Self-Assembled Supramolecular Materials for Substrate Transport by External Stimuli
Abstract
Substrate transport within biological tissues is diverse, with the most fundamental process being transport across cell membranes, which plays a crucial role in sustaining life. In this study, an artificial substrate transport system based on hydrogels by utilizing molecular recognition and stimuli-responsive substrates is developed. α- and β-Cyclodextrins are selected as host molecules, while adamantane serves as the guest molecule, enabling the adhesion of two hydrogels through self-assembly. Under light stimulation, the light-responsive dye, azobenzene derivative, is transported between the two hydrogels. This research provides new insights into the development of light-controlled substance transport systems, which can be applied to biological substance delivery and the creation of smart materials.
1 Introduction
Substrate transport represents one of the essential mechanisms by which organisms sustain life functions.[1, 2] Through active and passive transport, cells and tissues regulate the movement of nutrients, ions, and metabolic waste, thereby maintaining physiological homeostasis.[2] These highly sophisticated transport mechanisms depend on specific carriers, channel proteins, and energy-driven processes across biological membranes to ensure the precise balance of substances within different regions of the organism.[3] However, replicating these natural processes to design and develop efficient, controllable artificial substrate transport systems remains a significant challenge within the scientific community.
Recent advancements in molecular self-assembly[4-6] and nanotechnology[7, 8] have contributed to progress in artificial substrate transport systems. Systems utilizing molecular recognition[9-12] and host-guest chemistry[13-15] provide a promising strategy for mimicking the selective transport processes found in biological systems. For example, redox-reversible nanovalves for controlled release,[10] as well as biomimetic nanochannels formed through host-guest interactions,[13] offer new opportunities for achieving precise control of substrate transport in synthetic systems. However, these approaches often rely on chemical stimuli, such as changes in oxidation states or the presence of specific chemical triggers, which can limit their reversibility and flexibility.
This study seeks to develop a hydrogel-based substrate transport system that emulates biological transport mechanisms by integrating host-guest chemistry with light-responsive molecules, thereby facilitating controlled transport under external stimuli. Specifically, α-cyclodextrin (α-CD) and β-cyclodextrin (β-CD) are selected as host molecules, while adamantane (Ad) serves as the guest molecule, enabling the adhesion of two hydrogels through self-assembly. To investigate the transport behavior within this system, the photoresponsive dye azobenzene (Azo) is employed as a model substrate. The substrate transport is controlled through light-induced cis-trans isomerization of Azo (Figure 1). This innovative approach not only emulates biological transport processes but also provides a versatile platform for the controlled and stimuli-responsive transfer of substances. Such systems hold significant potential for applications in smart materials and drug delivery.
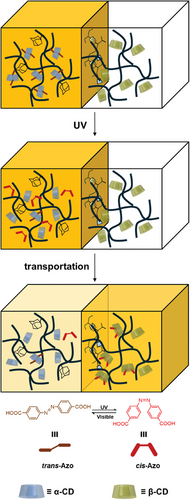
2 Results and Discussion
Two types of hydrogels, α-CD-Ad-gel and β-CD-gel were prepared as shown in Figure 2a,b, respectively (Schemes S1–S5 and Figures S1–S3, Supporting Information). NIPAAm-gel was prepared as a reference gel (Figure 2c; Scheme S1, Supporting Information).[16, 17] Azobenzene-4,4′-dicarboxylic acid was chosen as the photoresponsive molecule.[18, 19] Under UV irradiation, the trans-Azo undergoes isomerization to cis-Azo, which reverts back to trans-Azo upon exposure to visible light or heating.[20, 21] The binding constants of the different isomers with α-CD and β-CD also vary. The binding constants were calculated by fitting the Benesi-Hildebrand equation to the chemical shifts of azobenzene observed in the 1H NMR spectra as a function of CD concentration (Table 1 and Figures S9–S17, Supporting Information).[21] Ad was chosen as the adhesion molecule for the two hydrogels primarily because it exhibits negligible binding with α-CD but strong interactions with β-CD (Figure 2d).[22-26] The binding constants (Kas) of Ad with α-CD and β-CD are 9.8 × 101 M−1 and 1.5 × 103 M−1, respectively.[16]
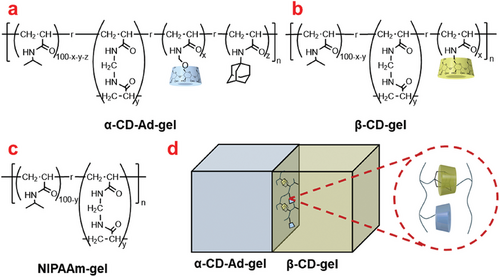
| Compound | α-CD | β-CD |
|---|---|---|
| Association constants (Ka) / M−1 | ||
| trans-Azo | 1.1 × 103 | 5.3 × 102 |
| cis-Azo | 1.6 × 102 | 3.6 × 102 |
2.1 Azo Transport in Different Hydrogels
Azo was dissolved in 0.05 M phosphate buffer (pH = 8.0). After immersing the α-CD-Ad-gel in the Azo solution (0.08 mM), the gel was taken out. The α-CD-Ad-gel was then adhered to the β-CD-gel and kept in a moist state. After irradiating the gels with 331 nm light, the intensity of the Azo color within the gel was measured.
Comparative experiments were conducted under the same conditions for α-CD-Ad-gel and NIPAAm-gel, as well as NIPAAm-gel and β-CD-gel. The samples were left to stand under moist conditions at 10 °C without irradiation. The color of Azo in each gel was monitored at 0.5 mm from the gel interface. The ‘0 mm’ position (zero point) represents the average value for the 0–1 mm distance. Changes in the concentration of Azo were indirectly indicated by variations in the “B-value” of the gel color in the data (Figure S18, Supporting Information). Through Figure S4 (Supporting Information), it was observed that the storage modulus (G′) of the gel consistently exceeded the loss modulus (G″), indicating that the elastic component dominates over the viscous component, and the system maintains stable solid-like behavior.
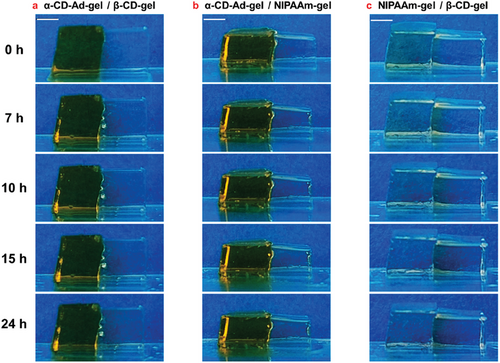
2.1.1 Azo Transport Before Irradiation in α-CD-Ad-gel / β-CD-gel, α-CD-Ad-gel / NIPAAm-gel, and NIPAAm-gel / β-CD-gel Systems
Figure 3 shows photographs of α-CD-Ad-gel / β-CD-gel, α-CD-Ad-gel / NIPAAm-gel, and NIPAAm-gel / β-CD-gel, respectively, taken at 0, 7, 10, 15, and 24 h without irradiation.
Simply adhering the α-CD-Ad-gel and β-CD-gel did not result in any Azo migration between the two gels without irradiation (Figures 3a and 4a,d). The same result was observed in the α-CD-Ad-gel / NIPAAm-gel system (Figures 3b and 4b,e). However, in the NIPAAm-gel/β-CD-gel system, the Azo dye moved from the NIPAAm-gel to the β-CD-gel. Over time, the amount of Azo transferred into the β-CD-gel increased (Figures 3c and 4c,f).
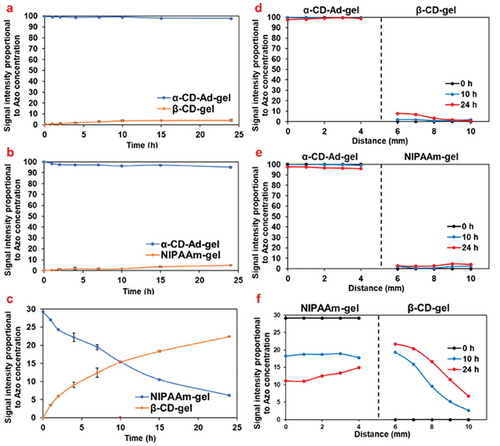
As shown in Table 1, the binding constants (Kas) of trans-Azo with α-CD (1.1×103 M−1) is greater than that with β-CD (5.3×102 M−1), resulting in the trans-Azo being immobilized within the α-CD-Ad-gel and remaining stationary.[21] In contrast, the NIPAAm-gel is unable to form an inclusion complex with trans-Azo, and thus cannot immobilize trans-Azo.
2.1.2 Azo Transport After 331 nm Light Irradiation in α-CD-Ad-gel / β-CD-gel, α-CD-Ad-gel / NIPAAm-gel, and NIPAAm-gel / β-CD-gel Systems
Figure 5 shows photographs of Azo transport in α-CD-Ad-gel / β-CD-gel, α-CD-Ad-gel / NIPAAm-gel, and NIPAAm-gel / β-CD-gel systems, respectively, taken at 0, 7, 15, 24, 48, 120, and 192 h. The samples were irradiated with 331 nm UV light after standing for 24 h. “0 h” refers to the time after the 24 h standing period, at which point the irradiation commenced.
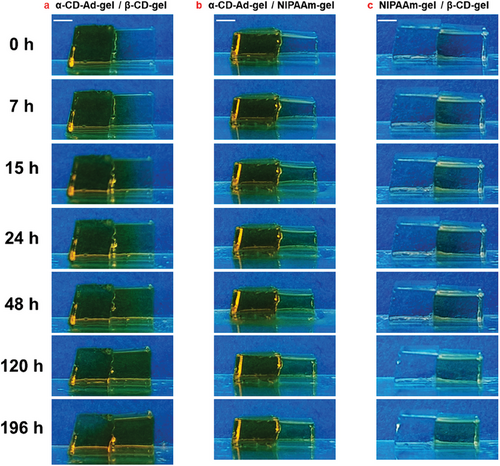
Under irradiation with 331 nm light, the Azo dye migrated from the α-CD-Ad-gel to the β-CD-gel, reaching the same concentration at 132 h, after which it continued to migrate toward the β-CD-gel, achieving transport from low concentration side to high concentration side (Figures 5a and 6a). In the β-CD-gel, the concentration of Azo dye was higher on the left end than on the right end, indicating that Azo migrates from left to right (Figure 6d). In the α-CD-Ad-gel/NIPAAm-gel system, the amount of Azo dye migration remained minimal, as shown in Figures 5b and 6b. There was no significant concentration difference of Azo dye between the left and right ends in the NIPAAm-gel (Figure 6e). In the NIPAAm-gel/β-CD-gel system, Azo dye continued to migrate into the β-CD-gel (Figures 5c and 6c), with the Azo dye consistently moving from the left end to the right end (Figure 6f).
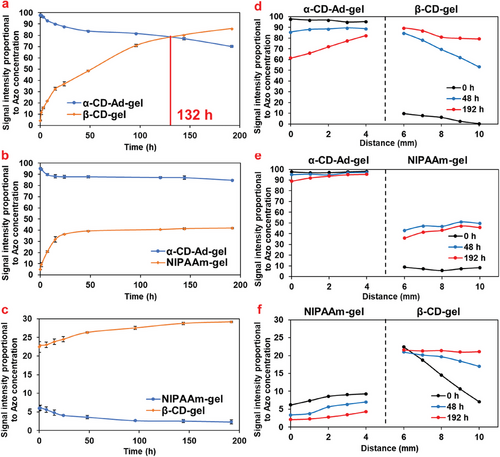
As shown in Table 1, under light irradiation, when trans-Azo isomerizes to its cis-form, β-CD (3.6×102 M−1) forms an inclusion complex with cis-Azo more readily than α-CD (Ka = 1.6×102 M−1), resulting in the migration of Azo dye toward the β-CD gel.[21] The migration of Azo from the left side into the β-CD gel during accumulation is also attributed to the formation of inclusion complexes between Azo and CD.
2.2 Transport of Azo between Gels with Different Degrees of Cross-linking
Similar comparative experiments were conducted for α-CD-Ad-gel (4 mol%) / β-CD-gel (4 mol%) system, α-CD-Ad-gel (6 mol%) / β-CD-gel (6 mol%) system, as well as the α-CD-Ad-gel (8 mol%) / β-CD-gel (8 mol%) system (Figure S19, Supporting Information). The samples were immediately irradiated with 331 nm light after contact. “0 h” refers to the time point when irradiation was initiated immediately after contact. All of these gels with different degrees of cross-linking had similar rheological properties (Figures S5 and S6, Supporting Information).
The degree of crosslinking in a gel refers to the number and density of crosslinking points within the gel network. Variations in crosslinking density can influence the pore structure and permeability of the gel.[27, 28] These pore structures and permeability are critical for determining the diffusion, transport, and water absorption properties of the gel. The Azo transport rates in gels with different crosslinking densities vary. For α-CD-Ad-gel / β-CD-gel gels with a 4 mol% crosslinking density, the Azo concentrations in both gels equalized at t = 148 h (Figure 7a). In contrast, for gels with 6 mol% and 8 mol% crosslinking densities, the Azo concentrations equalized at 157 and 176 h, respectively (Figure 7b,c). The reduction in crosslinking density resulted in an acceleration in the rate of Azo migration: 4 mol% > 6 mol% > 8 mol%.

As the degree of cross-linking increases, the transport speed of substances decreases. New cross-linking points are formed at the gel interface, where adhesion is facilitated through host-guest interactions, resulting in an increased crosslinking density of the gel and a corresponding reduction in transport speed.[29] Therefore, if faster substrate transport is desired, a lower crosslink density is considered necessary. Among the systems with 4 mol%, 6 mol%, and 8 mol% crosslinking density, the 4 mol% system showed the fastest migration rate. Therefore, the 4 mol% system used in this study was found to give the highest transport efficiency.
3 Conclusion
This study successfully developed an artificial substrate transport system based on hydrogels, utilizing the host-guest interactions of CDs with Ad and the photoresponsive molecule Azo. The results of this study demonstrate the critical role of host-guest interactions in controlling the substrate transport process, allowing for regulation of substrate transport under external stimuli, such as light. Additionally, it was shown that the transport efficiency between hydrogels varies with the degree of cross-linking, with lower cross-linking leading to faster transport rates.
4 Experimental Section
Materials
α-CD and β-CD were obtained from Junsei Chemical Co., Ltd. N-isopropylacrylamide, N-(hydroxymethyl) acrylamide, acryloyl chloride, triethylamine, N, N′-methylenebis (acrylamide) (MBA), 2,2′-azobis(isobutyronitrile) (AIBN) and p-Toluenesulfonic acid monohydrate were obtained from Tokyo Chemical Industry. Water used for the preparation of the aqueous solutions (except for NMR measurements) was purified with a Millipore Elix 5 system. Other reagents were used without further purification. α-CD-AAm,[17] β-CD-AAm,[16] Ad-AAm[16] were prepared according to the previous reports and reference.
Preparation of α-CD-Ad-Gel
NIPAAm (0.23 g, 2.00 mmol), MBA (y = 4 mol%, 6 mol%, 8 mol%), α-CD-AAm (42.2 mg, 0.04 mmol), and Ad-AAm (12.3 mg, 0.03 mmol) were dissolved in dimethyl sulfoxide (DMSO) (2.00 mL) and radical copolymerized with 2,2′-azobis(isobutyronitrile) (AIBN) (4.92 mg, 0.03 mmol) at 65 °C for 24 h. The resulting hydrogel was purified by immersing it in water for 3 days, with repeated washing to remove unreacted components.
Preparation of β-CD-Gel
NIPAAm (0.23 g, 2.00 mmol), MBA (y = 4 mol%, 6 mol%, 8 mol%), and β-CD-AAm (47.4 mg, 0.04 mmol) were dissolved in DMSO (2.00 mL) and copolymerized with AIBN (4.92 mg, 0.03 mmol) at 65 °C for 24 h. The resulting hydrogel was purified by immersing it in water for 3 days, with repeated washing to remove unreacted components.
Preparation of NIPAAm-Gel
NIPAAm (0.23 g, 2.00 mmol) and MBA (12.3 mg, 0.08 mmol, y = 4 mol%) were dissolved in DMSO (2.00 mL) and copolymerized with AIBN (4.92 mg, 0.03 mmol) at 65 °C for 24 h. The resulting hydrogel was purified by immersing it in water for 3 days, with repeated washing to remove unreacted components.
Measurements
The NMR spectra were obtained using a JEOL JNM-ECS 400 and 500 MHz NMR spectrometer. MALDI−TOF MS spectra were recorded in the linear positive mode on a (BRUKER DALTONICS, Bruker-Ultraflex III and autoflex). UV–vis measurements were performed using a JASCOV-650 spectrometer at room temperature. The oscillatory rheological behavior was obtained using an Anton Paar modular compact rheometer MCR 302.
Photoisomerization
Azo moieties were isomerized by photoirradiation using a 300 W Xenon lamp (Asahi spectra MAX-301) equipped with suitable mirror modules (ultraviolet mirror module, λ = 270–390 nm) as a function of irradiation wavelength. The distance between the sample cell and the lamp was 30 cm.
Acknowledgements
This work was partly supported by Iketani Science and Technology Foundation (No. 0351024-A) and JSPS KAKENHI grants (JP20K21225 and JP24K01539).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
X.L. performed investigation, wrote the original draft, wrote, reviewed and edited; Y.K. wrote, reviewed and edited; A.H. performed supervision, wrote, reviewed and edited; H.Y. performed supervision, wrote, reviewed and edited, and funding acquisition.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




