Effect of heat stress on seed protein quality in mungbean [Vigna radiata (L.) Wilczek]
Abstract
Abiotic and biotic stresses impair the productivity of agricultural crops. Among abiotic stresses, the higher temperature (i.e., heat stress) is unfavourable for plant growth and development. In recent years, the mungbean [Vigna radiata (L.) Wilczek] demand has been increasing, which can satisfy human protein requirements. However, its productivity and quality are negatively impacted by heat stress due to climate change. This requires a broadening scope of mungbean adaptation to warmer climates. Hence, the objective of this study was to assess the effects of heat stress on various mungbean genotypes for their seed protein characteristics (total seed protein content, proportion of four protein fractions, and electrophoretic patterns on SDS-gels). The 13 mungbean genotypes were grown under normal and heat-stressed conditions by sowing seeds at two different times, that is, the normal sowing time in the last week of March and late sowing in the last week of April in the experimental plots. In late-sown plants, the total seed proteins decreased by 4.1% to 9.3%. In addition, the relative proportion of glutelins and prolamins increased significantly while globulins and albumins decreased at high temperatures. Moreover, the intensity of polypeptides decreased under high temperatures. In our studies, some polypeptides appeared, and others disappeared in late-sown genotypes. The disappearance of bands reveals a higher rate of protein degradation than synthesis under heat stress. The detrimental effects of heat stress on seed protein characteristics studied were more prominent in MH 318, IPM 02-3 and PM-5 and less noticeable in MH 125, MH 421 and PDM 139 genotypes.
1 INTRODUCTION
Worldwide, the food supply is being impacted by extreme climate changes and a quick growth in the world population. In developing countries, the population is estimated to rise from current 8.0 billion to 9.7 billion by the year 2050 (United Nations [UN], 2023). Iqbal et al. (2006) stated that for the global population huge protein gap is predicted to rise linearly with the expected increase in population and with changes in dietary habits. Abiotic and biotic stresses immensely reduce plants' productivity. Among abiotic stresses, heat stress is the main factor disrupting the growth and yield performance of plants. Plants' response to heat stress depends on the type of species, growth stages, timing, duration and the intensity of heat stress. Hasanuzzaman et al. (2013) observed that heat stress distresses morphological, physiological, molecular and biochemical characteristics of plants, resulting in poor growth and yield. High temperatures can cause numerous changes at the cellular and subcellular levels. At a cellular level, heat stress leads to membrane damage, protein denaturation, enzyme inactivation in mitochondria and chloroplasts, impaired carbohydrate and protein synthesis, protein degradation, impaired carbon metabolism and synthesis of new defence-related proteins.
Food legumes ranked third after cereals and oilseeds in global production besides having a major impact on the agro-ecosystem and nutrition balance for humans and animals (Popelka et al., 2004). Legumes are a rich source of proteins, and diverse nutrients like dietary minerals, antioxidants, vitamins, fibres and other bioactive compounds (Ozga et al., 2017). Therefore, legumes have the potential to serve as an environmentally friendly and sustainable substitute for animal proteins. However, abiotic stress like salinity, drought, heat stress and heavy metals impact the quality of legume grains. Usually, seed protein content in the legumes is affected by drought, heat and other environmental stresses (Sita et al., 2018). Under heat stress, total oil content increases while protein content decreases in various legumes, with few exceptions (Dornbos & Mullen, 1992). Seeds of crop plants provide the major proportion of the protein consumed by humans and their livestock. Therefore, several approaches have been adopted to enhance their tolerance to various abiotic stresses. Unfortunately, the effect of heat stress on grain quality has not been undertaken in the past research. As legumes are chiefly consumed for proteins and sensitive to heat stress, a major goal of the field research should be to increase the plant's resistance to heat stress and improve the grain protein content and quality.
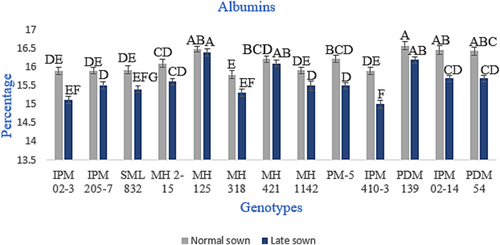
Among legumes, mungbean has an acceptable primary tolerance mechanism (HanumanthaRao et al., 2016), but most of the agro-physiological characteristics of the mungbean have not been researched extensively. Mungbean is a vital leguminous crop that supplements cereal-based diets. In India, it is grown in summer, Rabi (sown in autumn) and rainy seasons. It is also grown as an intercrop with pigeon peas, maize, orchards, sugarcane and poplar. Mungbean seeds are good sources of proteins, carbohydrates, antioxidants, phenolics, fibres and minerals (Guo et al., 2010). Though some proteins present in mature seeds have structural or metabolic roles, most have a storage role and serve as a source of amino acids during seed germination and seedling growth. Because the storage proteins determine the total seed protein content, they are of specific importance for their end uses. In view of the importance of proteins in nutrition, seed storage proteins were among the earliest proteins that have been characterised. The seed storage proteins are nonenzymatic and have the sole purpose of providing nitrogen and sulphur required during seed germination. Globulins and albumins are mungbean seeds' main storage protein fractions, typically, accounting for 60%–70% and 15%–20% of total proteins, respectively, followed by glutelins and prolamins (Gupta et al., 2018).
HanumanthaRao et al. (2016) reported that despite being an economically important crop, the total mungbean production is low due to various abiotic and biotic stresses. Moreover, mungbean is more sensitive to heat stress. High temperatures have a significant impact on the metabolism and biochemistry of mungbean plants. Under heat stress, the formation of reactive oxygen species (ROS), such as hydroxyl radical, singlet oxygen, superoxide radical and hydrogen peroxide, increases, causing protein degradation, membrane damage and enzyme inactivation, and hence increases oxidative stress (Liu & Huang, 2000). Seed shrivelling in legumes is primarily linked to reduced synthesis of carbohydrates and storage proteins due to elevated temperature. Heat stress is known to adversely affect the nitrate reductase enzyme activity, which is more detrimental for leguminous crops affecting protein biosynthesis during grain filling and reproductive stages (Farooq et al., 2017; Klimenko et al., 2006). The nutritional value of seed is impaired mainly due to adverse impact of heat stress on synthesis and accumulation of protein and carbohydrates (Taiz & Zeiger, 2010). However, extensive studies have been carried out only in some other legume crops like chickpeas (Awasthi et al., 2014; Upadhyaya et al., 2011), soybean (Krishnan et al., 2020), lentils (Sita et al., 2018) and peas (Mession et al., 2013). But research is still lacking on the effects of heat stress on seed protein quality in mungbean. Therefore, the main objective of this study was to analyse the impact of high temperatures on seed protein characteristics such as total seed protein content, the proportion of four protein fractions and the electrophoretic pattern on SDS gels of mungbean.
2 MATERIAL AND METHODS
2.1 Growing the plants
Seeds of 13 mungbean genotypes (IPM 02-3, IPM 205-7, SML 832, MH 2-15, MH 125, MH 318, MH 421, MH 1142, PM-5, IPM 410-3, PDM 139, IPM 02-14 and PDM 54) were grown in the experimental plots at the Department of Botany, Kurukshetra University, Kurukshetra, India, under normal and high temperatures (heat stress) conditions, with three replicates for each genotype. To expose the plants to normal temperature and high temperature, they were grown in the last week of March (34–36°C) and April (38–40°C), respectively. In both conditions, 10 seeds of uniform size were sown in each pot, keeping at a distance of 5 cm. Crop thinning was performed to keep five healthy plants/pot, with three replicates each. Before sowing, the seeds were primed with Rhizobium spp to ensure the formation of root nodules. Plants were watered when needed and screened for their tolerance to heat stress. The seeds harvested at maturity were analysed for total seed protein content, the proportion of four protein fractions (albumins, globulins, prolamins and glutelins) and their polypeptide patterns on SDS-PAGE gels. For different analyses in the present study, most of the chemicals used were from HiMedia Laboratories (Thane west, Maharashtra).
2.2 Seed proteins characteristics
2.2.1 Preparation of defatted seed meal
The seeds harvested at maturity were ground into a fine seed meal, by using a pestle and mortar passing through a 250-μm mesh size screen. One gram of seed meal was stirred with 10 mL of hexane at 4°C for 2 h and centrifuged at 10,000 rpm in a bench centrifuge for 10 min. The supernatant was decanted and discarded, and the process was repeated. The resulting seed meal pelleted at the bottom was spread on a petri plate lined with butter paper and dried in an oven at 35°C. The dried seed meal was sealed in polyethene bags and stored at 4°C until analysed.
2.2.2 Protein content of seed
For protein estimation, the total nitrogen was determined in the seed using the method of Peach and Tracey (1956). Seed meal was digested with concentrated sulphuric acid in the presence of a catalytic mixture, that is, copper sulphate, selenium dioxide and potassium sulphate (1:1:2). In distillation assembly; the digest was heated with 40% NaOH and the ammonia so released was fixed in boric acid solution followed by its titration against N/40 HCl to estimate the total nitrogen. The total nitrogen so calculated was multiplied by 6.25, the standard multiplication factor, to get the percentage of total proteins in the sample.
2.2.3 Estimation of four protein fractions
The method given by Croy et al. (1984), with slight modifications, was used for the separation of four protein fractions—albumins, globulins, glutelins and prolamins. For combined extraction of albumins and globulins, 100 mg of defatted seed meal was extracted twice with 1 mL of 50-mM borate buffer (pH 8) by continuous stirring for 2 h at 4°C. This was followed by centrifugation at 23,000 × g in a Remi C-24 high-speed refrigerated centrifuge at 4°C. The supernatant containing albumins and globulins was collected, which were separated by dialysis membrane against a 33-mM acetate buffer. The residue was then extracted with 0.1-N NaOH for glutelins and 70% ethanol for prolamins. Then the proportion of four protein fractions was determined by using the method of Bradford (1976).
2.2.4 SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-polyacrylamide gels were prepared as per Laemmli's (1970) formulations and run under dissociating conditions. The gels were prepared using 24 × 21 cm glass plates separated by 1.5-mm-thick Perspex spacers. Electrophoresis in the stacking gel (pH 6.8) was carried out at a constant current of 18 mA, which was increased to 32 mA when the dye's front entered the separation gel (pH 8.8). The gel was analysed by GelAnalyzer 19. To simplify our data and graphs, all the collected raw values were divided by a thousand in our computations.
2.3 Statistical analysis
The data of 13 genotypes with three replicates were analysed by one-way ANOVA using SPSS 16 statistical software. In addition, a post hoc test (Tukey) to compare means was done using the software. The means values, along with standard errors and least significant differences (P < .05) for interaction (genotype × date of sowing), are presented in the figures.
3 RESULTS
3.1 Total seed proteins
The genotype MH 125 had the highest total seed protein content and the lowest content was observed in genotype MH 318 and IPM 02-3 in normal sown plants. Under late sown (heat stress) conditions, the highest seed proteins were also noticed in genotype MH 125 and the minimum in MH 318, as shown in Figure 1. Due to high temperature, total seed protein content reduced from 4.1% to 9.3% in late sown genotypes. In late-sown genotypes, the highest reduction was noticed in genotype MH 318, and the lowest reduction was in genotype MH 125.

3.2 The proportion of four protein fractions
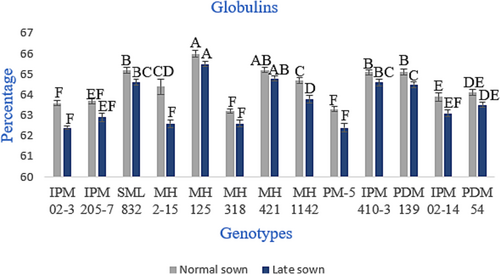
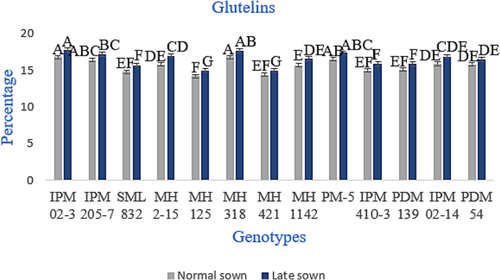
The relative distribution of four protein fractions, that is, albumins, globulins, glutelins and prolamins in seeds of different genotypes, is given in Figures 2-5. The relative proportion of albumins and globulins was shown to decrease while glutelin and prolamins increase at high temperatures or under heat stress. The globulins represented the highest proportion in all genotypes, and prolamins were the lowest. Whereas the relative proportion of globulins ranged from 63.2% to 66.0% in normal-sown plants and 62.4% to 65.5% in late-sown (heat stress) plants, as shown in in Figure 3. The proportion of prolamins varied between 3.1% and 4.2% in normally sown genotypes. By contrast, it varied from 3.5% to 4.7% genotype in late-sown conditions (Figure 5). The relative proportion of other fractions, albumins and glutelins in the seed protein did not show any significant variations, as shown in Figures 2 and 4.

3.3 Polyacrylamide gel electrophoresis
The electrophoretic analysis of total seed protein extract of normal and late-sown genotypes was carried out under reducing conditions on 14% polyacrylamide gels, as shown in Figures 6 and 7. Figures 8 and 9 show lanes 15 and 13 of the standard protein molecular marker showing the intensity peaks of different bands on SDS-gel, respectively. As shown in Figure 6a–g, the band intensities decreased under heat stress conditions. In Figure 6a, the intensities of the band of molecular weight (MW) 98, 75, 69, 54, 35, 30, 21 and 19 KDa in normally sown genotypes were found to be 0.79, 1.57, 1.09, 1.52, 1.10, 1.63, 1.92 and 1.55 and in late sown genotypes was observed 0.72, 1.29, 1.01, 1.45, 1.04, 1.52, 1.65 and 1.54, respectively. It was further observed that a new band of MW 112 KDa having an intensity of 0.83 appeared in the late sown genotype. In Figure 6b, two bands of MW 100 and 83 KDa with intensities of 1.03 and 1.27 disappeared, and one band of MW 37 KDa with 1.44 intensity appeared. The intensities of the rest of the bands decreased in late-sown conditions. In genotype, SML 832, the intensities of all bands decreased under heat stress conditions, as shown in Figure 6c. In the normal sown genotype, the intensity of band having MW 79, 74, 63, 57, 32, 23 and 20 KDa decreased from 1.18 to 1.09, 1.07 to 1.04, 3.99 to 3.80, 1.29 to 1.11, 1.22 to 1.20, 1.75 to 1.52 and 1.59 to 1.21, respectively, and two bands of MW 41 and 37 KDa having intensities 2.35 and 1.22 disappeared in late sown genotypes, as shown in Figure 6d. As shown in Figure 6e, the intensities of all bands decreased except one band of MW 88 KDa, having an intensity of 1.26 disappeared in late sown condition. In genotype IPM 205-7, the intensity of band 0.97 having MW 104 KDa disappeared, and the intensities of the rest of the bands decreased in the late-sown genotypes, as shown in Figure 6f. In genotype MH 421, the intensities of all bands decreased under heat stress conditions, as shown in Figure 6g.
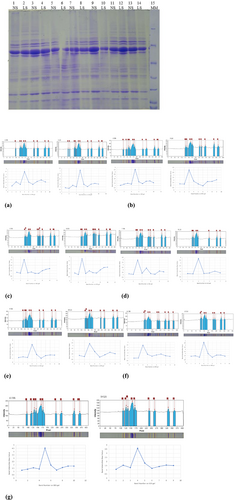


Figure 7a–f displays the intensity peaks and intensities values of polypeptides of normal sown and late sown genotypes. In genotype IPM 02-14, the intensities of bands having MW 102, 89, 75, 63, 57, 40, 37, 28 and 25 KDa decreased from 1.13 to 1.01, 1.31 to 1.28, 2.04 to 1.59, 3.49 to 3.40, 1.04 to 1.11, 1.35 to 1.23, 1.48 to 1.23, 2.32 to 1.94 and 1.88 to 1.72, respectively, in late sown condition as shown in Figure 7a. In genotype PM-5, the intensities of all bands decreased under heat stress, as shown in Figure 7b. In genotype, MH 1142, intensities of bands having MW 73, 56, 39, 36, 27, 24 and 29 KDa decreased from 0.83 to 0.80, 1.44 to 1.40, 1.30 to 1.19, 1.27 to 1.13, 1.68 to 1.61, 1.42 to 1.39 and 1.32 to 1.28, respectively, under heat stress as shown in Figure 7c. Under the late sown condition, the intensities of all bands of genotypes IPM 410-3 decreased, as shown in Figure 7d. The intensities of bands having MW 75, 72, 56, 39, 36, 28, 24 and 20 KDa of normally sown genotypes were observed as 1.022, 0.86, 1.41, 1.20, 1.18, 1.46, 1.30 and 1.08 that were decreased to 0.96, 0.68, 1.34, 1.15, 1.15, 1.33, 1.26 and 1.01, respectively, in late sown condition as shown in Figure 7e. The intensities of all bands of the late sown genotype decreased compared with the normal sown genotype, as shown in Figure 7f. Based on the results of this study, genotypes MH 318, IPM 02-3 and PM-5 were deemed heat sensitive and MH 125, MH 421and PDM 139 were noted to be heat tolerant genotypes.
4 DISCUSSION
The mungbean plants were exposed to heat stress by sowing the seeds 1 month late of the suggested sowing time. Late sowing is a commonly used approach to screen genotypes for heat tolerance of crop plants such as chickpeas (Awasthi et al., 2014; Upadhyaya et al., 2011) and lentil (Sita et al., 2018). In the present study, less damage to seed quality and seed yield was observed in tolerant plants compared with sensitive genotype, revealed by this comparison, which was most likely associated with more stability of leaves in terms of relative water content to stress conditions in tolerant plants, less damage to the cell membrane and more photo-assimilation ability (Sita et al., 2018). The sharp decline in the duration of pod formation is another reason for the low seed yield in late-sown mungbean plants. The tolerant genotypes also performed better under heat stress than the sensitive ones. These results were similar to those reported in previous studies on chickpeas (Awasthi et al., 2014) and lentils (Sita et al., 2018), mainly due to their potentiality to continue high leaf water conditions. Future studies, including mechanisms of turgor maintenance, sucrose transport and seed metabolism, are required to gain further insight into the dynamics of the improved stability of the tolerant lines compared with the sensitive lines.
High temperatures also considerably affect grain composition (Hasanuzzaman et al., 2013; Kumar et al., 2013). In our study, the total seed protein content and its quality are adversely influenced by the higher temperatures caused by heat stress. Heat stress significantly reduced protein content in the sensitive genotype, whereas this effect was minimal in the tolerant genotypes. The decrease in seed protein quality and its accumulation under heat stress has also been reported in various crops like wheat (Begcy & Walia, 2015), pea (Mession et al., 2013), lentil (Sita et al., 2018) and soybean (Krishnan et al., 2020). In chickpeas, similar research exhibited a significant reduction in protein accumulation during late pod-filling stages due to heat stress (Awasthi et al., 2014). The decrease in protein content may be associated with a decline in the protein synthesis rate or an enhanced rate of protein degradation (Balestrasse et al., 2003). In the present study, it was observed (spectrophotometrically) that high temperatures also affect the accumulation of four seed protein fractions, such as albumins, globulins, glutelins and prolamins, with higher reduction in sensitive lines than that in tolerant lines. The relative proportion of albumins and globulins decreased while glutelin and prolamins increased under high temperatures, and this agrees with the conclusions of Sita et al. (2018) in lentils and Krishnan et al. (2020) in soybean. The globulins fraction was found to be the dominating, as reported by Duranti (2006), followed by albumins and glutelins spreading over the same range. In contrast, prolamins were the lowest in all the investigated mungbean genotypes. Albumins and globulins are richer in the content of essential amino acids, so higher content of albumins and globulins is desirable for a better seed protein quality. However, their decreased accumulation under heat stress, as observed in our study, indicates a reduction in seed protein quality at high temperatures. The impaired protein synthesis, particularly albumins and globulins, can be attributed to the inactivation of the proteins' biosynthesis pathway and the low availability of precursors at high temperatures. The storage proteins act as sources of nitrogen, carbon and sulphur and are thus essential for plant growth and development and provide resistance against pests, pathogens and desiccation. The content of seed storage proteins influences seed germination and post-germination seedling establishment. The decreased accumulation of total seed protein content at higher temperatures in the present study matches with the studies on seed proteins in heat-stressed wheat (Begcy & Walia, 2015), chickpea (Behboudian et al., 2001) and common beans (Ghanbari et al., 2015), where nitrogen and protein contents in seeds declined significantly in the heat stressed plants.
A range of variations was observed in band patterns on SDS gel. Variation in any trait can be a result of genetic as well as environmental factors. The seeds used were harvested from the plants grown in the same season, and thus the possibility of environmental factors being the primary cause of observed variation seems unlikely. In our studies, some polypeptides appeared, and others disappeared in late-sown genotypes. The disappearance of bands reveals a higher rate of protein degradation than synthesis under heat stress, as Elavumoottil et al. (2003) observed. Plants quickly produce and accumulate heat shock proteins and heat shock factors in response to heat stress, which is considered one of the most important adaptation mechanisms to neutralise the negative effects of high temperatures (Keller & Simm, 2018; Wahid et al., 2007). The newly appeared bands may be considered as heat shocked proteins (HSPs), which help plants withstand heat stress (Gupta et al., 2010; Kotak et al., 2007) or may be the product of newly induced genes whose product is needed for enduring the impact of the stress (Zeevaart & Creelman, 1988). Both heat shock factors and heat shock proteins are essential to heat stress response and in the acquisition of heat tolerance in plants (Ohama et al., 2017). HSPs act as molecular chaperones, that is, preventing protein denaturation and aggregation. Previous studies have revealed that HSPs enhance the thermotolerance of legumes. Krishnan et al. (2020) reported that the accumulation of HSPs closely correlates with the thermotolerance of soybean seedlings. The production and accumulation of heat HSPs is a rapid response after exposure to heat stress. It is considered one of the most significant adaptive strategies to overcome the deleterious effects of high temperatures. It is well known that HSPs play a vital role in plant heat tolerance by maintaining the function of proteins and the integrity of various bio-membranes under heat stress (Maestri et al., 2002). Thus, the information about total seed protein content and four fractions could help breeding programmes and genetic engineering to improve the quantity and quality of seed proteins in mungbean.
5 CONCLUSION
The study has shown that heat stress negatively affected the seed protein characteristics. High-temperature stress reduced total seed protein content compared with their respective control. In addition, the intensity of polypeptides decreased under late-sown conditions. The screening results showed that genotypes MH 318, IPM 02-3 and PM-5 were heat sensitive, and MH 125, MH 421and PDM 139 were heat tolerant. Our research on different genotypes showed that thermotolerant genotypes suffered a minor injury under high-temperature stress than sensitive genotypes. Depending on the tolerance level, the plants respond to heat stress by increasing/decreasing the expression of some specific proteins, which are either metabolic or protective in function. The reduction in seed proteins, the proportion of albumins and globulins has revealed that abiotic stresses negatively affect seed protein quality. Therefore, these genotypes would be helpful in breeding programmes and serve as a standard plant source to gain more insight into high temperature-induced effects on cell metabolism.
AUTHOR CONTRIBUTIONS
Divya Batra: Investigation; methodology; formal analysis; writing—original draft. Sanju Bala Dhull: Conceptualization; technical guidance; proof reading of final draft. Jyoti Rani: Data curation; validation; editing. Meenakshi Meenakshi: Writing—review and editing; visualisation; software. Yogesh Kumar: Conceptualization; supervision; resources; technical guidance; editing. Joyce Kinabo: Resources; writing—original draft; editing.
ACKNOWLEDGEMENTS
The first author is thankful to Rashtriya Uchchatar Shiksha Abhiyan (RUSA) 2.0 for providing fellowship to conduct this study.
CONFLICT OF INTEREST STATEMENT
No conflict of interest exists.
Open Research
DATA AVAILABILITY STATEMENT
The data will be available on request.




