Quantitative membrane protein expression at the blood–brain barrier of adult and younger cynomolgus monkeys
Abstract
Cynomolgus monkey has been used as a model for the prediction of drug disposition in human brain. The purpose of this study was to clarify protein expression levels of membrane proteins affecting drug distribution to brain, such as transporters, receptors, and junctional proteins, in cynomolgus monkey brain microvessels by using liquid chromatography tandem mass spectrometory. In adult monkeys, three ATP-binding cassette transporters (multidrug resistance 1 (MDR1), breast cancer resistance protein (BCRP), and multidrug resistance protein 4 (MRP4)), six solute carrier transporters (glucose transporter 1 (GLUT1), GLUT3/14, monocarboxylate transporter 1 (MCT1), MCT8, organic anion transporting polypeptide 1A2, and equilibrative nucleoside tranporter 1), two junctional proteins (claudin-5 and vascular endothelial cadherin), and two receptors (insulin receptor and low-density lipoprotein receptor-related protein 1) were detected. Comparison of the expression levels with those in mouse, which we reported previously, revealed a pronounced species difference. BCRP expression in monkey was greater by 3.52-fold than that in mouse, whereas MDR1 and MRP4 expression levels in monkey were lower by 0.304- and 0.180-fold, respectively, than that in mouse. This study also investigated the developmental changes in expression of membrane proteins in neonate and child monkeys. Expression of MDR1 was similar in neonate and adult monkeys, whereas in rat, P-glycoprotein expression was reported to be significantly lower in brain microvessels of neonate as compared with adult rat. These results will be helpful to understand and predict brain concentrations of drugs in different species and at different ages of primates. © 2011 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 100:3939–3950, 2011
INTRODUCTION
Cynomolgus monkey has been considered as a model animal for humans because monkeys are the second nearest animal to humans in the evolutionary tree, and consequently, it has been used for the prediction of drug disposition in human brain. Substrates for P-glycoprotein (P-gp), such as verapamil and GR205171, were reported to be distributed more extensively in monkey brain than in rat brain,1 and it was suggested that there are species differences in drug permeability across the blood–brain barrier (BBB) between primates and rodents. Such species differences would have an impact on the usefulness of animal studies to predict the brain distribution of drugs in humans, and this is considered to be a major cause of the low success rate of central nervous system (CNS)-acting drugs in clinical studies. At present, the molecular basis of species differences in the transport systems at the BBB is poorly understood.
Species differences in P-gp during brain development have also been reported. In rats, P-gp expression in neonate brain was much lower than in adult brain,2,3 whereas in human, immunoreactivity of P-gp was detected in microvessels of fetal brain and, in some cases, the immunoreactivity was comparable to that in adult brain.4 This implies that the BBB function and expression of membrane proteins during development are different between primates and rodents. Therefore, it is important to consider species differences in drug distribution in infant brain between primates and rodents during the animal testing stage of pediatric drug development.
There have been several reports on mRNA expression of ATP-binding cassette (ABC) transporters in human brain microvessels. Warren et al.5 found that mRNA expression (percent of glucose transporter 1 (GLUT1)) of multidrug resistance protein 4 (MRP4/ABCC4) in isolated brain microvessels was lower in human than in mouse, whereas no significant difference was reported in the case of multidrug resistance 1 (MDR1/mdr1a/ABCB1) mRNA expression. It was also reported that the relative mRNA expression of breast cancer resistance protein (BCRP/ABCG2) to MDR1/mdr1a/ABCB1 was higher in human brain microvessels than in rat brain microvessels.6,7 However, mRNA expression levels do not necessarily correlate with protein expression levels. Indeed, it was reported that the protein expression of MDR1 and BCRP was downregulated by activation of estrogen receptor α-related pathways without any change of mRNA expression.8,9 Therefore, it is necessary to determine protein expression levels, rather than mRNA, to characterize and understand the differences in transport systems that underlie species differences in drug permeability across the BBB.
Recently, we have developed multiplexed multiple reaction monitoring (MRM) analysis using liquid chromatography tandem mass spectrometory (LC–MS/MS) to quantify absolute protein expression amounts of membrane proteins, and we used this method to investigate the absolute protein expression levels of 34 transporters in mouse brain microvessels.10 The objectives of the present study were to clarify the protein expression levels of membrane proteins affecting drug distribution, such as transporters, receptors, and junctional proteins, in cynomolgus monkey brain microvessels by means of multiplexed MRM analysis and to compare the results with those that we previously reported for mouse,10 in order to clarify species difference between primate and rodent BBB. In addition, the expression amounts of membrane proteins in cynomolgus monkeys of different ages (neonate, child, and adult) were examined to clarify developmental changes in the expression of membrane proteins in monkey BBB.
MATERIALS AND METHODS
Reagents
All peptides listed in Supplementary Table 1 were purchased from Thermo Electron Corporation (Sedantrabe, Germany). Peptide purity (>95%) was provided by the manufacturer, using reversed-phase high-performance liquid chromatography with UV detection (RP-HPLC–UV, with a detection wavelength of 215 nm) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analyses. Other chemicals were commercial products of analytical grade.
| Monkey | Age | History of Drug Administration Experiment | Blood Removal by Perfusion Before Excising Brain | Fasting | Body Weight (kg) |
|---|---|---|---|---|---|
| Indonesian neonate monkey | 1 day | None | + | None | – |
| Indonesian child monkey | 16 months | None | + | None | 1.7 |
| Indonesian adult monkey | 4 years 3 months | None | + | None | 2.8 |
| Chinese adult monkey #1 | 3 years 6 months | Loperamide | None | + | 2.7 |
| Chinese adult monkey #2 | 4 years | Paclitaxel | None | + | 3.8 |
| Chinese adult monkey #3 | 4 years 1 month | Quinidine | None | + | 2.6 |
| Chinese adult monkey #4 | 4 years 3 months | Diazepam | None | + | 3.2 |
| Chinese adult monkey #5 | 5 years 2 months | Indinavir | None | + | 3.6 |
- One day and 16 months after birth were categorized in neonate and child monkey, according to Beck et al.11
Animals
Brains of cynomolgus monkeys, which had originated from Indonesia (Indonesian neonate, child, and adult monkeys), that is, one neonate (1 day after birth, male), one child (16 months after birth, male), and one adult (4 years 3 months after birth, male) were purchased from Shin Nippon Biomedical Laboratories, Ltd. (Kagoshima, Japan; Table 1). One-day-old and 16-month-old monkeys were categorized as neonate and child, respectively, according to the classification by Beck et al.11 This classification categorized 0–0.5-month-old monkey as neonate and 6–36-month-old monkey as child; these ages correspond to 0–28days old and 2–12 years old in human, respectively.11 All procedures for brain excision were performed by Shin Nippon Biomedical Laboratories, Ltd.; they were performed in accordance with the animal welfare bylaws of Drug Safety Research Laboratories, Shin Nippon Biomedical Laboratories, Ltd., and had been approved by the Institutional Animal Care and Use Committee. Briefly, the monkeys were anesthetized with pentobarbital (32.4 mg/kg weight for neonate monkey, 25.9 mg/kg weight for child and adult monkey) and perfused with saline containing sodium heparin to remove blood. After the perfusion, the brains were excised and frozen in liquid nitrogen. The isolated brains were stored at −80°C until shipping and shipped to Tohoku University with dry ice. The brains of five adult cynomolgus monkeys (3 years 6 months–5 years 2 months after birth, male), which had originated from China (Chinese adult monkeys), were kindly provided by Banyu Pharmaceutical Co., Ltd. (Tsukuba, Japan; Table 1). All animal care and experimental procedures for these monkeys were approved by the Animal Care and Use Committee of Banyu Tsukuba Research Institute. Brains of these monkeys were excised after drug administration for pharmacokinetics analysis, performed independently of the present study. These monkeys had been confirmed to be negative for herpes B virus, Shigella, Salmonella, tuberculosis, and gastrointestinal parasites; however, no information was available about other infection or disease. The monkeys had been fasted overnight with access to water at the completion of drug administration experiments and then killed by exsanguination under anesthesia with isoflurane inhalation. Immediately, the brains were excised and frozen in liquid nitrogen. The isolated brains were stored at −80°C until shipping and shipped to Tohoku University with dry ice. These monkeys had not been perfused with any solution. Of the five monkeys, four had received a test drug (loperamide, quinidine, diazepam, and indinavir, one monkey each) by bolus administration followed by constant-rate intravenous infusion and one monkey had received paclitaxel by constant-rate intravenous infusion without bolus administration.
Selection of Target Peptides
Target peptides for 51 proteins (10 ABC transporters, 35 solute carrier (SLC) transporters, three junctional proteins, two receptors, and one membrane marker) were selected using the in silico criteria reported previously (Supplementary Table 1).10 Target peptides for MDR1, MRP1, MRP2, Sodium-dependent neutral amino acid transporter type 2 (ASCT2), L-type amino acid transporter (LAT1), monocarboxylate transporter 1 (MCT1), organic anion transporter 3 (OAT3), organic anion transporting polypeptide1C1 (OATP1C1/OATP-F), and equilibrative nucleoside tranporter 1 (ENT1) were selected based on the amino acid sequences of the cynomolgus monkey proteins in the National Center for Biotechnology Information (NCBI) database. In addition, we predicted the BCRP amino acid sequence from the cynomolgus monkey brain BCRP complementary DNA sequence and used this sequence to select a suitable target peptide for cynomolgus monkey BCRP. Target peptides for other proteins were selected based on the sequences in rhesus monkeys because no information was available for cynomolgus monkeys. It seems likely that the amino acid sequences are very similar in cynomogus and rhesus monkeys because they both belong to Macaca.
Isolation of Brain Microvessels from Monkey
Brain microvessels were isolated by combination of dextran density gradient separation and size filtration according to Dauchy et al.6 with minor modifications. Briefly, brain stored at −80°C was thawed in running water and a brain block including white and gray matter was obtained by lengthwise cutting of the middle part of the half brain, including the lateral cerebral ventricle. The brain block was dissected into small pieces and homogenized with 20 up-and-down, unrotated strokes in five volumes of cold solution A (101 mM NaCl, 4.6 mM KCl, 5 mM CaCl2·2H2O2, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, 15 mM HEPES; pH 7.4) per brain weight. The homogenate was centrifuged at 1000 × g for 10 min, and the pellet was resuspended in the same volume of solution A. The suspension was added to the same volume of 35% dextran solution, and the mixture was centrifuged at 4500 × g for 15 min. The pellet was resuspended in solution B (25 mM NaHCO3, 10 mM glucose, 1 mM pyruvate, 5 g/L BSA, 101 mM NaCl, 4.6 mM KCl, 2.5 mM CaCl2·2H2O, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, 15 mM HEPES; pH 7.4), and the suspension was passed through 210, 85, and 20 μm nylon mesh. The brain microvessels retained by the 20 μm nylon mesh were immediately collected as brain microvessel-rich fraction and frozen at −80°C. Protein concentrations were measured by the Lowry method using detergent-compatible (DC) protein assay reagent (Bio-Rad, Hercules, California).
Quantitative Analysis of Multiple Membrane Proteins by Using LC–MS/MS
Quantification of membrane proteins was performed according to our previous report.10 Isolated brain microvessels (50 μg) were solubilized in 7 M guanidine hydrochloride, reduced with dithiothreitol, and alkylated with iodoacetamide. The alkylated protein was precipitated with methanol–chloroform and resolubilized in 1.5 M urea, 0.1 M Tris–HCl (pH 8.0). The resolubilized protein was digested with sequence grade-modified trypsin (Promega, Madison, Wisconsin) at a trypsin/protein ratio of 1:100 at 37°C for 16 h. The stable isotope-labeled peptide mixture (500 fmol for each labeled peptide) was spiked in tryptic digests (25–50 μg) as an internal standard. The tryptic digests were acidified with formic acid and injected into the high-performance liquid chromatography (HPLC) system (Agilent 1100 system; Agilent, Santa Clara, CA), which was connected to an ESI–triple quadrupole mass spectrometer (API5000; Applied Biosystems, Foster City, CA). HPLC was performed with C18 capillary columns (Waters XBridge™ BEH130 C18, 1.0 mm ID × 100 mm, 3.5 μm particles; Waters, Milford, MA). Linear gradients of 1–50% acetonitrile in 0.1% formic acid were applied to elute the peptides at a flow rate of 50 μL/min for 50 min. The mass spectrometer was set up to run a MRM experiment for peptide detection, using 10 dwell times per MRM transition.The ion counts in the chromatograms were determined by using the quantitation procedures in Analyst software version 1.5 (Applied Biosystems).
In the MRM analysis, each peptide for a target protein was monitored with four kinds of MRM transitions specific for each peptide. The quantitative value was calculated from the peak area ratio of analyte and stable isotope-labeled peptide in each MRM transition. When more than two among four MRM transitions gave no signal peak, the mean quantitative value was not determined. The value of quantification limit of each protein (fmol/μg protein) was determined according to Kawakami et al.12 First, the value of the quantification limit of each MRM transition was defined as the value giving a peak area of 5000 counts for each MRM transition, calculated by using the peak area of the internal standard peptide. Finally, the value of the quantification limit of each protein was determined as the minimum value among the transitions in which the signal peak was not detected.
Statistical Analysis
Student's t-test was used to determine the statistical significance of differences between two groups. One-way analysis of variance followed by Dunnett's test or Tukey's honestly significant difference test was used to determine the statistical significance in comparisons of more than two groups. A p value of less than 0.05 was considered as statistically significant.
RESULTS
Isolation of Brain Microvessels from Monkey Brain
The brain microvessels were isolated by means of a combination of dextran density separation and size filtration from the brain of neonate, child, and adult monkeys. As shown in Figure 1, microscopic analysis revealed that brain microvessels were predominantly present in the preparations obtained from the brain at each age. The diameter of the microvessels was 4–8 μm, and there was no obvious age-related difference in the characteristics of the microvessels.
Microscopic view of brain microvessels isolated from cynomolgus monkeys of various ages. (a) Indonesian neonate monkey (1 day after birth), (b) Indonesian child monkey (16 months after birth), (c) Indonesian adult monkey (4 years and 3 months after birth), 10× magnification, scale bar = 60 μm.
Quantitative Protein Expression of Membrane Proteins in Brain Microvessels of Adult Monkeys
Protein expression amounts of membrane proteins were investigated in adult monkeys. One monkey was from Indonesia (Indonesian adult monkey) and the others were from China (Chinese adult monkeys #1–5) (Table 1). The Indonesian adult monkey had not been used for any experiment, including drug administration, and was perfused with saline to remove blood before excision of the brain (Table 1). The brains of Chinese adult monkeys were excised after drug administration experiments without removal of the blood by perfusion (Table 1).
First, the expression levels of 51 membrane proteins were quantified in brain microvessels from two monkeys: the Indonesian adult monkey and Chinese adult monkey #1 (Table 2). Fourteen proteins were detected in the monkey brain microvessels, and the other proteins were under the limit of quantification (ULQ; Table 2). Then, the detected membrane proteins were quantified in brain microvessels from four other Chinese adult monkeys (Table 3).
| Gene Name | Alias(es) | Quantification Result | |
|---|---|---|---|
| ABC transporters | ABCA1 | ABC1 | ULQ (<0.271) |
| ABCB1 | MDR1 | Detected | |
| ABCC1 | MRP1 | ULQ (<0.100) | |
| ABCC2 | MRP2 | ULQ (<0.249) | |
| ABCC3 | MRP3 | ULQ (<0.371) | |
| ABCC4 | MRP4 | Detected | |
| ABCC5 | MRP5 | ULQ (<0.111) | |
| ABCC10 | MRP7 | ULQ (<0.144) | |
| ABCC11 | MRP8 | ULQ (<0.0603) | |
| ABCG2 | BCRP | Detected | |
| SLC transporters | SLC1A5 | ASCT2 | ULQ (<0.114) |
| SLC2A1 | GLUT1 | Detected | |
| SLC2A3/14a | GLUT3/14a | Detected | |
| SLC6A6 | TAUT | ULQ (<0.236) | |
| SLC7A5 | LAT1 | ULQ (<0.326) | |
| SLC16A1 | MCT1 | Detected | |
| SLC16A7 | MCT2 | ULQ (<0.415) | |
| SLC16A8 | MCT3 | ULQ (<0.289) | |
| SLC16A4 | MCT5 | ULQ (<0.753) | |
| SLC16A2 | MCT8 | Detected | |
| SLC22A1 | OCT1 | ULQ (<0.457) | |
| SLC22A2 | OCT2 | ULQ (<0.166) | |
| SLC22A3 | OCT3 | ULQ (<0.374) | |
| SLC22A6 | OAT1 | ULQ (<0.044) | |
| SLC22A7 | OAT2 | ULQ (<0.125) | |
| SLC22A8 | OAT3 | ULQ (<0.404) | |
| SLC22A11 | OAT4 | ULQ (<0.153) | |
| SLC22A12 | URAT1 | ULQ (<0.195) | |
| SLC22A13 | OAT10 | ULQ (<0.247) | |
| SLC22A14 | OCTL2 | ULQ (<1.01) | |
| SLC22A15 | FLIPT1 | ULQ (<0.657) | |
| SLC22A23 | SLC22A23 | ULQb | |
| SLCO1A2 | OATP1A2/OATP-A | Detected | |
| SLCO1B1 | OATP1B1/OATP-C | ULQ (<0.308) | |
| SLCO1B3 | OATP1B3/OATP8 | ULQ (<0.135) | |
| SLCO1C1 | OATP1C1/OATP-F | ULQ (<0.365) | |
| SLCO2A1 | OATP2A1/PGT | ULQ (<0.363) | |
| SLCO2B1 | OATP2B1/OATP-B | ULQ (<0.135) | |
| SLCO3A1 | OATP3A1/OATP-D | ULQ (<0.165) | |
| SLCO4A1 | OATP4A1/OATP-E | ULQ (<0.397) | |
| SLCO4C1 | OATP4C1/OATP-H | ULQ (<0.454) | |
| SLCO5A1 | OATP5A1/OATP-J | ULQ (<0.250) | |
| SLCO6A1 | OATP6A1/OATP-I | ULQ (<0.637) | |
| SLC29A1 | ENT1 | Detectedc | |
| SLC29A4 | PMAT | ULQ (<0.167) | |
| Tight-junction proteins | Claudin-5 | Detected | |
| Occludin | ULQ (<0.285) | ||
| Adherence-junction protein | VE-cadherin | Detected | |
| Receptors | LRP1 | Detected | |
| Insulin receptor | Detected | ||
| Membrane marker | Na+/K+ ATPase | Detected |
- Expression levels of membrane proteins were quantified in brain microvessels from 2 monkeys: Indonesian adult monkey and Chinese adult monkey #1.
- a GLUT3 and GLUT14 could not be distinguished by the peptide used in this study due to its high-amino acid sequence similarity.
- b Quantification limit could not be determined because quantification was performed without standard curves for expression screening.
- c ENT1 was detectable in Chinese adult monkey #1, but not in Indonesian adult monkey.
- ULQ, under the limit of quantification; a value in brackets following “ULQ” represents the value of the quantification limit (fmol/μg protein).
| Quantitative values (fmol/μg protein) | ||||||
|---|---|---|---|---|---|---|
| Chinese Adult Monkeys | ||||||
| Alias(es) | Indonesian Adult Monkey | #1 | #2 | #3 | #4 | #5 |
| MDR1 (Peptide 1) | 2.65 ± 0.12 | 4.03 ± 0.38 | 4.65 ± 0.31* | 5.15 ± 0.50* | 6.49 ± 0.83* | 5.30 ± 0.39* |
| MDR1 (Peptide 2) | Not determined | 4.82 ± 0.47 | 6.08 ± 0.47 | 6.28 ± 0.22 | 7.65 ± 0.27 | 6.38 ± 0.39 |
| BCRP (Peptide 1) | 14.1 ± 0.3 | 13.5 ± 0.4 | 12.5 ± 0.2 | 16.0 ± 0.6* | 13.7 ± 0.4 | 15.2 ± 0.3 |
| BCRP (Peptide 2) | Not determined | 13.0 ± 0.7 | 14.3 ± 1.1 | 14.0 ± 0.5 | 14.6 ± 1.0 | 16.2 ± 0.6 |
| MRP4 | 0.201 ± 0.041a | 0.297 ± 0.012* | 0.304 ± 0.010* | 0.316 ± 0.020* | 0.304 ± 0.013* | 0.295 ± 0.015* |
| OATP1A2/OATP-A | 0.719 ± 0.097 | 0.732 ± 0.083 | 0.780 ± 0.121 | 0.754 ± 0.200 | 0.695 ± 0.052 | 0.664 ± 0.072 |
| GLUT1 | 118 ± 7 | 104 ± 2 | 133 ± 6 | 166 ± 6* | 123 ± 4 | 130 ± 1 |
| GLUT3/14 | 1.54 ± 0.05 | 0.986 ± 0.068* | 1.39 ± 0.09 | 1.13 ± 0.08* | 1.07 ± 0.03* | 1.20 ± 0.09* |
| MCT1 | 1.15 ± 0.22 | 0.683 ± 0.118a | 1.30 ± 0.41a | ULQ (<0.674) | 0.481 ± 0.082a | 0.555 ± 0.043a |
| MCT8 | 1.48 ± 0.04 | 1.41 ± 0.08 | Not determined | 1.16 ± 0.05* | Not determined | Not determined |
| ENT1 | ULQ (<0.417) | 0.622 ± 0.016a | 0.516 ± 0.044a | ULQ (<0.967) | ULQ (<0.349) | 0.484 ± 0.062a |
| Claudin-5 | 4.01 ± 0.22 | 6.64 ± 0.44* | 6.75 ± 0.48* | 7.93 ± 0.83* | 8.08 ± 0.27* | 6.44 ± 0.59* |
| VE-cadherin | 4.02 ± 0.23 | 2.07 ± 0.21* | 2.53 ± 0.25* | 2.69 ± 0.17* | 3.33 ± 0.18 | 3.22 ± 0.27 |
| Insulin receptor | 1.84 ± 0.10 | 1.11 ± 0.14* | 1.70 ± 0.31 | 1.52 ± 0.17 | 1.52 ± 0.24 | 1.44 ± 0.19 |
| LRP1 | 1.30 ± 0.08 | 1.21 ± 0.09 | 1.25 ± 0.08 | 1.31 ± 0.05 | 1.33 ± 0.08 | 1.33 ± 0.09 |
| Na+/K+ ATPase | 30.0 ± 1.2 | 27.8 ± 1.5 | 50.6 ± 2.1* | 36.5 ± 1.3 | 33.4 ± 3.7 | 32.2 ± 1.3 |
- *Significant difference of the quantitative value compared with Indonesian adult monkey. One-way analysis of variance followed by Dunnett's test for each MRM transition was used to determine the statistical significance of differences in quantitative values between Indonesian and individual Chinese adult monkeys (p < 0.05).
- a The reliability of the calculated values was considered to be lower than for other detected molecules because only two among four channels gave detectable peaks over 5000 counts.
- Isolation and quantification of brain microvessels was performed twice for Indonesian adult monkey. One isolated brain microvessels sample was quantified once or twice depending on the molecules to be examined for Chinese adult monkey. The amount of each molecule was determined as the average of three to eight quantitative values from four different MRM transitions of one or two analyses with signal peaks over 5000 counts (mean ± S.E.M., n = 3–8 MRM transitions).
- ULQ, under the limit of quantification; a value in brackets following “ULQ” represents the value of the quantification limit (fmol/μg protein).
Among the detected transporters, GLUT1 showed the greatest protein expression (average of all monkeys: 129 ± 21 fmol/μg protein; Table 4). In the ABC transporter family, MDR1, MRP4, and BCRP were detected, and BCRP exhibited the greatest expression (average of all monkeys: 14.2 ± 1.3 fmol/μg protein based on Peptide 1; Table 4). Both MDR1 and BCRP were quantified with two different peptides in Chinese adult monkey. Student's t-test was performed to determine whether or not there was a statistically significant difference of quantitative values obtained from each MRM transition between the peptides. The quantified values of BCRP and MDR1 were not significantly different between peptides in any of the Chinese adult monkeys, except MDR1 in Chinese adult monkey #2, suggesting that trypsin digestion proceeded efficiently. Among organic ion transporters, only OATP1A2/OATP-A was detected (Table 4). GLUT3 and/or GLUT14, which could not be distinguished by the peptide used in this study due to its high-amino acid sequence similarity, was detected in monkey brain microvessels in addition to GLUT1 (Table 4). MCT8 and ENT1 were also detected (Table 4).
| Average Quantitative Valuea (fmol/μg protein) | |||
|---|---|---|---|
| Alias(es) | Chinese Adult Monkeys #1–5 | All Adult Monkeys | Expression Ratiob (Indonesian Adult/Chinese Adult Monkeys) |
| MDR1 (Peptide 1) | 5.12 ± 0.91 | 4.71 ± 1.30 | 0.518 |
| MDR1 (Peptide 2) | 6.24 ± 1.01 | –c | –c |
| BCRP (Peptide 1) | 14.2 ± 1.4 | 14.2 ± 1.3 | 0.994 |
| BCRP (Peptide 2) | 14.4 ± 1.2 | –c | –c |
| MRP4 | 0.303 ± 0.008 | 0.286 ± 0.042 | 0.663 |
| OATP1A2/OATP-A | 0.725 ± 0.046 | 0.724 ± 0.041 | 0.992 |
| GLUT1 | 131 ± 22 | 129 ± 21 | 0.899 |
| GLUT3/14 | 1.16 ± 0.15 | 1.22 ± 0.21 | 1.33 |
| MCT1 | 0.755 ± 0.373 | 0.834 ± 0.368 | 1.52 |
| MCT8 | 1.29 | 1.35 ± 0.17 | 1.15 |
| ENT1 | 0.541 ± 0.072 | –c | –c |
| Claudin-5 | 7.17 ± 0.77 | 6.64 ± 1.46 | 0.559 |
| VE-cadherin | 2.77 ± 0.52 | 2.98 ± 0.69 | 1.45 |
| Insulin receptor | 1.46 ± 0.22 | 1.52 ± 0.25 | 1.26 |
| LRP1 | 1.29 ± 0.05 | 1.29 ± 0.05 | 1.01 |
| Na+/K+ ATPase | 36.1 ± 8.7 | 35.1 ± 8.2 | 0.831 |
- a Average quantitative values of Chinese adult monkeys #1–5 and all adult monkeys were determined as the average of the quantitative values of Chinese adult monkeys #1–5 and the average of the quantitative values of all adult monkeys, respectively (mean ± S.D., n = 3–6 monkeys), except for MCT8 in Chinese adult monkeys (n = 2 monkeys).
- b Expression ratio was determined by dividing the quantitative value in Indonesian adult monkey by the average quantitative value in Chinese adult monkeys #1–5.
- c The expression ratio could not be calculated because the quantitative value in Indonesian adult monkey was under the limit of quantification or not determined.
Among junctional proteins, claudin-5 and vascular endothelial cadherin (VE-cadherin) were detected in brain microvessels of all monkeys, whereas occludin was ULQ (Table 3). Insulin receptor and low-density lipoprotein receptor-related protein 1 (LRP1) were also detected (Table 4). This is the first report to quantify tight-junction and adherence-related proteins, and receptors in tissues.
There were significant differences in the expression amounts of MDR1, MRP4, GLUT3/14, and claudin-5 between Indonesian adult monkey and at least four of the Chinese adult monkeys (Table 3).
Comparison of Protein Expression Levels of BBB Transporters Between Monkey and Mouse
To investigate species difference of transporter expression amount in the BBB, the expression amount in adult monkeys was compared with that previously reported in mouse (Table 5).10 Among ABC transporters, the expression level of MDR1 in the monkeys was 0.304-fold that of in mouse (0.171- and 0.331-fold for Indonesian and Chinese adult monkeys, respectively; Table 5). In contrast, the expression level of BCRP in the monkeys was 3.52-fold that of in mouse, measured using peptide 1 (Table 5). MRP4 in the monkeys was about 0.180-fold that of in mouse (0.126- and 0.191-fold in Indonesian and Chinese adult monkeys, respectively; Table 5). Among organic ion transporters, the expression level of OATP1A2/OATP-A in the monkeys was 0.343-fold that of in mouse Oatp1a4/Oatp2, the mouse homolog of OATP1A2/OATP-A (Table 5). OAT3, OATP1C1/OATP-F, ASCT2, LAT1, and taurine transporter (TAUT) were only detected in mouse in our previous report,10 and not in monkey in this study (Table 5). The possibility cannot be ruled out that the failure to detect expression of some of the above molecules was due to differences in amino acid sequence between cynomologus and rhesus monkeys because rhesus monkey sequences were used to design target peptide sequences when the cynomologus monkey sequences were unavailable. However, the target peptides for OAT3, OATP1C1/OATP-F, LAT1, and ASCT2 were designed based on the amino acid sequences in cynomolgus monkey, indicating that the expression amounts of these transporters were indeed ULQ.
| Expression Ratio to Mouseb | ||||
|---|---|---|---|---|
| Alias(es) (Monkey/Mouse) | Expression Amount in Mousea (fmol/μg protein) | Indonesian Adult Monkey | Chinese Adult Monkeys | All Adult Monkeys |
| MDR1/Mdr1a (Peptide 1) | 15.5 ± 0.84 | 0.171 | 0.331 | 0.304 |
| BCRP (Peptide 1) | 4.02 ± 0.29 | 3.51 | 3.53 | 3.52 |
| BCRP (Peptide 2) | 4.80 ± 0.15 | Not determined | 3.00 | –c |
| MRP4 | 1.59 ± 0.07 | 0.126 | 0.191 | 0.180 |
| OAT3 | 1.97 ± 0.07 | ULQ in monkey | ULQ in monkey | ULQ in monkey |
| OATP1A2/Oatp1a4 | 2.11 ± 0.12 | 0.341 | 0.344 | 0.343 |
| OATP1C1/Oatp1c1 | 2.41 ± 0.16 | ULQ in monkey | UL.Q. in monkey | ULQ in monkey |
| GLUT1 | 90.0 ± 2.87 | 1.31 | 1.46 | 1.43 |
| MCT1 | 23.7 ± 0.87 | 0.0485 | 0.0318 | 0.0352 |
| ASCT2 | 1.58 ± 0.13 | ULQ in monkey | ULQ in monkey | ULQ in monkey |
| LAT1 | 2.19 ± 0.09 | ULQ in monkey | ULQ in monkey | ULQ in monkey |
| TAUT | 3.81 ± 0.60 | ULQ in monkey | ULQ in monkey | ULQ in monkey |
| Na+/K+ ATPase | 39.4 ± 1.01 | 0.761 | 0.916 | 0.890 |
- a The expression amounts in mouse brain microvessels were taken from Kamiie et al.10
- b Expression ratio to mouse was determined by dividing the quantitative value in adult monkey brain microvessels by the quantitative value in mouse brain microvessels reported previously.10
- c The expression ratio could not be calculated because the quantitative value in Indonesian adult monkey was not determined.
- ULQ, under the limit of quantification.
Developmental Changes in Expression of Membrane Proteins at the BBB in Monkey
Developmental changes in expression of ABCA1, MDR1, MRP1, MRP4, BCRP, ASCT2, GLUT1, GLUT3/14, TAUT, LAT1, MCT1, OATP1C1/OATP-F, claudin-5, occuludin, VE-cadherin, and Na+/K+ ATPase were investigated in the neonate (1 day after birth; Table 1) and the child monkeys (16 months after birth; Table 1), and compared with that of Indonesian adult monkey because the neonate and child monkeys were Indonesian. The neonate and child monkeys had not been used for any experiment, such as drug administration, and were perfused with saline to remove blood before harvesting the brain, as had been done with the Indonesian adult monkey (Table 1).
Among ABC transporters, the expression level of MDR1 in adult was significantly lower than that in child (0.680-fold; Fig. 2a), whereas no significant difference was observed between neonate and adult. The expression of BCRP was significantly increased during development: that in adult was 2.30- and 1.77-fold greater than those in neonate and child, respectively (Fig. 2b). The expression of MRP4 was unaltered during development (Fig. 2c). Among nutrient transporters, the expression of GLUT1 also did not change during development (Fig. 2d), whereas those of GLUT3/14 and MCT1 were significantly decreased during development (Figs. 2e and 2f). The expression of claudin-5 in adult was significantly lower than those in neonate and child (Fig. 2g). The expression of VE-cadherin was significantly higher in neonate than that in child, whereas no significant difference was observed between neonate and adult (Fig. 2h). The expression of Na+/K+ ATPase in neonate was significantly lower than those in child and adult (Fig. 2i). ABCA1, MRP1, ASCT2, TAUT, LAT1, OATP1C1/OATP-F, and occuludin were not detected in brain microvessels of neonate and child.
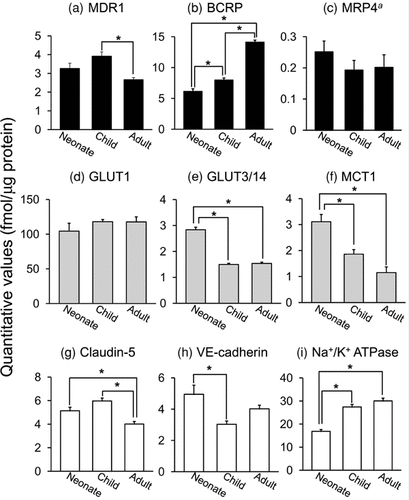
Developmental expression of membrane proteins in brain microvessels of cynomolgus monkey. Brain microvessels were isolated from brain of one Indonesian neonate monkey (1 day after birth), one child monkey (16 months after birth), and one adult monkey (4 years 3 months after birth). Protein quantification by LC–MS/MS was performed twice for each sample [(a) MDR1, (b) BCRP, (c) MRP4, (d) GLUT1, (e) GLUT3/14, (f) MCT1, (g) claudin-5, (h) VE-cadherin, (i) Na+/K+ ATPase]. The expression amount of each molecule was determined as the average of MRM transitions ( mean ± S.E.M., n = 4–8 from two to four different sets of MRM transition by two analyses). aThe reliability of the calculated values of MRP4 was considered to be lower than that for other detected molecules because only two among four channels gave the detectable peaks over 5000 counts in all ages. *Significant difference of the quantitative values between different ages. One-way analysis of variance followed by Tukey's honestly significant difference test was used to determine statistical significance (p < 0.05).
DISCUSSION
This is the first study to determine quantitatively the expression levels of membrane proteins in cynomolgus monkey brain microvessels, which constitute the BBB. Comparison with our previously reported results for mouse brain microvessels10 indicates that there is a pronounced species difference between monkey and mouse. BCRP expression in the monkey was higher than that in mouse, whereas MDR1 and MRP4 expression levels were lower than those in mouse (Table 5). OATP1A2/OATP-A expression in monkey was also lower than that of Oatp1a4/Oatp2 (Table 5), a mouse homolog of OATP1A2/OATP-A, in mouse. OAT3, OATP1C1/OATP-F, ASCT2, LAT1, and TAUT1 were not detected in monkey brain microvessels (Table 2), whereas these transporters or their homologs were detected in mouse in our previous report (Table 5). On the basis of the detection limits shown in Table 2, the protein expression of those transporters in monkey brain microvessels amounted to less than 20.5%, 15.1%, 7.22%, 14.9%, and 6.19% of those previously reported in mouse brain microvessels.10 These differences in protein expression presumably contribute to the differences in drug distribution to the brain and in the physiological function of the BBB between primates and rodents.
The lower MDR1 protein expression in monkey brain microvessels (Table 5) is consistent with a positron emission tomography (PET) study showing that the distribution of MDR1 substrates (brain to plasma ratio), including verapamil and GR205171, was 4.1- and 2.8-fold greater, respectively, in monkey than in rodents into cerebellum, where the receptor binding of GR205171 was negligible.1 It was also reported that the distribution of MDR1 substrates (brain to plasma ratio), such as Altanserin and GR205171, into cerebellum was 4.5- and 8.6-fold greater, respectively, in human than in rodent.1 Distribution into the cerebellum, where the levels of receptors for GR205171 or Altanserin were negligible, was evaluated to avoid the influence of receptor–ligand binding.1 In contrast, monkey brain microvessels showed higher BCRP expression than mouse brain microvessels (Table 5). Therefore, predictions of human brain distribution based on mouse studies could possibly underestimate the distribution into human brain for MDR1 substrates and overestimate it for BCRP substrates. The protein expression level influences the maximum transport rate (Vmax); however, the substrate transport capacity is related not only to Vmax but also to substrate affinity (Km). Hence, the species differences in drug distribution into the brain cannot be solely explained by the differences in transporter expression. Nevertheless, the present results suggest that the contribution of BCRP to drug permeability across the human BBB should be given greater consideration.
It was recently reported that the brain distribution of substrates transported by both P-gp and BCRP was dramatically increased in mdr1a/mdr1b/bcrp triple knockout mice compared with the increase observed in mdr1a/mdr1b or bcrp knockout mice, supporting a synergistic/cooperative effect of P-gp/BCRP at the BBB.13,14 Therefore, the impact of lower P-gp and higher BCRP expression in monkey brain microvessels on the brain distribution of P-gp/BCRP substrates may need to be considered further. Kodaira et al.14 reported that this synergy/cooperation could be explained kinetically in terms of transport activities of P-gp and BCRP at the BBB. The information about P-gp and BCRP expression presented here may be helpful to understand the synergistic/cooperative effect of P-gp and BCRP. Further, PET studies in primates and rodents would be valuable to understand species differences in the kinetics of P-gp/BCRP substrates.
There were remarkable species differences in the expression amounts of SLC transporters, including drug and nutrient transporters. Among drug transporters, Oat3 and Oatp1a4/Oatp2 proteins were detected in mouse brain microvessels in our previous study.10 These transporters are involved in blood-to-brain uptake and/or brain-to-blood efflux transport of organic anion compounds, such as homovanillic acid, 6-mercaptopurine (Oat3), and pravastatin (Oatp1a4/Oatp2) in rodents.15-17 However, OAT3 was not detected in monkey brain microvessels, and the protein expression of OATP1A2/OATP-A in monkey brain microvessels was less (0.343-fold) than that of Oatp1a4/oatp2 in mouse brain microvessels (Table 5). This suggests that the BBB permeability of substrates of these transporters might be less in primates than in rodents. Indeed, the concentration of homovanillic acid in brain striatum is higher in humans (3.24–7.20 μg/g brain) than that in mouse (0.31–1.45 μg/g brain),18-20 and the difference in OAT3 expression in brain microvessels is likely to contribute to this. Target peptides for quantifying OAT3 and OATP1C1/OATP-F were selected based on the amino acid sequences of cynomolgus monkey. However, target peptides for quantifying other OATs and OATPs shown in Table 2 were selected based on the amino acid sequences of rhesus monkey because the sequences are unavailable for cynomolgus monkey. Therefore, we cannot exclude the possibility that some proteins were not detected because of unknown differences in amino acid sequence within the target peptide between cynomolgus and rhesus monkeys.
Insufficiency of thyroid hormone, such as T3 and T4, during brain development results in neurological deficits and cognitive impairment.21 In mouse, MCT8 and Oatp1c1/Oatp14 are involved in the supply of thyroid hormones from blood to brain across the BBB.22-24 Monkey brain microvessels contained MCT8, whereas OATP1C1/OATP-F was under the detection limit, so that the level was at least 6.6-fold lower than in mouse (Tables 2 and 5). This difference in expression suggests that transport of thyroid hormones at the BBB is mediated by MCT8 and Oatp1c1/Oatp-f in rodents, and mainly by MCT8 in primates. It was reported that mutation of human MCT8 leads to thyroid hormone insufficiency in the brain and causes Allan–Herndon–Dudley syndrome (AHDS), an X-linked developmental disorder characterized by severe neurological abnormalities.21 In contrast to AHDS patients, only mild neurological abnormalities were observed in MCT8-null mice.21 It is likely that, in mouse, MCT8 deficiency is compensated by Oatp1c1/Oatp-f, resulting in the milder abnormalities.
There were differences between Indonesian and Chinese adult monkey microvessels in the expression levels of several proteins, especially MDR1, MRP4, GLUT3/14, and claudin-5 (Table 3). This difference presumably reflects the differences in the country of origin, drug administration, blood removal, and fasting before brain excision. It was reported that there are genetic differences among different colonies of cynomolgus monkeys from the Philippines, Indonesia, and Vietnam,25 suggesting that the genetic differences between Chinese and Indonesian adult monkeys may be the factor for the differences in the protein expression amount. It was reported that MDR1 protein expression was induced by MDR1 substrates, that is, rifampin and ritonavir in rat brain and isolated bovine brain microvessel endothelial cells, respectively.26,27 Furthermore, in cancer cells, MDR1 protein expression was reported to be induced by various MDR1 substrates including indinavir, which had been administered to one of the Chinese adult monkeys.28 Therefore, MDR1 expression might have been induced in the Chinese adult monkeys because of administration of MDR1 substrates, such as loperamide, paclitaxel, quinidine, diazepam, and indinavir.
The present study also identified species differences in the developmental expression of membrane proteins in brain microvessels between primates and rodents. In rat brain, it was reported that the P-gp (Mdr1a) protein levels were less than 20% from postnatal day 1 to 7 and less than 40% till postnatal day 14 compared with those in mature rats (postnatal days 42–84).2,3 In monkey, lower expression of MDR1 in neonate was not observed (Fig. 2a). Therefore, studies using younger rodents would not be appropriate for predicting the brain distribution of MDR1 substrates in primates, but would result in overestimation. Because a newborn rodent is premature,11 the difference in brain maturation at birth is likely to be one of the causes of the difference in developmental expression of P-gp between monkey and rodent.
Species differences in developmental changes of expression were also observed in nutrient transporters such as GLUT1. The expression level of GLUT1 in brain microvessels was similar in monkeys of all ages (Fig. 2d), whereas it was reported that the expression of GLUT1 in rat brain microvessels increases during development.29 These species differences in developmental expression may reflect differences in brain development between primate and rodent. It was reported that human brain weight increment reached a maximum in late gestation, whereas that in rat was reached a maximum at almost 10 days after birth.30 Therefore, developmental changes of transporters may occur earlier in primates than in rodents.
GLUT3 and MCT1 transport glucose and ketone bodies, respectively,31,32 which are important for brain development, and are being required for synthesis of brain lipids such as phospholipid and cholesterol.33,34 Considering that the maximum weight increment of human brain occurs just before birth,30 the higher expression levels of GLUT3/14 and MCT1 in neonate monkey (Figs. 2e and 2f) are likely to be associated with this prebirth development. In addition, MCT1 expression in the rodent brain is induced during the suckling period and by ketogenic diet.35,36 This induction is likely to function to supply lactate and ketone bodies to the brain as alternative energy sources. Therefore, it is conceivable that the higher expression of MCT1 in neonate monkey (Fig. 2f) represents preparation for supplying ketone bodies derived from milk to the brain. It was reported that GLUT3 was detected in human brain by reverse transcription polymerase chain reaction (RT-PCR), whereas GLUT14 was not detected.37 In addition, fivefold to 10-fold higher expression of GLUT3 was found in human brain microvessels compared with whole brain by means of an immunoblotting study.38 Therefore, it is conceivable that the expression level of GLUT3/14 mainly reflects that of GLUT3.
The expression of BCRP in neonate and child monkeys was significantly lower than that in adult (Fig. 2b). Methotrexate, which is a substrate of BCRP,39 has been used for the treatment of childhood acute lymphoblastic leukemia, but was reported to have an adverse effect on the CNS in methotrexate-treated children with acute lymphoid leukemia.40 The lower expression of BCRP in younger monkeys is consistent with the idea that CNS side effects of methotrexate in children may be due to higher distribution of methotrexate to the brain; however, the link between methotrexate neurotoxicity and its higher brain distribution has not been fully established, and multiple mechanisms such as vascular effects and dihydrofolate reductase interaction may also be involved in the neurotoxicity.40
It was reported that claudin-5 and VE-cadherin play key roles in the formation of tight junctions and adhesive junctions, thereby contributing to the barrier properties of the BBB.41 The higher expression levels of claudin-5 and VE-cadherin in neonate monkey (Fig. 2g and 2h) suggest that the BBB barrier properties may already be mature at birth. This would be consistent with previous reports of adult-like expression of claudin-5 in human brain microvessels during fetal development.42 In rat, it was reported that the brain distribution of [14C]-inulin at birth was 14-fold higher than that in adult, and one reason for this difference related to age was suggested to be immature BBB barrier function in neonate rat, in addition to larger extracellular space in the neonate brain.43 Considering the low expression level of MDR1 in young rodent, it should be noted that studies on drug distribution to the brain in young rodents would not be suitable to predict the human distribution because they would result in overestimation.
In conclusion, the present study has revealed substantial species differences in the quantitative expression amounts and developmental changes of membrane proteins between monkey and mouse brain microvessels, that is, the BBB. Further in vivo functional analysis, such as PET studies, based on this protein expression information should be helpful to understand and predict changes in the brain concentrations of drugs in different species and at different ages of primates.
Acknowledgements
This study was supported in part by a Grant-in-Aid for JSPS Fellows, Grant-Scientific Research (S), and a Global Center of Excellence (COE) Program grant from the Japan Society for the Promotion of Science. This study was also supported in part by the Industrial Technology Research Grant Program from New Energy and the Industrial Technology Development Organization of Japan. We are most grateful to Drs. Yasuyuki Ishii, Masato Chiba, Tomoyuki Ohe, and Kentaro Wakayama, Banyu Pharmaceutical Co., Ltd., for valuable discussions during this research. Tetsuya Terasaki and Sumio Ohtsuki are a full professor and an associate professor of Tohoku University, respectively, and are also directors of Proteomedix Frontiers. This research was not supported by Proteomedix Frontiers, and their position at Proteomedix Frontiers does not present any financial conflicts. The other authors declared no conflict of interest.




