Atmospheric corrosion behavior of 7A09 aluminum alloy exposed to an industrial environment
Abstract
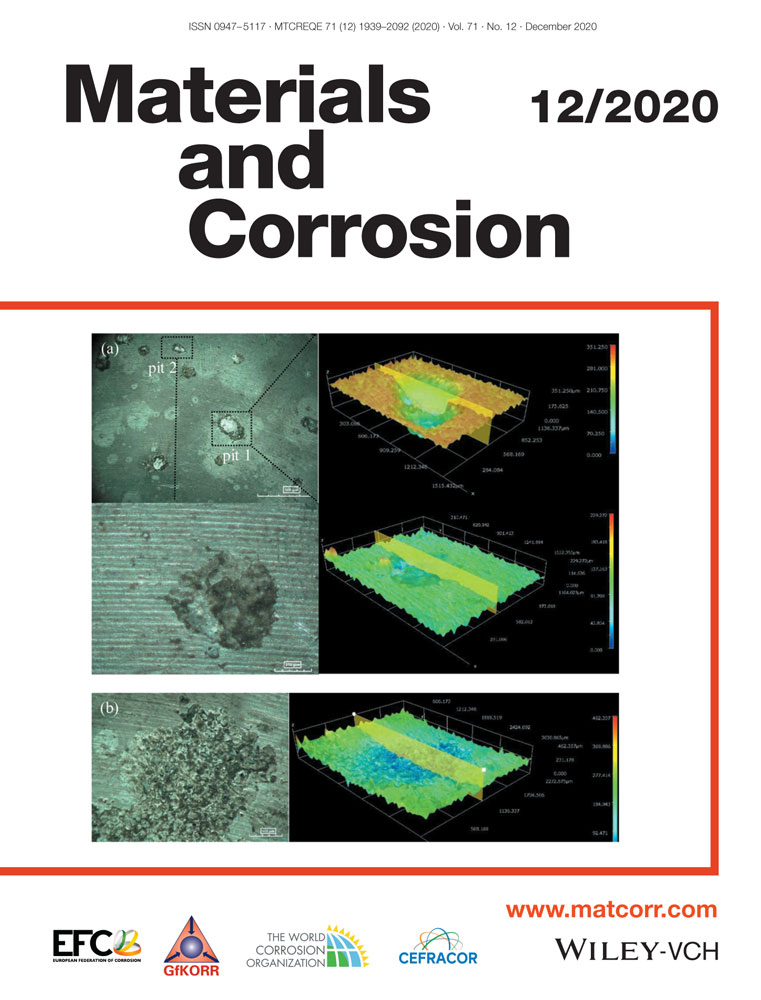
The corrosion behavior of 7A09 alloys exposed to an industrial atmosphere for 36 months was studied by weight loss method, morphology observation, electrochemical method, and loss in mechanical properties. Under the condition of exposure to an industrial atmosphere, the 7A09 alloy suffers obvious pitting corrosion and intergranular corrosion (IGC), and a charge transfer process controls the corrosion reaction. The corrosion rate presents a decreasing tendency with exposure time, which is mainly due to the enhancement of the protective ability of the corrosion product layer. The occurrence of IGC of this alloy is mainly caused by the preferential dissolution of the grain boundary precipitates Mg(ZnAlCu)2 and the precipitate-free zone along grain boundaries, and which leads to the reduction of the mechanical properties. In addition, the morphology and composition of the corrosion product layer were identified, and the corrosion mechanism was also discussed.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.