Gene expression profiling for metastatic risk in head and neck cutaneous squamous cell carcinoma
Data from this manuscript were provided, in part, within an accepted abstract for the AHNS 10th International Conference on Head and Neck Cancer.
Funding information: Castle Biosciences, Inc.
Abstract
Objective
Over 50% of newly diagnosed cutaneous squamous cell carcinoma (cSCC) lesions occur in the head and neck (cSCC-HN), and metastasis to nodal basins in this region further complicates surgical and adjuvant treatment. The current study addressed whether the 40-gene expression profile (40-GEP) test can predict metastatic risk in cSCC-HN with improved accuracy and provide independent prognostic value to complement current risk assessment methods.
Study Design
Multicenter, retrospective cohort study.
Methods
Formalin-fixed paraffin-embedded primary tumor tissue and associated clinical data from patients with cSCC-HN (n = 278) were collected from 33 independent centers. Samples were analyzed via the 40-GEP test. Cases were staged per American Joint Committee on Cancer, Eighth Edition (AJCC8) and Brigham and Women's Hospital (BWH) criteria after comprehensive medical record and pathology report review. Metastasis-free survival (MFS) rates were determined, and risk factors were analyzed via Cox regression.
Results
The 40-GEP test classified the cohort into low (Class 1, n = 126; 45.3%), moderate (Class 2A, n = 134; 48.2%), and high (Class 2B, n = 18; 6.5%) metastatic risk at 3 years postdiagnosis. Regional/distant metastasis occurred in 54 patients (19.4%). MFS rates were 92.1% (Class 1), 76.1% (Class 2A), and 44.4% (Class 2B; p < .0001). Multivariate analysis of 40-GEP results with AJCC8 or BWH tumor stage, or clinicopathologic risk factors, demonstrated independent prognostic value of the 40-GEP test (p < .03). Accuracy of predicting metastatic risk was also improved using 40-GEP classification (p < .02).
Conclusions
Improved metastatic risk stratification through the 40-GEP test could complement cSCC-HN risk assessment for better-informed decision-making for treatment and surveillance and ultimately improve patient outcomes.
Level of Evidence
3
1 INTRODUCTION
As the second most common form of skin cancer, cutaneous squamous cell carcinoma (cSCC) is diagnosed in approximately 1.8 million individuals each year in the United States,1-5 and this high and rising incidence1-7 corresponds to a substantial number of patients who develop metastasis (2%–6%).5, 8-13 Over 50% of newly diagnosed cSCC lesions occur in the head and neck region (cSCC-HN), posing unique challenges for surgical treatment,6, 14, 15 and cSCC-HN metastasis to nodal basins (e.g., parotid and cervical lymph nodes [LNs]) also complicates surgical and adjuvant treatment due to proximity of vital structures. In addition, regional and/or distant metastases in cSCC are associated with decreased survival; approximately 2% of cSCC patients die from the disease each year.10, 13 Whereas cSCC-HN is commonly treated via Mohs micrographic surgery (MMS), which is known to improve outcomes for high-risk tumors,16-18 accurate prediction of risk for metastasis in patients with cSCC-HN is paramount for determining risk-appropriate strategies for patient management, such as multidisciplinary consultation, nodal evaluation, and adjuvant therapy, for optimal outcomes.
Guidelines from the National Comprehensive Cancer Network (NCCN) utilize specific clinicopathologic factors to categorize a cSCC patient as low, high, or very high risk for local recurrence and/or metastasis. These guidelines characterize cSCC tumors as high risk when they are located on the head and neck (H&N) at any size and very high risk when ≥4 cm in any anatomical location.5 The American Joint Committee on Cancer (AJCC) Cancer Staging Manual, Eighth Edition (AJCC8), and Brigham and Women's Hospital (BWH) staging system also utilize clinicopathologic factors to categorize cSCC patients into tumor (T) stages that correlate with risk for poor outcomes.19-21 However, the accuracy of these systems for predicting metastatic risk in cSCC is low (positive predictive value [PPV] of 33% and 35% for AJCC8 [T3/T4] and BWH [T2b/T3], respectively) relative to what has recently been reported for a prognostic 40-gene expression profile (40-GEP) test (PPV, 60% for Class 2B).22, 23 Of importance, the AJCC8 staging system addresses cSCC of only the H&N region, whereas previous editions addressed cSCC in other anatomical sites as well.20, 24 Having a prognostic tool, such as the 40-GEP test, that provides molecular information from the primary tumor and has improved accuracy for metastatic risk stratification could complement risk assessment and tumor staging of cSCC-HN with potential for better-informed decision-making and more personalized, risk-appropriate patient management.
Herein, we describe risk classification of a cSCC-HN cohort (n = 278) via the 40-GEP test based on risk for metastasis at 3 years postdiagnosis: Class 1 (low risk), Class 2A (moderate risk), and Class 2B (high risk). In this high-risk cSCC patient cohort, we demonstrate that the 40-GEP test can improve upon accuracy of current risk stratification systems, provide additional prognostic value as an independent tool for metastatic risk assessment, and complement tumor staging systems and clinicopathologic factor–based assessment for better-informed decision-making in the management of cSCC-HN patients.
2 MATERIALS AND METHODS
2.1 Cohort tissue and data acquisition
Following institutional review board approval, archival formalin-fixed paraffin-embedded archival tissue from the primary tumor (from biopsy or definitive surgery) and associated clinicopathologic data from patients with cSCC-HN (n = 278) were obtained from 33 different clinical sites as part of an ongoing study. Informed consent was obtained from study participants as required. Tissue and data for this cohort are a subset (66.2%) of a previously reported high-risk cSCC cohort studied for clinical validation of the 40-GEP test,23 and inclusion/exclusion criteria have been previously described.22 Importantly, tumors from cSCC-HN (n = 87) that were used during the discovery of the 40-GEP22 were not included in the abovementioned clinical validation study,23 nor were they part of the current cSCC-HN cohort study. Tissue samples were analyzed via clinical standard operating procedures for the 40-GEP test, and patient data were monitored via centralized review of pathology reports and medical records as previously described.22
2.2 Cohort demographics, clinical characteristics, and risk stratification
Clinical characteristics of the primary tumor and patient characteristics were documented. Anatomical locations of the primary cSCC-HN tumor were categorized similarly to previous reports describing common sites of cSCC in the H&N region.5, 14, 25-28 Following comprehensive review of pathology reports and medical records, each case was completely staged via AJCC8 and BWH tumor staging systems19-21 and categorized as very high, high, or low risk per current NCCN guideline definitions.5 Characteristics and 40-GEP test results were analyzed for the cohort to determine significant differences between metastatic and nonmetastatic cases as previously described.22
2.3 Accuracy metrics, metastatic risk, metastasis-free survival (MFS), and hazard ratio (HR)
Sensitivity, specificity, PPV, and negative predictive value (NPV) were determined. Sensitivity and NPV were compared for 40-GEP Class 2 (Class2A/2B) and AJCC8 T3/T4 or BWH T2b/T3 to determine significant differences in accuracy of stratifying moderate- to high-risk tumors from tumors with low risk of developing metastasis (i.e., 40-GEP Class 1, AJCC8 T1/T2, or BWH T1/T2a). Specificity and PPV were compared for 40-GEP Class 2B and high-stage AJCC8 or BWH to determine significant differences in accuracy of stratifying high-risk tumors from tumors with low-to-moderate risk of developing metastasis.
The number of metastases observed (events) and metastatic event and MFS rates were determined for patients stratified as 40-GEP Class 1, Class 2A, and Class 2B and the whole cohort. To determine the significance of 40-GEP classification, T stage, and clinicopathologic risk factors for predicting metastatic risk, univariate and multivariate analyses were performed and relative HRs were calculated.
2.4 Statistical analyses
All statistical analyses were performed in R (v3.6.3) using standard methods as previously described.22 Chi-square, Wilcoxon F, and Fisher's exact tests were performed to determine statistically significant differences between cases with and without metastasis or between cases having different anatomical locations of the primary tumor. McNemar and generalized score statistics were performed to determine significant differences for accuracy metrics.29-31 Kaplan–Meier and log-rank tests were performed for MFS analyses. Univariate and multivariate Cox regression analyses were performed to determine significant differences across 40-GEP Class results, T stages, and clinicopathologic risk factors. Results from analyses were considered statistically significant if p < .05.
3 RESULTS
3.1 Demographics, clinicopathologic characteristics, and 40-GEP test results
As shown in Table 1, most of the individuals in the cSCC-HN cohort were male (82.4%) and Caucasian (99.6%), and the median age was 71 years. The cohort included 63 (22.7%) immunosuppressed patients, most of whom were transplant recipients. The mean diameter and thickness of the primary tumor were each greater in metastatic cases compared with nonmetastatic cases (p < .0001). The primary tumor was poorly differentiated in a larger number of metastatic cases relative to nonmetastatic cases (p < .001). Clark Level IV/V (p < .0001), perineural invasion (PNI; p = .002), and invasion into fat (p = .003) were also found to be significantly associated with metastases. The majority of the cases were treated definitively with MMS (n = 235) versus wide local excision (WLE, n = 40), and three cases were not treated beyond biopsy. Of the 54 patients who developed metastasis, 37 (68.5%) and 15 (27.8%) were treated with MMS and WLE, respectively, whereas the metastatic event rate was found to be lower for MMS- (15.7%) versus WLE-treated cases (37.5%; p < .004). Two of the three cases without additional surgery beyond biopsy developed metastasis.
| All (n = 278) | Non-met (n = 224) | Regional/distant met (n = 54) | p value | |
|---|---|---|---|---|
| Age: Median years (range) | 71 (34–95) | 71 (34–95) | 70.5 (44–90) | .689 |
| Male sex | 229 (82.4%) | 179 (79.9%) | 50 (92.6%) | .028 |
| Caucasian | 277 (99.6%) | 224 (100%) | 53 (98.2%) | .041 |
| Immunosuppressed | 63 (22.7%) | 47 (21.0%) | 16 (29.6%) | .173 |
| Tumor diameter: Mean cm (stdev)a | 1.90 (±1.63) | 1.65 (±1.25) | 3.08 (±2.47) | <.0001 |
| Tumor thickness: Mean mm (stdev)b | 3.96 (+/−6.35) | 3.10 (+/− 6.36) | 8.58 (+/− 3.99) | <.0001 |
| Poorly differentiated | 45 (16.2%) | 26 (11.6%) | 19 (35.2%) | <.001 |
| Clark Level IV/V | 42 (15.1%) | 31 (13.8%) | 11 (20.4%) | <.0001 |
| PNIc | ||||
Present (≥0.1 mm) Present (<0.1 mm or unknown caliber) Not present |
7 (2.5%) 37 (13.3%) 234 (84.2%) |
5 (2.2%) 22 (9.8%) 197 (88.0%) |
2 (3.7%) 15 (27.8%) 37 (68.5%) |
.002 |
| Invasion beyond subcutaneous fat | 38 (13.7%) | 24 (10.7%) | 14 (25.9%) | .003 |
| MMSd | 235 (84.5%) | 198 (88.4%) | 37 (68.5%) | .004 |
| WLEd | 40 (14.4%) | 25 (11.2%) | 15 (27.8%) | |
| NCCN risk group | ||||
High risk Very high risk |
171 (61.5%) 107 (38.5%) |
150 (67.0%) 74 (33.0%) |
21 (38.9%) 33 (61.1%) |
<.001 |
| AJCC8 T stage | ||||
T1 T2 T3 T4 |
160 (57.6%) 64 (23.0%) 44 (15.8%) 10 (3.6%) |
135 (60.3%) 55 (24.6%) 28 (12.5%) 6 (2.7%) |
25 (46.3%) 9 (16.7%) 16 (29.6%) 4 (7.4%) |
.004 |
| BWH T stage | ||||
T1 T2a T2b T3 |
141 (50.7%) 97 (34.9%) 30 (10.8%) 10 (3.6%) |
125 (55.8%) 75 (33.5%) 18 (8.0%) 6 (2.7%) |
16 (29.6%) 22 (40.7%) 12 (22.2%) 4 (7.4%) |
<.0001 |
| 40-GEP class | ||||
Class 1 (low risk) Class 2A (moderate risk) Class 2B (high risk) |
126 (45.3%) 134 (48.2%) 18 (6.5%) |
115 (51.3%) 101 (45.1%) 8 (3.6%) |
11 (20.4%) 33 (61.1%) 10 (18.5%) |
<.0001 |
- Note: Data were analyzed using the Chi-square test or Wilcoxon F test.
- Abbreviations: 40-GEP, 40-gene expression profile; AJCC8 T stage, American Joint Committee on Cancer, Cancer Staging Manual, Eighth Edition tumor classification; BWH T stage, Brigham and Women's Hospital tumor classification; cSCC-HN, cutaneous squamous cell carcinoma of the head and neck; met, metastasis, metastatic cases; MMS, Mohs micrographic surgery; NCCN, National Comprehensive Cancer Network; non-met, nonmetastatic cases; PNI, perineural invasion; stdev, standard deviation; WLE, wide local excision.
- a Tumor diameter reported (n = 258).
- b Tumor thickness reported (n = 77).
- c PNI with nerve caliper ≥ 0.1 mm or in nerves deeper than the dermis are upstaging factors for the AJCC8. Only nerve caliper ≥ 0.1 mm is an upstaging factor for BWH tumor classification system. All seven cases met AJCC8 upstaging and six of seven cases met BWH upstaging.
- d Definitive surgery by MMS or WLE (n = 275) with three cases not having additional surgery beyond biopsy, two of which developed metastasis.
According to current NCCN guideline definitions, 61.5% (n = 171) of the cases were high and 38.5% (n = 107) were very high risk (Table 1).5 A significant association was found between NCCN risk status and patients having metastatic events versus those without metastasis. Of cases having metastasis, 61.1% (n = 33) were very high risk and 38.9% (n = 21) were high risk, with event rates of 30.8% and 12.3%, respectively (p < .001). Per AJCC8 and BWH tumor staging, 57.6% (n = 160) and 50.7% (n = 141) of the cohort, respectively, were staged as T1. Associations between T stage and metastatic or nonmetastatic cases were significant for both AJCC8 and BWH (p = .004 and p < .0001, respectively). The 40-GEP test classified 45.3% of the cohort as Class 1 (low risk, n = 126), 48.2% as Class 2A (moderate risk, n = 134), and 6.5% as Class 2B (high risk, n = 18). Associations between 40-GEP Class and metastatic or nonmetastatic cases were significant (p < .0001).
3.2 Anatomical locations of the primary cSCC-HN tumor and metastatic events
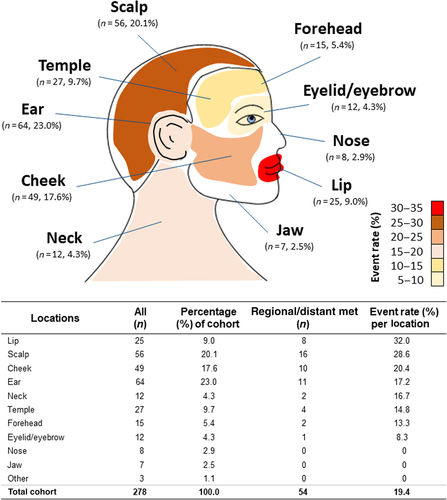
As shown in Figure 1, the ear (n = 64), scalp (n = 56), cheek (n = 49), temple (n = 27), and lip (n = 25) were the most common sites of the primary tumor, followed by forehead (n = 15), neck (n = 12), eyelid/eyebrow (n = 12), nose (n = 8), and jaw (n = 7). The lip had the highest metastatic event rate (32.0%) per location, followed by the scalp (28.6%), cheek (20.4%), ear (17.2%), neck (16.7%), temple (14.8%), forehead (13.3%), and eyelid/eyebrow (8.3%). The overall metastatic event rate for the cohort was 19.4%. No metastases were observed when the primary tumor was located on the nose, jaw, or other H&N locations. Using Fisher's exact test with simulated p values (based on 2000 replicates) to approximate chi-square, no significant differences were found for metastatic events between these different H&N locations (p = .4623).
3.3 Accuracy of the 40-GEP test compared to tumor staging
The sensitivity of a 40-GEP Class 2 (2A/2B) result (79.6%) was significantly increased compared with AJCC8 T3/T4 (37.0%) and BWH T2b/T3 (29.6%; p < .0001; Table 2), whereas the specificity of a 40-GEP Class 2B result (96.4%) was significantly higher relative to high-stage AJCC8 (84.8%) and BWH (89.3%; p < .01). The PPV of a 40-GEP Class 2B result was 55.6% compared to 37.0% and 40.0% for high-stage AJCC8 and BWH, respectively, although differences were not significant. For Class 2, the NPV (91.3%) was significantly higher relative to high-stage AJCC8 (84.8%) and BWH (84.0%; p < .02; Table 2).
| Accuracy metric | 40-GEP(Class 2) | 40-GEP(Class 2B) | AJCC8(T3/T4) | BWH(T2b/T3) |
|---|---|---|---|---|
| Sensitivity | 79.6%a | 18.5% | 37.0% | 29.6% |
| Specificity | 51.3% | 96.4%b | 84.8% | 89.3% |
| PPV | 28.3% | 55.6% | 37.0% | 40.0% |
| NPV | 91.3%c | 83.1% | 84.8% | 84.0% |
- Note: For accuracy calculations, a positive result for the 40-GEP test and AJCC8 or BWH T stage was defined as indicated in parentheses, with the corresponding negative result being that of the remainder of the cohort. Significant differences were determined using the McNemar test (sensitivity and specificity) and generalized score statistics (PPV and NPV).
- Abbreviations: 40-GEP, 40-gene expression profile; AJCC8, American Joint Committee on Cancer, Cancer Staging Manual, Eighth Edition; BWH, Brigham and Women's Hospital tumor staging system; cSCC-HN, cutaneous squamous cell carcinoma of the head and neck; NPV, negative predictive value; PPV, positive predictive value.
- a p < .0001 for sensitivity of 40-GEP Class 2 versus AJCC8 T3/T4 or BWH T2b/T3.
- b p < .01 for specificity of 40-GEP Class 2B versus AJCC8 T3/T4 or BWH T2b/T3.
- c p < .02 for NPV of 40-GEP Class 2 versus AJCC8 T3/T4 or BWH T2b/T3.
3.4 MFS, metastatic events per 40-GEP class, and HRs
As depicted in Figure 2, Kaplan–Meier analysis of the cases stratified as 40-GEP Class 1 (n = 126, 45.3%), Class 2A (n = 134, 48.2%), and Class 2B (n = 18, 6.5%) revealed significantly different 3-year MFS rates (92.1%, 76.1%, and 44.4%, respectively, p < .0001, log-rank). For the whole cohort, MFS was 81.3%. Metastatic event rates were 8.7%, 24.6%, and 55.6% for Class 1, Class 2A, and Class 2B cases, respectively; the cohort rate was 19.4%.
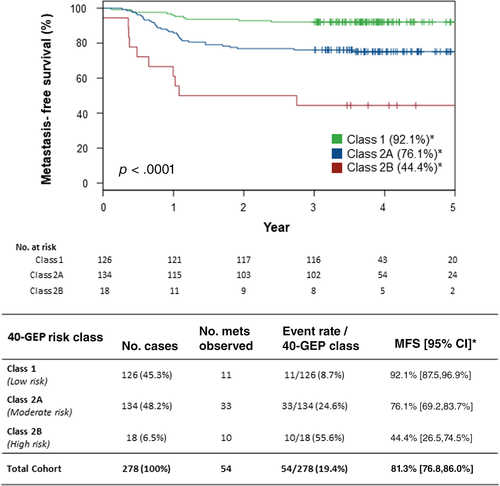
As shown in Table 3, both a 40-GEP Class 2B and Class 2A results had a significant HR (HR = 9.44, p < .0001 and HR = 3.07, p = .0013, respectively) relative to a Class 1 result. Binary AJCC8 T3/T4 and BWH T2b/T3 stages also had significant HRs (HR = 2.71, p = .0004 and HR = 2.84, p = .0005, respectively) relative to corresponding AJCC8 T1/T2 and BWH T1/T2a stages. Clinicopathologic risk factors found to be significantly associated with risk for metastasis in the cohort include tumor diameter (HR = 1.37, p < .0001), poor differentiation (HR = 3.28, P < 0.0001), PNI (HR = 2.63, P = 0.0010), deep invasion (HR = 2.86, p = .0002), and male sex (HR = 3.22, p = .0323). Immunosuppression was not a significant factor for predicting metastatic risk in this cSCC-HN cohort.
| Risk factor | n | Univariate | Multivariatea | ||
|---|---|---|---|---|---|
| Hazard ratio[95% CI] | p value | Hazard ratio[95% CI] | p value | ||
| 40-GEP test result | |||||
Class 1 (low risk) Class 2A (moderate risk) Class 2B (high risk) |
126 134 18 |
1.0 3.07 [1.55, 6.07] 9.44 [4.00, 22.26] |
— .0013 <.0001 |
1.0 2.88 [1.45, 5.71] 9.07 [3.85, 21.41] |
— .0025 <.001 |
| AJCC8 T stage | |||||
T1/T2 T3/T4 |
224 54 |
1.0 2.71 [1.56, 4.71] |
— .0004 |
1.0 2.51 [1.44, 4.37] |
— .0012 |
| 40-GEP test result | |||||
Class 1 (low risk) Class 2A (moderate risk) Class 2B (high risk) |
126 134 18 |
1.0 3.07 [1.55, 6.07] 9.44 [4.00, 22.26] |
— .0013 <.0001 |
1.0 2.86 [1.44, 5.69] 7.59 [3.14, 18.34] |
— .0027 <.001 |
| BWH T stage | |||||
T1/T2a T2b/T3 |
238 40 |
1.0 2.84 [1.58, 5.11] |
— .0005 |
1.0 2.10 [1.15, 3.83] |
— .0161 |
| 40-GEP test result | |||||
Class 1 (low risk) Class 2A (moderate risk) Class 2B (high risk) |
126 134 18 |
1.0 3.07 [1.55, 6.07] 9.44 [4.00, 22.26] |
— .0013 <.0001 |
1.0 2.28 [1.08, 4.81] 4.05 [1.34, 12.26] |
— .0311 .0134 |
| Clinicopathologic risk factors | |||||
Tumor diameterb Poor differentiation Perineural invasionc Deep invasiond Male sex Immunosuppression |
NA 45 44 49 229 63 |
1.37 [1.22, 1.53] 3.28 [1.87, 5.74] 2.63 [1.48, 4.67] 2.86 [1.64, 5.01] 2.96 [1.07, 8.20] 1.48 [0.82, 2.66] |
<.0001 <.0001 .0010 .0002 .0367 .1894 |
1.17 [1.01, 1.35] 2.42 [1.30, 4.50] — 2.06 [1.00, 4.23] 3.07 [1.08, 8.76] — |
.0391 .0051 — .0497 .0358 — |
- Abbreviations: 40-GEP, 40-gene expression profile; AJCC8 T stage, American Joint Committee on Cancer, Cancer Staging Manual, Eighth Edition tumor classification; BWH T stage, Brigham and Women's Hospital tumor classification; CI, confidence interval; cSCC-HN, cutaneous squamous cell carcinoma of the head and neck.
- a Multivariate analyses of 40-GEP Class with AJCC8 T stage and 40-GEP Class with BWH T stage used all cSCC-HN cases (n = 278, with 54 metastatic events), and multivariate analysis of 40-GEP class with clinicopathologic risk factors excluded cases not having tumor diameter reported (n = 20), leaving n = 258 cases (with 46 metastatic events) for analysis.
- b Tumor diameter was a continuous variable per centimeter.
- c Perineural invasion was considered positive regardless of nerve caliber; this variable was not significant when analyzed with other variables regardless of groupings and, therefore, was excluded from the multivariable analysis shown here to control for the event/variable ratio and maintain appropriate statistical power.
- d Deep invasion was invasion beyond subcutaneous fat, depth > 6 mm, or Clark Level V.
Factors found to be significant by univariate analyses were analyzed via multivariate Cox regression with 40-GEP test results (Table 3). The HRs for 40-GEP Class 2B and Class 2A were significant (HR = 9.07, p < .001 and HR = 2.88, p = .0025, respectively) as was the HR for AJCC8 T3/T4 stage (HR = 2.51, p = .0012). Similarly, when analyzed with BWH binary T stages, 40-GEP Class 2B (HR = 7.59, p < .001) and Class 2A (HR = 2.86, p = .0027) and BWH T2b/T3 stage (HR = 2.10, p = .0161) were significant indicators of metastatic risk. When clinicopathologic risk factors were included in multivariate analysis with 40-GEP Class, tumor diameter (HR = 1.17, p = .0391), poor differentiation (HR = 2.42, p = .0051), deep invasion (HR = 2.06, p = .0497), and male sex (HR = 3.07, p = .0358) were significant indicators for metastatic risk along with 40-GEP Class 2B (HR = 4.05, p = .0134) and Class 2A (HR = 2.28, p = .0311). PNI was not significant when analyzed with multiple factors (p < .05). In all, the 40-GEP test demonstrated significant independent prognostic value in multivariate analyses with current T staging systems and traditional clinicopathologic factors used in risk assessment (Table 3).
4 DISCUSSION
The majority of cSCC tumors arise in the sun-exposed H&N region.6, 14, 15 Successful treatment of primary cSCC-HN tumors can be complicated and extensive due to adjacent and underlying critical structures (e.g., skull base, eyes, facial nerve [CN VII], spinal accessory nerve, auditory canal, parotid gland).14, 32 High-risk tumors can have aggressive behavior with increased likelihood for metastasis and/or local recurrence.14 Common sites for metastasis from cSCC-HN are the parotid and cervical LN basins, which present anatomical challenges for treatment, and retrospective data suggest that LN dissection may be overutilized in this population.14, 25, 32 Thus, accurate prediction of risk for metastasis in cSCC-HN is essential to guide clinical decision-making for optimal patient management (e.g., nodal evaluation, enhanced surveillance). Previously, we demonstrated that molecular profiling using the clinically validated 40-GEP test provided significant independent prognostic value for metastatic risk assessment in a cohort of cSCC patients (n = 420), while also complementing clinicopathologic risk assessment methods.23 Here, we focus on the H&N subset (n = 278, 66.2%) from that cohort to demonstrate the 40-GEP test can also be a significant independent and complementary prognostic tool for determining metastatic risk in cSCC-HN.
Demographics for the cohort were typical of what has been previously described for cSCC; the majority of patients were Caucasian males, and median age was >60 years.4, 7, 22 Approximately 23.0% of the patients were immunosuppressed (e.g., transplant recipients), which is a controversial risk factor for cSCC occurrence, local recurrence, and metastasis.4, 7, 8, 13, 33, 34 Definitive surgery with MMS was significantly associated with more favorable outcomes (i.e., higher percentage of nonmetastatic relative to metastatic cases), and WLE was significantly associated with a higher rate of metastasis. This finding is in line with results from a large retrospective cohort study of high-risk cSCC managed with MMS and complete margin assessment and having low rates of nodal metastasis and disease-specific death (DSD) compared to cohorts treated primarily with WLE.35
Cases included in the current study were monitored and completely staged and, whereas 61.5% of the cohort had a high-risk and 38.5% a very-high-risk status per NCCN guidelines, <50.0% of the cases were staged as T2 or greater per AJCC8 and BWH criteria; 57.6% (AJCC8) and 50.7% (BWH) were T1. This could be due to understaging by pathologists or clinicians and/or could represent a true gap in staging and an opportunity to improve prognostication. The 40-GEP test classified 45.3% of the cohort as low (Class 1), 48.2% as moderate (Class 2A), and 6.5% as high (Class 2B) risk for metastasis at 3 years postdiagnosis. Similar to AJCC8 and BWH staging, the 40-GEP test classified a lower percentage of the cohort as high risk relative to NCCN guidelines. However, relative to previous findings for stratification of localized, high-risk cSCC (all sites) by the 40-GEP test,22, 23, 36 this cSCC-HN cohort has a higher percentage of cases at moderate risk (Class 2A) and lower percentage at low risk (Class 1) for metastasis. This demonstrates a shift toward higher risk for metastasis when cSCC is in the H&N region, which can be identified by the prognostic 40-GEP test.
The anatomical locations of the primary tumor in this cohort are similar to previously reported locations for cSCC of the H&N region.14, 25-27 The most common sites in this cohort were the ear, scalp, cheek, temple, and lip. Metastasis from the lip (32.0%) and scalp (28.6%) developed at a higher rate relative to other locations, and relative to the cohort overall (19.4%), which aligns with previous reports.37-40 No significant differences were found among the different locations with respect to metastasis. This may be partly due to relatively low numbers after categorizing per site. Together, these findings demonstrate and corroborate the high risk for metastasis from cSCC in the H&N region relative to non-H&N locations (e.g., trunk < 2 cm),5, 8, 9, 11 and underscore the need for accurate prognostication which, based on our study, can be improved with molecular profiling of the primary tumor via the 40-GEP test.
Accuracy of the 40-GEP test was enhanced over that of AJCC8 and BWH staging systems in this cohort. For a Class 2B result, specificity was significantly higher relative to high-stage binary AJCC8 and BWH classifications. For Class 2 results (Class 2A and 2B), sensitivity and NPV were significantly higher compared with AJCC8 T3/T4 and BWH T2b/T3 stages. As previously reported,22, 23 increased metastatic event rates were significantly associated with decreased MFS rates in this cohort. In addition, for 40-GEP Class 2A patients, the MFS rate in this cSCC-HN cohort was noticeably lower than that reported by Ibrahim et al. for the larger, all-site cSCC cohort (76.1% vs. 80.5%, respectively).23 Also, the event rate was higher in this cohort versus the all-site cSCC cohort for Class 2A patients (24.6% vs. 20.0%) and overall (19.4% vs. 15.0%).23 When compared with AJCC8 or BWH T staging in multivariate analyses, the 40-GEP test demonstrated significantly independent prognostic value for the cSCC-HN cohort as did AJCC8 and BWH binary T stages. More specifically, HRs for a Class 2B result were almost four-fold greater than those for AJCC8 T3/T4 and BWH T2b/T3, whereas HRs for a Class 2A result were similar to high-stage AJCC8 and BWH HRs. Tumor diameter, poor differentiation, deep invasion, and male sex were clinicopathologic factors in this cohort that had significant prognostic value along with 40-GEP Class 2A and 2B results in multivariate analysis. Whereas PNI was a significant risk factor in univariate analysis, when included with other risk factors, its significance as an independent predictor of risk for metastasis waned, which is consistent with previously reported studies.17, 41 Again, the HR for a Class 2B result was notably greater than the HRs for the other clinicopathologic risk factors analyzed.
Although immunosuppression is known to be a risk factor for developing cSCC with aggressive tumor behavior,4, 7, 8, 13, 33, 34 it was not found to be significantly associated with risk for metastasis in this cohort. Of potential relevance, immunosuppression is not included as a risk factor in AJCC8 and BWH tumor classification systems.21 Also, McLaughin et al.33 reported a rate of metastasis (5.4%) from cSCC-HN in a cohort (n = 130) of solid organ transplant recipients that is similar to that of the general cSCC population.5, 8-13 However, they also reported that many of the patients in that cohort had multiple primary tumors and the patients with regional metastasis (three parotid, four cervical LNs) had poor outcomes (DSD, 83.3% following multimodality treatment).33 Their study demonstrates the aggressiveness of cSCC in immunosuppressed individuals and the necessity of intensive patient management and surveillance. For the current study, ongoing enrollment will increase cohort size, allowing for further investigation of immunosuppression, primary tumor location, and other clinicopathologic factors in cSCC-HN.
5 CONCLUSION
The need for more accurate metastatic risk prediction has become more evident as a critical unmet need in cSCC-HN. High-risk cSCC-HN tumors can be aggressive with subsequent metastasis to parotid and cervical LN basins, where the presence of sensitive underlying structures can require extensive treatment. Our findings show the 40-GEP test is a significant prognostic tool for metastatic risk assessment in cSCC-HN that can complement current tumor classification systems and clinicopathologic factor-based assessment. This demonstrates the potential for better-informed decision-making (nodal evaluation, clinical workup, surgical management, and multimodality therapy considerations) for more personalized, risk-appropriate cSCC-HN patient management.
ACKNOWLEDGMENTS
The authors thank Clare Johnson, RN; Robert W. Cook, PhD; Aaron Farberg, MD; and Jennifer J. Siegel, PhD, of Castle Biosciences, Inc., for their intellectual contributions to this manuscript. Research and materials funding for this study were provided by Castle Biosciences, Inc.
CONFLICT OF INTEREST
Sarah T. Arron is an employee of Rakuten Medical and is a consultant for Castle Biosciences and Enspectra Health. Ashley Wysong is a board member for the American College of Mohs Surgery, Women's Dermatologic Society, and Dermatology Foundation and is the principal investigator on an institutional research grant from Castle Biosciences. Mary A. Hall, Christine N. Bailey, Kyle R. Covington, Sarah J. Kurley, and Matthew S. Goldberg are employees and option holders for Castle Biosciences, Inc. Sherrif F. Ibrahim has received advisor fees from Castle Biosciences, Regeneron Pharmaceuticals, and Sun Pharmaceutical Industries; research funding from Castle Biosciences, Galderma, and Regeneron Pharmaceuticals; and speaker fees from Castle Biosciences, Galderma, Genentech, Regeneron Pharmaceuticals, and Sun Pharmaceutical Industries. Shlomo A. Koyfman received research funding and consulting fees from Merck, research funding from Bristol Myers Squibb, and honoraria from UpToDate. Jason G. Newman is a consultant for Bolder Surgical, Inc. and Medtronic, Inc. and a steering committee member for Castle Biosciences The remaining authors served as investigators for this study, and there are no further conflicts to disclose.
AUTHOR CONTRIBUTIONS
Drs. Newman and Arron are senior authors and with the other coauthors take responsibility for the data integrity and accurate data analyses. Concept and design: Sarah T. Arron, Kyle R. Covington, Sarah J. Kurley, and Jason G. Newman. Acquisition, analysis, and interpretation of data: Mary A. Hall, Christine N. Bailey, Kyle R. Covington, and Sarah J. Kurley. Drafting of manuscript: Sarah T. Arron, Mary A. Hall, Sarah J. Kurley, and Jason G. Newman. Critical revision of manuscript for important intellectual content: Sarah T. Arron, Ashley Wysong, Mary A. Hall, Christine N. Bailey, Kyle R. Covington, Sarah J. Kurley, Matthew S. Goldberg, Julia M. Kasprzak, Ally-Khan Somani, Sherrif F. Ibrahim, David G. Brodland, Nathan J. Cleaver, Ian A. Maher, Yang Xia, Shlomo A. Koyfman, and Jason G. Newman. Statistical analysis: Christine N. Bailey and Kyle R. Covington.




