] While the thiol-ene reaction usually results in relatively better ideal network formation compared to typical free radical polymerization mechanisms, the considerations for the formation of non-ideal networks have also been investigated, establishing that thiol purity and intramolecular cyclization can contribute towards non-ideal networks.[60] An additional benefit of the thiol-ene reaction is that, unlike typical free radical polymerization reactions, it is relatively insensitive to oxygen retardation.[61]
While traditional photoinitiators for the thiol-ene reaction have already been firmly established as a viable route for thiyl radical generation and resultant step-growth polymerization, more recently the use of new catalysts is being explored. A simple example is the use of visible light photoinitiators, offering opportunities for low energy irradiance and for orthogonal wavelength-specific polymerizations.[62-64] Beyond this, more advanced examples of photoinitiators present exciting opportunities for nuanced polymer architecture control.[65-68]
3.2 Thiol-Ene Chemistry using Allyl (ether)s, Vinyl (ether)s, and Derivatives for AM
Allyls, allyl ethers, allyl urethanes, vinyl ethers, vinyl acetates, vinyls, and allyl carbonates are all relatively reactive towards the radical-mediated thiol-ene reaction and thus serve as appropriate ene groups for rapid photopolymerization. For example, the thiol-ene reaction with vinyl esters has been demonstrated to display comparable reactivity to acrylate free radical photopolymerization.[69] Several common commercially available monomers are frequently employed within AM (Figure 3).
3.2.1 Shape Memory, Self-Healing, and Degradable Polymers
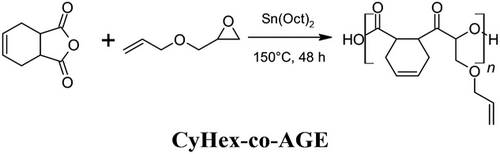
Polymers that can change geometry under stimulus (e.g., heat, light, pH magnetic field) are referred to as SMPs or 4D polymers in AM.[70-73] In particular, the use of covalently cross-linked polymers presents an appropriate strategy for achieving SMPs in vat photopolymerization AM.[74] For cross-linked SMPs, well-defined polymer architectures tailored through network homogeneity can result in narrow glass transition temperature (Tg) and thus facilitate rapid response and recoveries within small temperature windows. It is therefore crucial that the network contains evenly distributed netpoints and switching segments to achieve such.[75] Within this context, thiol-ene polymers can have relatively narrower Tg half-height peak widths compared to (meth)acrylates.[76] Furthermore, for thiol-ene polymers, it is well known that the weak and flexible thioether bonds often result in relatively low Tg polymers. In fact, this can be exploited to tailor SMPs that undergo geometric changes at useful temperatures for many applications. For example, various unsaturated poly(ester)s have been prepared between allyl glycidyl ether and 1,2,3,6-tetrahydrophthalic anhydride (CyHex-co-AGE, Figure 4), as well as incorporating pendant groups of 3-mercaptopropionic acid. These oligomers and co-poly(ester)s thereof were copolymerized with pentaerythritol tetrakis(3-mercaptopropionate) (PETMP, Figure 2) in ethylene carbonate via DLP printing using a 10 s layer cure time. The copolymers displayed a tensile modulus (ET) up to 600 MPa and Tg up to 67 °C, while the pendant addition of 3-mercaptopropionate groups decreased ET, but increased strain at break (εT) up to 52.5%. The CyHex-co-AGE and PETMP copolymer displayed excellent strain fixation release at 50 °C with rapid and complete strain recovery.[77] In an effort to control the (thermo)mechanical properties and associated shape memory capability of these SMPs, several organocatalysts were then explored for tailoring the molecular weight and polydispersity of the polyesters bearing pendant allyl ethers. Copolymerization using different molecular weight (0.4–25.5 kDa) and polydispersity oligomers with PETMP via DLP printing allowed tailorable thermomechanical properties (e.g., Tg 18–49 °C) of the polymers, thus facilitating targeted properties for specific applications.[78] Further to this, the effect of supramolecular thiourea co-catalysts were explored to tailor the polyester molecular weights. The DLP-printed SMPs displayed excellent strain fixations (>99%) and strain recoveries (>99%). However, rapid plateauing of Tg occurred for molecular weights above 1 kDa, rendering further tailoring of negligible effect.[79]
Achieving high-resolution fidelity of detailed features is highly attractive within AM. While achieving high-resolution 3D printing is in-part due to technological factors, the monomer system also plays an important role. For example, a comparative study between the use of acrylates and thiol-ene polymers using either triethylene glycol diallyl ether (TEGDAE) or triallyl-s-triazine-2,4,6(1H,3H,5H)trione (TATATO) and tris[2-(3-mercaptopropionyloxy)ethyl] isocyanurate (TMEICN) for VP 3D printing has been performed to establish optimal systems for high-resolution 3D printing. Here, it was shown that compared to analogous acrylate systems (tetraethylene glycol diacrylate (TEGDA, Figure 3) and tris[2-(acryloyloxy)ethyl] isocyanurate (TAEICN, Figure 3), greater spatial control could be achieved using thiol-ene chemistry.[80] Adding credence to this, SMPs were then produced via VP 3D printing using TEGDAE, TATATO, and TMEICN, whereby increasing the content of TEGDAE could effectively increase the polymers’ Tg to around 37–39 °C. Cycling the temperature 20 °C above and below the Tg under constant tensile strain conditions (9.4%) demonstrated that near-ideal shape recovery (>96%) could repeatedly be achieved. Furthermore, ideal shape fixity was achieved, with rapid shape recovery occurring at 80 °C. Here, the shape memory capability was also demonstrated for 3-arm robotic grippers.[81]
Various biobased polymers that exploit the unsaturation on naturally occurring platform molecules have been developed using thiol-ene polymerization. In most instances, the unsaturation on terpenes and terpenoids is insufficiently reactive for free radical polymerization under mild conditions, yet readily reacts via the thiol-ene mechanism.[82-84] The judicious choice of terpene/terpenoid has still found to be imperative, since examples such as limonene and linalool have been shown to rapidly crosslink (5 s) with PETMP, while nerol and geraniol crosslinked incredibly slowly (1 h) by DLP 3D printing.[85] As such, myrcene, linalool, and limonene have been used for thiol-ene polymerization in AM. Firstly, it was found that the synthesis of poly(myrcene) oligomers promoted polymerization kinetics with PETMP compared to using β-myrcene directly as a monomer. It was further established that either linear or branched oligomers could be prepared by anionic or free radical polymerization, respectively, and these oligomers were then copolymerized with PETMP using DLP printing. The resultant polymers could undergo post-fabrication surface modifications, whereby residual unsaturation on the polymer surface was coupled with either 1-hexadecanethiol or mercaptopropionic acid to produce hydrophobic or hydrophilic surfaces, respectively.[86] A similar approach of using limonene and β-myrcene oligomers has also been used to produce effective SMPs using DLP printing. Here, limonene and β-myrcene homo- and copolymer oligomers were prepared by bulk thermal free radical polymerization, followed by copolymerization with TATATO and PETMP via DLP printing using relatively short layer cure times (3–5 s). Adjusting the relative incorporation of limonene and β-myrcene components resulted in the ability to tailor mechanical properties, and higher contents of β-myrcene also displayed greater hydrophilicity. Furthermore, cell viability was investigated for potential biomedical applications as cellular scaffolds, with the highest cell viability observed for β-myrcene containing copolymers. These copolymers also displayed near-ideal shape fixity and strain fixity, with rapid strain recovery when heated above their Tg.[87]
Various biobased polymers have also been used for controlled degradable materials. For example, an allyl ether functionalized oligomer, allyl poly(acrylate ethyl lactate) (PAEL, Figure 5), has been synthesized using the renewably sourced ethyl lactate. Furthermore, a biobased thiol monomer was prepared through Fischer esterification between isosorbide and 3-mercaptopropionic acid, yielding isosorbide bis(3-mercaptopropionate) (IBMP, Figure 5). These monomers were then copolymerized with TTT and PETMP in varying ratios using DLP printing. The copolymer with the highest biobased monomer content (10 wt% PAEL, 5 wt% IBMP, 52 wt% PETMP, 33 wt% TTT) underwent rapid and complete degradation in basic conditions, while remaining relatively impervious to acidic conditions. Furthermore, the introduction of PAEL increased strength properties relative to a reference standard, with the aforementioned copolymer displaying a tensile strength (σT) of around 20 MPa and εT at around 9%.[88]
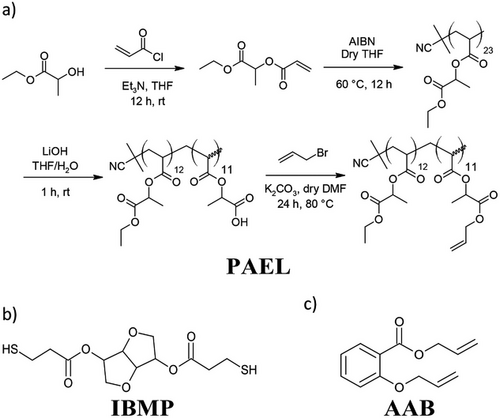
Derivatives of salicylic acid (bromosalicylic allyl ester, iodosalicylic allyl ester, allyl salicylate, allyl 2-(allyloxy)benzoate (AAB, Figure 5), and allyl 2-(((allyloxy)carbonyl)oxy)benzoate) have been prepared as prodrug monomers for soft tissue scaffold applications. The monomers were copolymerized with PETMP (monofunctional monomers with additional 25 wt% poly(trimethylolpropane allyl carbonate)) using DLP printing with a layer cure time of 5 s. Here, it was found that varying the functional group did not influence layer cure time. Interestingly, strain fixation was only achieved at low temperatures (-20 °C) for all polymers except polymers with iodosalicylic allyl ester, which was performed at 30 °C. A compressive elastic modulus as low as 65 kPa was achieved using AAB, making it comparable to many flexible hydrogels. All the copolymers were degradable in 1 N NaOH yet stable in phosphate-buffered saline (PBS) solution. The polymers successfully released the salicylic acid derivatives at a relatively sustained rate when degraded, which provided a prolonged release compared to other poly(aspirin)s and encapsulated forms of salicylic acid. Furthermore, layered structures composed of different polymer compositions could effectively produce spatially selective differential degradation rates. Lastly, the polymers displayed good cellular proliferations alongside diminished bacterial growth, with AAB displaying 110% ± 3% cell viability after 7 days. Interestingly, only the difunctional salicylic derivatives displayed inhibitory effects against E. coli and S. aureus.[89] In fact, several other allylated phenolic acids have also been shown as effective monomers within thiol-ene polymers, and thus may be of interest within the context of AM for biomedical contexts.[90-92] On that note, it is quite surprising that more biobased compounds containing allyl/vinyl groups have not generally been employed in AM to date. A variety of other highly promising biobased monomers present themselves as readily transferrable into AM, such as limonene,[93-97] eugenol,[98-106] vanillin,[107] rosin,[108] myo-inositol,[109] lignin,[110] levoglucosenone,[111] mangnolol,[112] glutamic acid,[113] isosorbide,[114, 115] and various other plant oils.[116-120]
Poly(glycidol) is a biocompatible material that can be exploited as a platform for further functionalization to yield crosslinked degradable materials.[123] For example, it has been partially functionalized with pendant allyl ether P(AGE-co-G), mercaptopropyl (PG-SHef), or propionate groups (PG-SHec) (Figure 6), which were then DIW 3D printed as hydrogels (15 wt% oligomer concentration) alongside 3.5 wt% hyaluronic acid (HA) to increase system viscosity. Human bone marrow-derived stromal cells could also be encapsulated in the hydrogels, which displayed acceptable cell viability after 24 h (78.9 ± 13.5%), although a high variability was observed. Here, it was established that controlled degradation could be achieved when PG-SHec was employed, owing to the hydrolytically labile ester moiety, and that compositional effects could be exploited to tailor cell survivals. These hydrogels displayed complete dissolution after 5 weeks in PBS solution at 37 °C.[121] Furthermore, the effect of positively charged species within thiol-ene hydrogels has shown promising results for the uptake of low molecular mass species, extending its scope within biomedical contexts.[124]
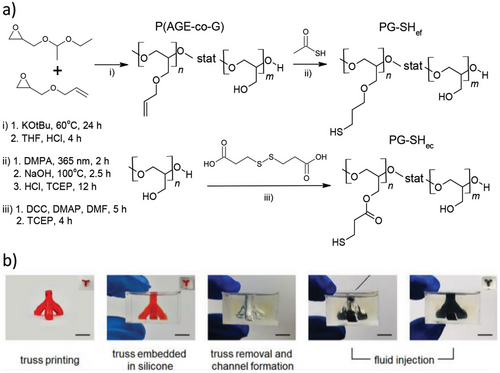
Aside from hydrolytic degradation, other mechanisms can be used for controlled degradable polymers. For example, tetraallyloxyethane (TAOE), 2,4,6-triallyloxy-1,3,5-triazine (TAT), ethylene glycol bis(3-mercaptopropionate) (EGBMP), and PETMP have been used to produce a series of shape memory and degradable thiol-ene polymers via DLP printing. Here, thermal treatment at 140 °C in hexylamine could effectively transform the polymer network through transamidation. Furthermore, the concentration of ester groups within the polymer could be tailored by partially replacing EGBMP with 2,2′-(ethylenedioxy)diethanethiol (EDDET, Figure 2), although it was found that a minimum of 50% EGBMP was required to revert the polymer into a liquid state, which could be used to create channels when the polymer object was molded within a silicone matrix (Figure 6). Treatment with hexylamine was further used to manipulate the Tg of the polymers, thus tailoring the shape memory recovery temperatures.[122]
The extrusion of high-viscosity monomer/oligomer gels in DIW 3D printing offers accessibility for incorporation of composite components into polymer networks such as SMPs. However, during extrusion, the oligomer is typically exposed to atmospheric oxygen, which can retard the kinetics of (meth)acrylate free radical polymerization and thus limit the processability of these systems. On the other hand, the thiol-ene reaction is generally impervious to oxygen sensitivity, making it a highly appropriate polymerization technique within this AM technology. For example, cellulose nanofibrils (CNF) have been modified with succinyl groups, followed by periodate oxidation to form aldehydes and hydrazide ligation using 6-azidohexanehydrazide, which were incorporated into polymers of allyl glycidyl ether functionalized gelatin (Gel-AGE) and PEG-2SH using DIW printing. Mechanical gradients were achieved for hydrogel by dual printing using two different concentrations of Gel-AGE and PEG-2SH (5 kDa). Furthermore, spatially selective functionally graded polymers were fabricated by additionally incorporating modified CNF-bearing azido moieties that could react through strain-promoted azide-alkyne reaction.[125] Given that Gel-AGE polymers have been identified as suitable materials for long-term cell survivals, their use within biomedical applications is promising.[126] In another example of 3D printing composite materials, biobased levoglucosan has been functionalized to form a trifunctional allyl ether monomer, which was copolymerized with PEG-3SH alongside 12-14 wt% fumed silica (SiF) via DIW printing. The polymer was shown to be fully degradable in 1N NaOH owing to the hydrolytically labile bonds within PEG, while no degradation was observed in 1N HCl or synthetic seawater.[127] This pH-specific degradation provides a useful feature for several applications, such as environmentally targeted biomedical applications.
The use of monomers and oligomers bearing carbonate groups have been established as useful within thiol-ene networks for biomedical applications.[128, 129] With this in mind, the DIW printing of poly(trimethyl propane allyl ether carbonate) oligomers and PETMP alongside a graphite filler resulted in SMPs that could be tailored to change geometry around 30 °C for in vivo biomedical applications. Furthermore, these polymers displayed electrical conductivity (resistivity of 1.99 × 10−6 Ω.m and conductance of 1.37 × 103 S at 30 wt% graphite), and high thermal conductivity, while they were also hydrolytically degradable. Interestingly, the addition of the graphite filler resulted in shear thinning behavior, which was beneficial for effective processing.[130] Continuing within the context of biomedical applications, triblock poly(carbonates) of butylene carbonate and cyclohexene carbonate oligomers have been copolymerized with PETMP using DIW printing. Incorporation of NaCl and dissolution of the oligomers in DMF resulted in thixotropic inks, allowing porous materials to be obtained by aqueous washing of the polymer post-fabrication. These porous structures could also be postfabrication modified by the addition of cell growth factors.[131] Further work using this oligomer for off-stoichiometric DLP printing demonstrated postfabrication modification by grafting alkylthiol chains to tailor hydrophobicity as well as halogenation of the polymer with molecular iodine, while still retaining shape memory capability.[132] In another example, a poly(carbonate) oligomer bearing pendant allyl group has been copolymerized with PETMP using 3D micro-stereolithography to produce degradable structures for tissue engineering.[133] With the aim to increase the toughness of polymers designed for bone replacements, an α,ω-allyl carbonate-functionalized PCL oligomer was incorporated into divinyl ether adipate (DVA, Figure 3) and TMPTMP for DLP printing. It was found that increasing the oligomer content from 0-20 wt% resulted in >250% increase in toughness (UT). Interestingly, no difference in photochemical or (thermo)mechanical properties was noticed between allyl, vinyl, or norbornene functionalization of the oligomer.[134] However, it is likely few examples employing allyl/vinyl carbonates are apparent in the literature given the typical cost associated with these monomers, although new synthesis strategies may open further opportunities.[135]
Semi-crystalline elastomeric polymers are an interesting class of polymers that can facilitate useful properties. For example, diallyl terephthalate (DAT) and 1,6-hexanedithiol (1,6-HDT) were copolymerized to form linear oligomers, which were then further crosslinked with low concentrations (<10 mol%) of various trifunctional allyl ether and allyl acetate monomers (TAT, TABT, TATATO, TATM) and either trimethylolpropane tris(3-mercaptopropionate) (TMPTMP, Figure 2) or PETMP to produce lightly crosslinked semi-crystalline polymers. Here, increasing TAT content decreased crystallinity, and thus after initial screening a monomer ratio of 1:0.95:0.05 of 1,6-HDT-to-DAT-to-TAT alongside 0.1 wt% carbon black was chosen for DLP and SLA printing. It was found that annealing the printed polymers above the melting temperature (Tm) of the crystalline component was imperative to achieve properties comparable to bulk specimens, attributed to interlayer adhesion that occurred during annealing. Indeed, the polymer εT increased from 36% to 532%, UT from 2 MJ m−3 to 59 MJ m−3, while σT increased from 7.4 MPa to 15.9 MPa after annealing.[136] Here, the influence of AM processing on the final polymer properties is highlighted as an important consideration for polymer network design and processing. Furthermore, the influence of dynamic bonds and catalytic control of crystallization has been investigated to gain a greater understanding of this interplay within thiol-ene networks.[137]
FFF 3D printing is typically reserved for thermoplastic and thermoplastic elastomeric polymers owing to its use of melt extrusion for manufacturing, which can present limitations in their achievable properties. On the other hand, a thermoplastic elastomer was prepared through the grafting of methyl thioglycolate to the butadiene blocks of poly(styrene-butadiene-styrene) (SBS) via thiol-ene reaction, which was melt extrudable with FFF printing. It was found that a high incorporation of the polar pendant moiety (98.5%) resulted in the interruption of poly(styrene) clustering typical of virgin SBS, owing to a CH···π interaction between adjacent δ+ CH2 or δ+ CH3 groups of the methyl thioglycolate and the δ- aromatic centre of styrene. The resultant microstructural disorganization, alongside abundant electrostatic interactions, resulted in self-healing capability.[138, 139]
Thermoplastic polymers are typically implausible for use in vat photopolymerization AM, since photopolymerization typically either results in low molecular weight polymers with poor mechanical properties, or the necessary irradiation to achieve acceptable polymers is unfeasibly time-consuming. However, 1,6-HDT and DAT have been successfully employed as bifunctional monomers to produce semi-crystalline linear thermoplastic thiol-ene polymers within vat photopolymerization AM. Indeed, the reaction between 1,6-HDT and DAT was investigated by FTIR, revealing near complete conversions in 3–5 s under low UV intensity. Interestingly, a delayed crystallization was observed that occurred after initial gelation. Given the structural similarities of the monomers used here to those of poly(ethylene terephthalate), many of its well-known molecular attributes such as dipole-dipole interactions of carbonyl groups and π–π stacking could be directly leveraged within this motif. Molecular weights of 104 g.mol−1 were achieved, which was interestingly attributed to near-ideal thiol-ene step-growth with some contribution of homopolymerization of DAT. The polymers also displayed somewhat remarkable mechanical properties, with ET of 75 MPa, σT around 24 MPa, εT of 793%, and UT of 102 MJ m−3. The polymers displayed classic necking and strain hardening in tension, while achieving elastomeric-like strains. The phenomenon of rapid crystallization and overcuring within these polymers initially posed difficulty within SLA and DLP printing, although it was found that the addition of carbon black could effectively act as a photoabsorber, thus mitigating the issue to produce relatively high-resolution objects (Figure 7). It was also noted that crystallization induced shrinkage in the AM-printed polymers. Nevertheless, as is the case for thermoplastic polymers, these could be effectively melt-processed, rendering them conventionally recyclable.[140, 141]

This work was further extended to demonstrate that the addition of a monothiol could be used to effectively decrease molecular weight and increase crystallization rate. Furthermore, the inclusion of 2 wt.% chromium oxide facilitated melting through induction heating, which was shown in fabricated dental molds.[142] This example denotes a remarkable paradigm shift in the molecular architecture achievable within vat photopolymerization AM.
The manufacturing of composite materials is often desirable to increase (thermo)mechanical properties, yet within the context of vat photopolymerization AM this can come with challenges of high viscosity and UV penetration inhibition. Nevertheless, TATATO alongside TMEICN have been used to SLA print composites of hydroxyapatite (HAp) for biomedical bone fixation implants. It was noted that the incorporation of these low-viscosity monomers was imperative to act in a diluent role, thus improving processing and printing resolution. Interestingly, it was established that the incorporation of either micro- or nano-HAp resulted in appreciably different polymers. Here, micro-HAp did increase the resin viscosity, but also successfully increased ET of the polymers. On the other hand, nano-HAp increased the gelation time from 5 min to 4 h, while making no impact on ET of the polymer. As such, a mixture of 0.4 wt% nano-HAp and 36 wt% micro-HAp was chosen for AM, and resulted in non-toxic composites with an ET of 2.4 GPa.[143]
3.2.2 (Thermo)Mechanically Excellent Polymers
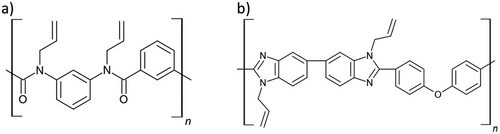
It is well known that protonated amides impart strong intermolecular interactions that can affect oligomer viscosity as well as influence solubility in polar solvents. As such, the secondary amides on poly(N,N′-(1,3-phenylene)isophthalamide) oligomers have been functionalized using allyl bromide, thus imparting functional groups to facilitate thiol-ene polymerization, while also reducing oligomer viscosity. This oligomer was copolymerized with PETMP via DLP printing, using N-methyl-2-pyrrolidone (NMP) as a solvent. The printed organogels were then dried under reduced pressure, leading to 30–35% isotropic shrinkage. The resultant polymers were thermally stable with a 10% degradation temperature of 380 °C. Mechanically, the polymers displayed σT of 88 MPa and εT of 22%, thus achieving strong yet relatively ductile polymers, owing to the incorporation of flexible thioether crosslinks.[144] In fact, similar strength with far greater ductility could be achieved here compared to similar 3D printed polymers that did not incorporate thiol-ene polymerization, although in these examples the use of dimethyl acetamide acrylate as a polar reactive diluent mitigated the necessity for organogel printing.[145, 146]
Poly(benzimidazole) polymers are capable of achieving high strength, extremely high Tg (>350 °C), and thermal stability, yet their application in processing technologies has remained limited. To harness the benefits of these polymers within AM, a poly(benzimidazole) oligomer was functionalized with allyl pendant groups through the facile coupling of allyl bromide to the imidazole groups present on the backbone (Figure 8). This oligomer was copolymerized with PETMP via DLP printing using 30 s layer cure time and N-methyl-2-pyrrolidone or N,N-dimethylacetamide as a solvent (6.7 mL/g monomer formulation). Evaporation of the solvent from the printed polymer resulted in 22% shrinkage, although geometric fidelity was reported to be preserved. Nevertheless, the copolymer displayed a σT of 164 MPa and T10% thermal stability of 397 °C.[147] Interestingly, while similar poly(benzimidazole) polymers have been demonstrated to display shape memory behavior, this research did not investigate such. Furthermore, the use of a polar reactive diluent such as vinyl N,N-dimethylacetamide has been shown as an effective alternative to the aforementioned solvents, which could be explored to mitigate the shortcomings of using a non-reactive solvent.[148, 149]
3.2.3 Incorporation of (Hydroxy)Urethanes
Poly(urethane) polymers and associated polymers containing urethane bonds benefit from strong intermolecular hydrogen bonding and environmental stability,[150-152] making it a logical progression to incorporate this group within monomer/oligomers for AM.[153] For example, an allyl urethane-functionalized poly(ethylene oxide-co-tetrahydrofuran) oligomer has been copolymerized with TMPTMP using DLP printing. A comprehensive investigation into the AM parametric optimization and initiator concentration was performed, establishing suitable printing parameters to minimize the risks of printing polymers for its intended application in energetic materials. Here, the use of the thiol-ene reaction was chosen to minimize UV exposures and potential thermal effects during fabrication.[154]
The incorporation of urethane bonds within thiol-ene polymer networks has also been investigated for biomedical applications using poly(δ-caprolactone) (PCL). PCL is well established as a biobased, biocompatible, and biodegradable polymer, making it a highly attractive candidate for these applications.[155-157] Various linear and star-shaped PCL oligomers have been prepared using polyol cores (ethylene glycol, 1,1,1-tris(hydroxymethyl)propane, pentaerythritol) which were terminally functionalized using allyl isocyanate. These oligomers were then copolymerized with PETMP or TMPTMP via DLP printing using 8–15 s layer cure times. Somewhat counterintuitively, using a pentaerythritol core alongside PETMP displayed the lowest mechanical properties, and also the most rapid degradation under basic conditions. On the other hand, using an ethylene glycol core copolymerized with PETMP displayed the highest mechanical properties, yet still degraded at an acceptable rate (100% within 30 days). This behavior was suggested to be due to differences in crystallinity within the polymer, whereby higher functionality oligomers and monomers led to a decrease in the polymers’ ability to crystallize.[158] This example of architectural control within the polymer network presents an invaluable insight into tailoring polymer morphologies for specific application performance, whereby semicrystalline components within the network could be exploited. Extending this motif using VP 3D printing, linear α,ω-terminated allyl urethane PCL oligomers of varying molecular weights (2.3–8.7 kDa) were copolymerized with PETMP to produce semi-crystalline elastomeric polymers. It was found that crystallite melting occurred below physiological temperatures for the short oligomer blocks, while melting was only observed around 50 °C for longer oligomer blocks, which was similar to thermoplastic PCL (80 kDa), suggesting that the longer oligomers with greater Mc could effectively crystallize to a greater degree. The polymers displayed controlled degradation in 5 m NaOH, with kinetics relating to cross-linking density (27–73% residual mass after 10 days), whereby higher residual mass was related to polymers incorporating smaller oligomers with higher crosslinking density. However, under simulated physiological conditions (37 °C, PBS solution) almost no degradation occurred within a month.[159]
A series of linalool-terminated diurethane monomers have been copolymerized with PETMP and propylene carbonate via DLP printing to produce SMPs. It should be noted that an extensive postfabrication photocuring for 24 h followed by thermal curing at 120 °C for 24 h was performed. Nevertheless, altering the isocyanate (isophorone diisocyanate (IPDI), hexamethylene diisocyanate (HDI), or methylene diphenyl diisocyanate (MDI)) could effectively tailor mechanical properties. Furthermore, the incorporation of hydrogen bonds from urethane components was noted to contribute to shape memory capability; the polymers were reported to achieve excellent strain fixation as well as up to 100% strain recovery within 30 s at 60 °C and 15 s at 80 °C. It was, however, noted that HDI (Tg 47 °C) and MDI (Tg of 35 °C) based polymers displayed more rapid rates at 40 °C than IDPI (Tg of 81 °C), given the difference in their Tg.[160]
Non-isocyanate poly(urethane)s (NIPUs) have been established as a sustainable avenue to impart the beneficial properties of the urethane bond without the use of isocyanates, thus mitigating the use of phosgene during synthesis.[161] Furthermore, the resultant β-hydroxy moiety can facilitate interesting physical properties or serve as a functional group for further synthetic modifications.[162] For example, a NIPU oligomer has been further coupled about the hydroxy moiety to yield allyl functional pendant groups (Figure 9). This oligomer was copolymerized with either PETMP, TMPTMP, ethanedithiol (EDT), or combinations thereof via DLP printing using 1-methyl-2-pyrrolidone as a solvent (2 mL/g oligomer). The polymers were targeted for application in biomedical implants, with biocompatibility studies examining its interaction with human fibroblasts, red blood cells human platelets, and platelet-poor plasma. Using thiols with greater functionality (i.e., PETMP) were shown to have good metabolic activity, reduced hemolysis and clotting times, and increased platelet adhesion.[163]

The effect of monomer functionality on controlling the polymer architecture and resultant mechanical properties has also been explored using NIPUs. Here, a diallyl functionalized non-isocyanate diurethane monomer was prepared using an allyl functionalized carbonate and diamine, which was then copolymerized with either PETMP, TMPTMP, 1,6-HDT, or combinations thereof using DLP printing. Varying the thiol monomer resulted in the ability to control the polymer architecture from linear to crosslinked networks, thus controlling the mechanical properties. Using these monomer systems, a multi-material compliant mechanical device was printed and demonstrated as a directionally biased semi-rigid gear with rotational dampening of acceleration, thus highlighting the control of rigid (using PETMP) and flexible (using 50% PETMP and 50% 1,6-HDT) tailorability of the polymers for functional application (Figure 9). Furthermore, the biocompatibility of the polymers was investigated and showed no acutely toxic effects, while low cell adhesion characteristics could make them suitable for applications of anti-fouling.[164] Within this context, thiol-ene polymers have been shown as appropriate polymers for hard and soft networks and dissimilar materials without suffering weak interfaces, making them highly suitable for this type of multi-material.[165-167]
Poly(propylene glycol) has been α,ω-functionalized with diallyl hydroxyurethane functional groups and copolymerized with TMPTMP via DIW printing, followed by postfabrication curing at 60 °C in a UV oven. It was observed that the allyl hydroxyurethane functionalization strongly increased the oligomer viscosity, attributed to hydrogen bonding. Furthermore, the oligomers demonstrated Newtonian shear viscosity behavior between 100 and 2.5 s−1, which alongside the high viscosity, made it a highly appropriate formulation for DIW printing. Interestingly, 3D printed specimens displayed higher gel content and tensile properties compared to bulk polymers, indicative that successful crosslinking between layers was achieved.[168] Lastly, an area of opportunity for the facile synthesis of urethane or amide-bearing monomers suitable for AM is using allyl-functionalized thiolactones, presenting opportunities for biobased developments that could be readily transferred into AM.[169-171]
3.2.4 Poly(Siloxane)s
Silicon-based polymers can be achieved in photopolymerization AM through the incorporation of thiol- and alkene-functionalized poly(siloxane) oligomers and silicon-based allyl and thiol monomers (Figure 10).[36, 172] An early example in AM used α,ω-vinyl terminated poly(dimethylsiloxane) (α,ω-vinyl PDMS) and poly(mercaptopropylmethylsiloxane-co-dimethylsiloxane) (PMMS-co-PDMS) (Figure 10) to SLA print soft robotic pneumatic actuators with self-healing capability. Self-healing was assisted by encapsulation of the pre-polymer, which could be released when a puncture occurred, thus rapidly polymerizing on exposure to sunlight. This work also investigated the effect of varying MW of α,ω-vinyl PDMS (186–43 000 Da) and thiol concentration of PMMS-co-PDMS (2–3% to 4–6% mercaptopropyl content), whereby properties could be tailored by adjusting these two oligomer components.[173] In another example, copolymers of α,ω-vinyl PDMS and PMMS-co-PDMS were produced with varying vinyl-to-thiol ratios. A ratio of 1:1.5 produced optimal curing kinetics and tensile properties (σT of 0.2 MPa and εT of 158%), which was used for SLA printing. The printed copolymer was also antimicrobial and biocompatible, hence providing viability for biomedical applications. The polymers were also mask photopatternable by incorporation of a red pigment, making them useful for applications such as chips and microfluidic devices. However, some issues were observed for printing resolution, and layer height discrepancies were reported, whereby for a programmed layer height of 50 µm a measured layer height of only 10 µm was produced.[174]
The effect of different pendant groups on poly(siloxane)s has been explored, showing that incorporating phenyl pendant groups imparted π–π stacking and thus facilitated high toughness.[175] Amorphous terpolymers have also been prepared using several pendant-substituted siloxanes (dimethylsiloxy, diphenylsiloxy, and diethylsiloxy) that allowed SLA printing of tailorable polymers. However, here it was noted that diphenylsiloxy incorporation had deleterious effects attributed to uncontrolled branching.[176] On the other hand, allyl- and thiol-functionalized poly(fluorosiloxane) oligomers have been shown as rapidly UV curable (5 s) with good hydrophobicity and corrosion resistance, making them promising candidates for AM.[177] Within the context of rapid polymerization, poly(siloxane) oligomers α,ω-functionalized with norbornyl groups have been copolymerized with PMMS-co-PDMS. Compared to α,ω-vinyl PDMS, the norbornyl functionalized oligomers displayed 3× faster curing and 5× less oxygen inhibition, with complete overall cure and without tacky surfaces.[178] Although this was not demonstrated within AM, it provides a promising platform for the use of functional groups with rapid kinetics for reducing layer cure times.
The viability of high molecular mass poly(siloxane) oligomers has been explored for DIW printing with the aim of producing soft elastomeric polymers with dielectric capability. Here, PMMS-co-PDMS (5% mercaptopropyl content) in the range of 7–16 kDa and poly(methylvinylsiloxane-co-dimethylsiloxane) (9% vinyl) (PMVS-co-PDMS) in the range of 55-260 kDa were assessed. High molecular masses, pendant functional groups, and not adding a filler were strategically chosen to enhance low modulus, highly elastic, and efficient dielectric properties. It was found that PMVS-co-PDMS of 55 kDa and PMMS-co-PDMS of 7 kDa was an effective formulation for AM. An optimal thiol-to-vinyl molar ratio of 0.047 was also established to ensure residual vinyl groups for interlayer adhesion. DIW printed grids were shown to withstand 100 compressive cycles to 50% strain without hysteresis, ET of 0.29 MPa, and <2% stress decay after loading to 200% strain for 100 min. Furthermore, the material was demonstrated as a dielectric, displaying an electric field of 41 kV mm−1 at 9.5% strain, which was unaffected by electric field frequencies up to 10 Hz.[179]
It is well known that the incorporation of a filler such as SiF can improve the mechanical properties of poly(siloxane) polymers. Additionally, the incorporation of fillers typically leads to an increase in system viscosity, which can be exploited in DIW printing. For example, DIW printing using poly(siloxane) oligomers with pendant thiol- and vinyl-functional groups of varying molecular weights and functional group concentrations were coextruded to produce multiple modulus poly(siloxanes). Here, by altering the relative ratio of coextruded components (up to three simultaneously), spatially selective property tailoring was achieved. Furthermore, the incorporation of 2 wt% SiF could be used to improve mechanical properties. The printed polymers were demonstrated to achieve high aspect ratio objects, overhanging structures, and discontinuous flow printing, making the fabrication of complex objects possible.[180] Another example of DIW printing developed a functionalized poly(siloxane) oligomer that could serve as both an extrudable ink, a medium for embedded printing of microfluidic devices, as well as a castable medium for a printing support bath. Here, PMMS-co-PDMS and α,ω-vinyl PDMS were copolymerized alongside hexamethyldisilazane-treated silica and a thixotropic agent. When employed as a medium for embedded printing, the polymer was employed as an organogel. Here, the optimization of self-recovery and yield stress was achieved by tuning the relative incorporation of oligomers and filler, which alongside its excellent optical transparency, enhanced its use for embedded printing. Next, it was found that the treated silica filler content was vital to optimize for use as a support bath. However, using a water-based ink within the hydrophobic polymer support bath resulted in resolution and geometric limitations. Nevertheless, microfluidic channels could be fabricated. Lastly, the ink was also demonstrated as an extrudable medium for DIW printing within a support bath. It was reported that the correct support bath medium (xanthan gum) was critical for successful printing, and this optimization led to the successful fabrication of soft robotic objects with embedded piezoresistive pressure sensor components.[181]
Despite the increase in system viscosity that is observed when silica is added as a filler, several examples have successfully employed these systems within vat photopolymerization AM. For example, α,ω-vinyl PDMS and PMMS-co-PDMS (4-6% thiol) oligomers alongside up to 33 phr SiF as a filler have been DLP printed using a 7 s layer cure time. Here, the system viscosity exceeded 100 000 Pa.s, which meant that a custom printer with rotational vat and wiper for recoating was necessary. Nevertheless, the printed copolymers were shown to have tuneable mechanical properties and εT up to 350%.[182] In another example, DLS printing was used to minimize flow pressure within viscoelastic suspensions incorporating SiF. The pressure difference (low pressure under print platform as head moves up) within a DLS printer was used to induce flow of the high viscosity system, and the non-dimensional Peclet number (Pe) for the system was calculated to understand processing limits. Here, α,ω-vinyl PDMS (7 kDa) and PMMS-co-PDMS (6 kDa, 4–6% mercaptopropyl) were copolymerized alongside SiF nanoparticles with 50–500 nm aggregate sizes. It was found that increasing SiF content increased toughness, until Pe was exceeded (μapp around 60 Pa·s, τy around 6 Pa) and printing was no longer viable. This work supported a hypothesis that liquid fracture caused breaking of the seal within the system during printing, and thus provided a basis to quantify the critical limit whereby the phenomenon occurs to mitigate deleterious printing parameters.[183] In another example employing silica as a filler, α,ω-vinyl PDMS and PMMS-co-PDMS were DLP printed alongside the addition of either SiF or precipitated silica (SiP). Increasing the content of SiP resulted in a dramatic increase in mechanical properties; unfilled polymers displayed σT of 0.30 MPa and εT 311%, while 15 wt% SiP displayed σT of 1.69 MPa and εT of 1107%, and 20 wt% SiP displayed σT of 2.59 MPa and εT of 1403%. The use of SiF displayed the same trend, although overall lower strength and elongations were observed, which was attributed to differences in morphology and surface functional groups. However, this trend was not observed when using lower molecular weight oligomers, whereby although σT increased, εT decreased with increasing silica content. The copolymers were then coated with carbon nanotubes and demonstrated as effective conductors for flexible electronics.[184] On the other hand, to overcome the high viscosity of many poly(siloxane) oligomers and addition of fillers that can negatively affect vat photopolymerization AM, the incorporation of a low viscosity chain extender monomer (1,6-HDT) has been used. Here, 50 wt% 1,6-HDT alongside 15 phr silica filler were incorporated into α,ω-vinyl PDMS and PMMS-co-PDMS, which rendered a system with sufficiently low viscosity (3.9 Pa·s) for DLP printing. The resultant polymers demonstrated εT of 601%, which was appreciably higher than polymers printed without 1,6-HDT and filler. Furthermore, acceptable resolutions could be achieved, although only qualitative examples were shown.[185]
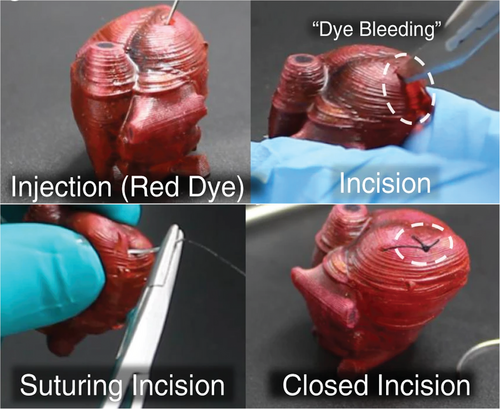
The use of multiple curing mechanisms of poly(siloxane)s has been demonstrated to synergistically enhance the properties of AM polymers and introduce the capability for shape memory and self-healing. Double network polymers were prepared by mixing a Sn-cured room temperature vulcanizing (RTV) poly(siloxane) alongside vinyl- and thiol-functionalized poly(siloxane) oligomers. Here, the mixture was SLA printed via thiol-ene polymerization, followed by slow room temperature condensation polymerization of the RTV poly(siloxane) component to form the double network polymer. These polymers displayed high εT (up to 400%) and UT (1.37 MJ m−3), while maintaining a moderate modulus. Furthermore, the properties were tailorable by changing the RTV poly(siloxane) formulations. Properties such as toughness, strength, and elongation were also magnitudes of order higher than the pure thiol-ene polymers and other analogous poly(siloxane)s. The polymers were used to print an artificial heart for educational surgical practice, whereby the tuneable mechanical properties could be exploited to match the properties of various tissue materials within the body, providing a versatile platform for tissue emulation Furthermore, the self-healing capability could be used to emulate sutured wounds (Figure 11). The polymers were also used to fabricate fluidic actuators for soft robotics and applied in several useful contexts, such as being adhered to orthotic gloves and incorporated onto heat-sensitive LEDs. Adhering of multi-materials could also be achieved through the incorporation of condensable groups at the surface, thus enhancing its adhesion properties between several materials such as glass, aluminum, thermoplastic polymers, and various thermosetting polymers.[186]
Dual network polymers combining an RTV poly(siloxane) alongside photopolymerisable thiol-ene poly(siloxane)s was further extended for use in DIW printing to fabricate multi-material polymeric foams. Here, three oligomer systems were kept in separate pumps: 1) containing α,ω-vinyl PDMS, PMMS-co-PDMS, and RTV poly(siloxane) part A, 2) containing RTV poly(siloxane) part B, and 3) containing α,ω-vinyl PDMS, PMMS-co-PDMS, RTV part A, and ammonium bicarbonate as a foaming agent. These pumps lead to a central nozzle, allowing for control of concentration of the relative oligomer systems to be supplied and varied during printing, thus facilitating spatially controlled multi-material printing. This system provided a highly versatile platform to achieve polymers with both varying thiol-ene and RTV contributions, as well as varied degrees of foaming.[187] The conceptual benefits of double network polymers synergistically enhancing the properties of the overall polymer beyond that of its individual components demonstrate an exciting potential within AM.[188]
The use of multiple crosslinking mechanisms has been extended to the incorporation of covalent thiol-ene bonds alongside physical crosslinks by ionic interactions. A system using a mixture of PMMS-co-PDMS, α,ω-vinyl PDMS, and pendant amine- and carboxylic acid-functionalized poly(siloxane) oligomers were SLA printed, leading to polymers that contained thiol-ene crosslinks and thermally reversible carboxy-amine ionic interactions. Interestingly, the incorporation of these ionic moieties did not strongly influence polymerization kinetics or overall conversion. On the other hand, the incorporation of these ionic interactions resulted in copolymers with relatively low temperature self-healing capability (100 °C), which were able to retain 98% tensile strength compared to original specimens. Here, greater concentrations of ionic moieties promoted self-healing, and slow room temperature self-healing was even demonstrated when sufficiently high concentrations were used. Additionally, the copolymers could be reprocessed, retaining 85% of their original strength. Lastly, they were also demonstrated as having good optical transparency (>90%) and as hydrolysis resistant.[189] Within the context of self-healing poly(siloxane) materials, a photoswitchable poly(siloxane) thiol-ene polymer has been produced that was capable of UV-activated self-healing. Here, a thiol oligomer was prepared between tri(ethylene glycol)divinyl ether and EDDET, followed by oligomerization with PETMP, which was finally copolymerized with α,ω-vinyl PDMS. Interestingly, self-healing, viscosity reduction, and remoulding was observed under UV irradiation. An excess of free thiols was necessary to observe photoswitching, where thiol-to-vinyl ratios ≥1:3 displayed these dynamic properties. It was considered whether disulfides or hydrogen bonding was responsible for this behavior, yet these mechanisms are thermally dependent, and this system did not exhibit such dependence. It was thus suggested that this was indeed a radically mediated mechanism.[190] While not demonstrated for AM, this poses an interesting contextual opportunity for inducing DCC both during and after the fabrication process, such as facilitating dynamic interlayer adhesion during AM.
Solid-solid phase change materials have been explored for regulation of latent heat, which can store and release thermal energy at near constant temperature during transfer. A series of poly(dimethylsiloxane-co-methylvinylsiloxane) oligomers of varying vinyl content were prepared and copolymerized with 1-octadecanethiol (ODT) and 1,6-HDT alongside 3 wt% hydrophobic SiF using SLA printing at 50 °C. The resultant polymers had a comb/bottlebrush morphology, which could be exploited for latent heat regulation. Here, the phase change behavior and latent heat could be tailored by ODT content, and the polymers displayed thermally actuated shape memory, attributed to the entropic elasticity of the ODT components melting. Chemical depolymerization and reprocessing were also possible with these polymers. Furthermore, the production of carbon fiber composites with the polymers displayed photonic/electrical responses, and skin-adhesive properties.[191] Another network polymer incorporating crystallizable alkyl thioether pendant groups has also been shown to achieve thermal energy storage capability, adding credence to this strategy of employing thiol-ene moieties.[192]
Beyond the context of AM, several poly(siloxane) thiol-ene copolymers have been shown to achieve luminescent properties. For example, a system employing allyl-functionalized poly(siloxane) oligomers has been further modified by thiol-ene coupling of several thioacids to enable coordination with Eu3+. Following this, the oligomers containing residual vinyl groups were copolymerized with PETMP to produce photoluminescent network polymers under 617 nm excitation.[193] This work was extended to investigate the incorporation of fluorescent dyes into poly(siloxane) network polymers, as well as the incorporation of imidazole into the poly(siloxane) backbone for switchable fluorescence.[194, 195]
3.2.5 Polymer Derived Ceramics
Polymer derived ceramics (PDC) present another area of opportunity for thiol-ene polymerization with silicon-based polymers.[196-199] For example, functionalized poly(carbosilane) and poly(carbosilazane) oligomers have been DLP printed, thus opening the door for a variety of additively manufactured PDCs. These PDCs were noted to show uniform shrinkage and high density, with manufactured SiOC lattices displaying exceptional specific compressive strength.[200, 201] Further work on SiCNO PDCs demonstrated 41% ceramic yield, a relatively low shrinkage of 24%, and hardness of up to 6.6 GPa.[202] Extending the scope of additively manufactured PDCs, a cyclosiloxane hybrid polymer (CHP) has been prepared through hydrosilylation reaction between 2,4,6,8-tetramethyl-2,4,6,8-tetravinylcyclotetrasiloxane and 2,4,6,8-tetramethylcyclotetrasiloxane using Karstedt's reagent as an organo-platinum catalyst.[203, 204] The resultant CHP retained residual ene functionality, making it useful for further thiol-ene reaction during AM. Here, PDMS-co-PMMS with varying thiol concentration (20–50%) was prepared, with photorheological investigations establishing an optimized oligomer system of 40% thiol concentration for PMMS-co-PDMS. This oligomer was copolymerized with CHP (1:1 ene-to-thiol) via DLP printing using 50 µm layer height and 2 s layer cure time. These polymers displayed a low critical energy for gelation of around 10 mJ cm−2, attributed to the high thiol branching degree. Furthermore, the oligomer system had a relatively low viscosity of 380 mPa·s, making it suitable for vat photopolymerization AM. The printed polymers qualitatively displayed low shrinkage, while pyrolysis of the polymer at 1000 °C resulted in 25% isotropic shrinkage, with residual weights up to 68% and without macroscopic voids or defects. Further pyrolysis was then performed at higher temperatures to obtain a semi-crystalline state, whereby the cubic phase appears from 1400 °C. It was unfortunate that no mechanical properties of the final PDC were reported. Nevertheless, the versatility of the polymer was extended beyond its use as a PDC by incorporating 0.5 wt% of Ti3C2Tx as a multilayered structure with light-to-heat conversion properties. This achieved a photothermal effect within the polymer, which was suggested as a useful feature for application in electromagnetic shielding devices. Lastly, a commercial fluorescent “invisible ink” was also incorporated into the oligomer system, which resulted in printed polymers that fluoresced under 365 nm light, which was suggested as a useful feature for anti-counterfeiting marks.[205]
3.2.6 Micro-Structured Polymers
Polymers with micro-level architectures have been identified as promising materials in several applications, including microfluidic devices and biomedical materials. As such, the thiol-ene reaction has been identified as an effective polymerization strategy for photolithography[206] and soft lithography[207-209] of microfluidic devices with 2.5D patterning of hydrophilic/hydrophobic polymers[210] and hydrophilic microfluidic devices.[211] Several early examples of 3D microfluidic devices were prepared using casting and contact liquid photolithographic polymerization (CliPP) fabrication, presenting a viable basis for transfer into other AM technologies.[211, 212] Within this context, an off-stoichiometric approach has been used to DLP print molds for casting microfluidic devices using PDMS.[213] Adding credence to this, a photostructuring effect in off-stoichiometric (excess thiol) monomer compositions can occur. Here, diffusion-induced monomer depletion at the photomask interface can allow intricate composition gradients, thus minimizing feature broadening and enhancing print resolution fidelity.[214] For example, an off-stoichiometric formulation of TATATO with either TMPTMP or PETMP (50% excess thiol) has also been used to produce microfluidic chips using photolithography and soft lithography. The material-induced oxygen scavenging of the off-stoichiometry could be exploited to tailor the oxygen concentration within the microfluidic devices to facilitate physiologically relevant conditions for immobilized enzyme reactors within the polymer.[215] In another example, an off-stoichiometric approach has also been demonstrated using allylsilanes for microfluidics.[216] Moving towards direct AM of microfluidic devices, an early example demonstrating the potential of thiol-ene chemistry within the field used PETMP, TMPTMP, 1,6-HDT, and TEGDAE copolymerized in varying molar ratios using DLS fabrication to produce tailorable, low modulus, microfluidic channels.[212] Within this context, it is known that photoblockers are often imperative to avoid feature broadening in high-resolution photomasking for vat photopolymerized thiol-ene systems, thus facilitating high-resolution fidelity for microfluidics.[207] Lastly, a custom DLP fluorescence microscope has been built that could achieve micro-3D printing. Here, thiol-ene polymers were chosen to obtain high resolutions, adding credence to the capabilities of thiol-ene chemistry for high-resolution AM.[217]
Porous materials offer an alternative mechanism to achieve microfluidic architectures.[218] Microporous structures have been prepared using AM alongside a porogen, which served as a solvent in which the monomer was miscible but the polymer insoluble, thus facilitating polymerization-induced phase separation (PIPS). Here, TMPTMP and TATATO were dissolved in methanol at 60% v/v. Initial runs using photolithography were performed, followed by DIW printing using poly(vinyl alcohol) (PVA) as a binder. Porous structures were then obtained by submersion of the polymer in water, obtaining pores with 66–310 µm diameter.[218] As a greater understanding of PIPS within AM is being established, it may bring more development of its application within the field.[219] Another area of current interest lies in the fabrication of aerogels, which can deliver exceptional specific strength and thermal properties. Given that bulk shape memory aerogels have been demonstrated using thiol-ene networks[220] and the advances in using AM for aerogel fabrication,[221-223] this presents and area of interesting development.
3.2.6.1 Incorporation of Thioesters
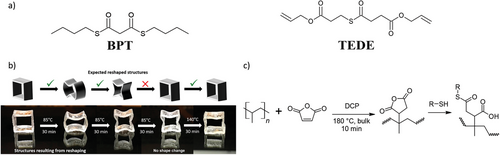
Thioester bonds present structural similarity to esters, yet the thioester is relatively more flexible owing to the increased bond length between carbon and sulfur due to the lower polarity of the sulfur atom compared to oxygen.[224, 225] Moreover, owing to their electronic orbital configuration, thioesters are susceptible to nucleophilic attack, making them effective acyl transfer agents.[226] Polymers that contain thioesters and free thiol groups can also undergo transthioesterification, amidation, hydrolysis, and ligation.[227] In an example that produced bulk polymers purely based on thioester bonds, transthioesterification occurred in the presence of a suitable catalyst, such as tin(II) 2-ethylhexanoate (Sn(Oct)2). Here, thioester moieties were introduced using S,S-dibutyl propanebis(thioate) (BPT, Figure 12), and copolymerized alongside PETMP. First, in the absence of Sn(Oct)2, no transthioesterification exchange was observed up to 110 °C. On the other hand, in the presence of 5 mol% Sn(Oct)2, exchange and resultant stress relaxation at temperatures above 100 °C were observed, yet the resultant polymers were creep-resistant at lower temperatures. The thioester-to-thiol molar ratio was adjusted between 0.5 and 0.7 equivalents of thiol, with an optimal ratio of 0.5 equivalents reported. It was also found that dynamic exchange was observed at room temperature in the presence of a tertiary amine and polar solvent. Furthermore, these polymers were depolymerizable for chemical recycling by utilizing a monofunctional thiol, butanethiol, and heating to 90 °C for 9 h. Lastly, the polymers were also biodegradable.[228] To gain a further understanding of the controlled degradation through thiol-thioester exchange this reaction has been modeled, revealing that the oligomer length played a significant role in degradation, while concentration and dispersity of thioesters within the oligomer structure played a less dominant role.[229] Extending this understanding toward AM, a thorough investigation into the thiol-thioester exchange in DLP-printed polymers was conducted. Here, DAA, several thiol comonomers (PETMP, TMPTMP, di-PETMP), alongside a thioester containing monomer (TEDE, Figure 12) were used. Initial kinetic model experiments were performed for the controlled degradation of the thioester polymers using TEA and BMP. This ascertained that an increase in initial free thiol concentration and ratio of base catalyst increased degradation kinetics, while increasing monomer functionality lowered kinetics. Furthermore, the influence of wall thickness on degradation rate of SLA printed polymers showed that for wall thicknesses <<3 mm the rate was independent of thickness as bulk degradation dominated, while at wall thicknesses >>3 mm surface degradation dominated; at 3 mm these models converged as a function of both bulk degradation and surface degradation.[230] The thiol-thioester exchange has also been exploited as a DCC in DLP printed polymers by incorporating TEDE, which was copolymerized with 1,10-decanedithiol (DDT) and PETMP. The printed polymers were semi-crystalline, attributed to a combination of the inclusion pyrogallol as a photoblocker, which could also behave as a nucleating agent, alongside using a decreased light intensity to retard polymerization rate and thus facilitate molecular ordering. Furthermore, adjusting the stoichiometric ratio of thiols, both “tough” (0.95:0.05:0.095 of TEDE-to-PETMP-to DDT) and “elastic” (1:0.15:0.085 of TEDE-to-PETMP-to DDT) polymer networks could be prepared. This molecular tailoring was attributed to an increase in crystallinity and decrease in crosslinking density when excess thiol was present. However, postfabrication annealing at 100 °C for 1 h was imperative to mitigate deleterious effects of crystallization at layer interfaces during 3D printing. After annealing, the polymer displayed UT in excess of 16 MJ cm−3. Alongside these excellent mechanical properties, the polymers were also chemically depolymerizable, reprocessable, shape memory, and self-healing. Here, the polymers were depolymerizable using 8× molar excess in PETMP and 1 molar equivalent TEA in acetone, whereafter the depolymerized mixture could then be directly repolymerized by DLP printing after solvent removal, since the thiol-thioester exchange preserves the functional groups on the monomers. The repolymerized printed polymers displayed only minor decreases in properties compared to original specimens.[231]
Thioesters have also been used to enable controlled degradation in hydrogels, which is of particular value within biomedical contexts.[232] For example, PEG-3SH and PEG 3-arm thioester norbornene monomers were copolymerized using DLP printing to fabricate sacrificial 3D organoid elastomeric hydrogel structures. The use of thioester functional groups within the polymers promoted controlled degradation, whereby dissolution occurred within 2 h when submerged in mercaptoethanol solutions.[233] In another example, DLP printed copolymers of PETMP and TEDE could be tailored by adjusting the stoichiometric ratio of thioester and thiols in the system to tune the depolymerization of the polymers into different molecular weight oligomers, where higher incorporation of thioesters resulted in low viscosity depolymerized oligomers. The depolymerized oligomers could then be repolymerized by DLP printing without appreciable losses in mechanical properties. This conceptual basis was also extended to introducing a variety of different functionality thiol monomers, allowing for tailorable mechanical properties.[234]
The reaction between cyclic acid anhydrides and thiols results in the ring opening of the cyclic acid anhydride to form a monothioester. Furthermore, the use of α,β-unsaturated cyclic acid anhydrides such as maleic anhydride and multifunctional thiols can result in a cascade reaction of anhydride ring opening to form the monothioester, followed by thiol-Michael addition reaction about the unsaturation. On the other hand, unsaturated cyclic acid anhydrides such as allyl succinic anhydride can form the monothioester, followed by controlled thiol-ene polymerization about the unsaturation. The resultant thioester bonds with proximate carboxylic acid groups have been demonstrated as a highly dynamic covalent linkage, where dehydration of the thioester and proximate pendant carboxylic acid reform the anhydride.[235-237] Furthermore, an extensive effort to understand and optimise the effect of thiol substitution and influence of base and nucleophile catalysts to promote the DCC has been reported, where it was found that primary substituted thiols and strong nucleophilic catalysts were most effective.[230, 235, 237, 238]
The relative contribution of both reversible addition and reversible exchange within these systems has also been established and investigated. Here, the basicity and/or nucleophilicity of the catalyst alongside the stoichiometry of the system both influenced the relative reversible addition and/or reversible exchange that occurred within the polymer.[241] An mSLA-printed polymer was successfully prepared using this chemistry with allyl succinic anhydride, TMPTMP, and diallyl carbonate. The polymers displayed rapid exchange kinetics at 80–140 °C, but were stable at temperatures below 60 °C. A remarkable feature of the polymer was that the exchange catalyst, 4-dimethylaminopyridine (DMAP), could be deactivated and thus erase the polymer's stimulus responsiveness. This was also remarked as a rare instance where DCC activation could be changed without overall detriment of the polymer network. Of particular interest was that geometric reconfiguration could be achieved after 30 min at 85 °C, whereby if the catalyst was then deactivated no further shape change could be reconfigured, but subsequent shape memory to the original geometry could be achieved by heating to 140 °C (Figure 12). While this catalyst deactivation demonstrates highly promising potential, the method of deactivation (swelling in acetone followed by immersion in HClg) may require refinement to less harsh conditions for practical application. Nevertheless, the polymer was also shown as depolymerizable at 120 °C by using an appropriate dithiol. The depolymerized oligomer/monomer could be repolymerized without appreciable loss in properties.[239]
Thioesters have been introduced into polymers for FFF AM by radical grafting of maleic anhydride onto the backbone of poly(propylene) using dicumyl peroxide at 180 °C for 10 min via reactive screw extrusion, followed by copolymerization with PETMP (Figure 12). The resultant polymer was semi-crystalline, endowed with thioester dynamic covalent crosslinks. A 6% crosslinked polymer was FFF processed at 220 °C, which is well within the acceptable temperature range for commercial 3D printers. Incorporation of the thioester crosslinks afforded a 25% increase in strength, while the polymer was also readily reprocessable, chemically recyclable, and reweldable.[240] This strategy could provide a promising platform for the grafting of maleic anhydride onto a variety of other commonly used thermoplastic polymers in FFF.[242-245] Indeed, the well-known shape memory properties of PLA could be further explored with the inclusion of dynamic crosslinks to extend its capabilities and applications.[246]
3.2.7 Incorporation of Boronic Esters
Boronic esters can undergo dynamic hydrolysis and re-esterification as well as transesterification reactions that provide highly dynamic covalent bonds, with activation of DCC occurring even under ambient conditions.[247, 248] While the ambient activation of DCC may be desirable, it can also lead to polymers with poor creep resistance. On the other hand, employing thiol- and ene-functionalized boronic ester monomers (vinyl dioxaborolane (VDB), allyl dioxaborolane (ADB), thiol dioxaborolane (TDB), Figure 13) has been demonstrated to provide polymers with improved creep resistance while retaining self-healing capabilities. Manipulation of the relative concentration of boronic esters and free 1,2-diol moieties has also been shown as an efficient method for tailoring the type of dynamic exchange mechanisms available within the polymer network. Here, dynamic hydrolysis and re-esterification could be achieved with 1:1 boronic ester-to-diol, while excess diol led to dynamic hydrolysis, re-esterification, as well as transesterification.[249, 250] Indeed, it was found that by combining “stable” thioether crosslinks alongside “dynamic” boronic ester crosslinks the temperature of self-healing could be tuned, while not compromising mechanical properties.[251] Further to this, copolymers of ADB, DEGVE, and EDDET could be used to efficiently produce “liquid-solid” plasticity polymers. Here, this “liquid” plasticity was found to be strain-rate dependent, whereby under low strain rates boronic ester association led to liquid-like flow, while high strain rates resulted in insufficient time for boronic ester association and resulted in a higher modulus elastic response. This behavior could also be tailored by the relative incorporation of ADB into the copolymers, which could be used to control shape programming and allowed them to be reshapable at room temperature by applying an external force for just 30 s.[252] While this room temperature reshaping capability may be useful in specific contexts, its implied lack of structural integrity may be limiting in other applications.
The incorporation of boronic esters into thiol-ene polymers has been investigated for AM-produced bone regeneration scaffold applications. Here, TMT was copolymerized with either VDB or ADB using a 1:1 vinyl-to-thiol ratio. An analysis of cytotoxicity was performed with EC50 values ranging from 2.5 (TMT) to >10 mM (VDB), suggesting low cytotoxicity. However, bulk polymerized specimens demonstrated an appreciable difference in mechanical properties between using VDB and ADB. ADB displayed ET of 1.31 GPa, σT of 67 MPa, εT of 11%, and UT of 4 MJ m−3, while ADB displayed ET of 0.31 GPa, σT of 14.6 MPa, εT of 313%, and UT of 20.8 MJ m−3. These differences were attributed to the less flexible functional groups on VBD. Subsequently, a copolymer of TMT and VDB was produced by SLA printing at 55 °C using 70 mW cm−2 power, 100 mm s−1 writing speed, and layer thickness of 100 µm. This increased temperature was necessary as VDB is insoluble in TMT at room temperature. Interestingly, the incorporation of a photoabsorber led to both improved printing resolution as well as increased mechanical properties. The polymers were shown to have low (<1%) swelling in PBS solution (pH 7.4), yet rapidly degraded by 57% and 55% after 7 days for ADB and VDB, respectively.[253] In another example, ADB, DAP, and PETMP have been DLP printed at 90 µm layer height and 25 s layer cure time. Varying the ratio of the “dynamic” ene (ADB) and “static” ene (DAP) monomers found that a) little stress relaxation occurred without the addition of ADB, b) there was a stretched exponential decay for stress relaxation time with increasing ADB content, and c) >60 mol% ADB resulted in little difference in stress relaxation times. For DLP printing, 15 mol% incorporation of ADB was found to be optimal with no visible creep at room temperature, whereas 30 mol% led to failed printing and creep occurring immediately after printing. Other notable properties of the DLP printed polymers included multipart welding at relatively low temperature (65 °C for 16 h) as well as the ability for orthogonal postfabrication modification by boronic ester exchange, evidenced by the selective addition of a boronic acid fluorophore, 3-(dansylamino)phenylboronic acid (DAPBA, Figure 13) into the polymer structure. Both a potential opportunity or challenge depending on the application, the boronic esters were shown to undergo facile hydrolysis in water for these systems.[254]
ABT, α,ω-vinyl PDMS, and 1,6-HDT have been used in DIW 3D printing to fabricate aortic root phantoms that were capable of self-healing and elastic properties, yet without displaying creep under service conditions. To achieve this, a complex layered multi-material architecture was attempted, which contained an inner grid-like crosslinked layer sandwiched between outer self-healing layers (Figure 13). This was achieved by using two different poly(siloxane) polymers, one of which was a thiol-ene elastomeric polymer containing α,ω-vinyl PDMS and 1,6-HDT, while the second incorporated TDB alongside α,ω-vinyl PDMS for dynamic exchange to facilitate self-healing. Furthermore, SiF was incorporated into both oligomer systems to optimise processing using DIW printing. Bilayer structures using 50:50 components demonstrated that self-healing at room temperature resulted in 40% recovery of strength after just 5 min and up to around 90% could be achieved after 24 h, while a minimum of 25% elastic layer was necessary to mitigate creep.[255]
In another example using poly(siloxane)s, octamethylcyclotetrasiloxane, DV4, and DTMS have been used to prepare oligomers containing both pendant and terminal vinyl functionality, which were then partially reacted with N-acetyl-L-cysteine. This oligomer was then copolymerized with TDB using DLP printing. The polymers displayed fluorescence under UV irradiance, attributed to the TDB within the network causing synergistic restricted energy dissipation. The polymers were also capable of self-healing and shape memory. For shape memory, shape programming was conducted at 100 °C, attributed to rearrangement of hydrogen-bonds from N-acetyl-l-cysteine moieties. On the other hand, the dynamic boronate ester exchange was noted to occur at a maximum rate at 150 °C. Near complete shape recovery could be achieved within 60 s and could then recover to its programmed shape on reheating. Shape fixation rates of 95% and shape recovery rates of 97% could be achieved repeatedly through cycling the polymer between room temperature and 100 °C. Interestingly, the shape fixation rate decreased slightly with increasing TBD content, while shape recovery rate increased dramatically. These phenomena were attributed to increased crosslinking within the polymers with increased TBD content. Self-healing was attributed to a combination of hydrogen bonds and dynamic boronate ester groups, whereby self-healing efficiencies up to 99% could be achieved, with a proportional increase in efficiency with increasing TBD content. Lastly, the polymers were also shown to be antimicrobial, attributed to the amphoteric ions of N-acetyl-L-cysteine to disrupt adsorption.[256]
Beyond the scope of AM, the use of proximate functional groups has been shown to influence the relaxation kinetics within poly(siloxane)s incorporating boronic esters, effectively inducing distinct dynamic chemistry (within an order of magnitude relative to one another) within the architecture.[257] Lastly, considering boronate esters’ potential for therapeutic bioconjugates, one may anticipate their further development within AM biomedical contexts.[258]
3.2.8 Incorporation of Orthoester(Acetal)s and Acetals
Although poly(orthoesters) benefit from cytocompatibility and rapid surface erosion, they are typically mechanically weak and suffer difficulty in effective processing. To overcome these limitations, poly(orthoester-thioether) polymers have been developed by using unsaturated orthoester monomers.[259] For example, SLA printing of an unsaturated orthoester (UOE, Figure 14) with PETMP using propylene carbonate as a diluent was performed, followed by thermal postcuring at 120 °C for 120 h under vacuum. However, it was noted that after approximately 5 h of printing, insufficient polymerization occurred due to in situ hydrolysis and separation of hygroscopic components. Extensive postcuring was also reported as a necessary step owing to this hydrolysis and incomplete polymerization. Furthermore, it was suggested that postcuring under air led to sulfoxide or sulfone introduction throughout the material and led to a decrease in the polymers’ mechanical properties. The polymers displayed little degradation in either neutral or acidic media, while there was selective alkaline degradation that was suggested to be due to the presence of carboxylic moieties that could be neutralized and become hydrophilic. Nevertheless, vacuum postcured polymers demonstrated the ability for tailored erosion rates, ranging from days to months. However, cytocompatibility of the polymers indicated poor cell viability, which was attributed to the acidic pH, and the authors suggested the inclusion of pH-adjusting compounds within the network to improve cell viability.[260, 261]
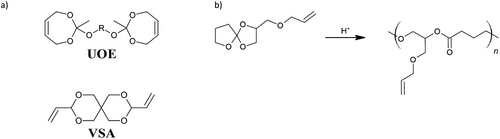
The incorporation of acetals within methacrylate monomers has been successfully demonstrated for achieving degradable polymers.[262] Furthermore, their incorporation within thiol-acrylate polymers has been identified as an effective mechanism to engineer pH-controlled degradable scaffolds.[263, 264] As such, the use of acetals within thiol-ene polymers for SLA printing has also been explored, formed through anti-Markovnikov thiol-ene addition to 2-methylene-1,3-dioxe-5-pene. However, the practicalities of printing with such acid-sensitive monomers and polymers proved challenging, whereby degradation occurred during the AM process.[259]
Spirocyclic acetals have been identified as useful constituents to tailor polymer morphology and Tg by inducing twisted polymer chains capable of crystallizing as well as incorporating bulky bicyclic groups within the polymer backbone.[265, 266] Beyond this, spirocyclic acetal moieties are pH sensitive, with the ability to produce chemically recyclable and pH solubility switchable materials,[267-270] while thiol-ene polymers can undergo dynamic transacetalation and metathesis.[270, 271] Furthermore, the degradation of poly(acetals) can be controlled by relative concentration and structure (linear versus cyclic) of acetal moieties, with degradation rates 80–200× faster than that of esters.[262] Moving towards their use in AM, TMPTMP, a vinyl spirocyclic acetal monomer (VSA, Figure 14), and 1,6-HDT have been copolymerized via DLP printing to produce semi-crystalline polymers. The crystallinity present in the polymer was attributed to the spirocyclic moieties within the linear portions of the polymer, finding an optimal concentration of 7.5–10% TMPTMP crosslinker to balance crystallinity, elasticity, and toughness. The polymers were annealed at various temperatures (22–60 °C) and times (0–504 h), whereby 40 °C for 120 h resulted in optimized mechanical properties. However, the crystallinity of the polymers was found to have a delirious effect on interlayer adhesion due to heterogeneity at the boundaries. Nevertheless, increased toughness (≈36 MJ m−3) relative to other thiol-ene polymers was observed, attributed to crystal dissipation and chain untangling events facilitated by the spirocyclic acetal moieties.[266, 272] Interestingly, no experiments were reported on its potential for stimulus responsiveness. Nevertheless, this work does provide viability for further studies incorporating these moieties for additively manufactured polymers.
Acetals and derivatives can undergo metathesis polymerization, opening opportunities for dual polymerization strategies.[273] For example, a spiro-orthoester (SOE) monomer susceptible to both cationic double ring-opening reaction and thiol-ene polymerization (Figure 14) has been DLP printed using DVE and PETMP as comonomers. To simultaneously induce both polymerization mechanisms during AM, both a free radical photoinitiator and cationic initiator were employed. Incorporating the SOE reduced polymerization shrinkage by 39% and exhibited 10–100 times higher ε″ values than the SOE-free polymer, with values as high as 104 at 0.1 Hz. Furthermore, the surface energies of the polymers were 52-60 mN m−1, while isoelectric points ranged from 3.7 to 4.1. These values are comparable to PEG-based polymers, making them promising candidates for biomedical applications.[274]
3.2.9 Incorporation of Imines
To both recycle and improve the properties of ABS, cysteamine has been grafted onto poly(butadiene) segments via thiol-ene reaction, resulting in pendant primary amine groups. Following this, glutaraldehyde was used to form imine crosslinking. The imine crosslinks underwent rapid dynamic exchange at 150 °C, which enabled the polymers to be utilized in FFF printing at an extrusion temperature of 230 °C. Varying the concentration of crosslinker could tailor thermomechanical properties; using 33% aldehyde/amine resulted in a 54% increase in σT (38 MPa) and doubling of UT (≈7.2 MJ m−3) compared to neat ABS. Furthermore, complete relaxation was observed after 1000 s at 170 °C, while the polymer's topological freezing transition temperature (Tv)[275-277] was observed at 54 °C, making them readily thermally reprocessable. However, as is typical of most FFF polymers, significant anisotropy was observed in the printed objects. Here, it may have been interesting to test whether there was a decrease in anisotropy after postfabrication thermal treatment to allow dynamic rearrangement between the layer interfaces.[278]
3.3 Thiol-Ene Chemistry using Norbornenes for AM
Norbornenes present ring-strained and electron-rich ene groups that typically facilitate rapid and near purely step-growth thiol-ene polymerization. It is therefore the case that the use of norbornenes can facilitate homogeneous and ideal network formation, and can afford rapid layer cure times within layer-by-layer AM.[75] Furthermore, developing high mechanically strong and high Tg polymers has been a challenge in many thiol-ene polymer networks, typically exacerbated by low crosslinking densities alongside flexible thioether bonds. However, the judicious use of the bulky norbornene structure has proved as an efficient method to achieve overcome such.[279] For example, this approach has been applied within poly(triazole) polymers in AM. These polymers formed glassy networks with high toughness alongside shape memory properties, making them attractive as engineering materials. Here, several norbornene functionalized triazole monomers were synthesized, including t-NBE (Figure 15). However, other norbornene functionalized triazole monomers that were prepared were waxy solids, thus necessitating dissolution in 40% (w/v) ethyl acetate (EtOAc), and were therefore not demonstrated within AM. Nevertheless, the SLA-printed copolymer of t-NBE with PETMP displayed ET of 1.06 GPa and σY of 31 MPa, while retaining a UT of 31 MJ m−3 and εT of 170%. Here, it was demonstrated that postfabrication physical aging for 16 h generally increased properties, with only minor decreases in toughness and ductility. This so-called “mechanical rejuvenation” phenomenon was used to attribute the high ductility and toughness that was observed. Interestingly, this aging could be reversed by heating the sample beyond its Tg. Furthermore, it was interesting to note that the polymer had a relatively low Tg of 38 °C, considering the triazole and norbornene groups within the polymer network.[280, 281]
3.3.1 Incorporation of O-Nitrobenzyl Esters
The o-nitrobenzyl ester (o-NBE) is a photolytically labile group that results in cleavage under UV exposure. o-NBE groups are useful in the fields of photoresists, photocleavable crosslinks, chemical amplification, photolithography, and tailoring of polymer morphology and surface properties.[283] The result of this cleavage is an increase in polar moieties, thus making chromophore active surfaces with tailorable wettability.[284] However, the presence of the nitro group is known to retard polymerization kinetics, thus potentially extending layer cure times; most (meth)acrylate systems present impractical polymerization kinetics for 3D printing. In fact, it was even found that using vinyl monomers in thiol-ene systems prohibited the feasibility of 3D printing.[285] On the other hand, the rapid kinetics of the thiol-norbornene reaction can be exploited to compensate for this effect. For example, a norbornene o-NBE monomer (norbornyl-NBE, Figure 15) was synthesized via Steglich esterification to yield a solid compound. An initial attempt to stoichiometrically copolymerise it alongside TMPTMP found that poor solubility necessitated the addition of other vinyl monomers (TATATO and/or TMPDE). Nevertheless, these monomers were then orthogonally DLP printed using visible light (450 nm) to prevent the photolysis of o-NBE groups, which could subsequently be photolyzed under UV stimulus (405 nm). The photolysis resulted in the formation of polar groups (free carboxylic acid and sulfonic acid) and thus increased the polymer's wettability (>100 down to <20) after just 1000 s of UV irradiation, evidenced by a relative decrease in water contact angle. Furthermore, gradual wettability could be spatially controlled by manipulating overall exposure. Although the use of norbornyl functionalization assisted in the layer cure time during DLP printing, these still necessitated relatively long exposures of 180 s for 50 µm layer height. Furthermore, presumably owing to high viscosity, slow lift (3 mm.s−1) and retraction (1 mm s−1) speeds were employed.[282] Other examples of embedding the o-NBE group into the polymer backbone via thiol-ene polymerization has further added to the potential for photodegradable networks,[286] while the photorelease of o-NBE has been shown as an effective method for photopatterning down to 4 µm resolutions.[285]
3.3.2 Bioinks, Hydrogels, and Biomedical
Bioinks are materials that serve as a scaffold to contain cellular materials such as single cells, spheroids, organoids, and microcarriers. Hydrogels are often employed as materials in bioinks, as the high aqueous content and viscoelastic properties are conducive to biocompatibility and mimicking biological tissue. While crosslinked hydrogels present several benefits, using (meth)acrylates generally suffer from heterogenous networks, side reactions with components of the cell structure, and harsh polymerization conditions leading to dead cells encapsulated in the hydrogel. Furthermore, the oxygen inhibition associated with methacrylates can lead to reactive oxygen species that are damaging to cells.[287] Furthermore, extrusion-based AM such as DIW printing typically requires rapid solidification upon deposition to ensure retention of structural integrity and feature definition. Although this can be achieved for methacrylates by increasing initiator concentration and/or UV intensity, in the case of bioinks, this can have deleterious effects on encapsulated cells and biocompatibility, and may demand a judicious choice of photoinitiator.[288] Furthermore, the rate of polymerization and resultant network structure have been shown to govern mechanical properties, which can impact cell viability in hydrogels.[289] Given these factors, the thiol-ene reaction poses itself as a highly appropriate strategy to circumnavigate many of these issues.[290] More specifically, the development of various bioinks bearing norbornene groups and exploiting thiol-ene polymerization has seen appreciable attention in recent years. Conceptually, the benefits of using thiol-ene polymerization in bioinks have been demonstrated through the functionalization of gelatin using allyl glycidyl ether. Comparison to methacrylated equivalents provided a platform demonstrating the viability and benefits of using thiol-ene polymerization within these systems, as well as viability for DIW printing.[291, 292] However, the use of norbornene groups has been demonstrated to have superior properties for these applications, such as low photoinitiator concentrations, stiffness control, and rapid kinetics.[293-295] An excellent example aiming to highlight the benefits of thiol-norbornene bioinks compared to methacrylate counterparts in DIW printing produced comparative methacrylate functionalized gelatin (Gel-MA) alongside using a combination of thiol functionalized gelatin (Gel-SH, prepared using N-acetyl-DL-homocysteine thiolactone, Figure 16) and norbornene functionalized gelatin (Gel-NB, prepared using 5-norbornene-2-carboxylic acid, Figure 16). The oligomers could be tailored over a large degree of functionalization range of the amine groups on the gelatin (20-97%). Here, it was found that a reduction of photoinitiator from 0.3% to 0.03% could be employed to minimise toxicity, while still retaining rapid polymerization kinetics (1-2 s) associated with the use of norbornene functional groups. Furthermore, it was found that mitigating the use of commonly employed DTT as a crosslinker also enhanced biocompatibility.[296] Indeed, beyond the biocompatibility issues associated with DTT, it is also well known to form disulfide bonds under physiological conditions as well as provide binding sites for growth factors. As such, several other thiol-functionalized macromolecular crosslinkers have been explored. For example, thiol-functionalized heparin (Hep-SH) was synthesized through the coupling of cysteamine to the free carboxylic acids. On the other hand, the primary amine groups on gelatin were coupled with norbornene anhydride by ring opening amidation (Figure 16), achieving 48% functionalization. These oligomers were directly compared against using DTT as a crosslinker, as well as Gel-MA, in extrusion-based printing. It was found that the Hep-SH could effectively mitigate issues associated with both methacrylate and DTT crosslinking, printed with comparative efficacy to the methacrylate counterpart, displayed excellent post-printing cell viability, as well as the added benefit that the heparin retained its VEGF-binding properties.[297]
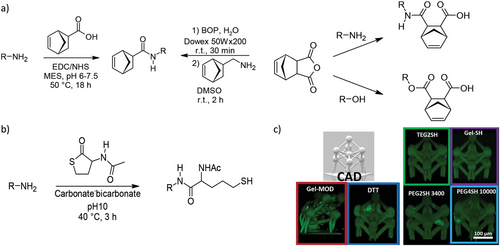
Another example compared the use of Gel-MA to Gel-NB and Gel-SH for AM of hydrogels. This study further compared the use of DTT, TEGDET, PEG-2SH (3.4 kDa), and PEG-4SH (10 kDa and 20 kDa) as crosslinkers. It was reported that there was a similar biological performance in metabolic activity between thiol-ene and methacrylate polymers, while DTT and TEG-2SH displayed high dark toxicity. It was also noted that the thiol-ene polymers promoted more ideal hydrogel network structures, leading to desirable hydrogel swelling properties. TPP printing with the thiol-ene systems resulted in faster printing speeds, and lower laser powers (20× lower polymerization threshold) could be used, thus both decreasing printing time and increasing cell viability. Furthermore, at higher laser powers, unexpected swelling occurred due to cleavage of amide bonds in the gelatin backbone, which could be mitigated by using the thiol-ene systems. Detailed structures were TPP 3D printed with the various copolymer formulations, establishing that using Gel-NB and Gel-SH presented the most suitable formulation for these applications (Figure 16).[298] The Gel-NB and Gel-SH oligomer combination has also been investigated for DIW printing of adipose-tissue-derived mesenchymal stem cells (MSCs). Here, varying crosslinking densities through concentration of oligomers in the hydrogels was demonstrated, and the resulting mechanical properties were investigated. It was reported that these tailorable properties led to the ability to optimise properties for different cells.[299]
Various other natural polymers have also been functionalized and used as bioinks in AM. For example, collagen has been functionalized with norbornene anhydride (Col-NBA) that was copolymerized with DTT or PEG-2SH (1 kDa). Here, collagen retained its triple helical structure, making it highly suitable for cellular incorporation. Furthermore, the additional carboxylic acid groups on the norbornene monoester were attributed to improving solubility.[300] Several examples using modified cellulose have also demonstrated it as an effective natural polymer for bioinks. For example, carboxymethyl cellulose has been coupled with norbornene triamine (a-CMC) or norbornene anhydride (e-CMC). 10 wt% and 15 wt% oligomer content in PBS solution were copolymerized with DTT in varying stoichiometric ratios (1:1, 1:2, 1:4 thiol-to-norbornene) using DIW printing. Generally, it was reported that e-CMC displayed better solubility than a-CMC, likely due to the presence of terminal carboxylic acid groups. Furthermore, increasing the thiol ratio resulted in increased viscosity for a-CMC, believed to be due to spontaneous crosslinking, which was also dependent on pH. Unfortunately, all formulations displayed autogelation of varying degrees, and only those with slow kinetics (1:2 and 1:4 formulations) could be used for DIW printing. It was reported that the polymers’ degradation was also affected by the choice of either ester or amide linkage, whereby greater degradation was observed for the amide containing a-CMC. On the other hand, comparable equilibrium swelling was observed between the two, while increasing the thiol-to-norbornene ratio from 1:4 to 1:2 decreased swelling 1.6–1.8-fold. However, relatively poor cell viability was reported, attributed to low cell adhesiveness.[301] In another example, hydrophobic components were exploited using methylcellulose, which is known for its thermoresponsiveness due to hydrophilic/hydrophobic interactions. Here, methylcellulose was functionalized with norbornene anhydride, achieving 2.7-4.4% conversion of hydroxyl groups. This was copolymerized with PEG-4SH via DIW printing, and the thermoresponsive nature of methylcellulose could facilitate orthogonal thermal physical crosslinking or photoinitiated chemical crosslinking induced gelation. However, relatively poor cell viability was reported, attributed to low cell adhesiveness of the polymers, but could be overcome through the introduction of Gel-NB within the system, which additionally enhanced printability.[302]
Interestingly, dynamic spatiotemporal modulation of the elastic modulus of alginate-based polymers has been demonstrated through crosslinking with PEG-2SH (2 kDa), where it was shown that stress relaxation could be tuned without influencing modulus. This was achieved by using a metal ion (Ca2+) neutralising residual carboxylic acid moieties for physical hydrogel creation, followed by spatially controlled thiol-ene covalent crosslinking. Through the combination of the physical and covalent network, spatial and temporal control of the 3D hydrogel viscoelasticity could be achieved by selective photo-tuning for the covalent component.[303] Conceptually, this has also been demonstrated within AM for DIW printed hydrogels. Here, alginate was functionalized with 5-norbornene-2-methylamine (12% functionalization) followed by partial thiol-ene coupling of RGD peptide sequence (CGGGRGDS). This functionalized alginate was then copolymerized with PEG-2SH (1.5 or 5 kDa) or PEG-4SH (5 kDa) in PBS solution. As expected, differing thiol functionality or PEG molecular weight could be used to tune mechanical properties. Furthermore, residual carboxylic acid groups on alginate could be neutralized using CaCl2 to incorporate ionic crosslinks, which resulted in tighter crosslinking and altered mechanical properties. It was noted that inclusion of the RGD facilitated cell adhesion and therefore high cell survivability (93% ± 2.8% after 1 day and 86% ± 4.6% after day 7).[304] The use of ionic contributions within hydrogels has also been demonstrated on hyaluronic acid (HA), which was functionalized with norbornene anhydride (HA-NBA) followed by conversion of the terminal carboxylic acid groups to the sodium salt. This oligomer was then copolymerized with Gel-SH (Figure 16) using DIW printing. Varying the degree of functionalization of Gel-SH, and therefore the thiol-to-norbornene ratio within the system, rendered tailorable mechanical properties. Compared to Gel-MA, the rapid thiol-ene polymerization facilitated lower photoinitiator concentrations and minimized reactive oxygen species generation, thus enhancing cell viability and cell proliferation.[305]
Aside from the use of functionalized natural polymers, synthetic polymers have also been prepared. For example, the residual hydroxyl groups on poly(glycerol sebacate) have been functionalized using 5-norbornene-2-carbonyl chloride and then copolymerized with PETMP using DIW 3D printing. The resultant polymers were partially degradable in PBS solution with controllable degradability based on thiol-to-ene ratios, and displayed adequate cytocompatability.[306] In another example, poly(glycerol sebacate-co-ethylene glycol) block copolymers bearing norbornene pendant groups was copolymerized with EDDET as hydrogels via DIW printing. The 3D printed hydrogels’ mechanical properties could be tuned by altering the concentration of monomers (20–40 wt%) or amount of EDDET crosslinker (0.65 to 1.33). The polymers were similarly partially degradable in PBS solution and presented acceptable cytocompatibility.[307]
Several bioinks and hydrogels bearing norbornene moieties have also been developed for vat photopolymerization AM. For example, poly(aspartic acid) functionalized with either norbornene or methacrylate groups (30% w/v in PBS solution) has been compared and employed in DLP and TPP 3D printing. Specific focus was paid to the comparison between the step-growth and chain-growth network formation using the respective functional groups. As perhaps could be expected, using methacrylates led to higher crosslinking densities, resulting in relatively lower swelling ratios and higher storage moduli.[308] In fact, this discrepancy in crosslinking density, and thus varied mechanical properties, has been exploited to create bilayer multi-materials for cartilage and osteochondral tissue engineering applications. Here, PEGDA (700 Da) and PETMP were DLP printed, then hydrogel systems composed of PEG-8NB (10 kDa), PEG-2SH (1 kDa), and combinations additionally incorporating matrix metalloproteinase sensitive polypeptide crosslinkers and/or nano-Hap, were developed for postfabrication infilling of the DLP printed structures.[309]
Oxidized sodium alginate has been functionalized using cysteine and cysteamine alongside norbornyl alginate for DLP-printed hydrogels. The printed hydrogels displayed porous structures with diameters around 200 µm and low cytotoxicity, indicating their potential for cellular activity.[310] A further example of DLP printing used HA functionalized with norbornene anhydride (Figure 16) to form norbornene monoamides (HA-NBA). Here, 1–5 wt% concentration of the HA-NBA alongside DTT (1:1 thiol-to-norbornene) were then used to prepare hydrogels in PBS solution. An optimized 40% functionalized HA-NBA at 5 wt% concentration was copolymerized with DTT via DLP printing using 6 s layer cure time. The hydrogels were hydrolytically degradable with tuneable rates controlled by concentration and degree of modification. Interestingly, it was found that while HA-NB was not degradable, the use of HA-NBA containing free terminal carboxylic acid groups promoted degradation as well as provided cell-adhesive sites with high cell viability.[311]
Interpenetrating network hydrogels have been developed by employing methacrylate functionalized HA (HA-MA), HA-NB, 1-adamantanethiol, and 6-mercapto-6-deoxy-β-cyclodextrin. Thiol-ene coupling of HA-NB with thiol compounds results in the functionalization of HA-NB with host-guest pendant moieties that can form physical crosslinks (HA-NB-GH), resulting in an interpenetrating network of covalently crosslinked Me-HA and physically crosslinked network HA-NB-GH, both formed using photoinitiation (Figure 17). This combination resulted in hydrogels with tuneable properties based on the relative concentration of components and overall polymer concentration within the hydrogel. The mechanical properties displayed notably high toughness and negligible hysteresis, attributed to using the combination of chemical and physical crosslinks. A formulation containing 0.6 wt% HA-MA and 4.5 wt% HA-NB-GH was demonstrated as a low viscosity system for DLP printing with rapid layer cure times of 2.5 s, and were able to print microporous structures with relatively complex geometries and fine resolutions (Figure 17).[312] A similar approach using HA oligomers containing pendant adamantane and norbornyl moieties, alongside HA oligomers containing pendant cyclodextrin moieties, have also been demonstrated for microchannel hydrogels using DIW printing.[313]
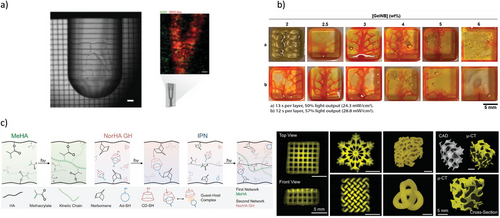
Pullulan has been functionalized using norbornene anhydride (Pul-NBA) with varying degrees of functionalization. Furthermore, varying concentrations (5–10% w/v) and using either DTT or 1,6-HDT as crosslinkers was investigated. An optimized 10 wt% Pul-NBA and DTT (1:1 thiol-to-norbornene) in PBS solution was then copolymerized via DLP printing using 40 s layer cure time and 10 µm layer height, achieving relatively complex geometries. A CCK-8 assay established that the hydrogels had OD values >95%, suggesting that metabolic activity and apoptosis of L929 cells were not affected.[314] Gelatin has also been functionalized by ring-opening reaction with norbornene anhydride (Gel-NBA, Figure 16), which was copolymerized with DTT at 5 wt% concentration in PBS solution using DLP printing. Here, a 1:4 norbornene-to-thiol stoichiometric ratio was used and a ruthenium/sodium persulfate photoinitiator system was employed to facilitate visible light (400-450 nm) photopolymerization. Cell viability studies were performed, establishing that cell viability decreased with increasing photoinitiator concentration. The low cell viability was attributed to a relatively low concentration of Gel-NBA that usually scavenges harmful free radicals. Furthermore, the formation of microcapillaries for vasculogenesis within the hydrogels was reported lower than a benchmark, and decreased junction density was observed with increasing photoinitiator content. These factors were attributed to the high crosslinking density and concentration of polymer that resulted in stiff polymers and pore shrinkage. To overcome this limitation, DLP printed microchannels of varying size (down to 200 µm diameter) and channel network designs were constructed within the polymer to establish optimized conditions for different cells. It was found that the channels successfully facilitated bridging of MSCs, and that co-cultures with human umbilical vein endothelial cells (HUVECs) also increased bridging capabilities.[315] This work demonstrated the importance and capability of using AM for geometric optimization of cell viability within these hydrogels. Gel-NBA has also been established as simple and effective oligomer for VP AM, with more effective physicochemical and biocompatibility properties compared to Gel-MA. Several Gel-NBA oligomers with a broad range of functionalization (3-50%) were copolymerized with either PEG-4SH (10 kDa), PEG-2SH (1 kDa), or EDDET. Owing to the rapid kinetics associated with norbornene thiol-ene polymerization, the entire object could be fabricated in just 10 s. Controlling the degree of substitution, the crosslinker functionality, and crosslinker molecular mass could effectively tailor the crosslinking density and resultant hydrogel stiffness. Of these formulations, Gel-NBA (50% functionalization, 2.5 wt% and 5 wt% concentration) and PEG-4SH could produce perfusable branched channels and other objects with complex geometries (Figure 17). Furthermore, the rapid photopolymerization with low light doses (80–90 mJ cm−2 and volumetric absorbed energy of 8.59–9.69 mJ cm−3) and using low photoinitiator concentrations (0.05% w/v) allowed a high cell viability (>95% after 7 days).[316] Gel-NBA (53% DS, 7.5 wt% concentration) has also been demonstrated as a suitable oligomer for TPP 3D printing. Here, copolymers of Gel-NBA and DTT could be used to fabricate microscale structures with pore sizes ranging from 10-40 µm loaded with L929 fibroblast cells, with good cell viability and proliferation.[317]
PEG presents one of the most accessible water-soluble synthetic polymers for hydrogels in biomedical applications, making it an obvious and attractive candidate for use in AM.[318, 319] For example, PEG-8 arm (20 kDa) has been functionalized with norbornyl moieties and copolymerized via DLP printing with PEG-4SH (10 kDa) at overall polymer concentrations of 3–9 wt% in PBS solution using a 10 s layer cure time. Furthermore, tartrazine was used as a photoabsorber to enhance print resolution fidelity. However, at concentrations over 2 mm it completely prevented gelation, thus an optimized 1.5 mm was employed to maximize resolution without completely inhibiting polymerization. This led to support-free 3D printing of high-resolution hydrogel objects up to 5 mm tall. Furthermore, the printing was capable of sub-millimeter diameter perfusable channels and microvascular models. Printing of microwell structures on the polymer surface facilitated a secondary thiol-norbornene reaction for conjugation of the integrin-binding motif reaction (CRDGS), rendering cell adhesive sites.[320] Indeed, the judicious use of excess norbornene groups can facilitate coupling with a variety of thiol-bearing biomolecules, making it an attractive opportunity within biomedical applications. To this end, a combination of Gel-NB alongside the use of functionalized PEG monomers has also been demonstrated for enhanced vascularization within hydrogels. Here, 3 wt% Gel-NB, 3 wt% PEG-4SH (10 kDa), and 0.5 wt% PEG-NB (20 kDa) in PBS solution were used for DLP printing. The incorporation of PEG monomers alongside Gel-NB improved the hydrolytic/enzymatic stability of the hydrogels, and a range of hydrogel properties were similar to several healthy human tissues such as brain, liver, and pancreas tissue. High gel fractions (85–95%) indicated efficient crosslinking within the network, although at high concentrations (5–6 wt%) high system viscosities caused bubbles within printed objects. Nevertheless, fabrication of complex geometry hydrogels (Figure 17) was achieved for lower concentrations (2–4 wt%). The incorporation of defined microchannels for vascularization was then investigated, producing perfusable channels with 300–1200 µm diameter and complex hollow branches, successfully achieving complex structures within soft matrices. Furthermore, VEGF-mimetic QK peptide binding was demonstrated during hydrogel crosslinking, allowing HUVECs that could be encapsulated on the surface of the perfusable surfaces. The seeded HUVECs were shown to adhere, sprout, and merge with encapsulated cells. Furthermore, secondary conjugation of QK peptides via residual norbornene groups also enhanced angiogenesis.[321] This research successfully demonstrated the potential to create tailored molecular architectures and mechanical properties, and further facilitate suitable morphologies via AM to enhance the biomedical potential of these systems. Indeed, it may also be argued that vat photopolymerization AM may be a more suitable fabrication method compared to DIW printing given that it affords high resolution and complex geometries.
The controlled degradation of bioinks containing thioester bonds can be exploited within hydrogels to facilitate both cellular activity and for controlled release of encapsulated entities.[233, 322] For example, 8-arm PEG (20 kDa) has been functionalized with norbornene using either ester, thioester, or amide coupling. Copolymerization with PEG-4SH (10 kDa) in PBS solution using electrospraying into a light paraffin oil bath yielded microgels suitable for extrusion printing, followed by postfabrication UV crosslinking. The printed scaffolds containing a ratio of amide-to-ester-to-thioesters of 3:1:1 could be treated with l-cysteine methyl ester and NaOH to induce degraded fractions. Here, the thioesters degraded most rapidly, while esters degraded relatively slower, whereby the controlled degradation facilitated efficient cellular colonizations.[323]
Poly(vinyl alcohol) (PVA) is another hydrophilic synthetic polymer that presents itself as a suitable platform for the preparation of hydrogels.[324, 325] For example, PVA has been partially functionalized with NBA and neutralized into its sodium salt. This oligomer was copolymerized with PEG-2SH, using unmodified gelation as a filler, to produce hydrogels using VP 3D printing. Here, VP 3D printing enabled ultra-soft hydrogels to be successfully fabricated, where layer-by-layer technologies may lead to damage during fabrication. Warming up the hydrogel to physiological temperature induced the diffusion of gelation out of the hydrogel, assisting with spreading of encapsulated cells. The fabricated hydrogels could then also undergo volumetric photopatterning of bioactive molecules for site-specific immobilization.[326]
In another example of using synthetic polymers, α,ω-norbornene-functionalized poly(caprolactone-co-1,4,8-trioxaspiro-[4,6]-9-undecanone) and DTT have been DLP printed to form shape memory, resilient, and biodegradable elastomers suitable for tissue engineering. Here, the cyclic acetal moieties were found to impart flexibility and resilience into the networks.[327] This field has been extended into bioconjugation for dynamic patterning of signaling proteins, achieving reversible and repeatable tethering of the signaling proteins via the thiol-ene chemistry with allyl sulfides.[328] Another interesting example demonstrated DLP printing with Gel-NB and Gel-SH, followed by postfabrication spatially selective bioconjugation of thiol-bearing proteins, peptides or growth factors, leading to tailored photopatterned microenvironments of bioactive moieties (Figure 18) for precision tissue engineering. Here, altering the molar ratio of norbornene-to-thiol groups could be performed to tailor mechanical properties, and adjust the number of residual norbornene groups for bioconjugation.[329]
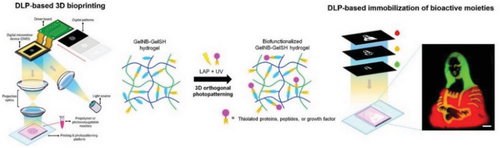
Aside from the incorporation of norbornene groups through pendant conjugation, norbornene moieties have also been embedded within the backbone of poly(ester) oligomers. Here, ABA block copoly(ester)s (Mn > 10 kDa) have been prepared through epoxy-anhydride ring-opening polymerization of norbornene anhydride with propylene oxide, followed by copolymerization with poly(1,3-propylene sebacate) (PPS). Notably, it was found that a triethyl borane-bis(triphenylphosphine)iminium chloride catalyst pair could precisely control the copoly(ester) architecture. The ABA copoly(ester) was then copolymerized with PETMP and limonene via DLP printing, using a 5 s layer cure time. However, it was found that low crosslinking density due to the long PPS blocks led to mechanical failure during postfabrication washing with organic solvents. Off-stoichiometric formulations with decreased PETMP were then explored to overcome this, but this strategy had deleterious effects on the mechanical properties. Nevertheless, the polymer displayed low cytotoxicity and cell viability with HUVECs was higher than that of PCL used as a reference standard. It was noted that off-stoichiometric formulations were planned to undergo postfabrication functionalization with cell adhesive sites in future work.[330]
In several of the previously presented examples, the incorporation of nucleic acid building blocks and polypeptides was shown to serve an important role in effective bioink formulations for biomedical contexts, whereby biocompatibility and biological recognition benefited cell viability. Within this context, the use of amino-acid-specific thiol-ene coupling has been shown to facilitate and govern the crosslinking mechanism and resultant cell behavior in hydrogels. Here, it was found that amino acids containing tryptophan promoted chain growth polymerization, while those without promoted step-growth, which was attributed to its aromatic group acting as a radical stabilizer (Figure 19). This presented an important finding, as it was previously established that there was an appreciable decrease in cell viability when tryptophan was present, and a difference in optical clarity within the hydrogels was also noted. Furthermore, the acidity of the peptides present during polymerization was investigated. Here, it was reported that under basic conditions the thiolate can form, leading to generation of disulfides and thus inhibiting the thiol-ene reaction due to consumption of free thiols. On the other hand, under neutral conditions the hydrogels polymerized rapidly and were far less optically turbid, yet under acidic conditions turbidity was observed along with different mechanical properties compared to the neutral hydrogels. This was attributed to the protonation of the carboxylate salt leading to hydrophobic components promoting norbornene homopolymerization as opposed to thiol-ene polymerization, thus promoting distinct heterogenous hydrogel networks.[331]

This work presents an important finding for the general mechanisms present in hydrogel bioinks, whereby careful consideration of peptide components needs to be evaluated to ensure that favorable mechanisms and resultant network formation occurs to promote cell viability. Furthermore, studies demonstrating the use of the thiol-ene reaction to facilitate the incorporation of tailored clickable nucleic acids within networks,[332] the modification of designer peptides,[24, 333, 334] and biochip-oriented surface immobilization of proteins[335] present opportunities to integrate these concepts into AM for advanced biomedical applications. Further examples of the potential of thiol-norbornene networks demonstrating advanced properties, such as hydrogels with tuned stress relaxation for cornea modeling,[336] polymers with excellent optical properties and photoelasticity,[337] and self-healing hydrogels[338] further highlight the potential of thiol-norbornene polymers within AM. Lastly, the use of norbornene thiolactones has also been established as an efficient pathway for monomer functionalization, which may provide viable pathways for monomer development.[171, 339-341]
3.4 Thiol-(Meth)Acrylate Polymerization and Properties
While (meth)acrylates and derivatives are technically considered enes of the previous classification, the polymer structures accessible with thiols are distinct from the previous ene examples due to the fact that most (meth)acrylates readily homopolymerizable via a free radical mechanism. Consequently, most thiol-(meth)acrylate polymers can form distinct binary networks that contain a combination of chain- and step-growth components (Figure 20). Here, the propensity for homopolymerization is generally far higher than the allyl and vinyl examples previously described, although non-homopolymerizable groups such as the maleate are known.[342] In fact, in certain instances, it has been found that methacrylate homopolymerization was more rapid than the thiol-methacrylate reaction, and thus the polymerization was limited by consumption of the methacrylate.[57]
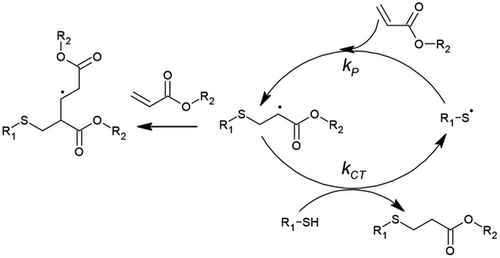
Minor thiol fractions (1–10 wt%) in (meth)acrylate systems have been demonstrated to supress oxygen retardation of the (meth)acrylate free radical polymerization, in part attributed to increased system viscosity effectively diminishing oxygen diffusion.[344] An in-depth comparative study between thiol-ene and thiol-acrylate polymers explored the differences in polymerization kinetics, conversion, and polymer (thermo)mechanical properties. Here, TATATO and TAEICN were employed as analogous ene and acrylate monomers, respectively, while several aliphatic and cycloaliphatic thiols based on esters of 3-mercaptopropionic acid were explored. Minor homopolymerization (6–13%) of the ene was observed in thiol-ene formulations, while appreciably higher (27–52%) homopolymerization was observed for thiol-acrylate formulations. Furthermore, in stoichiometrically equivalent thiol-acrylate formulations incomplete conversion of thiols (60-70% conversion) was observed owing to the contributions of homopolymerization, although there was overall higher ene conversion. Relatively lower mechanical properties and lower and broader Tg in the thiol-acrylate polymers was attributed lower thiol conversions, although similar gel contents were observed.[343] It is thus the case that judicious choice of the stoichiometry between thiol and acrylate components ought to be considered to achieve high overall functional group conversions and thus improve the overall polymer properties. Indeed, adjusting the stoichiometry within thiol-acrylate systems has been shown to effectively allow for equal high conversions for the thiol and acrylate functional groups, with resultant increases in thermomechanical properties.[345] Within this context, a scaling law has also been developed to account for the thiol-acrylate polymerization to establish optimal conversions.[346]
(Meth)acrylates and substituted derivatives (e.g., fumarates, maleates, crotonates) constitute α,β-unsaturated carbonyls and can therefore undergo thiol-Michael addition reactions, whereby the thiol acts as the Michael donor and the (meth)acrylate as the Michael acceptor.[27] The relative reactivity of the thiol-Michael reaction is in-part dependent on the electron-withdrawing nature of the (meth)acrylate substituent, with greater reactivity towards strongly electron-withdrawing groups, and the reaction can be base mediated or nucleophile catalyzed.[347] However, of the α,β-unsaturated carbonyls, (meth)acrylates display some of the lowest reactivity towards the thiol-Michael addition reaction, and are generally not autocatalytic.[27] An extensive variety of commercially available (meth)acrylate monomers are commonly employed in AM (Figure 21), while a number of other novel monomers have also been reported.

3.5 Thiol-(Meth)Acrylate Chemistry for AM
The residual acrylate groups present on DLP fabricated polymers have been exploited for thiol-acrylate postfabrication modifications. First, it was shown that hydrophilic or hydrophobic components could be coupled to the surface to tailor the polymer properties. This approach was then extended to utilize the excess thiols of the surface-modified polymers for subsequent anchoring of photogenerated Ag nanoparticles.[348] This strategy provides a convenient and versatile platform for conjugation with a variety of other thiol-bearing molecules.[349] Within this context, the combination of fabricating objects with complex geometries alongside metal ion/nanoparticle surface anchoring presents a promising platform for applications such as catalysis, energy storage, and biomedical contexts.[350] Adding credence to this, PEGDA (Mn 700 Da) and PETMP have been copolymerized via SLA printing, and here it was demonstrated that using a stoichiometric excess of thiols (1.1, 1.5, or 2) facilitated postfabrication grafting of hydrophilic, hydrophobic, or fluorescent moieties, as well as gold nanoparticle immobilization via reverse addition-fragmentation chain transfer. The versatility and functionality of the polymers were thus suggested for applications in adhesion, patterning, detection technologies, and catalysis. The polymers were also used to SLA print fully enclosed microchannels with internal diameters down to 250 µm. This was achieved by optimization of photoblockers using 0.05 wt% 2,4-dihydroxyazobenzene (Sudan Orange G), although this gave prints a distinctive orange color. On the other hand, 0.05 wt% 1,3-bis(4-methoxyphenyl)propane-1,3-dione (avobenzone D) was shown to produce similar resolution transparent objects, although more photoinitiator was required to account for the addition of a bandpass filter at the projector source.[351] In another example incorporating nanoparticles within the polymer network, the development of thiol-functionalized poly(siloxane) nanoparticles has been shown within DLP printing. These thiol-functionalized poly(siloxane) nanoparticles were incorporated at 10 wt% into commercial acrylate resins, and 3D printing could be successfully performed with just 0.5 s layer cure times.[352] This approach demonstrated an efficient methodology to mitigate the health and odor risks commonly associated with volatile thiol monomers, while not impacting on the polymer processing, and efficiently providing covalent bonds at the interface of the particles and polymer network.
Looking toward leveraging the thiol-acrylate polymerization to achieve high-resolution printing, a comparative analysis between using acrylate (di-TMPA) and thiol-acrylate (di-TMPA and PETMP) polymerization has been investigated for TPP 3D printing. Here, it was confirmed that using a thiol-acrylate system facilitated lower laser powers and higher writing speeds, resulted in a higher degree of monomer conversion, as well as achieving finer resolution fidelity and lower polymerization shrinkage (Figure 22). The critical speed of writing displayed a linear relationship to thiol content, while a reduction in Young's modulus and hardness displayed a maximum at 30 wt% thiol content.[353]
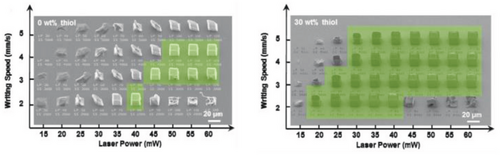
The use of controlled/living polymerizations have enjoyed increasing attention within AM, employed to gain greater control over the achievable molecular architectures.[354] Within DLP-printed polymers, it has been demonstrated that the addition of minor quantities of PETMP could effectively improve the toughness of an acrylate-terminated urethane-based PCL polymer through chain transfer during polymerization.[355] Indeed, thiols and derivatives (e.g., thioesters, thiocarbamates, or xanthanates) are well-known as chain transfer agents (CTAs) for reversible addition-fragmentation chain-transfer (RAFT) polymerizations.[356] A comparison between TEGBMP and oxybis(ethane-2,1-diyl)) bis(oxy))bis(ethane-2,1-diyl) bis(2-(tosyloxy)acrylate) (DVS, Figure 23) has also shown that both could effectively increase polymer toughness, attributed to their ability to act as CTAs and facilitate irreversible addition–fragmentation chain transfer (AFCT) polymerization. It was reported that the dithiol proceeded with more rapid polymerization kinetics and higher double-bond conversion. However, with increasing concentration of the CTA, DVS maintained higher (thermo)mechanical properties compared to TEGBMP.[357] Within this context, further investigations using DVS have elucidated its ability to act as an effective CTA, providing valuable insights into its scope within these systems.[358, 359]
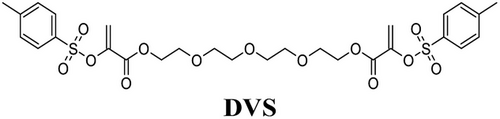
Within bulk polymers, the thiol-(meth)acrylate bond has been demonstrated as an effective polymerization strategy to prepare SMPs.[360] Additionally, the incorporation of several co-crystallizable domains within thiol-acrylate polymers can also provide tailorable two-way reversible shape memory actuation.[361, 362] With this conceptual basis in mind, PCL-diacrylate and poly(pentadecalactone)-diacrylate oligomers, and mixtures thereof, were copolymerized with PETMP via DLP printing using a layer cure time of 45 s and vat temperature of 70 °C. These polymers were shown to contain distinct crystalline domains with two Tm at 41 °C and 90 °C. The distinct crystalline domains, alongside the degree of curing, could be used to control triple shape memory at different transition temperatures associated to each region by differential melting and crystallization (Figure 24). Furthermore, spatially selective programming of the shape memory was accomplished.[363] A similar concept has been demonstrated using crystallizable side chains within DLP printed acrylate systems, whereby varying the crystallizable chains with distinct melting temperatures based on pendant hydrocarbon chain length could be used for multi-materials, providing an exciting basis for application within thiol-acrylate systems.[364]

The use of thiol-acrylate polymers has also been used to prepare self-healing polymers through DLP printing of HEA, EDDET, and TMADA using 5 s layer cure times. Here, only 1 wt% of EDDET and 1 wt% of TMADA were employed. Mechanically, the polymers displayed εT > 1000%, whereby varying the amount of EDDET and TMADA content could also tailor both ET and εT. Thermal treatment at 90 °C for 24 h led to successful self-healing, although the polymers’ toughness almost halved, which was thought to be due to loss of plasticizing species. It was further noted that self-healing only occurred when sufficient EDDET was incorporated into the polymers. Here, the polymers' self-healing was attributed to retro-Michael reactions facilitating thiol exchange.[365]
The maleate and fumarate groups are unsaturated cis/trans isomers, both well known for their incorporation into unsaturated poly(ester)s. ABA block copolyesters composed of γ-methyl-ε-caprolactone, maleic anhydride, propylene oxide, and 1,4-benzenedimethanol have been prepared in varying block ratios. The block copolyesters were then copolymerized with TMPTMP via DLP printing in thiol-to-ene ratios of 1:5, 1:2, and 1:1. Here, it was reported that varying of block length alongside changing the thiol-to-ene ratio effectively varied the crosslinking density and resultant mechanical properties of the polymers, providing a facile mechanism for their tailoring. The polymers also displayed slow degradation in both 0.25 n NaOH and PBS solution at 37 °C, with the latter maintaining a constant pH of 7.4 throughout degradation.[366] In another example using fumarates, a four-arm poly(propylene fumarate) (PPF) oligomer has been DLP printed with TMPTMP in varying fumarate-to-thiol ratios (5:1 to 30:1), using ethyl acetate as a solvent. Fully degradable polymers could be produced, whereby increasing fumarate-to-thiol ratio caused an increase in mass loss kinetics in 0.25N NaOH.[367] The same PPF oligomer has also been copolymerized with TMPTMP (10:1 alkene-to-thiol) alongside HAp particles to form DLP-printed composite materials. Here, it was noted that postfabrication processing strategies such as drying, post-curing irradiation, and thermal treatments played an appreciable role in the achievable mechanical properties. In fact, these were suggested as suitable strategies to impart tailorable properties for bone scaffold applications.[368] Interestingly, star-shaped PPF has been shown to effectively reduce system viscosity for AM, making its use within composite materials highly appropriate to mitigate processing constraints associated with high viscosities within vat photopolymerization AM.[369-372]
Within the context of biomedical applications, acrylate functionalized (4-5%) dendritic poly(glycerol) and PEG-2SH (10 kDa) hydrogels have been demonstrated as effective for streptavidin-encapsulation for biosensing applications. Here, allyl, acrylate, and acrylamide functionalities were explored, whereby thiol-acrylate networks had the highest encapsulation efficiency and degradation.[373] In another example of hydrogels, thiolated γ-polyglutamic acid (γ-PGA-SH), glycidyl methacrylate-conjugated γ-polyglutamic acid (γ-PGA-GMA) (Figure 24), and thiolated arginine-glycine-aspartate (RGDC) sequences have been prepared as all-peptide oligomers for DLP printing. These hydrogels were used for diabetic wound healing therapeutics, whereby the embedded RGDC integrin receptor supported encapsulated cell adhesion and spreading. An optimized concentration of 6% (w/v) was found to satisfy mechanical properties, facilitated rapid gelation, provided appropriate conditions for cell viability, and allowed controlled release of encapsulated HUVEC VEGF 165.[374]
The thiol-acrylate reaction has also been shown as an effective strategy for preparing polymerized high internal phase emulsions (polyHIPEs) as porous architectures for acoustic, cell culturing, and tissue engineering applications.[375-382] This has been extended to AM to fabricate polyHIPEs using PEGDA and TMPTMP for electroactive cellular scaffolds using DIW printing. The printed polyHIPEs were volumetrically responsive through cell deformation when an electrical potential was applied due to induced reduction and oxidation of species, with around 10% deformation achieved. Furthermore, the printed scaffolds were cytocompatible with rapid colonization and spreading of fibroblasts.[383] The fabrication of polyHIPEs with HDA and TMEICN has also been demonstrated using DLP printing, achieving pores less than 5 µm in diameter.[384]
3.5.1 Biobased
A variety of biobased platform molecules present themselves as suitable for functionalization into reactive monomers through (meth)acrylation. In the pursuit of developing sustainable polymers with acceptable properties for AM, vanillyl alcohol diacrylate and vanillyl alcohol dimethacrylate (VADM, Figure 25) have been copolymerized with 1,3-BDT using DLP printing. Curiously, the two polymers had very similar Tg for the acrylate (−5 °C) and methacrylate (−6 °C), yet VADM displayed a σt 5× greater than the acrylate (25 MPa and 5 MPa), with an even greater difference in ET (2953 MPa and 16 MPa), while εT decreased from 102% down to 6.8%. This may be owing to the difference in chain transfer constant between methacrylates and acrylates, leading to a difference in the amount of relative thiol-acrylate copolymerization and acrylate homopolymerization observed. However, further work would be necessary to elucidate this remarkable difference in polymer properties. Nevertheless, both of the vanillin-based polymers were also shown to possess antimicrobial properties.[385, 386] A terpolymer containing VADM, guaiacol methacrylate (GM, Figure 25), and 3,6-dioxa-1,8-octanedithiol eugenol acrylate (ODEA, Figure 25) exploited the rigid aromatic structures of these lignin model compounds to SLA print polymers with Tg up to 130 °C, ET of 1.23 GPa, and σt of 61 MPa.[387] Further work using VADM copolymerized with 1,3-BDT and tridecyl methacrylate has displayed shape memory capabilities, presenting and appealing avenue to explore within AM.[388] Similarly, a bulk copolymer of tetrahydrofurfuryl acrylate, 1,3-BDT, and tridecyl methacrylate has also displayed shape memory.[389]
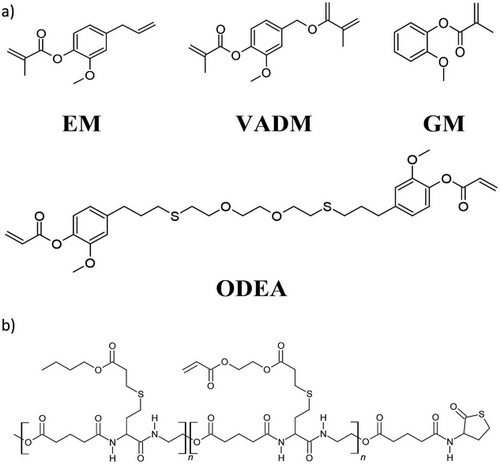
Itaconic acid is a biobased unsaturated diacid with analogous structural features to maleic acid and fumaric acid. As such, itaconic acid has been exploited to form unsaturated poly(ester thioether) oligomers (1.4–2.2 kDa) by transesterification polycondensation of dimethyl itaconate with thioether adducts of terpenes and mercaptoethanol. The oligomers were copolymerized with a reactive diluent (prepared by transesterification between dimethyl itaconate and 1,4-butanediol) using DLP printing. These formulations resulted in polymers with biobased carbon contents ranging from 74.5% to 88.2%. The 3D printed polymers displayed two Tg (−13 to −31 °C and 51 to 57 °C), attributed to some degree of phase compartmentalization within the polymers, and a range of tensile properties could be achieved based on tailoring of the components within the unsaturated poly(ester thioether) oligomers.[390] A similar approach to functionalizing vegetable oils has also been shown, whereby the unsaturation on fatty acid moieties was coupled with mercaptoethanol via thiol-ene reaction, followed by coupling with a methacrylate functionalized isocyanate to form methacrylated urethane monomers. These were copolymerized with isobornyl acrylate via DLP printing, with the polymers shown to be self-healing, weldable, chemically recyclable, and reprintable.[391] In another example exploiting itaconic anhydride, enzymatic coupling with perillyl alcohol to form the monoester was achieved. The perillyl itaconate monoester was then copolymerized alongside TMPTMP in varying thiol-to-ene ratios and several terpenes (e.g., limonene, linalool, perillyl alcohol) as reactive diluents via DLP printing using a relatively long layer cure time of 120 s. Addition of the terpene diluents decreased polymer Tg marginally, yet increased εT, while decreasing the relative incorporation of thiols from 1:1 to 1:0.33 also resulted in an increase in εT as well as maintaining or increasing the UTS.[392]
Thiolactones are a useful biobased platform for synthesizing urethanes and amides.[394] Here, the ring-opening reaction of functionalized thiolactones provides an appealing route to achieve functional monomers through a sustainable synthetic pathway. For example, D,L-homocysteine thiolactone has been used as an effective coupling moiety to achieve sequence-defined polymers in TPP AM (Figure 25).[393] Further examples of biobased polyamides,[395] thiol-functional urethanes,[396] epoxy-functionalized thiolactones capable of dual curing,[397] and fluorescent poly(siloxane)s[398] facilitated through thiolactone functionalization present opportunities for sustainably synthesized polymers suitable to employ within AM. Furthermore, examples showing this approach for bioconjugation[399-401] and gene delivery[402] highlight its potential for biomedical applications. Generally, it is surprising to find that relatively few established biobased monomers have been transferred to AM for thiol-acrylate polymers to date, as a broad variety of examples present themselves as suitable candidates.[403-408]
3.5.2 Incorporation of Michael Adducts
The thiol-Michael reaction between α,β-unsaturated carbonyls and thiols occurs via 1,4-conjugate addition and results in the formation of a stereoselective thioether bond.[409, 410] The reversibility of the thiol-Michael adduct has long been known, first reported in 1964,[411] which can be triggered under thermal or pH stimulus, thus making it suitable for CANs capable of self-healing and chemically recyclable materials.[412-417] A systematic empirical investigation exploring controlling factors of the autocatalytic reaction found that the thiolate concentration (thus thiol acidity) is the primary determinant for reaction kinetics, that retro-Michael exchange reactions are most dominant for maleimides, and that acrylamides combined with acidic thiols provided a balance between stability and reactivity. It was, however, further noted that more acidic thiols display a greater propensity for disulfide formation, which needs to be carefully addressed in certain instances.[418] Given that the thiol-Michael reaction is typically base or nucleophile mediated by homogeneous catalysis, it is unsuitable for direct photopolymerization-based AM technologies.[419] Nevertheless, the thiol-Michael adduct can be incorporated within the monomer structure to exploit these beneficial properties within the polymer network for AM, or catalyzed through the use of a photobase generator (PBG) such as 3-nitrophenylpropyloxycarbonyl 1,1,3,3-tetramethyl guanidine (NPPOC-TMG, Figure 26).[238, 420]

The use of the thiol-Michael adduct within monomer and oligomer structures has been extensively exploited. For example, the use of stereocontrol between the isomers of maleate and fumarate groups along unsaturated poly(urethane thioether) oligomers has been shown to control mechanical properties. Here, the ability to control the stereochemistry of these oligomers was facilitated by the mild and orthogonal reaction conditions required for the thiol-Michael reaction. The oligomers were DLP printed using 50 wt% 1-methyl-2-pyrrolinidone as a non-reactive diluent, and it was found that the short periods of UV irradiation and thermal treatment did not induce significant (<1%) isomerism of either the cis or trans bonds. On the other hand, the differences in stereochemistry resulted in remarkable differences in the polymers’ mechanical properties, with the trans isomer network displaying an ET 50× greater than the cis isomer network. This phenomenon was attributed to differences in packing efficiency between the isomers, with the cis isomer being less able to crystallize due to its less ordered structure. Additionally, the rate of degradation in 1N NaOH was higher for the cis isomer However, the difference in mechanical properties decreased with increasing oligomer molecular weights, which was attributed to the ability for the material to crystallize more effectively at higher molecular weights.[421, 422] This conceptual motif of controlled stereochemistry has also been extended to investigate the use of sugar-based stereoisomers (isosorbide, isomannide, and isoidide), but has yet to be extended into AM.[423-425]
The thiol-Michael adduct has also been exploited within monomers for AM of polymers with various interesting properties. For example, the design of polymers with the ability to respond to the formation of reactive oxygen species (ROS) presents an opportunity for identifying their formation within biological systems. As such, monomers containing thioether bonds formed through thiol-Michael reaction have been used in DLP-printed hydrogels. It was demonstrated that the thioether groups responded to the presence of ROS (e.g., H2O2) through swelling, thus permitting encapsulated chemotherapeutic drug release upon the presence of ROS.[426]
In another example, PETA has been coupled with nine different monofunctional thiols via thiol-Michael reaction in a 4:1 acrylate-to-thiol ratio, thus bearing 3 residual acrylate groups. These monomers were then polymerized via TPP AM, whereby the various types of thiol monomer (e.g., fluorinated, silanes, amino acid) allowed a broad range of functionalities to be achieved within μ-3D fabricated structures. Furthermore, Boc-protected amines could be postfabrication deprotected and reacted with an N-hydroxysuccinimide ester fluorecein molecule for fluorescent structures.[427]
The thiol-Michael addition reaction is also amenable for coupling of thiols with vinyl sulfones, which has been used in the synthesis of TMSDA (Figure 27). TMSDA has been copolymerized with HEA, n-butyl acrylate, and EDDET using DLP printing. The thiol-Michael adduct was thermoreversible within the polymer and thus provided triggered adhesive properties as a useful property within soft robotics applications. Furthermore, the materials were shown as self-healing with near-complete healing efficiency. Here, formulations with less EDDET displayed the poorest ability for self-healing.[428]
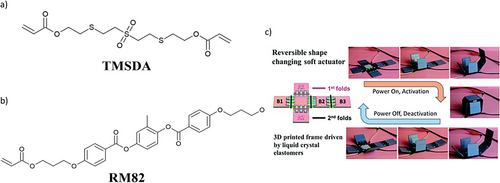
Liquid crystal elastomers (LCEs) are particularly attractive materials that can exploit dynamic covalent chemistries for various functionalities, such as shape memory.[429-433] The development of LCEs has extensively relied on the use of thiol-Michael adducts within the monomer structure. Indeed, an excellent example is the thiol-Michael addition between a commercially available mesogenic compound 1,4-bis[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene (RM82, Figure 27) and EDDET to form linear oligomers (RM82-EDDET), which can be used for copolymerization with multifunctional thiols such as PETMP.[434] Given the success of this strategy for preparing LCEs, the motif has been extended into AM. For example, RM82-EDDET has been copolymerized with PETMP via DIW printing to produce smart textiles and artificial muscles.[435] RM82-EDDET was also DIW printed through copolymerization with soft elastomeric resins or glassy polymer resins, as well as using a conductive formulation. The polymers were shown to be SMPs that could be employed as actuators.[436] A series of LCE SMPs have also been DIW printed using a multi-material approach by extruding multiple inks of differing thiol monomers (EDDET, EDT, or EGBMP) and concentrations alongside TATATO and RM82 via two-stage thiol-Michael and thiol-ene reactions. This approach allowed control of both actuation temperature and actuation strain, as well as facilitating sequential shape changes. This provided a valuable basis for understanding the effect of each component within the system to precisely control nematic-to-isotropic transition temperatures (TNI) and thus formulate intricate SMPs. Here, different formulations allowed varying TNI, whereby transition from optically scattery to transparency was observed by heating through the TNI. It was also shown that the thiol-ene LCEs exhibited 2× higher actuation strains and sharper transitions compared to acrylate equivalents, attributed to their homogeneous network formation. Furthermore, printing of the polymers within the nematic phase allowed the polymers to undergo reversible shape changes in the primary print direction, with the temperature and magnitude of strain dependent on monomer structure. This anisotropic behavior was attributed to the mesogenic monomer contributions within the polymer.[437]
Electrical actuation of SMPs has been achieved through inkjet printing of LCE copolymers of RM82-EDDET and PETMP that were incorporated as hinges within DIW printed structures (Figure 27). Here, silver nanoparticle circuits were embedded within the multi-material structures to facilitate electrical actuation. The bending angle of the hinges could be controlled by the applied electrical current, which induced Joule heating of the silver nanoparticle circuits and thus actuated the LCEs.[438] It may be anticipated that control of the TNI through the molecular architecture could be exploited to precisely engineer the electrical current required for actuation. Extending the mechanism by which SMP actuation is achieved, RM82-EDDET has been copolymerized with PEG methyl ether thiol while incorporating gold nanorods, yielding DIW-printed photothermally actuated SMPs. Photothermal actuation was performed by irradiation using an 800 nm NIR laser, achieving a temperature of 158 °C in the actuator after just 40 s at 1.55 W cm−2. Spatially selective photothermal actuation facilitated selective bending, which could be used to induce soft robotic locomotion within bilayer structures. Furthermore, the illumination time could be used to control the bending angle, and hence speed of locomotion.[439]
RM82-EDDET has been sequentially coupled with hexamethylene diisocyanate and then 1,6-hexanedithiol to yield liquid crystal thiourethane thermoplastic polymers.[440] This polymer could be extrusion printed without the need for photopolymerization, and exploited the thermoreversible hydrogen bonding to achieve SMPs that were also melt recyclable.[441] This polymer was also prepared into a drawn filament capable of rotating actuation, extending the scope of its usefulness.[442] Further work incorporating charge transfer complexes into the thiourethane polymers has also extended the scope of photothermal actuation for these polymers, although use within AM was not demonstrated here.[443] This work was then furthered by the addition of azobenzene derivatives within the backbone of the thiourethane polymer, whereby the polymer network displayed photoactuated SMP reversibility between UV and blue light by cis-trans isomerism of the azobenzene derivatives as well as thermal SMP actuation owing to the liquid crystal components and hydrogen bonding.[444] Indeed, several other thiol-ene polymers incorporating azobenzene derivatives have also been exploited for photoswitching bridges[190, 445] and diazocines,[446] with investigations into optimization of conditions for photomechanical response within LCEs.[447] Alternatively, the incorporation of surface-functionalized nitrogen-doped TiO2 nanocrystals has allowed photochromism and photoactuation within DIW-printed LCEs. Here, 1,4-bis-[4-(3-acryloyloxypropyloxy) benzoyloxy]−2-methylbenzene (RM257) and EDDET were coupled, followed by 15 wt% or 25 wt% incorporation of TiO2 nanocrystals. The printed LCEs displayed reversible photochromism when subjected to UV irradiation due to the photoreduction of Ti4+ to Ti3+ species. Additionally, NIR irradiation induced photothermal heating, leading to SMP capabilities. A synergistic mechanical actuation was observed with concurrent UV irradiation owing to the induced photochromism effect causing the material to turn black, which could be exploited for spatially selective differential actuation of the material. This was exploited for masking regions to be photochromically actuated within bilayer structures to act as “hinges” for NIR activated for soft robotic locomotion.[448]
Achieving acceptable printing resolutions has been an ongoing challenge for DIW printing. Attempts to improve printing resolution have been performed by using printing support baths. For example, RM257-EDDET was copolymerized with PETMP via DIW printing into a catalyst bath containing 2 vol.% 1,5-diazabicyclo[4.3.0]non-5-ene dioxane solution, whereby shape memory was programmed by UV irradiation followed by thermally actuated shape memory.[449] In another example, high fidelity printing was achieved by wet printing in a support bath, followed by freeze drying to obtain the final polymer.[450]
On the other hand, vat photopolymerization AM technologies usually facilitate improved printing resolution fidelity compared to most DIW 3D printing technologies. As such, RM82-EDDET has been further coupled with PEGDA (200 Da) and copolymerized with PETMP using DLP printing at 70 °C and using toluene as a solvent. Printed objects with complex geometries and relatively good resolution were achieved, while the relative incorporation of the LCE network could be exploited to achieve a range of TNI between 46.1-93.5 °C. Furthermore, by partially replacing PEG-DA with poly(pentadecanolide) diacrylate, a dual phase polymer with crystalline components could be achieved, resulting in reversible actuation and rapid reprogramming due to the additional crystalline transitions. In the examples performed, programming was performed at 140 °C, while shape memory actuation was achieved by changing the temperature between 25 °C and 65 °C, with a shape fixity ratio of 98.1% and shape recovery ratio of 99.9%.[451] Another example has used hybrid DLP/DIW AM to achieve LCEs by DIW printing the LCE within a DLP resin vat, such that simultaneous selective curing of the extruded polymer and resin within the vat could be achieved. This approach allowed for either the introduction of rigid DLP-printed components incorporated alongside LCE DIW-printed components for multi-materials, or the use of a dissolvable support that was DLP-printed around the DIW-printed LCE to facilitate intricate structures. A benefit of the latter approach was that large strain (40%) actuation could be achieved within printed lattices.[452] Within the context of intricate truss structures, composite materials have been fabricated using DIW printing by coextrusion of continuous poly(aramid) fibers alongside RM257, RM6, and 1,4-benzenedimethanethiol. The fiber position could be controlled to off-center positions within the extrudate, thus facilitating controllable shape memory deformations. Furthermore, the addition of the high modulus fibers allowed high load-bearing capacities and intricate truss structures to be achieved.[453]
Aside from SMPs, LCEs incorporating the thiol-Michael adduct have also been explored for impact-absorbing devices. For example, RM257-EDDET was copolymerized with PETMP via DIW printing, which showed soft elasticity large monodomain LCE for impact-absorbing devices. Here, the ability for the LCE to dissipate energy was relatively higher than that of reference formulations based on bisphenol-A dimethacrylate or commercial polyurethanes.[454] Given the success of this system, different dithiol monomers were studied to elucidate the relationship with properties,[455] the trade-off between stress and strain through crosslinking density,[456] the effect of strain rate and orientation on DIW printed specimens,[457, 458] the assessment of the real-time alignment and reorientation of mesogen chains to understand dissipation,[459] momentum transfer on impact dampening,[460] pressure-sensitive adhesion mechanism,[461] and relationships between molecular architecture and reorientation.[462] These investigations, along with other developments of bulk polymerized thiol-Michael-based LCEs, have rapidly developed the understanding and potential for additively manufactured objects.[463-472]
The reaction between thiols and maleimides presents itself as a subsidiary of the thiol-Michael reaction, and is generally characterized by appreciably more rapid kinetics compared to using acrylates.[473, 474] However, the thiol-maleimide reaction is typically also base-catalyzed, limiting its direct use as a polymerization strategy within AM. Nevertheless, maleimide functional surfaces have been demonstrated as an effective mechanism for post-polymerization thiol-ene peptide conjugation.[475] Within this context, the thiol-maleimide reaction has been exploited for coupling of CM-RGD peptides within the monomer of DIW-printed silk fibroin and silica nanostructured aerogels. The resultant polymer could undergo release of the CM-RGD peptide from the thiol-maleimide adduct, thus imparting antibacterial properties.[476]
It is interesting to note that while the strategic use of a PBG for the photoinitiated thiol-Michael reaction has been demonstrated in a number of instances for bulk polymers, it is yet to effectively extend into AM.[67, 477-482] In particular, the ability to exploit visible light[477] and red light (663 nm)[483] presents exciting opportunities for affordable light sources and mild conditions, or employing orthogonal wavelength-specific reactions. Furthermore, the photopolymerized thiol-Michael polymerization has been shown as reversible, making it an attractive chemistry for imparting DCC within the polymer network.[484] Within this context, the thiol-Michael reaction has also been shown to undergo a chain-growth mechanism using a PBG, opening opportunities for achieving interesting molecular architectures.[485]
3.5.3 Incorporation of [xπ+xπ] Cycloaddition Adducts
Coumarin-based compounds can undergo photodimerization by [2π+2π] cycloaddition, which can be exploited as a mechanism for crosslinking within the polymer network. The photodimerization reaction is reversible, whereby higher wavelengths induce the forward reaction and lower wavelengths the reverse (Figure 28).[486] This has been shown as an independently effective crosslinking strategy to impart reversible bonds within DLP printed hydrogels and bulk poly(urethane)s, hence demonstrating its individual merit as a crosslinking strategy.[487-489] Similarly, cinnamoyl moieties have also been shown to undergo [2π+2π] cycloaddition for effective crack healing.[490] Beyond this, however, it can also facilitate a secondary mechanism of crosslinking that can be used for spatially controlled and reversible mechanical properties and controlled configurational deformation.[491-494] On that note, coumarins generally react more rapidly at higher wavelengths compared to cinnamoyls, making them more suitable for most AM technologies.[495] For example, several acrylate-functionalized coumarin monomers have been synthesized and copolymerized alongside PETMP and THFA by DLP printing to fabricate multi-material polymers. Here, the thiol-acrylate polymerization could be induced at 450 nm, while the [2π+2π] cycloaddition only occurred under 365 nm irradiation, thus allowing sequence-dependent orthogonality within the system. Additionally, systems polymerized using 365 nm irradiation led to higher Tg (ΔTg of 17 °C when photodimerization not activated), which could also be tailored by increasing PETMP content (Tg from 24 °C to 4 °C for 0–20 wt% PETMP). These systems were polymerized using a dual-wavelength (365 nm and 405 nm) DLP printer that allowed layer-by-layer spatially selective polymerization by using either only 405 nm or a combination of 405 nm and 365 nm irradiance. This approach allowed spatially selective soft (only 405 nm: no cycloaddition) and hard (405 nm + 365 nm: coumarin cycloaddition activated) regions within the multi-materials. Notably, it was also suggested that the use of a thiol-acrylate system was beneficial for homogeneous network formation, as well as PETMP increasing overall curing compared to systems with only the acrylates, with a notable increase in coumarin photodimerization using 20 wt% PETMP (53% to 78% conversion). Having successfully achieved the fabrication of the multi-materials, the spatially controlled properties could be exploited to selectively induce differential shape memory properties (Figure 28).[496] The incredible versatility and functionality that can be achieved through this strategy provides a promising platform for applying similar systems in AM.[416, 497] Furthermore, the combination of coumarins within polymer networks containing thermally activated DCC has also been established as a viable route to create networks with dual stimulus through both heat and light within LCEs. Here, TMSDA was used alongside an acrylate-functionalized coumarin, whereby the thiol-Michael bond was shown to be reversible under thermal stimulus and the coumarins reversible under light stimulus, thus providing orthogonal mechanisms for self-healing and reconfiguration.[416]
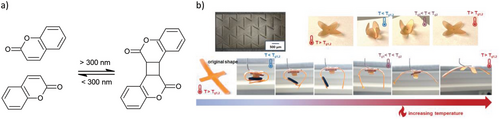
However, it is also well known that the cycloaddition is an equilibrium reaction that favors the reverse reaction at lower wavelengths (UVB range), begging the question of these multi-material properties’ stability under ambient light conditions where a full UV spectrum is present, which may limit their plausible environmental applications.
3.5.4 Incorporation of Diels-Alder Adducts
The Diels-Alder (DA) reaction is ubiquitous as a thermally reversible reaction via a dissociative mechanism, and has been exploited in numerous CANs.[498] However, the DA reaction alone has limited application in vat photopolymerization AM, owing to the fact that polymerization generally occurs autocatalytically at room temperature and thus cannot be selectively controlled using UV irradiation, but has seen development in FFF and SLS technology owing to the dissociative dynamic covalent chemistry at elevated temperatures.[499-503] Nevertheless, the incorporation of the desirable properties of the DA adduct within the structure of photopolymerisable monomers can afford this chemistry to be exploited within vat photopolymerization AM.[504] Furthermore, the incorporation of DA adducts within thiol-ene polymer networks has been shown to achieve polymer networks with both DCC as well as imparting flexible bonds and homogeneous networks. This concept has been demonstrated for thiol-acrylate systems with an acrylate-functionalized poly(urethane) oligomer containing maleimide/furan derivative DA adducts within the backbone, which displayed excellent thermal reversibility and self-healing capability.[505] Finally, an example using TPP 3D printing exploited the thiol-thioester exchange alongside a DA adduct in the backbone of the monomer. Here, tetrakis furfurylthiopropionate (TFTP, Figure 29) and 1,13-bismaleimido 4,7,10-trioxatridecane (BMTD, Figure 29) were copolymerized with PETMP. The polymers could be photofixated, followed by depolymerization in furfuryl alcohol at 105 °C to yield complex geometry objects (Figure 29).[506]
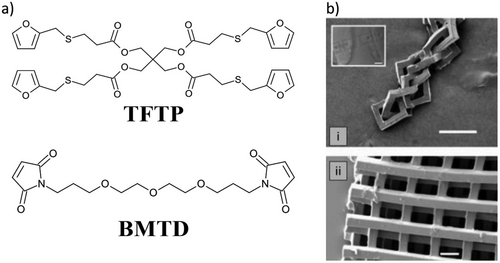
Despite there currently being few examples of incorporating DA chemistry within thiol-X polymers for AM, several potential opportunities present themselves. For example, the photoactivation of the DA reaction has been demonstrated using photocaged catalysts, enabling a potential pathway for direct photopolymerization within AM.[507] Furthermore, given the opportunities for interesting applications in post-polymerization conjugations, the development of polymers exploiting thiol-X and Diels-Alder chemistry within AM presents appealing opportunities.[473]
3.5.5 Incorporation of Oxime Esters
Visible light photoinitiators are incredibly useful within vat photopolymerization as they permit the use of affordable LEDs for illumination or can be exploited for multi-wavelength orthogonal chemistries. One of the most commonly employed visible light initiators is ITX. However, it is toxicologically harmful and has been found in food products, leading to its regulation and diminished use.[508] As such, benign photoinitiators that do not migrate out of the polymer are of growing interest. Oxime esters are known to undergo photolytic cleavage, typically in the visible light wavelengths (400–480 nm), making them useful as visible light photoinitiators.[486, 509, 510] Within this context, coumarin-based oxime esters (e.g., OEC-1 and OEC-2, Figure 30) with photobleaching have been demonstrated for thiol-ene and thiol-yne polymers using 400-480 nm.[511] Furthermore, these have been applied within SLA 3D printing by using oxime ester phenothiazines with TMPTA, which demonstrated their efficacy as photoinitiators using 405 nm irradiation with comparatively better results than commonly used TPO.[512] The incorporation of oxime esters within monomer structures has also proved an effective strategy to achieve low migrations, as they are covalently incorporated into the polymer network. For example, an acrylate functionalized oxime ester monomer was used for thiol-acrylate 3D printing using PETMP and TMPTA. The oxime ester successfully displayed low migration as well as imparting luminescence in the polymer.[513] This motif has been extended to demonstrate that photoinitiation could be embedded using an oxime ester coumarin photoinitiator (OEC-3, Figure 30) for thiol-ene polymerization in a “dandelion-like” manner with near-infrared radiation (980 nm). Here, thiol-functionalized silica was used to coat up-conversion nanoparticles (UCNPs), followed by functionalization with the photoinitiator via thiol-ene coupling of OEC-3. Polymerizations were noted to be most efficient for the thiol-ene polymerization, and present an exciting new method of polymerization particularly useful for instances when direct use of low wavelength irradiation is inappropriate within AM.[514] An additional advantage of the incorporation of oxime esters is that they can also undergo thermal dynamic transesterification. For example, bulk copolymerization between OE-2, PETMP, EDDET, and OEA has been shown to undergo rapid exchange kinetics, making the polymer depolymerizable, reshapable, and reprocessable.[515] These properties hold obvious desirability within AM, adding impetus towards further developments using oxime esters within vat photopolymerization AM technologies.
3.6 Ternary Thiol-Ene-(Meth)Acrylate Systems for AM
While the thiol-(meth)acrylate system can form distinct binary networks due to the combination of thiol-(meth)acrylate step-growth and (meth)acrylate chain-growth radical homopolymerization, ternary networks of thiol-ene-(meth)acrylate systems can further form complex distinct networks within the polymer. Here, electron-rich enes and thiols typically proceed almost exclusively via step-growth, while methacrylates can polymerize via the aforementioned combination of step-growth and chain-growth. The choice of thiol, ene, and (meth)acrylate allows for tailoring of propagation and chain transfer rates, thus manipulating the resultant network formation. The functional group stoichiometric ratio can be less sensitive in ternary systems, yet can facilitate reduction in controlling the half-width Tg, which is appealing for SMPs.[516] Ternary systems can also display phase separation through differential reaction kinetics, which has been observed to reduce polymerization shrinkage stresses.[517, 518] Ternary thiol-ene-acrylate systems have also been shown to optimize high conversions, low polymerization shrinkage, and bioavailability.[519-522] For example, TMPTA, TTT, and PETMP have been copolymerized in varying ratios using DLP printing. Here, it was demonstrated that the incorporation of thiol monomers to form ternary formulations could effectively reduce polymerization shrinkage, increase overall conversion, and tailor the (thermo)mechanical properties.[523] In another example, the incorporation of nitrile rubber particles (up to 10 wt%) within mixtures of commercial acrylate resin and PETMP (up to 7.5 wt%) successfully introduced the particles within the network using the residual unsaturation on the rubber. The LCD-type 3D printed polymers displayed low polymerization shrinkage and improved toughness with incorporation of the nitrile rubber particles and PETMP.[524] This approach has also been shown using TATATO, PETMP, and TMPEA alongside two acrylate- and thiol-functionalized quaternary ammonium salts as intrinsic antimicrobial agents within DLP printing. A minimum in shrinkage was found for 30 wt% thiol content and 70:30 ratio of TMPEA-to-TTT. The antibacterial agents demonstrated high efficiencies with S. aureus, although relatively high incorporation was necessary for E. coli.[525]
In another example exploiting thiol-ene-acrylate systems for network architecture control, acrylate-functionalized hydrogenated poly(butadiene) oligomers have been copolymerized with 1,6-HDT by mask projection micro vat photopolymerization AM and scanning-mask projection vat photopolymerization. Owing to the difunctionality of both monomers, controlled linear thiol-ene step-growth of the main chain with chain-growth only occurring for crosslinking was observed. This resulted in polymers that displayed an increasing strain at break (up to 64%) with increasing dithiol content (up to 0.75 eq. 1,6-HDT) that could be cycled up to 40% strain without hysteresis.[526] A similar approach to selective thiol-ene and thiol-acrylate polymerization strategy was employed to allow postfabrication metal plating of DLP-printed polymers. This system allowed a stoichiometric excess of thiol moieties on the surface to act as active sites for metal ion complexation. Furthermore, Cu, Ag, and NiP metal layers were successfully postfabrication grafted to the surface, demonstrating this as a viable strategy for additively manufactured electronic components.[527] In fact, thiol-bearing polymers have also been shown as useful within the context of silver nanoparticles[528] and gold nanoparticles.[23] Indeed, this provides a highly viable platform for the controlled release of nanoparticles from additively manufactured hydrogels, thus facilitating controlled drug delivery potential.[529] A further example exploiting postfabrication surface functionalization used a diallyl functionalized monomer derived from biobased levoglucosan that was copolymerized with PETMP and PEGDA via SLA printing to yield a transparent polymer. Postfabrication surface modification of off-stoichiometric (excess thiol) bulk formulations with 1-pyrenemethyl methacrylate resulted in polymers that fluoresced under 365 nm irradiation.[530]
Within the context of electronic component fabrication, an electrically conductive weather-resistant wearable device has been fabricated using LCD-type 3D printing. Here, the use of an allyl functionalized poly(siloxane) oligomer, poly(urethane) oligomer, TMPTMP, TMPA, alongside 10 wt% lithium trifluoride (LiTf3) (Figure 31) resulted in polymers with tuneable electrical conductivity. The electrical conductivity was both temperature and strain-dependent, with low hysteresis in both instances. Examples of its use as a gel keyboard for typing, detection of human motion of phonating, and use within wearable devices demonstrated its versatile applicability.[531] Another soft actuator has been developed using TMPTMP, TATATO, decanedimethanol diacrylate (TCMDA) alongside iron(II, III) oxide nanoparticles to produce multi-material shape memory hydrogels triggered only by combined stimulus of pH and temperature. Here, mSLA printed multi-layered actuators containing a hydrophilic pH-sensitive layer and a hydrophobic magnetic and temperature-responsive SMP layer resulted in the ability to design anisotropic multi-materials capable of advanced spatiotemporal responsiveness that were demonstrated for soft robotic grippers.[532]
Lastly, thiol-ene-acrylate systems have also been exploited to control the mechanical properties and nonlinear threshold response in VP 3D printing. This was achieved using a combination of acrylates TEGDA and TAICN, allyl ethers TAEICN and TEGDAE, and thiol, TMEICN. Here, by varying the relative incorporation of monomers the mechanical properties could be tailored within a wide range (ET from 0.12 to 421 MPa), while adjusting the amount of inhibitor (2,2,6,6-tetramethyl-1-piperidinyloxy, TEMPO) was used to effectively control the nonlinear threshold response. It was also noted that the sharpness of the threshold in these systems effectively improved print resolution fidelity.[80]
3.7 Thiol-Hydroxy(Meth)Acrylate Chemistry for AM
The transesterification of β-hydroxyesters is ubiquitous with many CAN systems, whereby free hydroxyl groups and proximate ester groups can undergo dynamic exchange.[533] Common examples of β-hydroxyacrylates include 2-hydroxy-3-phenoxypropyl acrylate (HPPA), glycerol 1,3-diglycerolate diacrylate (GGD), and bisphenol A-glycidyl methacrylate (bis-GMA) (Figure 33).
The benefits of thiol-hydroxyacrylate polymers have been demonstrated in effectively producing high Tg polymers with low polymerization shrinkage and improved impact properties. Here, bis-GA was copolymerized alongside a synthesized hyperbranched thiol (T-HBP, Figure 32) using DLP printing. Generally, it was reported that increasing weight fraction of T-HBP (0-30 wt%) decreased the system viscosity, increased polymerization kinetics, and increased impact strength, with the trade-off of a modest decrease in tensile strength (57.6 to 47.9 MPa). Additionally, it was observed that increasing the weight fraction of T-HBP resulted in lower volume shrinkage, from 8.95% for 0 wt% to 3.56% for 30wt%, as well as reducing polymerization shrinkage stresses. The use of T-HBP presents itself as a good example for achieving high crosslinking density within thiol-X polymers, without compromising on the viscosity (79.2 mPa.s) facilitating rapid photopolymerization, and reducing polymerization shrinkages.[534] The low viscosity that was achieved despite the monomer's high molecular mass has previously been attributed to its hyperbranched architecture.[535]
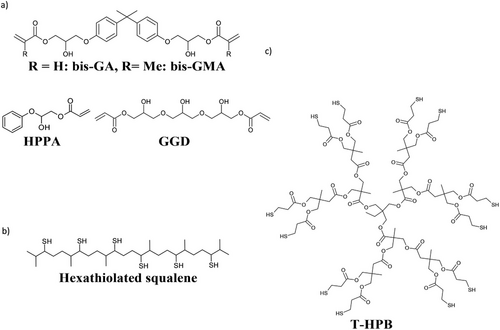
Excessive polymerization shrinkage during the fabrication of composite materials using reinforcing fillers can lead to antagonistic effects due to localized stresses at the interface.[536, 537] As such, the surface coating of TiO2 nanoparticles with T-HBP has been reported to effectively reduce polymerization shrinkage in DLP printed systems using bis-GA and dipropylene glycol diacrylate. The surface coating was achieved using a common coupling agent (3-(trimethoxysilyl)propyl methacrylate) followed by thiol-methacrylate coupling of the hyperbranched thiol monomer. Polymerization shrinkage was successfully reduced from 8.95% down to 4.18% through its incorporation. Furthermore, maxima in σt of 62 MPa and impact strength of 18 kJ.m2 were reported for 5 wt% filler, which were higher than the unfilled polymers.[538]
Acrylated epoxidized soybean oil (AESO) is a β-hydroxyacrylate that is arguably one of the most studied biobased vegetable oil-derived monomers for radical polymerization in contemporary literature. In fact, its use within thiol-acrylate photopolymerized polymers was demonstrated as early as 2010.[539] The AESO monomer has been extended in its use with a variety of thiols, including 1,3-BDT, PETMP, and hexathiolated squalene (Figure 32).[41, 540-542] Furthermore, of the aforementioned thiols, optimized mechanical properties using PETMP have been reported.[543] Within the context of AM, AESO has been copolymerized with either 1,3-BDT or 1,4-BDT via DLP printing. Here, using 1,4-BDT resulted in greater crosslinking density, possibly owing to lower steric hindrance.[540] In another example, AESO, VADM, and PETMP were mSLA printed in varying relative concentrations to yield tailorable mechanical properties, with AESO and VADM providing rigid contributions and PETMP flexible contributions.[544] AESO has been demonstrated as an effective monomer to produce CANs via dynamic transesterification, presenting an opportunity to achieve biobased 3D printable CANs,[545-547] where AESO has also been copolymerized with PETMP to produce self-healing polymers.[548] Moving towards its use within AM, AESO, vanillin methacrylate, methacrylate-functionalized lignin particles, and PEG-2SH (400 Da and 600 Da) have been copolymerized via DLP printing to form composite materials, incorporating Zn(acac)2 as a transesterification catalyst. However, it was noted that probe sonication was imperative to achieve even distribution of the lignin particles, Zn(acac)2 had low solubility in the resin, and that uncrosslinked methacrylated lignin acted as a plasticizer. Despite these issues, the polymers were capable of self-healing both scratches and complete fractures after 24 h at 140 °C, and the healed samples showed comparable, or even higher, mechanical properties. In this system, the methacrylate-functionalized lignin was able to participate in both step- and chain-growth within the polymer network, with the thiol-ene bonds presumably decreasing brittle interfaces.[549] This work highlights the potential of using thiol-X chemistry to enable the processing of lignin composites to optimize mechanical properties while retaining self-healing capability.[550]
3.7.1 Incorporation of Organic Phosph(on)ates
Organic phosph(on)ate esters have been employed in various polymers for their dynamic exchange ability, while the hydroxyl functionality on hydroxy(meth)acrylates presents these catalysts as highly effective.[551] Furthermore, organic phosphates that contain unsaturated moieties can contribute to the polymer network and thus become intrinsic catalysts. Common examples of such include bis(2-methacryloyloxy)ethyl phosphate (BMEP) and ethylene glycol methacrylate phosphate (EGMP) (Figure 33). Aside from their transesterification capability, many organic phosph(on)ates are also known for their flame retardant properties, which is often an attractive property to impart in polymers.[552] Hydroxyacrylate examples have demonstrated that mSLA printed polymers could afford high concentrations (15 wt% EGMP) owing to its participation in the polymer network. On the other hand, alternative catalysts have been shown to be unsuccessful; TBD retarded polymerization kinetics acting as radical scavenger, while triphenylphosphine displayed sluggish relaxation rates.[553] DLP-printed thiol-hydroxymethacrylate polymers were then prepared using several different organic phosph(on)ates to assess their ability to catalyze transesterification for dynamic exchange. Generally, it was determined that protic organic phosphates (e.g., EGMP, BMEP) provided superior dynamic exchange, and relaxation rates increased with increasing pKa of the catalyst, while incorporation of the catalysts increased the Tv by ≈10 °C.[554] It has also been shown that using EGMP resulted in 4× faster stress relaxation compared to using Zn(OAc)2 or Zn(acac)2 as a catalyst, while uncatalyzed polymers displayed very little stress relaxation.[553, 555] Using 5 wt% EGMP as organic phosphate catalyst, HPPA (50 mol%), GGD (25 mol%), and TMPTMP (25 mol%) were copolymerized using an LCD-type 3D printer with an 8 s layer cure time. The polymers displayed efficient triple-shape memory and excellent self-healing, with negligible difference in tensile properties after repair. Compared to the previously described analogous hydroxyacrylate polymers, relatively shorter layer cure times were achieved (10 s for hydroxyacrylate). On the other hand, the introduction of TMPTMP reduced the σT from around 20 MPa to 5 MPa, while εT increased dramatically from 7% for the hydroxyacrylate system to 80% for the thiol-hydroxyacrylate. Comparatively, the characteristic relaxation time (τ*) of the hydroxyacrylate polymer containing 15 wt% EGMP was 102 min at 180 °C, while the thiol-hydroxyacrylate polymer took only 7.5 min under the same conditions.[553, 556] The dynamic exchange kinetics were also shown to increase with an increase in concentration of ester and hydroxyl moieties in DLP printed systems containing unsaturated organic phosphates, allowing tailoring of properties through judicious monomer choice. Looking towards the influence of thiol crosslinker several dithiols (HDT, ethylene glycol bis-mercaptoacetate (EGBMA), and EGBMP) were compared. Here, HDT performed relatively poorer in both polymerization kinetics and displayed longer τ*. On the other hand, using thiols of higher functionality (e.g., PETMP, di-PETMP) did not affect τ* appreciably. The polymers were then applied as soft robotic actuators through thermal shape memory actuation.[557] The application of soft robotics was extended with a DLP-printed polymer containing bis-GMA, HPPA, TMPTMP, using EGMP as transesterification catalyst. The shape memory capability of the polymer produced an effective gripper device that could grasp both delicate and hard objects (Figure 33).[558] In another example, acrylated epoxidized linseed oil (AELO) has been copolymerized with TMPTMP or EDDET by DLP printing, incorporating BMEP as intrinsic transesterification catalyst. The dynamic capability of the polymer was demonstrated for a triple shape memory experiment by heating the sample relative to the Tv and Tg, and self-healing experiments also demonstrated promising results.[559] Furthermore, the synthesis of biobased phosphonate catalysts using either eugenol or citronellol (EUGP and CITP, Figure 33) has also been demonstrated on AELO for AM, although thiol-ene chemistry was not used here.[560] In fact, it is quite surprising that these were not incorporated into thiol-ene polymers as it is well known that the unsaturation on both eugenol and citronellol are sluggish towards free radical polymerization to effectively incorporate the catalysts into the acrylate polymer network.
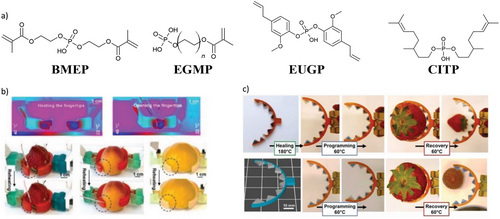
The incorporation of Fe3O4 nanoparticles for magnetoactive polymers has been demonstrated using DLP printing using HPPA, GDGDA, TMPTMP, and EGMP as catalyst, alongside various loading of Fe3O4 nanoparticles. It was found that above 10 wt% nanoparticle loading the cure rate decreased, presumably due to particles inhibiting light penetration. Interestingly, it was noted that the nanoparticles acted as catalyst for the thiol-Michael addition reaction, thus decreasing the pot life, yet the organic phosphate transesterification catalyst reduced this effect.[561] This work was extended into DLP printing of magnetoresponsive polymers that possessed magnetically driven movement and self-healing capability. However, some deleterious effects of the filler on the mechanical properties of the healed specimens were noted.[562]
Multi-materials can be fabricated by using dual vats in vat photopolymerization AM, whereby the print build plate is able to switch between the two vats, each containing different monomer systems. This approach has been performed using two different vats containing thiol-acrylate systems of a) EGBMP, GGD, HPPA, and EGMP and b) EGBMP, GGD, and TMPA. Here the individual resin formulations had distinct Tg a) 3 °C and b) 50 °C) and mechanical properties a) ET 9 MPa and b) 1472 MPa) owing to the difference in acrylate functionality of the monomers, and therefore differences in the ratio of thiol-to-acrylate groups (1:2 and 1:4). These difference in the polymer network architecture also resulted in differences in τ*, with formulation using a 1:2 ratio taking only 8.8 min, while formulation at 1:4 ratio took 83.5 min at 180 °C. As such, DLP printing using dual vats afforded fabrication of spatially controlled polymer properties, and was demonstrated as an effective method of constructing soft robotic devices with nuanced actuation and movement owing to the different polymer properties within the final object (Figure 33).[563]
Lastly, shape memory-assisted self-healing (SMASH) has been developed as a synergistic approach for geometric changes to facilitate self-healing.[564, 565] Thiol-hydroxyacrylate polymers incorporating an organic phosphonate catalyst have been demonstrated to facilitate SMASH, providing a promising basis for its translation into AM.[566]
3.7.2 Incorporation of Photoactivated Catalysts
While PBGs have previously been discussed as a promising method for photoactivation of catalysts to induce polymerization, various other photoactivated catalysts can present interesting opportunities within AM. Indeed, the photo-selective release of catalysts can also be used to orthogonally induce DCC within the polymer network. Within this context, further work examining the potential of organic phosphates as transesterification catalysts extended their use towards orthogonally activated photocatalysis by using a blocked organic phosphate, diethyl (2-nitrobenzyl) phosphate (DNBP, Figure 34), which was incorporated into a DLP printed polymer containing HPPA, GGD, and TMPTMP. DLP printing was performed using visible light (453 nm), while postfabrication spatially selective exposure to UVA (373 nm) induced release of the catalyst by rearrangement of the o-nitroaryl group. Subsequent thermal treatment resulted in rapid stress relaxation in areas where the catalyst had been activated, thus achieving spatially selective shape memory programming.[567] The potential of other photoactivated catalysts, such as photoacid and photobase generators, has also seen development in recent years.[568, 569] Specifically, ammonium salts of thioxanthone acetic acid (TXA) and tetraphenylborate (TPB) have been used as radically mediated PBGs.[570, 571] Interestingly, it was noted that for a proposed mechanism of decomposition of the TX-base, a methylene free radical after decarboxylation results, which can promote the formation of thiyl radicals.[571] It has been demonstrated that localized activation of catalysts can be performed at selective wavelengths (405 nm) using 1,1,3,3-tetramethylguanidine phenylglyoxylate (PGA-TMG, Figure 34). In this example, TMPDE and partially esterified PETMP were copolymerized using visible light (450 nm), while postfabrication activation of PLB-TMG could be spatially selectively performed to induce rapid dynamic transesterification, thus spatially controlling shape memory properties.[572] Triphenylsulfonium phosphate has been demonstrated as another latent photocatalyst, which undergoes photolytic cleavage at 365 nm to form a Brønsted “super acid”. One of the primary benefits of this catalyst is that it is latent at 405 nm, allowing conventional wavelength AM to be used. To their additional benefit, triphenylsulfonium salts do not promote radical-induced cationic polymerization, and radicals therefore cannot be oxidized by the species.[573] To demonstrate their efficacy, DLP-printed thiol-acrylate polymers were prepared using TMPTMP, HEA, and GGD alongside 10 wt% triphenylsulfonium sulfate. A dual wavelength DLP printer was employed with 365 nm and 405 nm projectors, and hence could be exploited to fabricate multi-materials. The catalyst was also shown to be thermally latent, yet postfabrication 365 nm irradiation facilitated dynamic covalent transesterification, evidenced by rapid stress relaxation. Indeed, this resulted in polymers with spatially selective exchange reactions that allowed spatially controlled remolding and shape memory properties. Here, shape recovery was achieved after just 10 min at 100 °C, with τ* at 180 °C taking 53.5 min when the photoacid generator was activated.[574] Given the success of this catalyst, work was extended to determine the efficacy of a series of other triphenylsulfonium salts. It was generally found that efficacy in transesterification was primarily dependent on the structure of the cation, which determines the absorption characteristics and quantum yields of released acid. DMS-borate was found to be the most efficient, although it did display thermal activation. PDS-triflate was used in a dual wavelength DLP printer, where specific regions were printed using either 365 nm or 405 nm irradiation, and local activation and shape recovery was achieved. Additionally, it was shown that these polymers were photopatternable.[575]
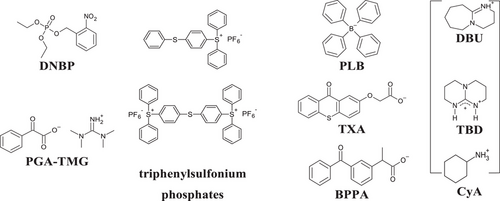
Coumarin photocages present another interesting area of PBGs suitable for vat photopolymerization AM.[477] For example, the molecular composition of coumarin photocages has been shown to govern the relative step-growth and chain-growth within the polymer network due to the congruent generation of radicals and organobase, whereby the composition of the PBG influences the relative chain-growth and anionic step-growth.[67] Another approach is the photocaging of thiol moieties using bromobimane to form photocleavable thioether bimane moieties. With this approach, nucleophilic thiol-X reactions could be initiated using visible light.[576]
3.8 Thiol-Acrylamide Chemistry for AM
The acrylamide group is an α,β-unsaturated carbonyl, implying it can also undergo thiol-Michael addition reaction, although it is approximately 24× slower than the thiol-acrylate reaction.[577] It is thus the case that radical-mediated thiol-acrylamide step-growth and acrylamide chain-growth homopolymerization are predominant. Furthermore, acrylamides have been shown to effectively impart hydrogen bonding within the polymer network of DLP-printed polymers, thus facilitating interesting properties.[578] For example, α,ω-thiol-functionalized PDMS and acrylamide-PDMS oligomers have been 3D printed using mask-projection scanning stereolithography. Here, using a low MW α,ω-thiol-PDMS alongside a high MW diacrylamide-PDMS oligomers meant that the thiol was less diffusion limited, hence promoting the thiol-acrylamide reaction. Comparatively, the thiol-acrylamide polymers displayed a 2× increase in εT compared to analogous thiol-acrylate polymers, attributed to this diffusion control.[579]
DLW 3D printing offers incredibly high x-y resolutions that can even surpass diffraction limits of around 30 nm in certain instances. As such, the thiol-acrylamide reaction has been exploited for DLW printing of photoresists using a chymotrypsin-cleavable crosslinker based on tyramine and L-phenylalanine copolymerized with PEG-2SH (1 kDa). Interestingly, the homopolymerized crosslinker was non-degradable with chymotrypsin, attributed to the high crosslinking density hindering access to the cleavable ester bonds. On the other hand, the incorporation of PEG-2SH successfully increased the molecular mass between crosslinks, resulting in enzymatic degradation after 4 h with 1 mg mL−1 chymotrypsin in PBS solution at 37 °C. Furthermore, the polymer was degradable under basic conditions (1N NaOH) and underwent aminolysis with ethanolamine, while it remained undegraded in acidic (1 n HCl) and neutral (PBS solution) conditions. It was further mentioned that the thiol-acrylamide polymerization enhanced fabrication parameters such as laser power and fabrication speed. The polymers were also established to be biocompatible with good cell viability and proliferation. Lastly, multi-material structures with degradable bridges could be fabricated (Figure 35) and establish the versatility of the polymer system.[580]
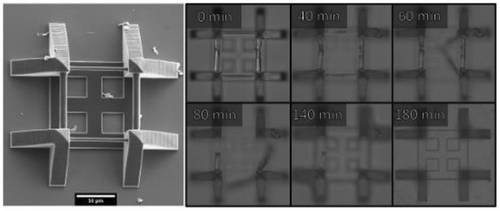
Beyond thiol-X polymers, several acrylamide functional monomers have also been prepared using α-amino acids for DLP printing, highlighting the ease with which biocompatible and biodegradable polymers could be synthesized using this approach.[581] Furthermore, the use of acrylamides has also been shown as an effective method to exploit orthogonal thiol-ene and thiol-acrylamide reactions using allyl- and N-vinyl acrylamides to accomplish sequence-defined polymers such as nucleic acids, extending the scope of bioconjugations within these polymer networks.[332, 582] Another underexplored area is in the use of N-vinyl acrylamides that can undergo orthogonal thia-Michael, radical thiol-ene, and Markovnikov hydrothiolation reactions useful for thiol-thiol bioconjugations in biomedical applications.[583]
4 Thiol-Yne
4.1 Polymerization and Properties
The alkyne functional group is an oft-neglected yet useful platform for a variety of reactions within polymer science, including the thiol-yne reaction.[584-587] The thiol-yne reaction is radical-mediated by a radical generator or UV irradiation to form a thiyl radical, and initially results in the formation of an alkenyl sulfide bond. This reaction is anti-Markovnikov with a mixture of E/Z alkenyl sulfide isomers, and has also been identified as a stereoselective reaction, thus imparting interesting properties to the polymer network with tuneable mechanical properties and shape memory.[425, 588, 589] One of the significant differences between the thiol-ene and thiol-yne reaction is that the residual unsaturation on the alkenyl sulfide product can result in further diaddition of a thiol to result in 1,2-disulfides or 1,1-dithioacetal formation (Figure 36). However, relative to thiol-ene reaction kinetics, the thiol-yne reaction is slower, while the subsequent reaction between the formed alkenyl sulfide and another thiol is once again relatively higher than the initial thiol-yne reaction.[590] The consequence of this two-step reaction is an increase in maximum theoretical crosslinking density within the polymer network.[591, 592] Additionally, by limiting the thiol stoichiometric ratio, residual unsaturation of the alkenyl sulfide bond can be exploited to provide a platform for further post-polymerization modifications or interesting surface functional applications.
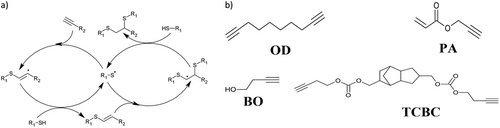
Similar to thiol-ene polymerization, the thiol-yne polymerization also promotes ideal network synthesis,[61] while ternary thiol-yne-(meth)acrylate systems reduce polymerization shrinkage stresses[593], and thiol-yne-epoxy systems can be leveraged for accessible dual curing strategies.[594] However, relative to its thiol-ene counterpart, far fewer developments have been demonstrated within AM. Likewise, far fewer alkyne monomers are commercially available (Figure 36) compared to alkene monomers, which may partially be the cause for less development seen within thiol-yne polymers in AM. Nevertheless, examples of monomers containing multiple functional groups, such a propargyl acrylate (PA, Figure 36), have also been shown to present opportunities for orthogonal chemistry.[595]
4.2 Thiol-Yne Chemistry for AM
A tetrafunctional pendant alkyne poly(carbonate) oligomer (BCO, Figure 37) has been synthesized and copolymerized with EDDET, TMPTMP, or PETMP. Polymers DLP printed using PETMP displayed rapid hydrolytic degradation in 1N NaOH solution at room temperature, with complete dissolution achieved in just 120 min.[596] In another example of degradable polymers, poly(4-methyl-ε-caprolactone) dialkynylate oligomers, PEG-dialkynylate, and PETMP were copolymerized via DLP printing to form elastic scaffolds. Here, varying the relative incorporation of oligomer or PEG components could effectively tune the mechanical properties, while rapid degradation (200 min) in 1N NaOH was achieved. Furthermore, the polymer scaffolds displayed good cytocompatibility.[597] Unsaturated poly(ester) oligomers containing fumarate, succinate, and propylene components with terminal propargyl groups have been copolymerized with TMPTMP using CLIP 3D printing. The relative incorporation of succinate and fumarate components could be exploited to tune the mechanical and swelling properties that displayed degradation in 0.25N KOH at 37 °C.[598] Oligomers formed between limonene oxide and cyclic acid anhydrides containing terminal propargyl groups have been copolymerized with eugenol methacrylate and PETMP using DLP printing. The polymers displayed shape memory behavior and were capable of chemical recycling. Furthermore, degradation in 1N NaOH was achieved, and higher rates of degradation were observed for thiol-yne-methacrylate networks compared to analogous free radical polymers.[599]
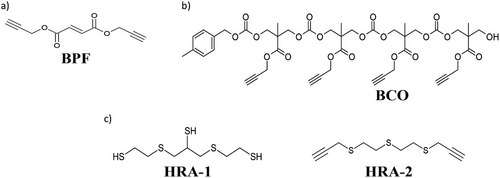
DLP printing of a thiol-yne polymer using visible light (470 nm) has been achieved through dual photoredox catalysis. Here, 1,7-octadiyne (OD, Figure 36) and PETMP were copolymerized using two different photoredox catalysts to optimize printing resolution and reaction kinetics.[600] The higher radiant efficiency of visible light LEDs implies that these systems could benefit from higher printing speeds with cost-effective systems. Furthermore, various other efficient photoredox catalyst exist, which could be viable within AM systems.[601]
4.2.1 Surface Functional Polymers
Owing to the residual ene functionality that can be retained within thiol-yne polymers, one of the primary focus areas within AM has been for biomedical devices that can be postfabrication surface modified. A series of butyne-1-yl carbonate oligomers (BCO, Figure 37) and various thiol monomers (e.g., PETMP, di-PETMP, TMPS, EDDET) were investigated as viable polymers for DLP printing of tissue engineering materials. Here, tricyclo[5.2.1.02,6]decane-4,8-dimethanol dibut-3-yn-1-yl carbonate (TCBC, Figure 37) and di-PETMP were identified as promising candidates owing to rapid kinetics, low cytotoxicity, and acceptable mechanical properties, which were therefore chosen for DLP printing of medical device structures. Printed specimens with x-y resolutions down to 40 × 40 µm2 could be achieved using these monomers.[602] Off-stoichiometric polymerization between bis(propargyl) fumarate (BPF, Figure 37) and TMPTMP via DLP printing was performed for materials suitable for postfabrication functionalization. It was determined that thiol-limited stoichiometry could lead to chain-growth mechanisms of alkyne/vinyl sulfide, while alkyne-limited stoichiometries were predominantly step-growth, thus enabling the tailoring of network formation from a stoichiometric perspective. Furthermore, the Tg of alkyne-limited polymers was 8 °C, while the thiol-limited polymer was 34 °C; the difference was thought to be due to the rigidity of residual alkyne moieties in the thiol-limited polymers. DLP printing afforded x-y resolutions down to 200 µm, and good layer adhesion was qualitatively noted. Following printing, azide-terminated squarine dye could be successfully grafted onto the thiol-limited polymer via the residual alkyne moieties of thiol-limited polymers.[603] A similar study showed that the incorporation of PA into SLA-printed polymers of PEG-divinyl ether and PETMP could be used for postfabrication functionality. Here, the relatively lower reactivity of the propargyl group was exploited to retain its functionality in the polymer, thus allowing postfabrication functionalization of the polymer with an azide dye. However, cytotoxicity diminished its prospects for tissue engineering.[604]
Dye pollutant removal has also been investigated as an interesting application of thiol-yne polymers; as has previously been seen, azide dyes are able to graft onto thiol-yne polymer surfaces that contain residual alkyne moieties. SLA-printed polymers from PETMP and 3-butyn-1-ol (BO, Figure 36) were demonstrated to effectively remove malachite green from water, and could be used repeatedly without appreciable loss in efficacy (>90% dye extraction after 10 consecutive dye extractions).[605] The residual functional groups on thiol-yne polymers have also been exploited within biomedical contexts for postfabrication functionalization/conjugation. For example, FFF printing of poly(ester urea) oligomers containing phenylaniline and propargyl pendant groups resulted in the ability to postfabrication graft azide-derivatized peptide moieties onto the polymers.[606] Indeed, the ability to impart postfabrication bioconjugations poses great potential within biomedical contexts.[607]
4.2.2 High Refractive Index Polymers
A series of propargyl ether monomers were synthesized and copolymerized with different thiols via DLP printing to prepare high-refractive index polymers. Both the propargyl ethers and thiols were endowed with thioether bonds within their structures (HRA-1 and HRA-2, Figure 37). It was noted that using primary thiol monomers was important to ensure high conversion and acceptable Tg materials. Refractive indices >1.6 were achieved, and it was demonstrated that high concentrations of sulfide bonds within the polymer allowed refractive indices >1.68. These polymers were also two-stage printed onto a poly(urethane-thiourethane) matrix to assess their capability as holographic materials.[608] This provides an exciting platform for further transfer of other thiol-yne high refractive polymers into AM.[609, 610] Furthermore, several other thiol-X polymers have been investigated as holographic materials, alongside computational elucidation of the liquid crystal phase separation by periodic laser-induced photopolymerization; it thus may be anticipated that further examples incorporated into AM could become evident.[611-615]
5 Thiol-Isocyanate
5.1 Polymerization and Properties
The thiourethane bond is reversible through dynamic dissociation and also introduces hydrogen bonding into the material.[616, 617] Here, hydrogen bonding in poly(thiourethanes) can induce adhesive properties with improved adhesion to metals, and undergo thermal dissociative exchange (Figure 38).[618-620] Furthermore, poly(thiourethane)s have been demonstrated as effective SMPs, whereby the isocyanate structure can be used to tailor its performance and chemical recyclability,[621] while thiol-ene polymers containing free isocyanates displayed DCC to facilitate self-healing.[622] Incorporation of thiourethane bonds within polymer networks have also been demonstrated to improve toughness and reduce shrinkage stresses.[623] Being a base-catalyzed reaction, the use of PBGs has been shown as an effective method to spatially selectively catalyze the reaction for photopolymerization.[624-626]
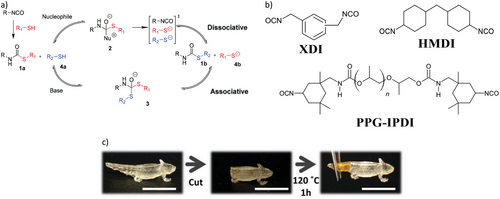
5.2 Thiol-Isocyanate Chemistry for AM
Poly(thiourethane) polymers have been prepared using ethoxylated TMPTMP, CHDI, and TEMPO as a thermal base catalyst, which were cured in bulk at 80 °C for 6 h. Depolymerization into oligomers could be achieved using excess (3-9 equivalents) of ethoxylated TMPTMP and 4 wt% TMG for 4 h at room temperature. Pragmatically, 9× molar excess was chosen to reduce system viscosity for further investigations. These oligomers could be repolymerized and retained the same mechanical properties (σT 25 MPa, εT 200%) as original specimens. Furthermore, adjusting the monomer composition with xylene diisocyanate (XDI), 4,4′-methylenebis(cyclohexyl isocyanate) (HMDI) (Figure 38), and TMPTMP could tailor mechanical properties. Next, it was demonstrated that the recycled thiol oligomers could be employed within DLP printing by using TATATO to yield thiol-ene polymers, extending the useful lifespan of the chemicals.[627, 628] While this example highlights the beneficial properties of poly(thiourethane) polymers and extends into the context of AM, it does not demonstrate the direct use of this chemistry within AM. On the other hand, the direct use of thiol-isocyanate reaction as a polymerization strategy has been accessed using PBGs. For example, a number of different PBGs have been explored, including NPPOC-TMG, BPh4-TBD, BPh4-DBU, as well as conjugate ammonium ions of DBU, TBD, and cyclohexylamine (CyA) (Figure 34). Here, it was found that NPPOC-TMG had a poor dark shelf-life, while 0.5 wt% TBD-HBPh4 with 0.25 wt% ITX was the optimal combination owing to the higher pKa of TBD. Initial studies on this system noted that the resin had a good dark stability, remaining uncrosslinked for over a month in a dark dry environment. Furthermore, it was shown that both a sensitizer (ITX) and photobase generator (BPh4-TBD) were required for effective rapid polymerization under UV irradiation. After screening several isocyanate-terminated oligomers, α,ω-IPDI functionalized poly(propylene glycol) (2 kDa) (PPG-IPDI, Figure 38) was chosen for AM owing to its relatively high polymerization kinetics. Here, it was reported that using greater molecular mass isocyanate functionalized oligomers (400-1000 Da) effectively decreased gel time. Hence, PPG-IPDI and TMPTMP were successfully copolymerized using BPh4-TBD and ITX using DLP printing. DLP printing was performed using a 20 s layer cure time and 30 wt% acetone, resulting in shrinkage of 10% after postfabrication solvent evaporation. On the other hand, DLP printing without a solvent resulted in acceptable printing up to a z-height of 3 mm; thereafter the achievable printing resolution diminished, attributed to catalyst migration. This work was then extended to TPP 3D printing, which was performed in an oil immersion using 0.3 µm layer height, 0.2 µm hatching, a laser power of 40-50 mW, and scan speed of 2000 µm s−1. Both the DLP and TPP printed polymers were self-healing at 120 °C (Figure 38), and chemically recyclable by dissolution in a mixture of acetone, virgin TMPTMP, and a basic catalyst, affording yields >95% after 4 h at room temperature. Next, the depolymerized oligomers could be readily chemically recycled in 3D-printed formulations for up to two cycles. Here, effective recycling where mechanical properties were retained could only be achieved when a stoichiometrically equivalent ratio of thiol and isocyanates were used.[629]
The beneficial properties of poly(thiourethane)s have also been exploited within FFF AM. This was achieved through the reaction between EDDET and HDI to form a thermoplastic copolymer with Mw of 17.2 kDa, Tg of 10.5 °C, and Tm of 110.5 °C. FFF printing was performed using 0.2 mm layer height and 205 °C nozzle temperature. 3D printed specimens displaying σT of 27 MPa, εT of 284%, and UT of 53 MJ m−3. The high toughness was attributed to good interlayer adhesion that could be achieved due to the thiourethane groups. However, appreciable anisotropy was observed for the 3D-printed specimens.[630] Building on from this work, a poly(thiourethane) polymer composed of HDI, EDDET, and EGBMP was developed, resulting in a polymer with Tg of 7 °C and Tm of 107 °C. However, cold crystallization was observed in the as-printed polymers. On the other hand, postfabrication annealing at 60 °C for 3 h after printing resulted in near-isotropic properties, alongside an increase in strength and toughness and without a reduction in strain.[631]
6 Thiol-Epoxy
6.1 Polymerization and Properties
Thiols can react with epoxies to form β-hydroxythioethers through a base-catalyzed and thermally mediated reaction.[632] Although this makes the reaction inappropriate for vat photopolymerization AM, orthogonal dual polymerization can be exploited through postfabrication thermal thiol-epoxy polymerization. With this concept in mind, the thiol-epoxy reaction presents itself as a useful polymerization strategy for polymer networks.[633-635] Indeed, thiol-acrylate-epoxy bulk polymers displayed faster reaction kinetics, higher Tg, ET, hardness, and lower polymerization shrinkage, compared to analogous thiol-acrylate systems.[636] Furthermore, a study aiming to optimize the stoichiometry of these hybrid systems established an equal weight percentage of thiol-ene and thiol-epoxy constituents resulted in the highest Tg polymers.[637] On the other hand, it has also been shown that various off-stoichiometric formulations could be used to tailor thiol-acrylate-epoxy networks, thus being able to tune the (thermo)mechanical and hydrolytic properties.[638] In fact, off-stoichiometric formulations of thiol-ene-epoxy networks have been shown to possess exceptional properties, such as flexibility, multi-material adhesion, low permeability, and low cytotoxicity, thus having been posed as suitable materials for neural implants.[639] As is the case with most monomer systems, judicious choice of the monomer structure can also be exploited to tailor the polymer's properties.[640] Furthermore, the residual hydroxyl moieties on β-hydroxythioethers can be leveraged for post-polymerization modifications.[641] It is also worth noting that photopolymerized thiol-epoxy polymers have been demonstrated as chemically recyclable via “proton-coupled electron transfer (PCET) activation of hydroxy groups within the polymer network to generate key alkoxy radicals that promote the fragmentation of the polymer through C–C bond β-scission”.[642] Additionally, SMPs have been prepared using thiol-epoxy[643] and thiol-acrylate-epoxy[644, 645] polymers, and exploited within thiol-ene-epoxy LCEs,[646] while other thiol-epoxy polymers have also been used as reversible adhesives.[647] Beyond the thiol-epoxy reaction, it is also possible to exploit anionic or cationic epoxy-epoxy reactions within the polymer network for dual polymerization strategies.
6.2 Thiol-Epoxy Chemistry for AM
The sequential polymerization of thiol-ene/(meth)acrylate-epoxy monomer systems via initial thiol-ene/(meth)acrylate photopolymerization followed by thermal thiol-epoxy polymerization has generally been established as a robust method to enhance polymer properties.[648-651] With this in mind, a system containing AESO, epoxidized linseed oil, 1,3-BDT, and PETMP was shown to be viable for laser direct writing 3D lithography, employing 1-methylimidizole as the thiol-epoxy base catalyst. However, these formulations were reported to have poor long-term storage due to thiol-Michael reaction and thiol-epoxy reaction promoted by the strong base catalyst.[652] A monomer system containing TATATO, PETMP, EGBMP (9:1 molar ratio of PETMP-to-EGBMP), and diglycidyl ether of bisphenol A (DGEBA, Figure 39) in stoichiometrically equivalent quantities has been used to prepare polymers capable of undergoing thiol-ene, thiol-epoxy, and epoxy-epoxy polymerizations. Here, the thiol-ene polymerization was achieved using photopolymerization, while the thiol-epoxy reaction occurred at 80 °C using imidazole as thermal base catalyst, and finally the epoxy-epoxy anionic reaction occurred at 120 °C. Remarkably, the mechanical properties of the polymers from this single formulation could be tailored over a large range by choice of thermal treatment schedule (Figure 39). Here, the thiol-epoxy and epoxy-epoxy reactions resulted in soft and flexible crosslinks, respectively, and thus could be selectively induced through temperature of favored reaction. Furthermore, the concentration of residual thiol groups for these subsequent reactions could be tuned through the initial photopolymerization irradiance dosage. This strategy was also claimed to provide an added benefit that all the reactive groups were consumed through the three reactions, thus successfully mitigating aging and long-term changes in mechanical properties. The polymers were then SLA printed using a 250 µm layer height. Interestingly, a photopatterned gradient was observed, attributed to diffusion of epoxy species prior to thermal treatment(s) leading to differential interpenetrating network formation, resulting in spatially gradient mechanical property transitions. A wearable pneumatic braille assistance display device was produced using various components of differing mechanical properties from the formulation (Figure 39). However, the use of an ene instead of an acrylate may assist in the long-term storage, although this was not reported.[653]
A thiol-ene-epoxy approach has also been demonstrated as an effective method to minimise anisotropy in DLP printed polymers. Indeed, weak interfacial bonds in layer-by-layer fabrication can lead to anisotropy when this mode dominates mechanical deformation.[654] TEGDMA, glycidyl methacrylate (GMA, Figure 39), TDD, and PETMP, alongside ITX and BPh4-TBD for photobase generation, were copolymerized via DLP printing using a 20 s layer cure time and 50 µm layer height, followed by postfabrication thermal treatment at 120 °C for 5 h. Anisotropy less than 5% in each orientation was achieved after thermal treatment, attributed to improving interlayer adhesion through thiol-epoxy crosslinking at the interfaces. Additionally, up to 500% increase in strength and toughness was also reported. This approach also successfully mitigated undesired polymerization during storage as the strong base catalyst was only released during AM.[655] This strategy also has potential for highly pigmented monomers or opaque composites where UV penetration suffers, and because the reaction does not occur at room temperature it can be employed orthogonally without deleterious effects on print resolution fidelity.[656] Within the context of achieving isotropic polymers, the use of the thiol-epoxy reaction has been exploited within FFF AM. Here, DGEBA was partially crosslinked using a commercial hardener (Locktite, containing amine and thiol monomers). Next, crosslinking with a bisphenol A-based benzoxazine was performed, and 4 wt% carbon nanotubes were incorporated. FFF printing of this material could be achieved at just 100 °C, followed by postfabrication thermal treatment. The resultant printed objects displayed excellent mechanical properties with ET above 2 GPa, σT above 60 MPa, and <5% anisotropy, as well as efficient shape memory capability.[657]
Dual wavelength DLP printing has been exploited to achieve spatially selective thiol-ene and thiol-epoxy polymerization. Here, using longer wavelength (405 nm) irradiation facilitated the thiol-ene polymerization, while exposure to 365 nm wavelength facilitated PBG release. As such, post-fabrication thermal treatment resulted in spatially controlled thiol-epoxy reactions only where the PBG had been activated, alongside the base catalyst promoting dynamic transesterification. Using this approach, multi-materials that had spatially controlled mechanical properties and stimulus responsiveness were successfully achieved.[658] As such, the use of red light using TMG-methylene blue for thiol-nucleophilic polymerizations has been established to effectively catalyze thiol-acrylate, thiol-yne, and thiol-epoxy reactions, extending the scope for wavelength specific multi-materials.[483] The use of dual-wavelength DLP printing for orthogonal free radical acrylate polymerization (405 nm) alongside a photoacid generator for cationic epoxy reaction (365 nm) has also been demonstrated as an effective strategy to achieve multi-materials with spatially controlled hard and soft polymer networks.[659] Additionally, PLB-TMG has also been demonstrated as an effective catalyst for dual curing of acrylate and epoxy polymers via free radical and cationic mechanisms, respectively, to achieve spatially controlled interpenetrating network polymers, but this strategy has yet to be transferred into thiol-X systems.[659] Given that this strategy has been successfully shown with thiol-epoxy-acrylate bulk polymers using ITX and TPB-TBD, it would be reasonable to expect that a similar approach could be applied for thiol-acrylate-epoxy systems within AM.[636] Within this context, thiol-isocyanate-epoxy polymers have been shown to successfully facilitate the incorporation of two DCC with highly different exchange kinetics, allowing for tailored CANs.[660]
Several other PBGs have also been shown as effective initiators for the thiol-epoxy reaction, such as thioxanthone acetic acid ammonium salts, where TXA-TBD was found to be particularly effective.[571] Furthermore, methyl 4-((hexahydropyrrolo[1,2-a]pyrimidin-1(2H)-yl)methyl)benzoate and 1-benzyloctahydropyrrolo[1,2-a]pyrimidine photolatent amidine bases have been shown to effectively initiate the thiol-epoxy polymerization and additionally activate dynamic transesterification, furthering the potential for achieving CANs within these systems. Of particular interest, these examples were also shown to completely mitigate undesirable thermally induced release of the base catalyst.[661]
The combination of using cationic, thiol-ene, and free radical polymerization has been demonstrated to produce complex and tailorable polymer architectures.[662] Within this context, the use of 2-amino-4,6-diphenyl-benzene-1,3-dicarbonitrile derivatives alongside diphenyliodonium salts has been established as a versatile photoinitiator to facilitate cationic, free radical, and thiol-ene polymerizations over a range of UV and visible wavelengths.[663] This system was extended to exploit complex interpenetrating network polymers obtained via these three polymerization mechanisms under low light intensities. Here, a series of naphthalene derivatives were used as effective photosensitizers alongside iodonium salts for combined cationic, free radical, and thiol-ene polymerizations for DLW 3D printing.[664]
7 Disulfide
7.1 Polymerization and Properties
The incorporation of disulfide bonds within polymers can enable several dynamic exchange mechanisms. First, an anionic thiol-disulfide exchange is well known. Mechanistically, the exchange involves the attack of a thiolate anion on the disulfide, leading to nucleophilic displacement of a thiolate anion from the disulfide. It is hence typically catalyzed under basic conditions using amine bases.[665] However, this mechanism suffers the susceptibility for oxidation of free thiolates, leading to reduced thiolate concentrations and hence diminished exchange kinetics.[666] Nevertheless, the thiol-disulfide exchange has been shown as an effective mechanism to facilitate chemical recycling, reprocessing, and self-healing of polymers.[667-670] On the other hand, it has been found that gold(I) capping of the thiolate is an effective protection strategy, while not compromising the exchange kinetics,[671] and the properties of the Au(I)-thiolate bond have been noted for several promising applications.[350, 672, 673] Alternatively, a disulfide-disulfide dynamic exchange mechanism is also possible. Interestingly, it was established that aryl disulfide exchange occurs through a [2+1] radical-mediated mechanism, whereby the low bond dissociation energy of aryl disulfides facilitates free radical generation even under low irradiation (Figure 40).[674] Here, aromatic disulfides generally have lower bond dissociation energies compared to alkyl disulfides, thus facilitating relatively higher exchange kinetics,[675] with examples of it facilitating self-healing,[676, 677] reversible anchoring,[668] and spatial patterning.[678]
7.2 Disulfide Chemistry for AM
The dynamic disulfide-disulfide exchange has been exploited for UV-mediated disulfide metathesis in AM polymers for postfabrication exchange to address anisotropy and warping. This was achieved in DLP-printed polymers by incorporating 2-hydroxyethyl disulfide dimethacrylate (HEDMA, Figure 41). It was found that with just 10 wt% incorporation of HEDMA the z-axis σT was around 98% relative to the x-axis, while without HEDMA only 74% was measured. This degree of isotropy was attributed to superior interlayer adhesion due to disulfide rearrangement introducing covalent bonds at the interface between layers. UV irradiation also led to reshaping of polymers, which could be used to rectify warping and deformation during printing.[679] The dynamic disulfide exchange has also been exploited within poly(siloxane) polymers, whereby PMMS-co-PDMS (4-6% thiol) was partially oxidized using iodobenzene diacetate to form disulfide bonds. This oligomer was then copolymerized with α,ω-vinyl PDMS using μ-DLP printing. The printed copolymers could self-heal within 2 h at 60 °C with comparable strength to the original polymers, which could be repeated up to 10 times while retaining 90-100% strength of the original polymer. Furthermore, a control polymer without disulfide bonds only achieved 40% strength after similar self-healing conditions, thus supporting the active role of the disulfide exchange for self-healing. It was noted that the degree of oxidation and quantity of free thiols for photopolymerization were competing, and hence judicious balance between the two was necessary. Alternatively, it may be reasonably suggested that a higher concentration of thiols in PMMS-co-PDMS could also be used. Nevertheless, a self-healing pneumatic actuator was demonstrated, and multi-materials were also demonstrated using these polymers alongside HDDA and carbon grease to create electronic devices.[680] In another example, ethylene glycol phenyl ether methacrylate and PEG-MA (280-320 Da) were copolymerized with either S2MA (Figure 41) or HEDMA via DLP printing to produce chemically recyclable and reprocessable polymers. Polymers containing only disulfide bonds (HEDMA) and those containing both disulfide and β-hydroxyesters (S2MA) were compared to ascertain whether both the disulfide exchange and transesterification reactions could be exploited. Polymers containing only disulfide bonds displayed τ* of 2.38 min and at 110 °C with complete relaxation, whereas the combination of both disulfide exchange and transesterification displayed τ* of 0.74 min at 100 °C but non-zero final stress relaxation. Mechanical recycling at 120 °C and 5 MPa for 4 h were achieved for the polymers only containing disulfides with only a modest reduction in mechanical properties, while polymers containing hydroxyesters could not be recycled.[681] In another example of self-healing polymers, a canola oil fatty acid derivative was functionalized using HEA to prepare a diacrylate monomer, which was then copolymerized alongside partially oxidized PMMS-co-PDMS using inkjet printing followed by UV curing. The resultant polymer could be both self-healed and reprocessed with 86% and 124% efficiency relative to original mechanical properties, respectively.[682]
Various unsaturated poly(urethane) derivatives exploiting disulfides within the oligomer backbone have also been demonstrated. For example, α,ω-diacrylate poly(urethane) oligomers containing disulfides within the backbone (1.7-3.3% incorporation) have been prepared using IPDI, PEG, bis(2-hydroxyethyl) disulfide, and HEA. DLP printing using HEA as a reactive diluent resulted in polymers that displayed self-healing at 80 °C for 12 h with an efficiency of around 95% relative to original tensile properties. Oligomers without disulfide bonds were also prepared, which did not display these self-healing properties, indicating that the disulfide bond was integral to its ability for self-healing.[683]
In an example using poly(urethane)s, IPDI, poly(tetrahydrofuran) (1 kDa), and 4,4′-dithiodianiline were copolymerized to form polymers containing disulfides within the backbone. Composites of the polymer with hydroxylated multiwalled carbon nanotubes (1–3 wt%) were DIW printed to form polymers capable of photothermal self-healing and recycling. This was achieved through the ability of the hydroxylated multiwalled carbon nanotubes to absorb and convert near-infrared light into heat energy, thus activating the disulfide exchange, with temperatures up to 170 °C for 2 wt% composites achieved after 60 s irradiation. As a result, spatial control of these properties could also be achieved.[684]
In another example using isocyanates, PCL (2 kDa), IPDI, and 2,2′-bis(hydroxymethyl) propionic acid oligomers were copolymerized with IPDI, bis(2-aminophenyl) disulfide, castor oil glycerides, and the glycerol ester of citric acid using DIW printing. The resultant semi-crystalline polymers displayed elastomeric properties with high tensile strain at break, while also capable of self-healing (110 °C for 2 h, up to 80% healing efficiency), reprocessing (80 °C, 6–8 MPa, 1.5 h), and shape memory (shape recovery at 80 °C for 3 min). These polymers were then explored for application as artificial muscles by printing alongside partially solidified gelatin, resulting in polymers with acceptable hemocompatibility, cell viability, and biodegradability, making this a promising material for the application.[685] Within this context, the FFF printing of “electronic skin” has been demonstrated using hydrogels composed of thiol-functionalized pullulan (using 3,3′-dithiopropionic acid followed by DTT reduction), 200-500 nm molybdenum disulfide particles as crosslinking agent, and polydopamine nanoparticles to facilitate thiol-Michael addition. Here, sodium trimetaphosphate was introduced to induce ionic crosslinking.[686] Poly(disulfide) hydrogels that could undergo self-healing and controlled degradation have been prepared through the oxidative copolymerization of 2,3-dimercapto-1-propanol and meso-2,3-dimercaptosuccinic acid at 45 °C using 5 mol% HBr in DMSO, followed by immersion of the product in Ca2+ solution. The formed hydrogel was then extrusion 3D printed either in air or within a water vat. The combination of disulfides along with ionic crosslinks resulted in rapid self-healing (within 5 s). It was noted that equivalent hydrogels prepared without disulfides displayed appreciably poorer self-healing rate and performance. The hydrogels were also electrically conductive and displayed strain-dependent electrical resistance.[687] In a further example of additively manufactured hydrogels, methacrylate-terminated triblock copolymers of isopropyl glycidyl ether, ethyl glycidyl ether, and PEG (8 kDa) have been copolymerized with pyridyl disulfide-urethane methacrylate (PDS-UM, Figure 41) using DIW printing. The amphiphilic polymer displayed a lower critical solution temperature-driven sol–gel transition at 15 °C, resulting in reversible temperature-dependent equilibrium swelling ratios. Furthermore, the printed hydrogels displayed release of 2-pyridothione that was both temperature and pH-dependent; through this release, additional postfabrication conjugations could be afforded, such as functionalization with RGD-SH and a fluorescent dye.[688] The disulfide on cystamine has been leveraged by incorporation into NIPU hydrogels in DIW 3D printing. In this work, PEGDA was coupled with 1-thioglycerol, followed by conversion to a cyclic carbonate using dimethyl carbonate, and then finally polymerized with cystamine to form a NIPU oligomer. An aqueous mixture of quaternized chitosan, NIPU oligomer, and TEMPO-CNF was DIW printed to yield hydrogels with good cell proliferation and viability.[689] Cystamine has also been incorporated into DLP printed hydrogels, prepared using HEA, PEGDA (700 Da), and N,N′-bisacryloyl cystamine. The additively manufactured hydrogels could take up peptides lanreotide or calcitonin within the network structure, whereby they could then undergo redox-triggered release of the peptides by thiol-disulfide exchange. Importantly, the peptides did not diffuse out of the hydrogel without stimulus, highlighting the efficacy of the exchange for triggered release.[690]
The use of 1,2-dithiolanes as functional groups for polymerization has shown potential for CANs.[691, 692] For example, a poly(propylene oxide) and poly(ethylene oxide) ABA block copolymer has been terminally functionalized with lipoic acid ((PPO-co-PEO)-LA, Figure 41). Here, hydrogels containing Au nanoparticles facilitated the Au-thiolate bond for the redox reaction between the strained cyclic disulfide groups and Au ions. Furthermore, the incorporation of nanosilicate laponite (1.5 wt%) facilitated ionic absorption, rendering the hydrogel stable for extended periods of time. DIW-printed hydrogels displayed self-healing capability by dynamic exchange between disulfides and the Au(I)-thiolate, while the Au(I)-thiolate bonds successfully protected thiols from atmospheric oxidation.[693] In fact, lipoic acid has also been shown to radically-mediated form either disulfides (only 1,2-dithiolane present) or thioethers ((1,2-dithiolane and ene present).[694] The Au-thiolate bond has also been used within FFF-printed polymers for immune-sensing applications. In this example, a commercially available PLA filament with graphene-based nanocomposite filler was decorated with Au nanoparticles on its surface, followed by sequential Au-thiolate coupling of cysteamine and then imide coupling of glutaraldehyde. The free aldehyde groups of glutaraldehyde were then exploited for anchoring of a biomarker via imide bond formation with a recombinant protein of COVID-19. The resultant immunosensors were demonstrated to selectively detect COVID-19 with electronic readouts from the device.[695]
Several opportunities within the use of polymers containing disulfides have been demonstrated for bulk polymers that could have potential application in AM. For example, the disulfide exchange mechanism has also been embedded into monomers bearing boronic acid ester groups, thus incorporating two distinct DCC mechanisms within the polymer network.[696] Additionally, the use of two-stage thiol-ene and disulfide-ene semi-orthogonal sequential polymerizations has been demonstrated when an excess of enes was employed, making a versatile platform for molecular architecture control.[697-699] The reaction between disulfides and ynes has been found to result in dithioether and vinyl dithioether adducts, thus presenting an alternative mechanism possible to exploit within photopolymerization AM.[700] Lastly, disulfide degradation using a PBG has also been established, extending itself for use as a mechanism for wavelength-selective control of degradability.[701]
8 Cyclic Thioether
Cyclic thioethers can be prepared by alkyl halide exchange through the reaction between 3-chloro-2-(chloromethyl)-1-propene and 1,2-ethanedithiol (6-methylene-1,4-dithiepane (MDP), Figure 42) or 3-propanedithiol (3-methylene,1-4-dithiacyclooctane (MDC), Figure 42) to yield photopolymerizable monomers. Here, the radical-mediated ring-opening polymerization of the cyclic allyl sulfide monomers (Figure 42) has been demonstrated in DLP printing to produce thermoplastic polymers. Once again, the unusual application of thermoplastic polymers within vat photopolymerization AM presents a paradigm shift in the achievable molecular architectures within this technology. Bulk polymers were prepared using varying ratios of MDP and MDC, resulting in semicrystalline polymers with melting points between 91 °C and 116 °C. Comparatively, the semicrystalline polymers displayed moduli several orders of magnitude greater than analogous amorphous polymers. The bulk semi-crystalline polymers displayed ET of 974-1260 MPa and σT of 18-24 MPa for varied ratios of cyclic thioether monomers. Here, it appeared that predominant quantities of either monomer presented better properties compared to mixtures thereof, with the poorest properties for the 50:50 ratio, which was attributed to the low capacity for crystallization. Next, a monomer formulation of 25% MDP and 75% MDC was SLA printed. Considering that thermoplastic polymers were being polymerized in situ, relatively short layer cure times of 50 s were used for 50 µm layer heights. 3D printed polymers displayed ET of 368 MPa and σT 16 MPa. The relatively lower properties of the 3D printed specimens were attributed to poor interlayer adhesion due to curing between layers restricted to chain ends at the surface.[702, 703] Further examples of using cyclic thioethers have also demonstrated the ability to exploit the residual unsaturation for postpolymerization modifications and thiol-ene crosslinking, extending the scope of their use.[704] Furthermore, crosslinked thiol-ene polymers containing allyl sulfides analogous to those produced using cyclic thioethers have been demonstrated to display photoinduced stress and/or strain relaxation.[705]
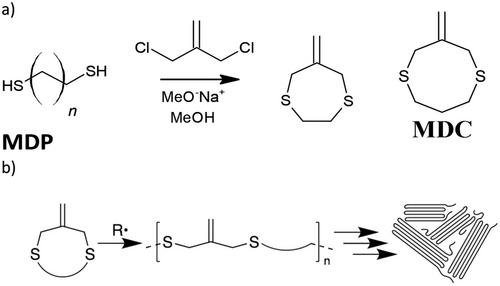
9 Conclusions
When considering the extensive variety of polymers that thiol-X chemistry has been used for, within a number of different AM technologies, it is unquestionable that it has firmly embedded itself as a robust and versatile avenue to achieve additively manufactured advanced polymers. Indeed, thiol-X chemistry has served two purposes within this context: it has opened the door for both facilitating the additive manufacturing process via “click” polymerization as well as introducing bonds capable of imparting desirable properties onto the polymers. Furthermore, the diversity of thiol-X chemistry, as well as its functional group tolerance to incorporate other chemistries into the polymer, presents a highly attractive toolkit. Put together, its usefulness has already been shown to extend into a host of different engineering applications. However, a shortcoming of thiol-based chemistry is the odor of typical sulfur-based compounds, alongside toxicity issues. This is particularly relevant for relatively volatile small monomers containing free primary thiols. One of the challenges of moving thiol-based additive manufacturing out of the convenient fume hoods of research laboratories and into the commercial sector will be the control and mitigation of odors that may pose an additional constraint on the choice of monomers at one's disposal. Additionally, the judicious choice of monomers to ensure adequate shelf-life may also impose challenges. This is particularly relevant considering that thiols can undergo thiol-thiol oxidation to form disulfides, which can result in a loss of functional groups on monomers, as well as lead to viscosity increases and crosslinking within multifunctional monomers.
Moving towards the future, the continuous development of monomer systems, developed in conjugation with their intended AM technology, ought to see the refinement and progress of advanced polymers that can satisfy contemporary engineering applications. It is also may be reasonably anticipated that bespoke additive manufacturing technologies tailored to specific monomer chemistry and adapted for advanced polymer fabrication could also develop in the following years.
Thiol-X chemistry is not new, it has stood the test of time and proved its value within classical organic chemistry, and so it is reasonable to expect the same for its application in AM into the future.
Acknowledgements
T.H. Becker is graciously acknowledged for guidance in the manuscript preparation.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
J.A.D. performed conceptualization, visualization, and wrote the original draft. C.W. reviewed and edited the final manuscript. All authors have read and agreed to the published version of the manuscript.