Acute exposure to doxorubicin results in increased cardiac P-glycoprotein expression
Abstract
Doxorubicin is a frequently used anticancer drug, but its use is restricted due to the occurrence of severe side effects, namely strong cardiotoxicity. It is known from cancer cells that doxorubicin enhanced the expression of its efflux pump P-glycoprotein (P-gp), which may modulate local drug concentrations. We therefore studied the cardiac expression of P-gp in doxorubicin-treated mice. Mice were treated with doxorubicin, and P-gp expression was studied after 1, 3, and 5 days. Thereby, we could show a significant upregulation of abcb1a (162 ± 15% of control) and abcb1b (418 ± 110% of control) mRNA transcripts after 3 days. On protein level, western blot analysis and immunofluorescence staining revealed a similar finding 5 days after doxorubicin administration. In addition, these results could be confirmed by in vitro models using primary rat cardiomyocytes and the murine cardiomyocyte-like HL-1 cells. Besides an enhanced mRNA and protein expression, doxorubicin-treated HL-1 cells also demonstrated an enhanced P-gp function as assessed by a daunorubicin accumulation assay. Our in vivo and in vitro results demonstrate a cardiac upregulation of P-gp in doxorubicin-treated mice on expression and functional level. This finding may be relevant for cardiac tissue concentrations of P-gp substrates and may represent a mechanism in cardiac self-protection against xenobiotics. © 2011 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 100:3951–3958, 2011
INTRODUCTION
Drug-induced cardiotoxicity is a serious problem in pharmacotherapy. Among others, especially anticancer drugs often exhibit serious cardiac side effects. For example, antimetabolites and taxanes are associated with an enhanced risk for myocardial infarction and drugs such as trastuzumab can lead to contractile dysfunction. Another group of cardiotoxic anti cancer drugs are anthracyclines. These compounds can lead to arrhythmias, electrocardiogram changes, and myocarditis, which in turn may result in cardiomyopathy.1 Among the anthracyclines, cardiotoxicity of the frequently used doxorubicin (Adriamycin) has been analyzed in a variety of different studies. Despite all benefits in therapy, doxorubicin treatment is still restricted due to severe cardiotoxic side effects, which lead to left ventricular dysfunction often resulting in congestive heart failure with poor prognosis.2 Although numerous underlying mechanisms have been proposed, the pathogenesis of doxorubicin-induced cardiomyopathy is still poorly understood. Many studies indicate an involvement of increased reactive oxygen species (ROS), lipid peroxidation accompanied by reduced levels of antioxidants, and a variety of other factors.3-6 In addition, there are some indications that the pharmacokinetics of doxorubicin is crucial for the cardiotoxic side effects. For example, cardiotoxicity could be diminished when doxorubicin intravenous bolus application was replaced by slow but continuous intravenous application. In addition, a comparable experimental setting using rabbits demonstrated reduced doxorubicin peak concentrations in plasma and heart.7,8 Beside the application method, other pharmacokinetic parameters may also influence cardiac doxorubicin concentration and in turn its cardiotoxicity. In this context, drug transporters of the ATP-binding cassette (ABC) family are of special interest because its members such as P-glycoprotein (P-gp) and multidrug resistance-associated protein 1 (MRP1) have been shown to transport doxorubicin.9,10 These transporters are not only expressed in cancer cells but also expressed in a variety of cells under physiological conditions. Here, such transporters are often localized to barrier structures such as the gut wall, the blood brain barrier, and the placenta.11,12 Furthermore, both proteins are expressed in the human heart. MRP1 localization was most prominent for cardiomyocytes,13 whereas P-gp was detected in vascular structures as well as in cardiomyocytes.14-16 Interestingly, a reduced cardiac P-gp expression was described for patients with dilative cardiomyopathy, whereas the expression of MRP1 was unchanged by heart failure.14,17 Against this background, it was not surprising that cardiac MRP1 could be identified as a protecting factor in doxorubicin-induced cardiomyopathy.18 In contrast, similar studies for P-gp were rather limited; however, pharmacokinetic data indicate an influence of cardiac P-gp expression on local doxorubicin concentration. For example, coadministration of P-gp inhibitors such as verapamil and ketotifen resulted in enhanced cardiac anthracycline concentrations and similar findings were made for abcb1a knockout mice.19-21
Therefore, we studied left ventricular expression of P-gp in doxorubicin-treated C57Bl/10 mice, in the murine HL-1 cell line, and in primary adult rat cardiomyocytes. We could demonstrate an upregulation of both murine abcb1 mRNA transcripts and protein. In addition, accumulation assays using doxorubicin-treated HL-1 cells demonstrated a functional relevance of this finding. Our results suggest that doxorubicin administration induced cardiac P-gp expression, which in turn may influence cardiac drug accumulation and toxicity.
MATERIALS AND METHODS
Animals
C57Bl/10 mice aged between 8 and 10 weeks were treated with doxorubicin (Doxo Cell® (Cell Pharm, Bad Vilbel, Germany) 20 mg/kg single dose i.p.) or saline as control. Animals were killed 1, 3, and 5 days after doxorubicin injection. Hearts were removed and immediately frozen in liquid nitrogen or fixed in formalin. Animal samples used in this study were taken from a former study.22 All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (publication no. 85-23, revised 1985).
Cell Culture and Chemicals
The HL-1 cells derived from AT-1 mouse atrial cardiomyocyte tumor lineage were a kind gift from Professor W. Claycomb (Department of Biochemistry and Molecular Biology, Louisiana State University Medical Center, New Orleans).23 HL-1 cells were maintained in Claycomb medium (Sigma–Aldrich, Munich, Germany) supplemented with 10% fetal calf serum (FCS), 2 mmol/L l-glutamine, 100 µmol/L arterenol (Sigma–Aldrich), and 100 U/mL penicillin/streptomycin (Biochrom, Berlin, Germany). Primary adult rat myocytes were isolated as described previously.24 Immediately after preparation, cells were placed on laminin-coated 12-well plates using creatine (5 mmol/L), l-carnitine (2 mmol/L), and taurine (5 mmol/L) supplemented M199 medium as described by Piper et al.25
Doxorubicin was obtained from Sigma–Aldrich and dissolved in dimethyl sulfoxide. Radio-labeled substances were obtained as indicated in the respective section; all other substances were obtained from Sigma–Aldrich.
Cell Viability
HL-1 cells were cultured as previously described. Cell viability in HL-1 cells was measured by Alamar blue assay26 before and after doxorubicin treatment. Cell viability was determined in 96-well plates for different doxorubicin concentration after 24 and 48 h by adding 15 µL Alamar blue solution and incubation for 2 h at 37°C in humidified atmosphere with 5% CO2. Subsequent fluorescence intensity was determined with a fluorescence counter (1420 Victor Multilabel Counter; Wallac, Boston, Massachusetts) for the emission wavelengths of 570 and 595 nm. The survival index was calculated as percent viability after incubation with drug compared with untreated control (100%).
RNA Preparation and Complementary DNA Synthesis
For RNA preparation, the left ventricles of murine hearts were taken. Tissue samples were homogenized and total RNA was prepared using TRIZOL® reagent [Life Technologies (Invitrogen), Darmstadt, Germany] according to the manufacturer's protocol. RNA from HL-1 and primary rat cardiomyocyte cell culture was isolated using peqGold RNA Pure (PeqLab, Erlangen, Germany). In both cases, total RNA was reverse transcribed using the TaqMan Reverse Transcription Kit [Life Technologies (Applied Biosystems), Darmstadt, Germany] with random hexamer primers.
Quantitative Real-time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (qPCR) was performed on ABI Prism® 7900HT sequence detection system [Life Technologies (Applied Biosystems)]. For detection of murine and rat abcb1 transcripts, primer and probe oligonucleotides were designed on the basis of the published complementary DNA sequences (mouse: abcb1a NM_011076—abcb1a_for, 5′-TGCAAGTGTAGGAAACGTCTCTAAAA-3′; abcb1a_rev, 5′-TGTGTATCTGTCTTCCAGCTGCCA-3′ and abcb1a probe, 5′-6FAM-AAGAAATGACCACGTACGCCTACTATTACACCGT-3′-TAMRA; rat: Abcb1b NM_012623—rat_Abcb1b_for, 5′-CCCATGGCCGGAACAGT-3′; rat_Abcb1b_rev, 5′-ATGGGCTCCTGGGACACA-3′ and rat_Abcb1b_probe, 5′-6FAM-TGGCTCCGCGCCCACCTGT-3′-TAMRA) or commercially ordered as TaqMan®Gene Expression Assays (mouse abcb1b Mm00440736_m1; Applied Biosystems). For normalization, 18S ribosomal RNA (rRNA) expression was determined using a predeveloped housekeeping gene assay [Life Technologies (Applied Biosystems)]. Analysis of real-time PCR data was obtained by the comparative delta–delta CT-method using 18S rRNA expression levels for normalization.
Protein Preparation
Cells were collected in phosphate-buffered saline (PBS) and centrifuged by 12,000 g for 5 min. Membrane fractions were prepared by resuspending the respective pellets in 5 mmol/L Tris–HCl (pH 7.4) supplemented with protease inhibitors (1 mg/L aprotinin, 0.5 mg/L leupeptin, and 100 µmol/L phenylmethanesulfonylfluoride (PMSF)). Samples were snap frozen in liquid nitrogen for repeated cycles, and crude membranes were enriched by a following 100,000 g centrifugation at 4°C for 30 min. The resulting pellets were resolved in 5 mmol/L Tris–HCl supplemented with protease inhibitors. Protein concentration was measured according to the bicinchoninic acid protein assay.
Immunoblot Analysis
Membrane fractions (50 µg each) were separated by a 7.5% sodium dodecyl sulfate (SDS) gel and immunoblotting was performed using a tank blotting system (Bio-Rad, Munich, Germany). Blotting efficiency was checked by Ponceau S staining. Primary antibodies were diluted in Tris-buffered saline containing 0.05% Tween 20 and 1% bovine serum albumin to the following final concentrations: P-gp antibody (C219 (sc-1517); Santa Cruz Biotechnology, Santa Cruz, California), 1:1000; secondary horseradish peroxidase-conjugated rabbit anti-goat IgG antibody (Bio-Rad) was used at a 1:2000 dilution. Detection was performed using the enhanced chemiluminescence (ECL-plus) detection system (GE Healthcare, Munich, Germany) and X-ray films (Kodak, Rochester, New York). For quantification, the P-gp band and a representative Ponceau S band were quantified by densitometric analysis using the Kodak ID scientific imaging systems software.
Accumulation Experiments
P-gp function was assessed by daunorubicin (daunomycin) accumulation assay. HL-1 cells were cultured up to 90% confluence in 24-well tissue plates as described above. Cells were treated with different doxorubicin concentrations for 48 h. After washing with PBS, incubation buffer containing the P-gp-substrate [3H]daunomycin (American Radiolabeled Chemicals, St Louis, Missouri; 1 µCi/mL) was added to the cells for 30 min. Transport was stopped by washing the cells three times with ice-cold PBS. After that, cells were lysed in 500 µL 0.2% SDS (1 mM ethylenediaminetetraacetic acid); an aliquot of 150 µL was dissolved in 2 mL scintillation cocktail (Rotiszint; Roth, Karlsruhe, Germany) and measured in a scintillation beta-counter (type 1409; LKB Wallac, Turku, Finland). To determine P-gp-specific transport, all accumulation studies were performed in the presence and absence of the P-gp inhibitor cyclosporin A (100 µmol/L) and transport rates were normalized to the respective protein concentration measured by the BCA method.
Immunofluorescence Staining
For immunofluorescence staining, primary adult rat myocytes were seeded on laminin-coated coverslips and cultured as mentioned before. Cells were incubated with doxorubicin or control as indicated. Thereafter, cells were fixed using ethanol, blocked with 5% FCS in PBS, and staining for P-gp using the JSB-1 antibody was performed. P-gp detection in murine heart was carried out using paraffin-embedded sections, which were deparaffinized and boiled in citrate buffer (pH 6.0) in a microwave for 10 min for antigen retrieval. Sections were blocked using 5% FCS and incubated overnight with C-19 anti-P-gp antibody. Primary antibodies were detected using AlexaFluor488-labeled antibodies (Life Technologies (Invitrogen)), and nuclear counterstaining was performed using the TOTO-3-iodid dye [Life Technologies (Invitrogen)].
Statistical Analysis
For statistical analysis and presentation, Microsoft Excel (Microsoft, Redmond, Washington) and Prism 5.02 (GraphPad, La Jolla, California) have been used. Statistical calculations were made using the one-way analysis of variance (ANOVA) in combination with the Newman–Keuls multiple comparison test, the two-way ANOVA followed by the Bonferroni posttest, and the Student's t-test.
RESULTS
P-gp Expression in Doxorubicin-treated Mice
C57Bl/10 mice were treated with doxorubicin for 1, 3, and 5 days. Cardiac function was monitored after 1 and 5 days by hemodynamic characterization. Parameters such as left ventricular pressure, dP/dt, and cardiac output were impaired after 1 day with a much more profound effect at day 5 (for original data see Bien et al.22). To determine P-gp expression, western blot analysis and immunofluorescence staining were performed on hearts of saline- and doxorubicin-treated (5 days) mice. In addition, mRNA expression of both P-gp mRNA transcripts, abcb1a and abcb1b, were measured 1, 3, and 5 days after doxorubicin injection. Western blot analysis demonstrated a significant enhancement in P-gp protein expression levels of 180 ± 18% in doxorubicin-treated mice (Figs. 1a and 1b). This effect was also visible by immunofluorescence staining that demonstrated the endothelial wall and small capillaries as main expression sites of cardiac P-gp (Figs. 1c–1e). The mRNA of both transcripts was measured for all time points by real-time PCR and normalized to 18S rRNA expression values. In general, mRNA expression of both abcb1a and abcb1b confirmed the data on protein level; however, some differences between abcb1a and abcb1b were detected. The abcb1a mRNA was slightly increased at days 1 and 3 (139 ± 16% and 162 ± 15% of control) but not at day 5 (80 ± 13% of control). The second P-gp transcript abcb1b showed a similar increase at days 1 and 3 with an even more pronounced effect (day 1, 126 ± 18.3%; day 3, 279 ± 73.7%; day 5, 105 ± 31.2% of untreated control, respectively) (Fig. 2a).

P-gp protein expression in murine heart. Cardiac P-gp expression was detected by western blot analysis (a and b) and immunofluorescence staining (c–e) using the left ventricle of control and doxorubicin-treated (5 days, 20 mg/kg) mice. Densitometric analysis (b) of the respective bands was performed and P-gp signals were normalized to the band intensity of Ponceau S staining. Protein expression was displayed as mean ± SEM (n = 5), and statistical testing was performed by Student's t-test. Immunofluorescence staining of a representative, paraffin-embedded heart was shown in panels (c) to (e). P-gp expression in untreated (c) and doxorubicin-treated (d) mice was indicated by the green fluorescence, whereas nuclei counterstaining was indicated by the blue fluorescence (insert indicates P-gp staining in blood vessels of doxorubicin-treated mice). Specificity of the staining was demonstrated by peptide competition (e).
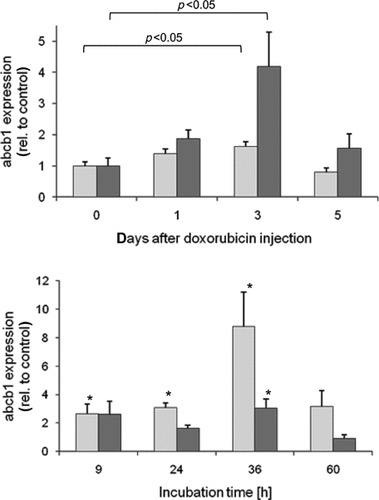
mRNA expression of abcb1a and abcb1b in murine heart (upper panel) and HL-1 cardiomyocytes (lower panel). mRNA expression of abcb1a (light gray columns) and abcb1b (dark gray columns) as well as 18S rRNA was analyzed in heart samples of untreated (n = 5), and doxorubicin-treated (20 mg/kg) mice (1 day after doxorubicin, n = 6; 3 days after doxorubicin, n = 9; and 5 days after doxorubicin, n = 5) by TaqManTM real-time polymerase chain reaction. Transporter expression was normalized to 18S rRNA and depicted in relation to untreated animals (mean ± SEM), statistical testing was performed by one-way analysis of variance followed by the Newman–Keuls multiple comparison test. A similar RNA analysis of abcb1a and abcb1b was performed on doxorubicin (333 nmol/L)-treated HL-1 cells. Transporter expression was depicted in relation to the expression of untreated cells at the respective time points (mean ± SEM; n = 3). Statistical analysis was performed between treated and untreated cells for the respective time point by Student's t-test (*p < 0.05).
P-gp Expression in Doxorubicin-treated HL-1 Cells
To further elucidate the effect of doxorubicin on P-gp expression, in vitro studies were performed on the murine cardiomyocyte-like HL-1 cell line. In the first step, we tested the viability of HL-1 cells in the presence of different doxorubicin concentrations (10–1000 nmol/L) for incubation times of 24 and 48 h; thereby, demonstrating that doxorubicin concentrations up to 333 nmol/L did not significantly alter the viability of adherent HL-1 cells after 48 h of treatment (data not shown). Next, the time-dependent effect of doxorubicin (333 nmol/L) on P-gp expression was studied. Like in the animal model, we could detect a significant increase in P-gp expression for both mRNA transcripts with a maximal effect after 36 h (Fig. 2b). In contrast to the murine heart, the induction was much higher for both transcripts and even more pronounced for abcb1a. These findings could be confirmed by western blot analysis on HL-1 cells after treatment with different doxorubicin concentrations for 48 h (Figs. 3a and 3b). Besides the expression level, we also studied the effect of doxorubicin treatment on P-gp function. Therefore, HL-1 cells were treated for 48 h with different doxorubicin concentration (100–1000 nmol/L) prior to the functional assay; P-gp function was studied using a daunorubicin accumulation assay in the presence and absence of the P-gp inhibitor cyclosporin A (100 µmol/L). Here, the intracellular accumulation of daunorubicin was enhanced in the presence of cyclosporin A after doxorubicin preincubation. The daunorubicin accumulation in presence of cyclosporin A compared with the respective control (100%) was 112 ± 15%, 162 ± 7%, 544 ± 108%, and 198 ± 26% (mean ± SEM), respectively, for 0 µmol/L, 100 nmol/L, 333 nmol/L, and 1000 nmol/L doxorubicin (Fig. 3c).
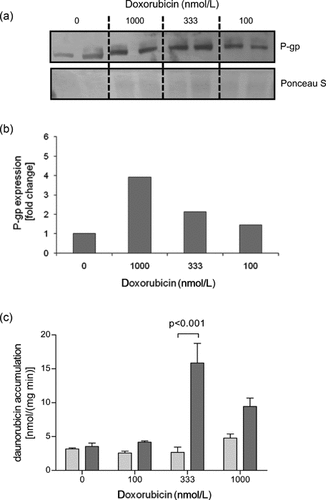
P-gp protein expression and function in HL-1 cells. (a) Representative western blot for P-gp in doxorubicin-treated (48 h) HL-1 cells. (b) Densitometric analysis. P-gp signals were normalized to the band intensity of Ponceau S staining. (c) P-gp function. HL-1 cells were treated with different doxorubicin-concentration for 48 h. P-gp function was studied by measuring intracellular accumulation of 3H-labeled daunorubicin after 30 min of incubation in the presence (dark gray columns) and absence (light gray columns) of the P-gp inhibitor cyclosporin A (100 µmol/L, dark gray columns). Data were presented as mean ± SEM (n = 3; statistic: two-way analysis of variance followed by the Bonferroni posttest).
In addition to HL-1 cells, the effect of doxorubicin on P-gp expression was also examined in primary adult rat cardiomyocytes as a less artificial in vitro model. Like in HL-1 cells, P-gp expression was also induced by doxorubicin as demonstrated by mRNA expression studies (Fig. 4a) and immunofluorescence staining (Figs. 4b and 4c). However, these cells seem to be more sensitive to doxorubicin than HL-1 cells.
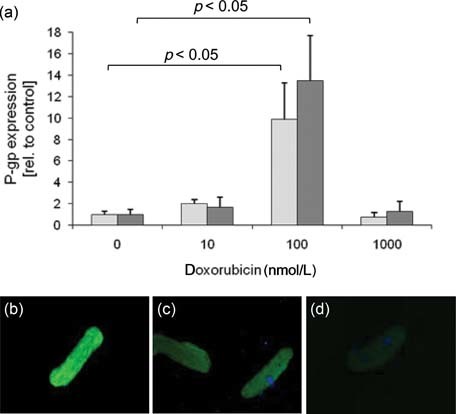
P-gp expression in rat cardiomyocytes. (a) Primary adult rat cardiomyocytes were treated with different doxorubicin concentrations for 24 h (light gray columns) and 48 h (dark gray columns). P-gp mRNA transcripts were measured by real-time polymerase chain reaction and results were normalized to 18S rRNA expression (mean ± SEM; n = 4 independent preparations, statistic: Student's t-test). (b–d) P-gp immunofluorescence staining (JSB-1 antibody) on rat cardiomyocytes treated with doxorubicin (0.1 µmol/L) (b) or control 48 h (c), [(d) secondary antibody control].
DISCUSSION
Doxorubicin-induced cardiotoxicity is a severe problem in anticancer therapy and characterized by left ventricular dysfunction with impaired systolic and diastolic function.27 Although the underlying molecular mechanisms are still a topic of intense research, there is evidence that parameters affecting cardiac bioavailability of doxorubicin are important risk factors for cardiotoxicity.28 In this context, uptake and efflux transporters for doxorubicin may modulate cardiac concentrations. Concerning uptake transporter, in vitro results indicate a possible role of OCT6 and OCTN1 for doxorubicin transport, but data on their role for cardiac uptake are missing.29,30 In contrast, efflux transporters of the ABC transporter family have already been shown to affect pharmacokinetics of their substrates and are present and functionally active in the heart.31,32 Among them MRP1 and P-gp are capable to transport doxorubicin, thereby possibly affecting cardiac drug concentrations.9,10 Therefore, it was not surprising that cardiac MRP1 expression and function have been associated with doxorubicin-induced cardiomyopathy.18,33 These findings were underlined by a human study indicating an association between a MRP1 polymorphism (Gly671Val) and acute anthracycline-induced cardiotoxicity.34 In contrast to MRP1, the effect of doxorubicin on cardiac P-gp expression has not been investigated so far.
An acute anthracycline-dependent cardiomyopathy was induced in a murine model by application of a single dose of doxorubicin.22 mRNA expression of both murine transcripts, abcb1a and abcb1b, was determined by quantitative real-time PCR. Here, a significant increase in the expression could be observed, this finding could be verified on protein level by western blot and immunofluorescence analysis. It is very likely that this effect is directly caused by doxorubicin and not by the doxorubicin-induced development of cardiomyopathy itself because P-gp expression levels were reduced in other forms of cardiomyopathy.14 Moreover, short-term regulation of P-gp by doxorubicin has already been demonstrated in tumor cell lines.35,36 The underlying molecular mechanism has not been investigated in further detail, but there is evidence for an interaction of doxorubicin and the abcb1 promoter.37 A recent study investigating the doxorubicin-mediated P-gp induction in leukemic cells indicated an involvement of the transcription factor FOXO2a.38 This forkhead box protein is also expressed in cardiomyocytes and there is evidence for an activation by ROS-dependent mechanisms.39 Another explanation for our finding may be an interaction of doxorubicin with nuclear receptors. Recently, a study identified doxorubicin as an agonist for the aryl hydrocarbon receptor.40
In addition to the in vivo results, we could show a doxorubicin-dependent upregulation of abcb1a and abcb1b in the cardiomyocyte-like murine HL-1 cell model on mRNA and protein level. These results could be verified in primary adult rat cardiomyocytes, excluding a cell line-specific effect. Interestingly, the primary cardiomyocytes seem to be much more sensitive to doxorubicin because much lower drug concentrations were necessary to achieve a comparable effect. Compared with the in vivo results, the mRNA induction was more pronounced in cell culture, which could be explained by general differences between “cells” and “tissue” as well as pharmacokinetic processes. Furthermore, there was a different response of abcb1a and abcb1b to doxorubicin treatment in vivo and in vitro. This finding may be explained by different cardiac cell types such as cardiomyocytes and endothelial cells compared with cell culture.
The functional relevance of this finding was demonstrated by a daunorubicin accumulation assay. Whereby, we could show that in HL-1 cells, the cyclosporin A-sensitive accumulation of the P-gp substrate daunorubicin was enhanced after pretreatment with doxorubicin. Interestingly, this effect was reduced at high doxorubicin concentrations, which may be explained by a competitive P-gp inhibition through intracellular doxorubicin. In general, this finding was in line with a previous study on rat cardiomyocytes demonstrating an enhanced intracellular daunorubicin accumulation in the presence of P-gp inhibitors such as verapamil or PSC833.15 In addition, animal studies on knockout mice support the suggestion that cardiac P-gp expression affects local drug concentrations. For example, van Asperen et al.21 could demonstrate that intravenous application of doxorubicin to abcb1a knockout mice resulted in prolonged presence of doxorubicin in heart compared with wild-type animals. Moreover, there is evidence that P-gp inhibition results in enhanced cardiotoxicity of doxorubicin because in mice, coadministration of the P-gp inhibitor verapamil and doxorubicin resulted in a lower survival rate than doxorubicin alone.19
Taken together, these data indicate a protective role of P-gp not only in physiological barriers such as the blood–brain barrier or the intestinal wall but also in target compartments such as the heart. Here, several findings indicate that P-gp may limit the uptake of the doxorubicin, an anticancer drug associated with severe cardiac site effects. A lack of P-gp expression and induction during doxorubicin treatment may enhance the individual susceptibility to anthracycline-induced cardiotoxicity.
Acknowledgements
The authors acknowledge the excellent technical assistance of Tina Sonnenberger (Department of Pharmacology, University of Greifswald, Greifswald, Germany). The work was supported in part by a grant from the Deutsche Forschungsgemeinschaft, Bonn, Germany (grant #SFB-TR19), and by the German Federal Ministry for Education and Research [grant #03IP612 (Innoprofile)].




