Characterization of human OCT1-mediated transport of DAPI as a fluorescent probe substrate
Abstract
The present study was conducted to assess the functional characteristics of human organic cation transporter 1 (hOCT1) for the transport of 4′,6-diamidino-2-phenylindol (DAPI), a fluorescent compound that may be used as a probe substrate for rapid assays of its functionality. The specific uptake of DAPI by hOCT1 heterologously introduced into Madin–Darby canine kidney II cells by stable transfection was found to be, when assessed by DAPI-derived fluorescence intensity, rapid and saturable with a Michaelis constant of 8.94 µM, indicating that DAPI is a good substrate of hOCT1. The specific uptake of DAPI was insensitive to the membrane potential and extracellular pH, indicating a mode of operation different from that for typical cationic substrates such as tetraethylammonium (TEA), for which hOCT1 has been suggested to be driven by an inside-negative membrane potential and favor higher pH for optimal operation. However, many organic cations were found to inhibit the specific DAPI uptake with extents well correlated with those of inhibition of the specific uptake of [14C]TEA, indicating comparable performances of both substrates as probes in identifying inhibitors. Thus, DAPI can be an alternative probe substrate that enables fluorometric rapid assays of the functionality of hOCT1. © 2011 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 100:4006–4012, 2011
INTRODUCTION
Organic cation transporter (OCT)1/SLC22A1 is a liver-specific member of OCTs and is suggested to be involved in the hepatic uptake of various cationic compounds.1,2 Owing to its polyspecific nature, evaluations of its functionality, including those for identifying its substrates and inhibitors, have been of great interest in various aspects of drug development and drug therapy.3-6 For such evaluations, simple and efficient methods are preferred generally and especially when many compounds are enrolled to be tested, for example, in drug development. It is expected that the use of a fluorescent probe substrate, if available, can help that because the fluorometric method using a plate reader is easier and faster than ordinary methods such as high-performance liquid chromatography (HPLC) analysis and liquid scintillation counting analysis of a radiolabeled probe compound.
We had recently reported that 4′,6-diamidino-2-phenylindol (DAPI) can be used as a fluorescent probe substrate for rapid assays of the functionality of human multidrug and toxin extrusion protein 1 (hMATE1/SLC47A1) and hMATE2-K/SLC47A2.7 It was also suggested that human organic cation transporter 1 (hOCT1) can be another candidate for which DAPI is effective as a probe substrate. DAPI, which is widely used for nuclear staining, is unique as a fluorescent probe in that it can emit fluorescence only when intercalated, with a high capacity of irreversible binding characteristic, into DNA double strands.8 This characteristic enables it to be retained at high levels in the cells after transport and, hence, it is advantageous in fluorometric detection over 4-(4-(dimethylamino)-styryl)-N-methylpyridinium,9 [2-(4-nitro-2,1,3-benzoxadiazol-7-yl)aminoethyl]trimethylammonium,10 and berberine,11 which are ordinary fluorescent agents transported by hOCT1. It is also notable that DAPI enables real-time visualization of its uptake process because it does not emit fluorescence in the extracellular medium.
The present study was therefore conducted to demonstrate that DAPI can be used to assess the functionality of hOCT1, characterizing the hOCT1-mediated transport of DAPI in detail and comparing it with that of tetraethylammonium (TEA), which is available in 14C-labeled form as a typical organic cation.
MATERIALS AND METHODS
Materials
[14C]TEA bromide (55.0 Ci/mmol) was obtained from GE Healthcare (Little Chalfont, Buckinghamshire, UK) and DAPI was purchased from Sigma–Aldrich (St. Louis, Missouri). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Invitrogen (Carlsbad, California). All other reagents were of analytical grade and commercially obtained.
Preparation of Madin–Darby Canine Kidney II Cells Stably Expressing hOCT1
Madin–Darby canine kidney (MDCK) II cells were transfected with the plasmid carrying the complementary DNA of hOCT1, which was prepared in our previous study,7 by using Lipofectamine 2000 (Invitrogen) as a transfection reagent, according to the manufacturer's instructions, and cultured in DMEM supplemented with 10% FBS and 400 µg/mL G418 for 2 to 3 weeks. Antibiotic-resistant clones were selected and tested for the transport of DAPI as a probe substrate.
Uptake Study in MDCKII Cells Stably Expressing hOCT1
For the assays of DAPI uptake, MDCKII cells stably expressing hOCT1 (0.5 × 105 cells/well initially) were grown on 96-well plates for 48 h at 37°C and 5% CO2 in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. The cells were used without polarization. In regular uptake assays, the cells in each well were preincubated in 0.25 mL of substrate-free uptake buffer (5.36 mM KCl, 0.441 mM KH2PO4, 0.812 mM MgSO4·7H2O, 136.7 mM NaCl, 0.385 mM Na2HPO4·12H2O, 0.952 mM CaCl2, 25 mM d-glucose, and 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), pH 8.5) for 20 min. In uptake assays at a low extracellular pH of 6.0, 10 mM HEPES was replaced with 10 mM 2-(N-morpholino)ethanesulfonic acid. Uptake assays were started by replacing the substrate-free uptake buffer for preincubation with uptake buffer containing DAPI (0.1 mL). All the procedures were conducted at 37°C. Assays were stopped by addition of ice-cold substrate-free uptake buffer (0.25 mL) and the cells were washed two times with 0.25 mL of the same buffer. Then, each well was filled with 0.25 mL of ice-cold substrate-free uptake buffer and the intensity of fluorescence from DAPI was measured with a fluorescence plate reader, using the wavelengths of 360 nm for excitation and 460 nm for emission, for the evaluation of uptake. Fluorescence intensity was measured in arbitrary units and it is indicated to be FI in data presentation.
For the assays of [14C]TEA uptake, MDCKII cells stably expressing hOCT1 (1.5 × 105 cells/well initially) were grown on 24-well plates for 72 h. The cells in each well were preincubated in 1 mL of substrate-free uptake buffer for 20 min and uptake assays were started by replacing the substrate-free uptake buffer for preincubation with uptake buffer containing [14C]TEA (0.25 mL). All the procedures were conducted at 37°C. Assays were stopped by addition of ice-cold substrate-free uptake buffer (2 mL) and the cells were washed two times with 2 mL of the same buffer. The cells were solubilized in 0.5 mL of 0.2 M NaOH solution containing 0.5% sodium dodecyl sulphate at room temperature for 1 h and the associated radioactivity was measured by liquid scintillation counting, using 3 mL of Clear-sol I (Nakarai Tesque, Kyoto, Japan) as a scintillation fluid, for the evaluation of uptake.
Cellular protein content was determined by the method of Lowry et al.,12 using bovine serum albumin as the standard. Uptake assays were also conducted in mock cells, which were transfected with empty pCI-neo vector, to estimate nonspecific uptake.
Data Analysis
The specific uptake by hOCT1 was estimated by subtracting the uptake in mock cells from that in the hOCT1-transfected cells. The saturable transport was analyzed by assuming Michaelis–Menten type carrier-mediated transport represented by the following equation: v = Vmax × s/(Km + s). The maximum transport rate (Vmax) and the Michaelis constant (Km) were estimated by fitting this equation to the experimental profile of the uptake rate (v) versus the substrate concentration (s), using a nonlinear least-squares regression analysis program, WinNonlin (Pharsight, Mountain View, California), and the reciprocal of variance as the weight. The parameters are presented as the computer-fitted ones with standard error (SE).
Experimental data are presented as the means ± SE. Statistical analysis was performed using analysis of variance followed by Dunnett's test, with a p value of less than 0.05 was considered significant.
RESULTS AND DISCUSSION
Time Course of DAPI Uptake
The uptake of DAPI (1 µM) was greater in MDCKII cells stably expressing hOCT1 than in mock cells (Fig. 1), consistent with our earlier finding in human embryonic kidney 293 cells transiently expressing hOCT17 and indicating its efficient uptake by hOCT1. The uptake in mock cells remained minimal throughout the experimental period of 60 min. Because DAPI uptake increased in proportion to time up to 20 min in cells expressing hOCT1, we set the uptake period to be 20 min in subsequent experiments to evaluate hOCT1-mediated transport across the plasma membrane within the initial uptake phase. Although DAPI can emit fluorescence only after intercalation into DNA double strands, it can be assumed that the fluorometrically evaluated uptake process would mainly represent transport across the plasma membrane as the primary barrier in the whole process including the subsequent intracellular distribution and binding to DNA. As we discussed previously,7 such intracellular events could occur swiftly and the binding capacity of DNA for DAPI could be high enough.
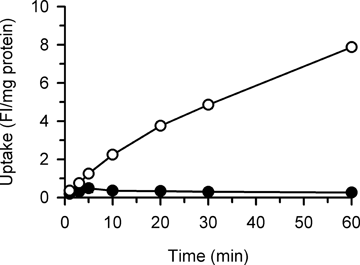
Time courses of DAPI uptake in MDCKII cells stably expressing hOCT1 (○) and mock cells (•). The uptake of DAPI (1 µM) was evaluated at 37°C and pH 8.5. Data are presented as means ± SE (n = 4). Error bars are mostly invisible as they are small compared to symbols.
Functional Characteristics of hOCT1 for DAPI Transport
The specific uptake of DAPI by hOCT1 was saturable, conforming to Michaelis–Menten kinetics with a Km of 8.94 µM (Fig. 2a). It was much smaller than that of 1.75 mM for TEA as a typical organic cation (Fig. 2b), indicating that hOCT1 has a higher affinity for DAPI than for TEA.
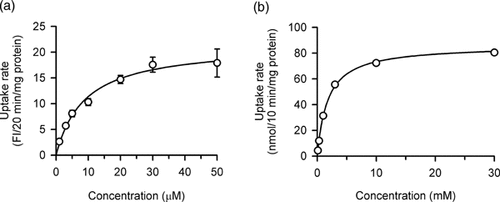
Concentration dependence of the uptakes of (a) DAPI and (b) TEA by hOCT1 stably expressed in MDCKII cells. The specific uptake rate was evaluated at 37°C and pH 8.5 for 20 min (DAPI) or 10 min ([14C]TEA). Solid lines represent the computer-fitted profiles. The Km and Vmax are 8.94 ± 1.26 µM and 21.5 ± 1.0 FI/20 min/mg protein, respectively, for DAPI, and 1.75 ± 0.07 mM and 86.1 ± 0.7 nmol/10 min/mg protein, respectively, for TEA, as the computer-fitted parameters with SE. Data are presented as means ± SE. (n = 4). Error bars are mostly invisible as they are small compared to symbols.
hOCT1 has been characterized as a membrane potential-dependent transporter, as reported for TEA13 and MPP.2 This transporter has also been reported to favor higher extracellular pH for TEA transport.14 We, therefore, examined these aspects with regard to DAPI transport. The concentrations of DAPI (1 µM) and TEA (3 µM) in this and all the subsequent sets of experiments were set to be much smaller than the respective Km values for these compounds, to have their hOCT1-mediated uptakes most efficient and sensitive to various treatments and inhibitors. Interestingly, the depolarization of the plasma membrane by replacement of NaCl with KCl was found to have no impact on the specific uptake of DAPI by hOCT1 (Fig. 3a), in contrast to an extensive reduction in the specific uptake of TEA (Fig. 3b). However, specific DAPI uptake was found to be reduced by elimination of Cl−, by replacement of NaCl with Na gluconate, and further by elimination of Na+ by replacement of NaCl with K gluconate or iso-osmotic mannitol. Specific TEA uptake was found to be reduced similarly by the same replacements. These results suggest that hOCT1 requires Na+ and Cl− for its optimal operation. The membrane potential seems to be additionally involved in TEA transport because the effect of the replacement of Na+ with K+ was greater for TEA transport than for DAPI transport.
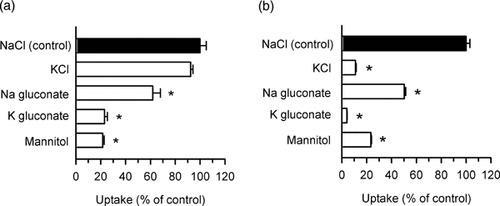
Effect of extracellular ions on the uptakes of (a) DAPI and (b) TEA by hOCT1 stably expressed in MDCKII cells. The specific uptake was evaluated at 37°C and pH 8.5 for 20 min (DAPI, 1 µM) or 10 min ([14C]TEA, 3 µM). NaCl was iso-osmotically replaced as indicated. The control values were 3.20 FI/mg protein and 132 pmol/mg protein, respectively, for DAPI and TEA. Data are presented as means ± SE (n = 4);*p < 0.05, significantly different from the control.
The effect of extracellular pH on hOCT1-specific DAPI uptake was found to be modest, being almost constant particularly in the range of pH 7.0–8.5 (Fig. 4a). In contrast to this, hOCT1-specific TEA uptake increased remarkably with an increase in pH (Fig. 4b), in agreement with the previously reported characteristic.14
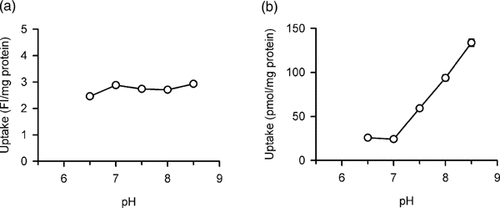
Effect of extracellular pH on the uptakes of (a) DAPI and (b) TEA by hOCT1 stably expressed in MDCKII cells. The specific uptake was evaluated at 37°C for 20 min (DAPI, 1 µM) or 10 min ([14C]TEA, 3 µM). Data are presented as means ± SE (n = 4). Error bars are mostly invisible as they are small compared to symbols.
Thus, the mode of hOCT1 operation for DAPI was suggested to be different from that for TEA in terms of dependences on the membrane potential and extracellular pH. Although the mechanism behind it is unclear, the ionization status does not seem to be a factor involved in the difference because DAPI (pKa 11.65)7 and TEA (pKa 11)15 are both presumed to be almost completely dissociated in the entire pH range. Also for unknown mechanisms, similar cases have been reported for several other transporters such as hMATE1, hMATE2-K, rat MATE1 (rMATE1), human novel organic cation transporter 2 (hOCTN2/SLC22A5), and human H+/sialate cotransporter (hSIALIN/SLC17A5). Although MATEs act as H+ antiporters for organic cations such as TEA and cimetidine,16-19 they do not require countering H+ for the transport of DAPI7 and fluoroquinolones.20 hOCTN2, which is a Na+-dependent carnitine transporter, can also operate as an organic cation/H+ antiporter.21,22 hSIALIN, which is a lysosomal H+-coupled sialate transporter, can transport aspartate and glutamate in a facilitative mode.23
Effect of Various Compounds on hOCT1-Specific DAPI Uptake
Although a difference was indicated between the modes of hOCT1-mediated transport for DAPI and TEA, many cationic compounds were found to be similarly effective as inhibitors for the hOCT1-specific uptake of DAPI as well as that of TEA (Table 1) and the extents of inhibition were in good correlation, close to a 1:1 relation between them (Fig. 5). Therefore, DAPI may share the substrate recognition site with organic cations despite the difference in the mode of transporting operation, and can be an alternative probe substrate that enables fluorometric rapid assays of the functionality of hOCT1.
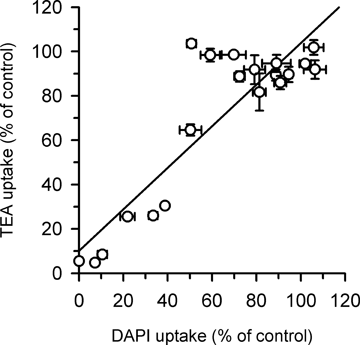
Correlation between the uptakes of DAPI and TEA by hOCT1 stably expressed in MDCKII cells. Data shown in Table 1 were examined for correlation. The regression equation is as follows: y = 0.937x + 10.1 with r2 = 0.782, where x and y represent DAPI uptake and [14C]TEA uptake, respectively.
| Uptake (% of Control) | ||
|---|---|---|
| Compound | DAPI | TEA |
| None (control) | 100.0 ± 4.0 | 100.0 ± 3.4 |
| Desipramine | Not detectable | 5.5 ± 0.3* |
| Verapamil | 7.2 ± 1.0* | 4.7 ± 0.1* |
| Diphenhydramine | 10.6 ± 2.1* | 8.4 ± 0.2* |
| Propranolol | 21.9 ± 3.3* | 25.5 ± 0.4* |
| Corticosterone | 33.4 ± 2.0* | 26.0 ± 0.6* |
| Trimethoprim | 38.8 ± 1.4* | 30.4 ± 0.2* |
| Colchicine | 50.3 ± 4.9* | 64.6 ± 2.5* |
| Chloramphenicol | 50.7 ± 2.1* | 103.5 ± 0.5 |
| Glibenclamide | 59.2 ± 4.3* | 98.3 ± 2.9 |
| Hemicholinium-3 | 69.8 ± 5.5* | 98.5 ± 1.2 |
| Fluconazole | 72.3 ± 2.4* | 88.7 ± 2.3 |
| Pyridoxine | 79.2 ± 4.8* | 91.8 ± 6.5 |
| Phenytoin | 81.3 ± 2.9* | 81.7 ± 8.3* |
| Nicotinate | 88.7 ± 1.3 | 89.4 ± 2.5 |
| Catechin | 89.0 ± 6.5 | 94.6 ± 3.9 |
| Diaminobiotin | 90.7 ± 2.7 | 86.0 ± 3.0* |
| GABA | 94.6 ± 1.7 | 89.6 ± 3.4 |
| Benzylpenicillin | 102.0 ± 2.6 | 94.5 ± 0.9 |
| NMN | 105.7 ± 4.4 | 101.8 ± 3.3 |
| Nicotinamide | 106.4 ± 5.1 | 91.8 ± 4.2 |
- GABA, γ-aminobutyrate; NMN, N-methylnicotinamide.
- The specific uptake was evaluated at 37°C and pH 8.5 for 20 min (DAPI, 1 µM) or 10 min ([14C]TEA, 3 µM) in the presence of a test compound (0.2 mM) or in its absence. The control values were 3.20 FI/mg protein and 132 pmol/mg protein, respectively, for DAPI and TEA. Data are presented as means ± SE (n = 3–4).
- *p < 0.05, significantly different from the control.
We finally used the DAPI uptake assay to assess the effect of a wide variety of compounds on hOCT1 (Table 2). As expected, various types of cationic compounds were found to inhibit hOCT1-specific DAPI uptake, indicating that they are recognized as substrates or inhibitors. Among them, neurotransmitter monoamines such as dopamine, serotonin, and norepinephrine would be of particular interest. Endogenous steroids such as progesterone, cortisone, and hydrocortisone, in addition to corticosterone, which was included in the selected compounds for the initial assessment (Table 1), were also suggested to be recognized by hOCT1 with fairly high affinities, even though these steroids are neutral compounds. hOCT1 may play physiological roles in the disposition of those monoamines and steroids. It is also notable that several flavonoids such as quercetin and kaempferol were found to be potent inhibitors, although they are neutral compounds, and a few anionic compounds such as sulfasalazine and estrone-3-sulfate were suggested be recognized, although weakly.
| Compound | Uptake (% of Control) | Compound | Uptake (% of Control) |
|---|---|---|---|
| None (control) | 100.0 ± 4.0 | α-Hydroxybenzamide | 78.5 ± 2.9* |
| Progesterone | 2.7 ± 1.6* | Azathioprine | 79.5 ± 1.6* |
| Quercetin | 2.8 ± 0.2* | Thymidine | 79.7 ± 4.8* |
| Dopamine | 5.1 ± 0.5* | Phenobarbital | 80.4 ± 6.1* |
| Disopyramide | 7.0 ± 1.0* | Cimetidine | 83.4 ± 0.9* |
| Diltiazem | 7.9 ± 1.7* | Histamine | 83.5 ± 2.7* |
| Perphenazine | 8.1 ± 1.4* | Sulfamethoxazole | 86.7 ± 2.2 |
| Propantheline | 9.8 ± 1.5* | Caffeine | 88.2 ± 8.9 |
| Serotonin | 16.8 ± 1.8* | Dithioerythritol | 88.7 ± 2.0 |
| Norepinephrine | 25.7 ± 2.3* | l-3-Methoxytyrosine | 89.1 ± 4.0 |
| Metoclopramide | 25.8 ± 1.0* | Furosemide | 90.1 ± 5.1 |
| Berberine | 28.8 ± 6.5* | Tranexamate | 91.2 ± 3.8 |
| Clonidine | 34.0 ± 2.1* | TPP | 93.1 ± 4.6 |
| Kaempferol | 35.3 ± 1.0* | 5-Aminovalerate | 93.4 ± 2.1 |
| Cortisone | 35.6 ± 0.8* | Sulfinpyrazone | 93.9 ± 2.5 |
| Omeprazole | 35.9 ± 1.8* | Ethosuximide | 94.2 ± 2.5 |
| Pindolol | 37.6 ± 1.4* | Cyclosporine A | 94.5 ± 4.7 |
| Rutin | 42.7 ± 2.3* | Tolbutamide | 96.1 ± 2.3 |
| Hydrocortisone | 50.0 ± 1.4* | Cephradine | 97.1 ± 3.2 |
| MPP | 54.3 ± 3.5* | Allopurinol | 97.4 ± 6.7 |
| Carbamazepine | 56.2 ± 4.0* | PABA | 98.2 ± 8.5 |
| Naringenin | 58.7 ±1.1* | Valproate | 98.8 ± 5.0 |
| NBMPR | 59.1 ± 2.5* | Guanosine | 99.3 ± 2.6 |
| Procainamide | 58.9 ± 1.7* | Taurocholate | 100.0 ± 2.0 |
| BSP | 60.0 ± 4.3* | Biotin | 101.2 ± 7.4 |
| Lidocaine | 60.4 ± 3.5* | N-Ethylmaleimide | 101.8 ± 2.4 |
| Prednisolone | 63.7 ± 1.6* | TEA | 103.9 ± 3.9 |
| Phloridzin | 66.9 ± 3.8* | Acyclovir | 104.2 ± 3.9 |
| Sulfasalazine | 67.7 ± 6.0* | Imidazole | 104.5 ± 1.1 |
| Primidone | 68.3 ± 1.7* | Pyridoxal 5′-phosphate | 104.8 ± 1.2 |
| Ouabain | 74.3 ± 1.5* | Theophylline | 105.3 ± 6.1 |
| Uridine | 74.8 ± 3.9* | Tolbutamide | 110.0 ± 1.9 |
| Estrone-3-sulfate | 75.6 ± 4.3* | l-Carnitine | 114.9 ± 5.7 |
| Mefenamate | 77.1 ± 7.4* | PAH | 121.4 ± 3.6* |
| Guanidine | 79.3 ± 0.5* | TMA | 127.6 ± 2.5* |
| d-Desthiobiotin | 77.8 ± 2.0* | Spermine | 140.6 ± 5.2* |
- MPP, 1-methyl-4-phenylpyridinium; NBMPR, nitrobenzylthioinosine; BSP, sulfobromophthalein; TPP, thiamine pyrophosphate; PABA, p-aminobenzoate; PAH, p-aminohippurate; TMA, tetramethylammonium.
- The specific uptake of DAPI (1 µM) was evaluated at 37°C and pH 8.5 for 20 min in the presence of a test compound (0.2 mM) or in its absence. The control value was 3.20 FI/mg protein. Data are presented as means ± SE (n = 4).
- *p < 0.05, significantly different from the control.
The DAPI uptake assay is thus simple and efficient in identifying potential substrates or inhibitors, but not without limitations. Fluorescence from a tested compound is a factor that may be needed to be taken into consideration. Some of the hOCT1 substrates that can emit fluorescence might accumulate in hOCT1-expressing cells to extents that they add fluorescence to DAPI-derived one, affecting the assay results. That possibility can be examined separately in the absence of DAPI. A test compound identified to be so can at least be a substrate of hOCT1 and, hence, may not necessarily be tested by the DAPI uptake assay. Estimating the intensity of fluorescence from the test compound in the presence of DAPI, which could be different from that in its absence due to potential alteration in the hOCT1-mediated uptake of the test compound by DAPI, is not possible, thus making it not possible to estimate the DAPI-derived fluorescence intensity corrected for the test compound-derived one. All the compounds tested in the present study were examined for the possibility and only dipyridamole was identified as a compound having that problem and was excluded from the test compounds. Not many compounds can emit fluorescence and only a few of hOCT1 substrates among them seem to accumulate in hOCT1-expressing cells to emit fluorescence at levels not negligible. Reduction of DAPI-derived fluorescence due to the interference of a tested compound with the intercalation of DAPI into DNA is another factor that may affect the assay result. There also may be some test compounds that can quench DAPI-derived fluorescence by interacting with DNA-bound DAPI and may similarly affect the assay results. Such possibilities can be examined in the cells permeabilized with digitonin,7 but it is not possible to evaluate the extents of reduction in DAPI-derived fluorescence by such mechanisms in intact hOCT1-expressing cells in which the uptakes of DAPI and test compounds are limited to varied extents, and to make any corrections to observed fluorescence intensities. However, for tested compounds to exert such effects, they need to enter the cells most likely by hOCT1-mediated transport. Therefore, the apparent inhibition of DAPI-derived fluorescence is still effective as an indication that the test compound may possibly be recognized as a substrate or inhibitor, although a possibility that non-hOCT1 pathways might be involved in its cellular entry cannot be fully excluded. The test compounds that may interfere with the DNA intercalation of DAPI include quercetin and dopamine.24,25 To ensure that the correlation of the inhibition of DAPI uptake with that of [14C]TEA uptake (Table 1 and Fig. 5) was not biased by such effects, we examined the effect of the compounds selected for that assessment on DAPI-derived fluorescence in digitonin-permeabilized cells and confirmed that they were without such kinds of problems.
In conclusion, we have successfully demonstrated that DAPI can be efficiently transported by hOCT1. Although the mode of hOCT1 operation for DAPI was suggested to be different from that for TEA, hOCT1-mediated DAPI transport was inhibited by many organic cations with extents well correlated with those of inhibition of hOCT1-mediated TEA transport, indicating comparable performances of both substrates as probes in identifying inhibitors. Taking advantage of its fluorescent nature, DAPI can be used as a probe substrate for rapid assays of the functionality of hOCT1.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (#21790155).




