Photodynamic Therapy Minimally Affects HEMA-DMAEMA Hydrogel Viscoelasticity
Abstract
Soft matter implants are a rapidly growing field in medicine for reconstructive surgery, aesthetic treatments, and regenerative medicine. Though these procedures are efficacious, all implants carry risks associated with microbial infection which are often aggressive. Preventative and responsive measures exist but are limited in applicability to soft materials. Photodynamic therapy (PDT) presents a means to perform safe and effective antimicrobial treatments in proximity to soft implants. HEMA-DMAEMA hydrogels are prepared with the photosensitizer methylene blue included at 10 and 100 µM in solution used for swelling over 2 or 4 days. Thirty minutes or 5 h of LED illumination at is then used for PDT-induced generation of reactive oxygen species in direct contact with hydrogels to test viable limits of treatment. Frequency sweep rheological measurements reveal minimal overall changes in terms of loss modulus and loss factor but a statistically significant drop in storage modulus for some PDT doses, though within the range of controls and biological variation. These mild impacts suggest the feasibility of PDT application for infection clearing in proximity to soft implants. Future investigation with additional hydrogel varieties and current implant models will further detail the safety of PDT in implant applications.
1 Introduction
Implant infections are an unfortunately common occurrence and are often more severe with longer-lasting health effects than non-implant infections.[1, 2] A key factor in infection outcomes is the implant material, chosen to best match the mechanical properties of adjacent tissues. Hard implant materials such as those used in orthopedics can be treated more directly as they are more durable and can be constructed of naturally antimicrobial materials such as titanium.[3] The resilience of hard implants also enables further treatments such as photothermal therapy without significant risk of changing the material.[4] Soft implants, such as those used in reconstructive and aesthetic surgery, are more difficult to treat due to their more delicate nature and the short-term potential of antimicrobial material dopants.[5] Antimicrobial treatment of implant infections often includes antibiotic therapy, but recent research supports potential improvement by concurrent photodynamic therapy (PDT) to decrease resistance and required dosage.[6] However, any treatment in proximity to implanted materials must be well-studied to guarantee the stability of materials and continued function.[7] Dental implant infections have utilized PDT to great effect,[8] but use with soft materials is focused on planned degradation rather than long-term implant survival.[9] As such, this study aims to determine the efficacy of PDT in proximity to hydrogel materials to improve patient outcomes and preserve implant lifespan.
Due to the novelty of hydrogel usage in medical implants and tissue engineering, there are not many global statistics to draw upon. However, the most common soft implant, breast augmentation, is well monitored and documented. Though implantation is standardized and safe, the incidence of implant infection, considering all types of infection and implants, is 5% per year in USA.[10] These infections range from the most common, acute following surgery, to the most dreaded, chronic, and formidable to eliminate. Unlike infections in otherwise healthy patients, association with implants drastically affects the proper integration and acceptance by surrounding tissue and the recipient's immune system.[11] Current techniques in soft material implants focus on improving patient outcomes immediately following implantation as acute infection is by far the most common. Oral antibiotics are a standard safety net. More advanced is the use of antimicrobial material dopants that are becoming a popular focus of research.[5] These dopants could be antibiotics or photoactive materials such as silver nanoparticles to perform photothermal therapy as is done on metal implant surfaces, as well as many novel approaches in recent literature.[12] However, the use of any dopants trades qualities in implant biocompatibility, mechanical characteristics, and biodegradability and requires the calculation of drug release rates.[13] Furthermore, these methods are often short-lived and best suited to preventing or treating acute infections rather than chronic. As such, there is something missing in the medical toolbelt to aid the elimination of chronic infections in proximity to soft material implants, while maintaining tissue-implant stability.
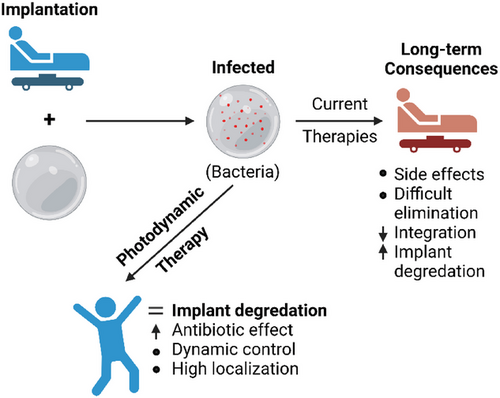
Photodynamic therapy (PDT) is a technique that generates reactive oxygen species (ROS) upon the coincidence of a photosensitive molecule (PS), molecular oxygen, and a tuned light source. In a biological environment, these ROS find nearby oxidation targets and cause diverse OS. A large factor in determining the PDT effect is the response to OS and whether the threshold for cellular recovery has been surpassed. As such, the applications of PDT change drastically with different dosages. High doses are the most common implementations where it is used to eradicate tumor tissues.[14] Moderate doses are used in the elimination of bacteria[6, 15] and viruses,[16, 17] both promising medical tools in development. At the lowest doses, PDT is commonly used for aesthetic treatment of photoaging and altered pigmentation in the skin.[18, 19] The proposed use of PDT in implant infection clearing would take place in the moderate range, where all biological material receives damage near the threshold of OS recovery. Recovery mechanisms are the key advantage over bacteria and viruses when applying a therapy of this type.[20] The most fatal cellular damage caused by PDT is to nucleic acids, causing alteration in function and often failure. In bacteria, this nucleic acid damage can be recovered somewhat by means of non-homologous end-joining and homologous recombination. Human cells have the same systems, but also the support of surrounding tissues to ease the burden of OS. The key factor against bacteria is the combination of antibiotic therapy, which reduces antibiotic resistance to a more manageable range.[6, 21] The treatment of viruses is very different, as virions are harder to accurately target with OS but easier to damage since there are no inherent nucleic acid repair systems. Regardless, bacterial infections are more common at implant sites.[22] This study assumes future use as a therapy for acute and chronic infection in combination with antibiotics to reduce infection time and improve patient outcomes, as demonstrated in Figure 1.
The PS, light, and generated ROS all have the potential to interfere with hydrogel properties that are specially tuned for the chosen tissue environment. Particular causes for altered material properties include PS effects of molecular integration and chemical transformation, light effects of local heating and photochemical activity, and PDT effects of molecular oxidation from ROS. Hydrogels and soft material implants are developed with specific compounds to maintain a desired range of physical properties suited to the body.[23] The integration of additional molecules within a polymer network is known to alter physical properties[24] and increase osmotic pressure, leading to increased hydrogel swelling.[25] Exposure of hydrogels to light can potentiate further cross-linking, increasing storage modulus,[26] or heating local regions causing a reduction in swelling capacity and subsequent loss of storage modulus.[27] Finally, chemical oxidation increases hydrogel susceptibility to hydrolysis, which degrades the crosslinked bonds, breaking down the large-scale molecular structure and characteristics.[28] Each of these factors is involved in the process of PDT making a full evaluation of individual effects necessary to determine the impact on hydrogel degradation and thus potential efficacy for medical use.
The hypothesis is that PDT causes minimal changes in hydrogel material characteristics at doses suitable for medical applications. This hypothesis is verified by frequency-sweep viscoelastic testing of hydrogels given moderate to extreme doses of PDT, far beyond what would be experienced in medical applications. Additionally, it is expected that hydrogel swell time, photosensitizer concentration, and illumination time may contribute individually to changes in viscoelasticity, based on the research discussed above. Storage and loss moduli are tested by direct application of PDT and its individual components to HEMA-DMAEMA hydrogels for extended periods and evaluation by rheometric measurements. Viscoelastic measurements reveal a statistically significant but biologically insignificant change in hydrogel storage moduli following PDT but also in PS, light exposure, and swell time controls. Though implanted hydrogel materials vary widely, HEMA and DMAEMA compounds are common in the more matured field of dental implants.[29] It is anticipated that this evidence will support future development in applications of PDT to implant infection sites to improve microbial clearing and thus patient outcomes.
2 Experimental Section
2.1 Sample Preparation
Samples were prepared as described by Prabhakaran and Benjamin until the preconditioning stage.[30] The pre-polymer solution for a HEMA-DMAEMA hydrogel was prepared by mixing two monomers 2-hydroxyethyl methacrylate (HEMA) and 2-(dimethylamino) ethyl methacrylate (DMAEMA), a cross-linker ethylene glycol dimethacrylate (EGDMA), and a photo-initiator 2,2-dimethoxy-2-phenylactophenone (DMPA) at weight percentages of 80, 16, 1, and 3, respectively.[30]
An SYLGARD 184 Silicone Elastomer Kit was used to prepare polydimethylsiloxane (PDMS). Prepolymer solution was placed into 1-inch circular wells made of PDMS. This solution was held in place by two glass coverslips which ensure surfaces are smooth and free of bubbles. A 1-inch circular photomask was placed on top to reveal only prepolymer solution and no PDMS. UV light curing was then completed with an OmniCure series 1500 with an irradiance of for 120 s.[30]
2.1.1 Preconditioning
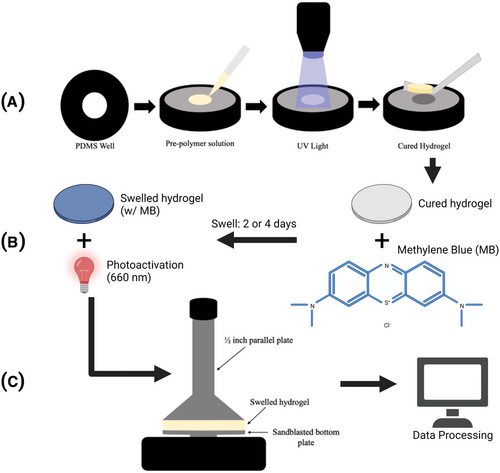
All cured samples were transferred to phosphate-buffered saline (PBS) with or without methylene blue, as described in Section 2.3, to swell for 48 or 96 h (diffusive equilibrium).[31] One glass coverslip remained connected to samples during this swelling process. All samples were enclosed in aluminum foil to prevent photoactivation of the photosensitizer and maintain neutrality between all sample groups. After preconditioning was completed, three half-inch circular samples were punched from each one-inch hydrogel. This size was selected based on the rheometer plates available for testing. Figure 2 demonstrates the steps for sample creation, testing, and evaluation.
2.2 Rheometric Evaluation
An Anton Paar Rheometer MCR 302 was used to conduct frequency sweep experiments on the viscoelastic properties of HEMA-DMAEMA with a ½-inch parallel plate setup (Figure 2C). 1.5 N normal force was applied to samples between the parallel plates to reduce slippage during testing. PDT and light control groups received additional light exposure within the swelling media as denoted in Section 2.3. After any desired light exposure, those samples were tested under matching conditions. The hood temperature was reduced and maintained during evaluation, with hydrogels exposed to the atmosphere for only 6 min during testing. Samples were subject to a frequency sweep from 0.1 to 100 Hz at 0.5% shear strain.
2.3 Photodynamic Treatment
The photosensitizer methylene blue (MB) was dissolved in water and mixed with 10× PBS and water to generate 1× PBS with 10 or 100 µM concentration of MB (MB-PBS). These solutions were used for hydrogel swelling over 2 or 4 days as described above to reach equilibrium diffusion. If samples were to be illuminated, they were returned to the swelling media following cutting and exposed to an LED (THORLABS M660L4) centered at 660 nm with a measured irradiance of 9.20 mWcm−2 at the standardized sample distance for 30 min or 5 h, making the total delivered power 17 or 170 Jcm−2, respectively. These concentrations and illumination times ranged from moderate to extremely high-dose PDT compared to medical applications. In total, this made for a control swelled in PBS, a photosensitizer control swelled in MB-PBS, a light control swelled in PBS and exposed to the LED, and a test group swelled in MB-PBS and exposed to the LED.
Sample groups included the following numbers of replicates. Of samples that received 48-h swelling, 15 control, 15 MB control (10 µM MB), 15 MB control (100 µM MB), 12 light control (17 Jcm−2), 12 PDT (10 µM MB, 17 Jcm−2), and 13 PDT (10 µM MB, 170 Jcm−2). Of samples that received 96-h swelling, 21 control, 19 MB control (10 µM MB), 17 MB control (100 µM MB), 12 light control (17 Jcm−2), 15 light control (170 Jcm−2), 22 PDT (100 µM MB, 17 Jcm−2), and 18 PDT (100 µM MB, 170 Jcm−2).
It should be noted that hydrogels were maintained in liquid media at all times except during rheometric analysis to keep consistent moisture content. During light exposure, MB-PBS above the hydrogel samples might absorb some photoactivating light and reduce the total PDT effect possible at the sample location.
2.4 Data Handling & Statistical Methods
All data were collected electronically through the software provided with the Anton Paar Rheometer MCR 302. Data were exported to Excel and entered into GraphPad Prism for Windows for graphing and statistical analysis.[32] All data sets were analyzed first with one-way ANOVA then, if found to be statistically significant, were further evaluated with individual tests of Welch's t-test between relevant sample groups.
3 Results
Samples included HEMA-DMAEMA hydrogels divided into true controls, light exposure controls, MB exposure controls, and PDT test groups. True controls are hydrogels swelled in plain PBS and no additional light exposure. Light controls are swelled in plain PBS but are exposed to PDT illumination. MB controls are swelled in MB-PBS but are not exposed to illumination. PDT test groups are swelled in MB-PBS and exposed to illumination to activate the photosensitizer. Doses of PDT applied are in excess of standard medical application for microbial clearance to gauge a limit of effect on materials. Samples are evaluated by frequency sweep rheometric measurement to evaluate viscoelastic properties most pertinent to implant material viability and matching to surrounding tissue.
3.1 High-Dose PDT Moderately Alters HEMA-DMAEMA Storage Modulus
The tests described in Experimental Section were used to interrogate the mechanical properties of storage modulus, loss modulus, and loss factor. Briefly, this involves frequency sweep rheometry from 0.1 to 100 Hz under 0.5% shear strain. Figure 3 illustrates these changes for PDT test groups as compared to true controls and details the statistical results.
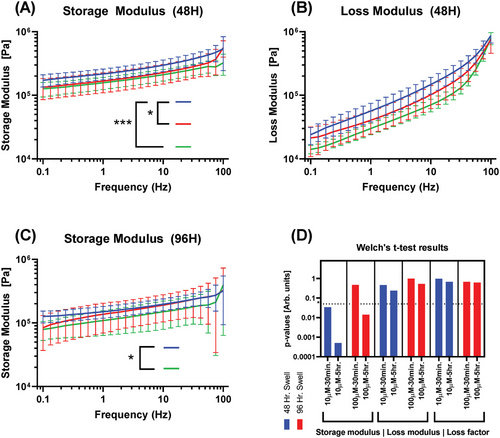
It is apparent that hydrogels treated with PDT have a slight variation in storage modulus but minimal changes in loss modulus and resulting loss factor. Notably, every PDT test group curve is less than controls on average, with longer light exposure maintaining the greatest depression in value. Increasing hydrogel swell time from 48 to 96 h also consistently decreases controls in both storage modulus and loss modulus by 34.5% and 39.7%, respectively, calculated using average modulus values over each dataset and the percent change formula.
Storage and loss modulus are consistently reduced on average by all tested doses of PDT. The greatest reductions in viscoelastic moduli due to PDT are observed in the 48-h swell, 10 µM, and 5-h exposure groups for all measured values. Storage and loss moduli are both decreased with longer exposure in each swelling group. The loss factor is less comparable due to its nature as a fraction of the moduli. The group least impacted by PDT is the 96-h swell, 100 µM, 30-min exposure.
3.2 Light Exposure and Methylene Blue Alter HEMA-DMAEMA Similar to PDT
It is necessary to compare the effects of PS and light exposure alone on the test media since the introduction of additional molecules and light exposure can alter hydrogel molecular structures and, in turn, macrostructural properties important in implant mechanical qualities. Figure 4 demonstrates the comparison of PDT controls (exposure to MB and 660 nm light independently) to true controls.
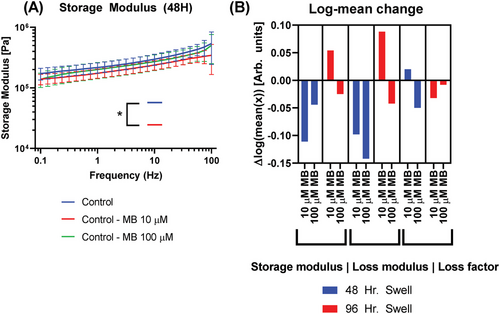
In general, the 48-h swell groups follow previous trends for storage and loss modulus with the addition of MB lowering mean values in a negative dose-response relationship in three of four groups. However, the 96-h swell group with 10 µM MB observes a notable increase in both storage and loss modulus that is once again reduced below controls at 100 µM MB. Increased swell time caused an increase in the log of mean values (Figure 4B) by 0.092 for storage modulus and 0.143 for loss modulus, averaged across MB doses. At both 48- and 96-h swelling times, the storage modulus data of 100 µM MB controls more closely aligns with true controls and PDT tests (Figure 4B), while 10 µM MB controls lie below controls at 48 h and above at 96 h. Potential sources of this variance are provided in Section 4.
The inclusion of MB into hydrogel swelling media at 10 µM caused a diverse response in storage modulus log mean values due to swell time differences, with the 48-h swell group matching closely to PDT group results. At 100 µM MB, more consistent but smaller reductions are present. There are similar results in terms of loss modulus, with 10 µM MB ranging from positive to negative changes and 100 µM MB maintaining a decreased value more akin to prior PDT results. The only statistically significant difference from the control was 48-h swell and 10 µM MB. As before, the loss factor is presented but less comparable due to its nature as an expression of the two moduli.
Finally, the light control is evaluated similarly in Figure 5, comparing 30 min and 5 h of direct 660 nm exposure to baseline controls with no exposure, as well as 48- and 96-h hydrogel swelling.
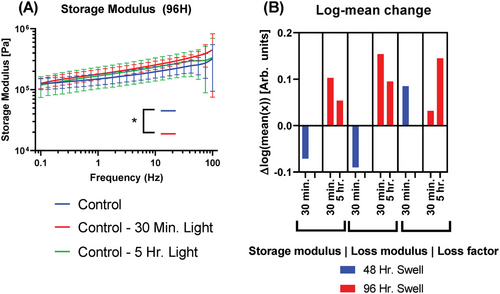
Changes are observed in viscoelastic moduli driven by exposure to 660 nm light at , but more so by hydrogel swell time. As in MB tests above, changes are driven largely by swell time. From 48- to 96-h swells, the 30-min exposure group experiences an increase in the log of mean storage modulus by 0.174 and likewise for loss modulus by 0.244.
A 48-h swell and 30-min exposure led to a moderate decrease in moduli relative to controls and closely matches prior PDT changes. At a longer swell of 96 h, both 30-min and 5-h exposure caused increases in storage and loss moduli relative to control. Still, of all sample groups, only the storage modulus of samples treated with a 96-h swell with 30-min exposure differed from the control in a statistically significant manner.
4 Discussion
Frequency sweep viscoelastic interrogation of hydrogels was used to determine changes imparted by PDT and individual factors of MB concentration, light exposure, and swell time. Reductions of storage modulus agree with existing literature for each of these groups and strongly suggest the source of the observed variation. ROS generated by PDT is known to oxidize compounds and, in a hydrogel, may be responsible for increased hydrolysis of crosslinked bonds,[28] reducing the macro-scale rigidity. MB are relatively small molecules at [33] and are thus expected to diffuse readily into gels, where they increase osmotic pressure and consequently swell volume.[24, 25] Light can change storage modulus in both directions, increasing it by photocatalysis of crosslinking,[26] and decreasing it by heat-induced degradation.[27]
Considering all test conditions, results indicate significant changes in only the storage modulus for PDT (Figure 3), MB (Figure 4), and light exposure (Figure 5). Both loss modulus and loss factor never differed from controls in a statistically significant manner. The following discussions will compare PDT doses and results to medically relevant applications to evaluate safety and efficacy.
4.1 High-Dose Photodynamic Therapy Minimally Alters Mechanical Properties in Hydrogels
PDT clearly alters the viscoelastic moduli of HEMA-DMAEMA hydrogels, generally with some yet-undefined negative dose-response relation. Though these changes may appear large when viewed linearly, viscoelastic properties of tissues within the body range orders of magnitude. In particular, joint tissues such as costal cartilage, found at the joining of ribs, exhibit storage moduli in the order of single to tens of MPa.[34] Highly dynamic tissues such as skin vary with bodily location and statuses such as temperature and hydration to a large degree, typically from lows around tens of kPa up to hundreds of MPa.[35] In muscle, often adjacent to soft material implants, the storage modulus can range from tens to hundreds of kPa throughout contractile movements.[36] Though the location of soft material implants changes, and thus the implant properties, the variation within even a single tissue group is substantial and often greater than an order of magnitude. Therefore, it is expected that the changes observed in hydrogel viscoelastic properties due to PDT are not biologically significant. Furthermore, the doses of PDT applied in these tests are greater than any used for antimicrobial applications in medicine. The lowest dose, 10 µM MB exposed for 30 min (48-h swell), is the most relevant directly to future implant clearing procedures and yielded an average reduction in storage modulus of 21.3% and loss modulus of 23.2% – substantially less than the 90% representing an order of magnitude reduction.
PDT is a complicated process in which any component can obfuscate the correlation of effects in experiments. As such, Section 3.2 was completed to detail the individual impacts of MB inclusion and light exposure. This is particularly relevant since hydrogels and many soft implant materials are sensitive to molecular integration[37] and light-based processes of curing,[38] both of which are often used to modulate material properties in testing. Interestingly, none of the data sets for components of PDT varied by statistically significant amounts relative to the respective PDT group (e.g., 30-min exposure and 10 µM MB controls vs PDT with those variables combined). This indicates that the impact on viscoelasticity observed in PDT data is likely due in part to individual effects of MB inclusion and light exposure, rather than relying solely on ROS generation for direct molecular damage. Though data from PDT and its components do not significantly differ, they exhibit different moduli responses to increasing variables. Increasing MB content in the swell media leads to decreasing loss modulus but a non-linear or biphasic effect on storage modulus. Increasing 660 nm light exposure decreased both viscoelastic moduli for 48-h swells but yielded a non-linear effect for 96-h swells.
4.2 Photodynamic Therapy Proposed for Antimicrobial Treatment in Proximity to Soft Matter Implants
In current medical practice, implant infection clearing is somewhat simple for hard materials and more difficult to gauge for soft materials. Hard materials benefit from higher durability and may consist of metal useful in phototherapies[4] but are not suitable for many applications. Soft material implants, ranging from softer silicone to more rigid cured hydrogels, are much more difficult to treat due to their lesser durability and generally increased likelihood of issues.[39, 40] As a result, the most common therapy for soft implant infections is antibiotic therapy. In severe cases with implant-integrated biofilm formation, surgery revision or resection are often necessary.[41] Though antibiotics are often effective, many implant infections must be entirely eliminated to avoid recurrence or complications arising from biofilm development, potentially requiring greater doses or more dangerous classes of therapeutic in conjunction with correcting surgery.
To address the issue of soft implant infection clearing, PDT is proposed as an alternative or combined therapy. PDT is well studied in the elimination of bacterial colonies both in vitro and in vivo as well as the reduction and control of biofilm development. Though useful in isolation, the antimicrobial effects of photodynamic and antibiotic therapies have been observed to be synergistic, suggesting a more useful application in combination. In combination with the preceding analysis of hydrogel damage, it is anticipated that medical doses required for implant clearing would lie far below the threshold of significant damage. Photosensitizer compounds have been integrated and used externally in a multitude of dental implants for in situ PDT and found success in microbial reduction.[42, 43] Beyond application in existing scenarios, integration with implant materials may aid in reducing post-implant infection probability. This approach would be preferable if photosensitizer can be reasonably included and sustained near implant surfaces, as PDT would require only the delivery of photoactivating light to the area. In addition to ease of applying therapy, this would also allow moderate PDT to be applied during implantation to reduce any surface bacteria. One standing hypothesis for delayed implant infection, occurring over 3 months post-surgery, involves the extremely minute presence of bacteria on implant surfaces[44] which may go undetected for a multitude of reasons. In the case that residual microbes on implant surfaces are a cause of delayed infections, rather than microbes arriving from elsewhere in the patient post-surgery, moderate applications of PDT during implantations may be effective in reducing delayed infection prevalence.
Alternative techniques in recent literature include developments in photoactive antibacterial materials, which may be used to coat implants in manufacturing. For example, nanofiber membranes specifically designed for efficient photocatalysis show high efficacy in eliminating common infectious bacteria Escherichia coli and Staphylococcus aureus.[45] The benefits of this type of material are its form as a membrane and its potential for tunability. The state of the material allows for standardized coating of implants during manufacturing, simplifying the implantation and treatment process. However, the coating may not always be necessary unless preventative measures are desired. PDT offers the benefits of a liquid state for PSs as well as proven bioelimination and FDA-approval for similar applications. Application of PDT can be applied as desired during procedures, complicating the process, but allowing more flexibility in treatment. A multitude of PS drugs have been FDA approved for different PDT applications. These PSs have known bioelimination pathways and well-defined characteristics, and may be more easily investigated and approved for implant infection-clearing potential.
4.3 Advancement and Limitations
There are many facets of PDT and implant infection worthy of further research efforts. The methods and applications used in this study would benefit from exploration with additional hydrogel types, photosensitizer compounds, and commercially available soft implant materials. An improved understanding of infection pathways and tissue-implant interactions is vital to the development of PDT as a means for implant infection clearing. For example, commercial implants could be used for in vitro study of infection-clearing efficacy by simple bacterial culture and elimination by PDT or combined therapies. Since novel therapy applications, such as those of post-implantation infections, require substantial efficacy evaluation and vetting, the application of PDT during implantation for further microbial clearing may be the path of least resistance and the least risk for clinical trial and evaluation of efficacy in vivo. It is hoped that these photodynamics research developments may contribute to the reduction of implant infections and improvement in patient outcomes post-treatment.
The immediate significance of this study reflects in the research focused on implant integration of PS as an antimicrobial mechanism.[46] These recent developments aim to dope materials with PSs to enable easy applications of PDT, both intra- and post-surgery for infection prevention and treatment, respectively. Research in hydrogel-aided wound healing is also considerate of PDT for improved bacterial elimination throughout the healing process.[47] Confirmation that PDT is not significantly altering the doped implants is vital to the continued development and testing of PS-doped soft implants. Additionally, this novel efficacy evaluation opens a wide avenue for PDT application and improvement of medical outcomes in existing and future implant procedures.
The impact of this work is centered on the improvement of implant patient outcomes and, currently, would include primarily breast implant recipients. As of 2020, over 280 000 augmentation surgeries are performed annually in USA alone,[48] 19% of which will develop implant-related infections.[49] The prevalence of breast cancer means that many do not have a better option than implants for continuing life than before cancer. Additionally, forthcoming research on the diverse utility of hydrogel implants warrants preemptive study and validation of PDT in these materials to form a better therapy from the start.
Future research should focus on the exploration of effects in different hydrogel and PS combinations including current soft material implants. This work will further expand the efficacy of PDT in experimental use on hydrogels and, hopefully, lead to safety determinations and medical implementation. In terms of material interrogation, additional tests could be considered including microscopy evaluation of large-scale degradation, transition electron microscope (TEM) evaluation of crosslinking architecture, and tensile strength. Each of these tests provides valuable information regarding material alteration, advancing the potential for implant infection clearing with PDT.
Acknowledgements
J.A.W. and V.V.Y. received partial funding from the National Science Foundation (NSF) (grant CMMI-1826078), the Air Force Office of Scientific Research (AFOSR) (grants FA9550-20-1-0366, FA-9550-20-1-0367), the National Institutes of Health (NIH) (grants 1R01GM127696, 1R21GM142107, 1R21CA269099), the DOD Army Medical Research program (grant W81XWH2010777), the Cancer Prevention and Research Institute of Texas (CPRIT) (grant RP180588). A.T. and C.C.B. received partial funding from Texas A&M Engineering Experiment Station (TEES). This material is also based upon work supported by the NASA, BARDA, NIH, and USFDA, under Contract/Agreement No. 80ARC023CA002. The copyright line for this article was changed on 6 November 2023 after original online publication.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.