Impact of implementing stricter criteria for blood transfusion in patients with head and neck cancer undergoing free tissue transfer
Abstract
Objective
Recent literature studying the impact of blood transfusion on outcomes in patients with head and neck cancer (HNC) have shown that blood transfusions are associated with increased risk of death and higher wound infection rates. The purpose of this study was to implement a lower transfusion threshold while comparing outcomes of free flap patients following initiation of a new transfusion guideline.
Methods
A retrospective study of all patients at a tertiary care academic center who underwent free tissue transfer after HNC resection between July 17, 2007 and June 7, 2021. Transfusion criteria were adjusted in 2014; the hematocrit threshold to transfuse was incrementally reduced from 30% in 2007 to 21% in 2017. The main outcomes of interest were overall survival (OS) and recurrence free survival (RFS).
Results
A total of 346 patients met the criteria for inclusion in the study. Groups 1 (less strict protocol – 30%) and 2 (stricter protocol – 21%) consisted of 171 and 175 patients, respectively. Fewer units of packed red cells were transfused per patient in group 2 (0.26 vs. 2.87 in group 1, p < .001). Group 1 was associated with worse OS (p = .01; hazard ratio [HR] = 1.7) and RFS (p < .001; HR = 2.5). Comparing only patients with SCC between the two groups also demonstrated poorer OS (p = .01; HR = 1.8) and RFS (p = .006; HR = 2.1) in group 1.
Conclusion
In HNC patients undergoing free tissue transfer, stricter transfusion criteria with threshold hematocrit of 21% was associated with improved OS, RFS, and complication rates with no negative impact on free flap survival.
Level of Evidence
Level IV.
1 INTRODUCTION
Patients undergoing head and neck cancer (HNC) resection often require free tissue transfer for reconstruction of their defects. The role of perioperative blood transfusion in these patients has recently come under question. Although no optimal hematocrit (Hct) has been defined for free flap reconstructions, patients were historically maintained with a Hct >30% based on data suggesting that a low hematocrit impairs oxygen delivery, thereby reducing flap perfusion and survival.1 However, recent literature studying the impact of blood transfusion on outcomes in patients with HNC have shown that blood transfusions are associated with increased risk of death as well as higher wound infection rates.2 Perioperative transfusions in HNC patients have also been associated with increased rates of hospital readmission.3 Moreover, transfusion in patients undergoing free flap reconstruction does not significantly affect flap survival but was associated with perioperative complications such as flap takeback, wound dehiscence, myocardial infarction, congestive heart failure, respiratory distress, and pneumonia.4
As such, the increased tendency to transfuse free flap patients in order to maintain a threshold hematocrit has come into question. The purpose of this retrospective study was to evaluate the impact of implementing stricter transfusion criteria on outcomes of free flap reconstruction in patients with HNC.
2 METHODS
2.1 Patient selection
This was a retrospective cohort study performed at a single tertiary care academic center in which IRB approval was obtained. All patients who underwent free tissue transfer after HNC resection within the Department of Otolaryngology–Head and Neck Surgery between July 17, 2007 and June 7, 2021 were included. Each microvascular free tissue transfer surgery was counted as a separate entry. A total of 346 patients were included. Pertinent demographic and clinical data were collected. Our institution began implementing updated transfusion criteria in 2014, as our Hct threshold to transfuse was incrementally changed from <30% (2007–2014) to <21% (2017 to present). Patients who underwent free flap reconstruction between 2014 and 2017 were not included in this study as the Hct threshold was gradually adjusted. The control group (group 1) represented patients before 2014 who were transfused when Hct fell below 30%. Patients after 2017 were included in the stricter transfusion criteria group (group 2); these patients were transfused when Hct fell below 21% or when Hct was below 24% with clinical evidence of poor perfusion. The demographic and comorbidity variables of sex, age, adverse pathological features, disease stage, length of hospital stay, and radiotherapy history were collected. Adverse pathological features included lymphovascular invasion, perineural invasion, extracapsular spread, and positive margins. Cancer staging was based on final histopathology and the American Joint Committee on Cancer (AJCC) seventh edition TNM staging. Additional variables assessed were the type of flap, operative time, and postoperative complications. Postoperative complications included flap loss, wound breakdown, or infection. Information was collected on preoperative hemoglobin and Hct as well as the total amount of units of blood that were transfused in each patient. Patients were followed to assess for death or disease recurrence. Rates of adjuvant therapy, patient refusal of adjuvant therapy, patient frailty precluding adjuvant therapy, and total treatment package time were also collected.
2.2 Statistical analysis
Chi-square test and student t-test were used for analysis of categorical and continuous variables, respectively. A p-value of <.05 was considered statistically significant. Overall survival (OS) and recurrence free survival (RFS) were determined using the Kaplan–Meier method and compared statistically using log-rank tests. Statistical analysis was performed using R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
3 RESULTS
A total of 346 patients met the criteria for inclusion in the study, 171 patients in group 1 and 175 patients in group 2. The average age of the overall group was 60.7 years. The majority of patients were male (70.9%). The average age was older in group 2 (63.5 vs. 57.9 years in group 1, p = .002). 84.4% of patients had squamous cell carcinoma (84.4%) and the most common tumor site was the oral cavity (67.9%). Group 2 had a statistically significant higher rate of stage III/IV tumors (71.7% vs. 60.5% in group 1, p = .028). The distribution of flap type was significantly different, with group 1 including more radial forearm free flaps and group 2 including more anterolateral thigh free flaps (p = .006). Fewer units of packed red cells (PRBCs) were transfused per patient in group 2 (0.26 vs. 2.87 in group 1, p < .001). Patients in group 1 had significantly longer follow up times (16.3 vs. 13.3 months in group 2, p = .036). These findings are summarized in Table 1. The mean length of stay was significantly shorter in group 2 (8.3 vs. 9.9 days in group 1, p = .001). Patients in group 2 experienced fewer postoperative wound complications or infections (14.3% vs. 26.3% in group 1, p = .006). These findings are summarized in Table 2.
| Group 1 (Hct <30) | Group 2 (Hct <21) | p-value | |
|---|---|---|---|
| n | 171 | 175 | |
| Mean age, years (SD) | 57.99 (12.01) | 63.5 (10.3) | .002* |
| Gender | .397 | ||
| Male (%) | 117 (68.4%) | 128 (73.1%) | |
| Female | 54 (31.6%) | 47 (26.9%) | |
| Mean units transfused (SD) | 2.87 (3.04) | 0.26 (0.72) | <.001* |
| Mean follow up time, months (SD) | 16.3 (14.35) | 13.3 (12.01) | .0355* |
| Tumor type | |||
| Squamous cell carcinoma | 148 (86.5%) | 144 (82.3%) | .7579 |
| Site | .883 | ||
| Oral cavity/mandible | 116 (67.84%) | 119 (68.4%) | |
| Oropharynx | 21 (12.28%) | 18 (10.3%) | |
| Larynx/hypopharynx | 15 (8.77%) | 14 (8.05%) | |
| Skin/other | 19 (11.11%) | 23 (13.2%) | |
| Stage | 157 patients | 159 patients | .024 |
| I–II | 62 (39.49%) | 45 (28.3%) | |
| III–IV | 95 (60.51%) | 114 (71.7%) | |
| Adverse path factors | 89 (52.05%) | 95 (54.3%) | .676 |
| Type of flap | .0063 | ||
| Radial forearm | 102 (59.65%) | 80 (46.0%) | |
| Fibula or scapula | 30 (17.5%) | 33 (19.0%) | |
| Latissimus or rectus | 15 (8.77%) | 13 (7.5%) | |
| Alt | 24 (14.0%) | 48 (27.6%) |
- Note: Bold indicates significant values p < 0.05.
| Group 1 (Hct <30) | Group 2 (Hct <21) | p-value | |
|---|---|---|---|
| n | 171 | 175 | |
| Mean length of stay, days (SD) | 9.89 (4.37) | 8.3 (7.86) | .001 |
| Wound breakdown or infection | 45 (26.3%) | 25 (14.3%) | .005 |
| Flap loss | 6 (3.5%) | 3 (1.7%) | .29 |
- Note: Bold indicates significant values p < 0.05.
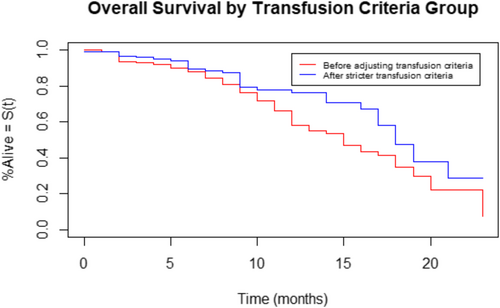
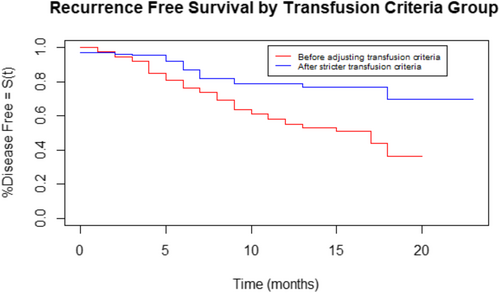
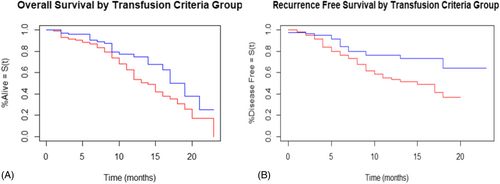
The OS and RFS are shown in Figures 1 and 2, respectively. Patients in group 1 had poorer OS (p = .01; hazard ratio [HR] = 1.7) and RFS (p < .001; HR = 2.5) compared to patients in group 2. Comparing only patients with squamous cell carcinoma (148 patients in group 1 vs. 144 patients in group 2) between the two groups also demonstrated poorer OS (p = .01; HR = 1.8) and RFS (p = .006; HR = 2.1) in group 1 compared to group 2. The OS and RFS for patients with SCC are shown in Figure 3A,B, respectively. Free flap failure rates were similar in both groups (3.5% group 1 vs. 1.7% group 2; p = .29).
Post hoc analysis revealed that the rate of receiving adjuvant therapy was significantly greater in group 2 compared to group 1 (p = .0088). However, rates of patient refusal of adjuvant therapy, and patient frailty precluding adjuvant therapy did not significantly differ between the groups (p = .15 and p = .62, respectively). Additionally, the total treatment package time did not significantly differ between the groups (p = .35), suggesting that treatment delay did not significantly impact outcomes.
4 DISCUSSION
When performing retrospective comparison of two groups defined by differing transfusion thresholds in patients with HNC requiring reconstruction with free tissue transfer, a higher rate of blood transfusion was associated with poorer OS and RFS, and with higher postoperative wound infection rates. After implementation of a stricter transfusion threshold of Hct <21%, OS, RFS, and wound infection rates improved with no negative impact on free flap survival. The two groups were statistically similar with regard to gender, tumor type and site, and presence of adverse pathological features. Although average age was older in group 2 (63.5 vs. 57.9 years in group 1, p = .002), this difference is likely not relevant as studies have previously shown that prognosis becomes significantly worse in patients older than 70 years.5 Mean follow-up times were longer in group 1 (16.3 vs. 13.3 months in group 2, p = .036), given that they were operated on much earlier. This difference however, is not likely relevant as we chose to evaluate our outcomes of OS and RFS within 24 months given the consistency of available follow up time through 2 years. Additionally, group 2 had more advanced stage tumors (stage III/IV); one would expect this difference to bias group 2 towards poorer survival outcomes.
The post hoc analysis revealed that the rate of receiving adjuvant therapy was significantly greater in group 2 compared to group 1. This finding suggests that patients in group 2 were more likely to receive timely adjuvant therapy, which could contribute to their improved overall and disease-specific survival. However, rates of patient refusal of adjuvant therapy, patient frailty precluding adjuvant therapy, and salvage surgery did not significantly differ between the groups. Additionally, the total treatment package time did not significantly differ between the groups, suggesting that treatment delay did not significantly impact outcomes.
Based on these findings, we propose that the increased disease recurrence in the group with more blood transfusions (group 1) may be related to factors other than treatment delay or patient-related factors such as refusal or frailty. It is possible that the immunomodulatory effects of blood transfusions may play a role in the observed differences in disease-free survival between the groups. Previous studies have suggested that blood transfusions can lead to immunosuppression, which may potentially impact cancer recurrence rates.2
In 2014, prior to adjusting our transfusion criteria from a Hct <30%, our institution compared patients with HNC who received 0–2 units of PRBCs to patients who received ≥3 units after reconstruction using free tissue transfer. Danan et al. demonstrated that patients who received ≥3 units of blood had significantly poorer OS and RFS after controlling for age, preoperative hemoglobin and albumin, cancer stage, and adverse pathologic features. Increased volume of transfusion was also associated with higher wound infection rates.2 Similarly, Szakmany et al., when adjusting for relevant prognostic factors in Cox regression, found that in a cohort of 559 patients, the hazard ratio for patients having 3 or more transfused units relative to those not transfused was 1.52 (95% confidence interval (CI) 0.93–2.47) for disease-specific mortality and 1.52 (95% CI 1.05–2.22) for overall mortality. They suggested that receiving transfusion of 3 or more units of PRBCs might confer a worse prognosis in patients undergoing primary surgery for oral and oropharyngeal squamous cell carcinoma.6
These studies served as pilot studies, questioning the tendency to liberally transfuse patients with HNC undergoing free tissue transfer in order to maintain a threshold Hct. Our institution has since incrementally altered our transfusion criteria from Hct <30% to Hct <21%. After completion of this transition, patients are undergoing substantially fewer transfusions. Group 2 received a mean of 0.26 (SD = 0.72) units compared with 2.87 (SD = 3.04) units in group 1. The higher transfusion rate in group 1 was associated with poorer OS (p = .01; HR = 1.7) and RFS (p < .001; HR = 2.5). Additionally, group 1 was associated with higher rates of wound breakdown and infection (26.3% vs. 14.3% in group 2, p = .005). In order to ensure consistency in pathology between the two groups, we compared only patients with squamous cell carcinoma and also found poorer OS (p = .01; HR = 1.8) and RFS (p = .006; HR = 2.1) in group 1. Free flap failure rates were unaffected by blood transfusion amounts, as both groups had similar rates (3.5% group 1 vs. 1.7% group 2; p = .29). Our findings support those found by Puram et al. When comparing head and neck free flap patients who were transfused under a theoretically restrictive criterion Hct <21% to those transfused under a liberal criterion of Hct <27%, they found no differences in LOS, flap survival, or postsurgical complications.4 Similarly, Rossmiller et al. compared a head and neck free flap patients transfused for Hct <30% with another group transfused for Hct <25% and found that complication rates between the two groups were not significantly different aside from higher rates of fistula and respiratory failure in the former group.7 Our findings corroborate these studies and further show that even stricter transfusion threshold of Hct <21% does not negatively impact free flap outcomes and may actually improve them. More importantly, our study shows that tightened standard transfusion criteria are associated with improved cancer survival. Interestingly, these improved outcomes when transfusing less blood come despite patients in group 2 having a significantly higher rate of stage III/IV tumors (71.7% in group 2 vs. 60.5% in group 1, p = .016).
It is notable that, in the present study 150 patients (85.6%) in group 2 required no blood transfusion compared with only 32 patients (18.7%) in group 1. This would be expected to result in reduced treatment cost.8
This study shares the typical limitations of retrospective analyses. Some patients had limited follow-up or missing data on recurrence and death. The groups also differed in the proportion of patients receiving adjuvant therapy. Additionally, changes in transfusion criteria over time, rather than a randomized control trial, may attribute improved outcomes to advancements in surgical technique, postoperative care, or surgical providers. However, our protocol for free flap surgery and postoperative care remained largely consistent, except for the adjustments in transfusion thresholds. Despite these limitations, this study, alongside existing literature, strongly suggests that stricter transfusion thresholds may improve patient survival and reduce postoperative wound infections without adversely affecting free flap survival in the HNC population.
5 CONCLUSION
After implementing a stricter transfusion threshold of Hct <21% for HNC patients undergoing free flap reconstruction, improved OS, RFS, and wound infection rates were observed with no negative impact on free flap survival.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Deafness and Communication Disorders (NIDCD) within the National Institutes of Health, (Grant/Award Number: 5R01DC014070-08; 5R01DC019632-02) and National Center for Advancing Translational Sciences, (Grant/Award Number: UL1TR002529).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




